Abstract
Cocaine dependence is characterized by compulsive drug taking and reduced involvement in social, occupational, or recreational activities. Unraveling the diverse mechanisms contributing to the loss-of-interest in these ‘non-drug' pursuits is essential for understanding the neurobiology of addiction and could provide additional targets for treating addiction. The study objectives were to examine changes in cocaine-induced dopamine (DA) overflow in the nucleus accumbens (NAc) over the course of self-administration and determine the roles of α1- and β-adrenergic receptors (AR) in the loss-of-interest in food rewards following the development of an addicted phenotype in male and female rats. Subjects were given access to cocaine and palatable food pellets in a choice self-administration paradigm to identify ‘addicted' cocaine-preferring (CP) individuals and resistant pellet-preferring (PP) individuals based on their patterns of self-administration over 7 weeks. Cocaine-induced DA overflow in the NAc was examined with microdialysis early and late during self-administration (weeks 2 and 7). Subjects were treated in counter-balanced order with propranolol (β-AR antagonist), terazosin (α1-AR antagonist), or vehicle for an additional 3 weeks of self-administration. CP rats displayed increased motivation for cocaine and attenuated motivation for pellets following the development of cocaine preferences. In females, the estrous cycle affected pellet, but not cocaine, self-administration. CP rats displayed attenuated cocaine-induced DA overflow in the NAc. Propranolol enhanced cocaine reinforcement and reduced pellet intake, whereas terazosin enhanced motivation for pellets and reversed preferences in a subset of CP rats. The implications of these results for the treatment of addiction are discussed.
INTRODUCTION
Addiction is characterized by persistent drug-seeking and use despite serious physiological, medical, and social consequences (Chen et al, 2011; Degenhardt and Hall, 2012; Sommers et al, 2006). Neurobiological addiction models primarily focus on reinforcement mechanisms and alterations in mesocorticolimbic pathways (Koob and Volkow, 2010; Volkow et al, 2010). However, an additional feature of addiction is reduced involvement with friends and family, jobs, and recreational activities as consequences of drug use. These secondary consequences may be particularly relevant to perpetuating the addiction cycle, as they involve the loss-of-interest in alternative pursuits/rewards that might otherwise compete with drugs and promote abstinence.
Numerous preclinical studies demonstrate that alternative rewards, including palatable food, social partners, and running wheels, reduce drug use and seeking (Liu and Grigson, 2005; Peterson et al, 2014; Westenbroek et al, 2013). However, it is unclear whether alternative rewards promote resilience, or just unmask the fact that many individuals find drugs less rewarding than these alternatives (Lenoir et al, 2007; Perry et al, 2013). We have developed a paradigm that captures several features consistent with diagnostic criteria for addiction, including escalation of cocaine intake and reduced interest in palatable food rewards that were previously highly preferred (Perry et al, 2013). Thus, we can examine mechanisms contributing to the enhanced reinforcing effects of drugs and reduced involvement in alternative pursuits, and directly test whether reinvigoration of interest in alternative rewards reduces drug use.
The primary objectives of this study were to examine the contributions of dopamine (DA) and norepinephrine (NE) to cocaine preferences in male and female rats, as alterations in these monoamines underlie many aspects of addiction (Baik, 2013; Schmidt and Weinshenker, 2014). Chronic cocaine use is associated with reduced DA neurotransmission in the nucleus accumbens (NAc) and striatum in human and non-human animal models (Calipari et al, 2014; Gerrits et al, 2002; Volkow et al, 1997). Attenuated cocaine-induced DA in the NAc may underlie escalation of intake (Willuhn et al, 2014; Wise et al, 1995), whereas reduced basal DA may contribute to anhedonia and loss-of-interest in alternative rewards. Therefore, we monitored changes in cocaine-induced DA overflow in the NAc across self-administration to determine whether CP rats developed this neurochemical feature of addiction.
Chronic cocaine use also enhances central noradrenergic function (Beveridge et al, 2005; Mash et al, 2005), which may contribute to the loss-of-interest in alternative rewards. Adrenergic receptors (AR) are implicated in a variety of stimulant-induced behaviors (Schmidt and Weinshenker, 2014). α1-AR antagonists reduce motivation for cocaine and cocaine-induced DA overflow in the NAc (Mitrano et al, 2012; Wee et al, 2008). β-AR antagonists also reduce cocaine responding, but potentiate cocaine-induced DA in the NAc (Harris et al, 1996). Activation of α1- or β-AR in several brain regions underlies stimulant-induced hypophagia (Leibowitz, 1975; Levone et al, 2015; Wellman et al, 2002; Wellman, 2000). Therefore, we treated animals with the α1-AR antagonist (terazosin) and non-selective β-AR antagonist (propranolol) to examine effects on cocaine preferences. We predicted that terazosin would reinvigorate interest in the food reward, reduce motivation for cocaine (ostensibly by further attenuating cocaine-induced DA) and that this would shift the preferences of CP rats back to the alternative reward. We predicted that propranolol would also reduce cocaine intake (ostensibly by potentiating DA neurotransmission). However, β-blockade can also increase NE overflow and preferential α1-AR activation (Tuross and Patrick, 1986), which could augment cocaine-induced hypophagia and further exacerbate cocaine preferences.
MATERIALS AND METHODS
Sprague Dawley rats (n=50/sex; Charles Rivers, age 70–80 days upon arrival) were housed in same-sex pairs in standard laboratory cages and maintained on a 14L:10D light-cycle (‘lights-off' at 0800 hours) with ad lib chow and water. Procedures were conducted between 0900 and 1600 hours and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and preapproved by the University of Michigan IACUC.
Animals were fitted with indwelling jugular catheters as previously described (Westenbroek et al, 2013), with some also undergoing stereotactic surgery for microdialysis guide cannula implantation (SciPro; MAB 6.14G) in the left NAc (AP: +1.7 mm and ML: +0.8 mm from Bregma, DV: −6.0 mm from skull)—an obturator extended an additional 2 mm. Rats were single housed post surgery and catheters were flushed daily with bacteriostatic saline containing gentamicin (3 mg/ml) and heparin (20 U/ml). Patency was verified weekly with 0.05–0.1 ml methohexital (7.5 mg/ml). Animals were weighed daily and the estrous cycle was monitored by daily vaginal lavage.
Self-Administration and Estrous Cycle Effects
Self-administration was conducted in standard operant chambers (Med Associates) using a fixed ratio (FR) schedule 4 days a week (FR1 during Week 1 and FR5 thereafter) and a progressive ratio (PR) schedule once a week (Supplementary Figure S1; Perry et al, 2013). On the FR schedule, the house light was illuminated during three 30-min active sessions (pellet-only, cocaine-only, and choice), and was extinguished during two intervening 30-min ‘off' sessions. Reward availability (pellet and/or cocaine) was indicated by illumination of the cue-light inside each nose port, which was inactivated for 40 s after reward delivery. Nose pokes in the active pellet hole resulted in the delivery of one food pellet (F0059, 45 mg, Banana Flavor, BioServ); nose pokes in the active cocaine hole resulted in an infusion (cocaine hydrochloride, 0.4 mg/kg per infusion). Reward delivery in choice sessions inactivated both holes for the 40-second timeout. The sequence of cocaine-only and pellet-only sessions was alternated daily. Cocaine preference was defined as the maintenance of infusion ratios twice as large as pellet ratios for 3 consecutive sessions. PP rats earning ⩽2 infusions/day by the end of self-administration were labeled ‘abstaining' (ABST).
Motivation, as defined by the final completed ratio or breaking point (BP), was examined simultaneously for both rewards using a concurrent PR schedule that independently increased through an exponential series for each reward: 1, 3, 6, 9, 12, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208… (Richardson and Roberts, 1996). The concurrent PR schedule produces comparable results as ‘single-reward' PR schedules (Perry et al, 2013). Ratio completion on each hole resulted in delivery of the associated reward and 5-s inactivation of that hole. Failure to complete the current ratio within 60 min resulted in inactivation of that hole for the remainder of the test, which lasted 6 h or until both holes were inactivated (whichever occurred first).
Numbers of infusions and pellets earned during cocaine-only and pellet-only sessions were aligned to the proestrous/estrous (PE) stage for each female for each week. P and E stages were combined because most females only displayed one or the other per cycle, which is typical for this phase of the light cycle (Becker, 2005). In the few estrous cycles with both, data were aligned to P and E was designated +1. Body weight change from the previous day was also aligned to estrous cycles. Mean were obtained for the last 5 weeks of self-administration (Weeks 1–2 omitted due to acquisition-related variable responding).
Microdialysis
Microdialysis probes (SciPro; MAB 6.14.2) were inserted and flushed with Ringer's (1.5 μl/min, 60 min) in microdialysis chambers (Med Associates, PHM-125B) containing food and water. Afterwards, the inlet/outlet tubing was disconnected while probes remained in the guide protected by custom-built stainless-steel caps, and animals were returned to their home cages.
Inlet/outlet tubing was reconnected ~24 h later (half before, half after self-administration). Six 10-min baseline samples were collected at least 45 min post reconnection. Subjects received a non-contingent cocaine infusion (1.2 mg/kg, i.v.) and six additional 10-min samples (C1–C6) were collected. Microdialysis was conducted over 4 consecutive days during Weeks 2 and 7 to examine changes over time (Supplementary Figure S1B). Raw values for each day were converted to percent baseline (mean of three last baselines). Cocaine-induced DA overflow was consistent across days and unaffected by the estrous cycle (Supplementary Figure S3). Therefore, means of the multiple days were used for analyses.
Dialysate DA content was determined by HPLC with electrochemical detection (C-18 column, ESA Biosciences, HR-80X3.2, 3 μm particle size, 80 mm length) with mobile phase (1.4 mM octanesulfonic acid and 20% methanol, pH 4.6–4.8) pumped at 0.7 ml/min at 27ºC. Potentials (−75/+100 mV) were applied to a dual coulometric analytical cell (ESA model #5014B) connected to a Coulochem II/III detector, with the latter potential used to determine [DA] against a 1-pg standard. Probe placement was examined in horizontal sections. Only data from animals with track marks within the NAc were included (Supplementary Figure S4).
Pharmacological Manipulations
After 7 weeks, animals were treated in counter-balanced order with the non-selective β-AR antagonist ((S)-(−)-propranolol, 10 mg/kg, i.v., Tocris Bioscience), the α1-AR antagonist (terazosin, 2 mg/kg, i.v., Tocris Bioscience), or vehicle (1 ml/kg saline, i.v.). Doses were chosen for being well-below lethal thresholds and biologically active (Alonso-Galicia et al, 1996; Kyncl, 1986). Treatments were administered 15 min before self-administration for 5 consecutive days (Supplementary Figure S1C). Subjects shifting preferences during treatment received vehicle the next week to determine whether it was permanent. In such cases, treatments were repeated and only data from the final period were used.
Statistics
Analyses were performed with SPSS (IBM, v21.0). Q–Q plots, Shapiro–Wilk, and Levene's tests were used to examine data for normality and homoscedasticity. Box–Cox power transformations identified optimal transformations (Supplementary Table S1). Thirty-one rats did not complete the 7 weeks of self-administration due to patency loss or complications related to the craniotomy surgery/microdialysis.
Cocaine and pellet BP were analyzed by repeated-measures ANOVA with between-subject (preference and sex) and within-subject (week) variables. General changes in behavior across three time blocks (Week 1, Weeks 2–6, and Week 7) were examined with pre-planned contrasts. Fisher's Exact tests were used to analyze whether the proportion of CP females was greater than males after Week 7 and during the final week of vehicle. Raw data aligned to the estrous cycle were analyzed with repeated measures ANOVA with between-subject (preference) and within-subject (stage) variables.
Microdialysis data were analyzed by repeated-measures ANOVA with between-subject (preference and sex) and within-subject (time, early vs late, and sample, C1–C6) variables. Pair-wise comparisons were only made between preferences within a time period, or between time periods for each preference using Fisher's LSD test.
Pharmacological manipulations were analyzed with repeated-measures ANOVA with between-subject (preference) and within-subject (treatment) variables. Pair-wise comparisons were only made against the vehicle treatment within each preference, and between all three preferences within each treatment using Fisher's LSD test. Choice session data were analyzed with Friedman's test followed by Wilcoxon signed-rank tests to identify treatment effects within preferences, and with Kruskal–Wallis tests to identify preference effects within treatments. Preference shifts during treatments were examined with McNemar's test.
To simplify analyses, FR data were summed each week to collapse 4 consecutive days into single weekly measures. Repeated-measures ANOVA were conducted with sphericity assumed modeling, and Greenhouse–Geisser and Huynh–Feldt adjustments were applied as appropriate. Results were considered significant at p<0.05 for all analyses.
RESULTS
Self-Administration, Sex Differences, and Estrous Cycle
Self-administration data on the FR schedule (Supplementary Figure S2) were consistent with our previous report (Perry et al, 2013). By Week 7, 42% of females (13/31) and 26% of males (10/38) had developed cocaine preferences. Additional 6 animals met CP criteria during the final vehicle week, resulting in 58% CP females (18/31) and 29% CP males (11/38). While the proportion of CP females was greater than males at both Week 7 and the final vehicle week, only the latter reached statistical significance (p=0.027 by Fisher's Exact test).
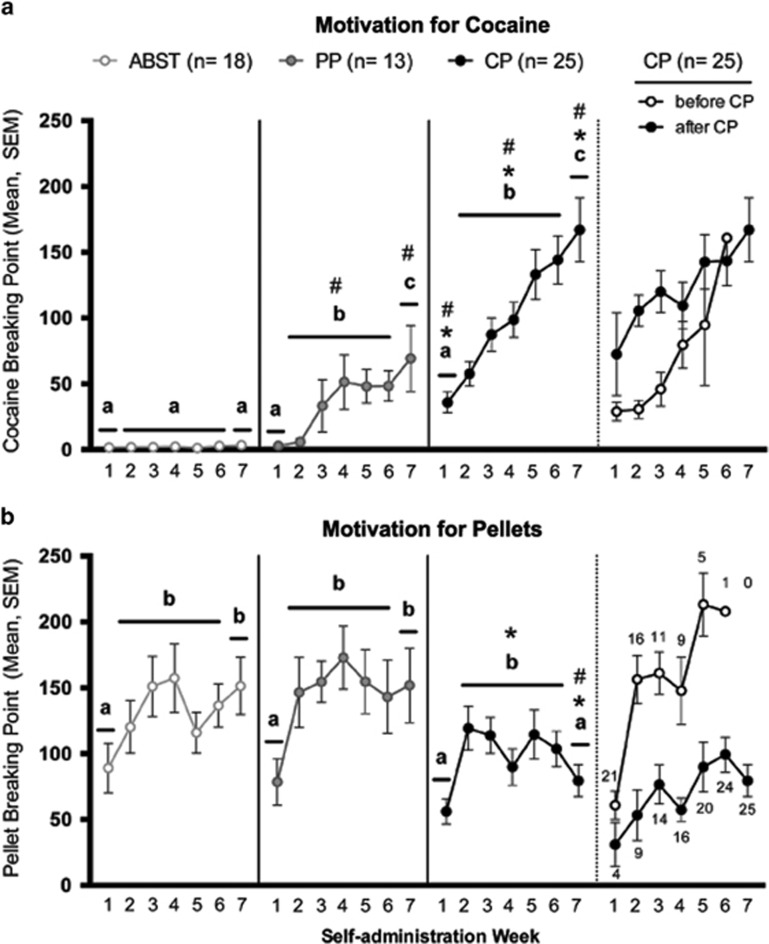
Cocaine motivation (Figure 1a) was affected by preference (F2,50=84.43, p<0.001), week (F6,300=19.55, p<0.001), and week × preference interaction (F12,300=5.15, p<0.001). ABST rats showed consistently low cocaine BP that did not change over time. In contrast, cocaine motivation increased in PP and CP rats, such that motivation in Weeks 2–6 was greater than Week 1 (p<0.001 for both), and motivation in Week 7 was greater than Week 1 (p<0.001 for both) and Weeks 2–6 (p=0.019 and p<0.001, respectively). CP rats always maintained higher cocaine BP than PP and ABST rats (p<0.001 for all), whereas cocaine BP of PP rats were only greater than ABST rats during Weeks 2–6 and Week 7 (p<0.001 for both).
Figure 1.
Changes in motivation for cocaine and food pellets over the course of self-administration. (a) Cocaine BP. (b) Pellet BP. Time blocks that do not share a letter are different from one another within a give preference group (p<0.05). The far right section in each panel represents the same data from the 25 CP rats segregated by whether individuals were still ‘pellet preferring' (open circles, ‘before CP') or were displaying cocaine preferences (closed circles, ‘after CP') at each time. The numbers associated with each point represent the number of CP individuals in each subgroup in each given week. Significant difference from PP (*p<0.05) and ABST rats (#p<0.05). Vertical lines represent SEM. ABST, abstaining; BP, breaking point; CP, cocaine preferring.
Pellet motivation (Figure 1b) was affected by preference (F2,50=3.82, p=0.029) and week (F6,300=7.68, p<0.001). ABST and PP rats showed an asymptotic increase in pellet BP, such that Weeks 2–6 (p⩽0.003 for each) and Week 7 (p⩽0.002 for each) were greater than Week 1, but not different from each other. CP rats also showed increased pellet BP from Week 1 to Weeks 2–6 (p<0.001); however, by Week 7 their motivation was lower than Weeks 2–6 (p=0.047) and not different from Week 1. Pellet BP were similar among all preference groups during Week 1, whereas CP rats had lower pellet BP than PP rats during Weeks 2–6 (p=0.018) and both ABST and PP rats during Week 7 (p=0.005 and p=0.012, respectively).
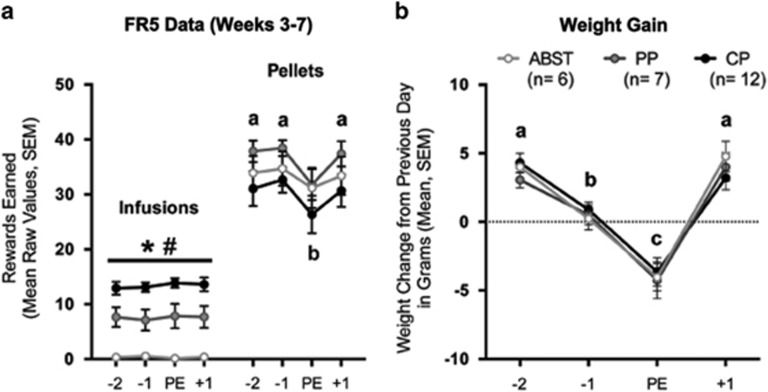
There were no effects of sex or interactions involving sex and any factor on FR or PR measures. When FR data were aligned to the estrous cycle (Figure 2), there was a robust stage effect on pellet intake (F3,66=21.16, p<0.001), but not infusions. Females earned fewer pellets during PE compared with the other stages (p<0.001 for each), which were not different. There were no effects of preference and no interactions between preference and stage on pellet intake, whereas preference affected infusions (F2,22=27.19, p<0.001), with ABST, PP, and CP rats all displaying different levels of intake (p<0.003 for each). The same results were obtained with ranked data (Supplementary Figure S5).
Figure 2.
The estrous cycle affected pellet intake and weight gain, but not cocaine intake. (a) The number of pellets earned in the pellet-only sessions was affected by the estrous cycle, whereas there was no effect of preference. In contrast, infusion number was affected by preference, but not the estrous cycle. The lack of a preference effect on pellet intake likely reflects averaging across multiple weeks, during which not all CP females were displaying cocaine preferences. The same effects were observed for intake during choice sessions (data not shown). (b) Weight gain was robustly affected by the estrous cycle in all preference groups, which confirms that females continued to cycle normally throughout self-administration. Therefore, the lack of estrous cycle effects on cocaine intake cannot be attributed to alterations in hormone fluctuations across the cycle (at least in regard to those affecting weight and food intake). Data were averaged across the last 5 weeks of self-administration and aligned to the proestrous/estrous (PE) stage of the cycle. The two days before PE (−2 and −1) correspond with diestrus I and diestrus II, whereas the day after PE (+1) corresponds with metestrus or sometimes estrus (see text for details). Stages without common letters are significantly different from one another. Intake in CP rats was significantly different from PP and ABST rats (*p<0.05 for each). Intake in PP rats was significantly different from CP and ABST rats (#p<0.05 for each). Vertical lines represent SEM. ABST, abstaining; CP, cocaine preferring.
The estrous cycle influenced weight gain (F3,66=38.60, p<0.001), whereas there was no effect of preference or interaction between preference and stage (Figure 2b). Weight gain leveled off the day before PE, with females losing even more weight in PE, such that these stages were significantly different from all others (p<0.001 for all).
Microdialysis
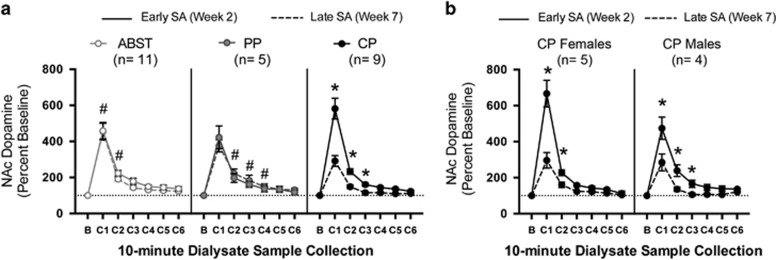
Analysis of cocaine-induced DA overflow in the NAc revealed main effects of time (F1,19=5.40, p=0.031) and sample (F5,95=194.62, p<0.001), time × sample interaction (F5,95=4.91, p=0.032) and time × sample × preference interaction (F10,95=4.05, p=0.027). The main effect of sex and all interactions involving sex were not significant.
Early during self-administration, there were no differences in cocaine-induced DA between preference groups (Figure 3). Late during self-administration, cocaine-induced DA was attenuated in CP rats compared with their earlier response (C1: p<0.001, C2: p=0.001, C3: p=0.042) and the late responses of ABST (C1: p=0.007, C2: p=0.021) and PP rats (C2: p=0.002, C3: p=0.002, C4: p=0.011). There were no differences between early and late DA responses in ABST or PP rats, and the late responses of ABST and PP rats were not different.
Figure 3.
Changes in cocaine-induced DA overflow in the NAc over self-administration. (a) Rats received a single non-contingent cocaine infusion (1.2 mg/kg, i.v.) early (Week 2, solid lines) and again late during self-administration (Week 7, dashed lines). Cocaine-induced DA overflow was significantly attenuated in CP rats during Week 7 compared with their earlier response (*p<0.05) and the late responses of ABST and PP rats (#p<0.05 compared with late CP). Data are repeated measures from 11 ABST rats (5 female, 6 male, white circles), 5 PP rats (2 female, 3 male, gray circles) and 9 CP rats (5 female, 4 male, black circles). Basal DA values in pg per 10 min sample were not different between groups at either time point: ABST rats (early: 7.2±1.9, late: 11.4±2.7), PP rats (early: 6.3±1.6, late: 4.4±1.3), and CP rats (early: 9.3±1.2, late: 11.9±3.2). (b) Both CP males and females displayed attenuated cocaine-induced DA overflow during Week 7 compared with Week 2 (*p<0.05). Vertical lines represent SEM. ABST, abstaining; CP, cocaine preferring; DA, dopamine; NAc, nucleus accumbens; PP, pellet preferring.
Pharmacological Manipulations
FR5 schedule
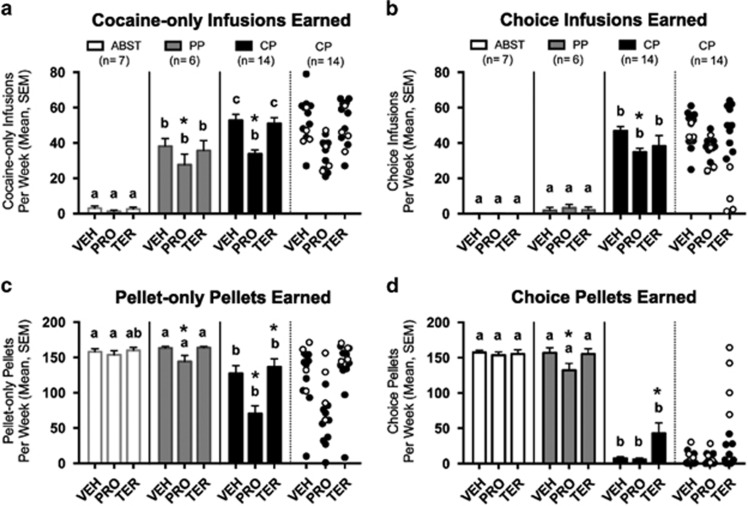
Cocaine-only session infusions (Figure 4a) were affected by preference (F2,24=82.68, p<0.001), treatment (F2,48=26.94, p<0.001), and preference × treatment interaction (F4,48=4.59, p=0.003). Propranolol reduced cocaine intake in CP and PP rats (p⩽0.004 for each). Compared with their intake during vehicle, propranolol reduced infusion number similarly in PP and CP rats (32±9.8% and 35±3.7%, respectively). PP and CP rats earned more infusions than ABST rats during all treatments (p<0.001 for all), whereas CP rats only earned more infusions than PP rats during vehicle (p=0.015) and terazosin (p=0.012).
Figure 4.
Effects of propranolol (PRO) and terazosin (TER) on cocaine and pellet intake. (a) Cocaine-only sessions: PRO reduced cocaine infusions in both PP and CP rats. (b) Choice session infusions: PRO reduced cocaine infusions in CP rats. The intake of the four CP rats most affected by TER (n=4, white circles in far right section of each panel) was substantially lower than their intake during VEH and PRO treatments, which is not apparent in the overall CP group mean and SEM. (c) Pellet-only sessions: PRO reduced pellet intake in PP and CP rats, whereas TER increased pellet intake in CP rats. (d) Choice session pellets: PRO reduced pellet intake in PP rats, whereas TER increased pellet intake in CP rats, which was largely due to the four CP individuals that reverted to pellet preferences during TER. For all panels, preference groups without common letters are significantly different from one another within a given treatment (p<0.05). Treatment differs from vehicle (VEH) condition within a given preference group (*p<0.05). Vertical lines represent SEM. Comparisons were not made between preference groups with different treatments. ABST, abstaining; CP, cocaine preferring; PP, pellet preferring.
Choice session infusions (Figure 4b) were also affected by preference (VEH: H(2)=20.66, p<0.001; PRO: H(2)=20.69, p<0.001; TER: H(2)=19.76, p<0.001), with a treatment effect in CP rats (k=3, F=13.13, p=0.001). Propranolol reduced cocaine intake in CP rats (p<0.001), reflecting a 24±4.4% decrease from vehicle. CP rats earned more infusions than ABST and PP rats during all treatments (p⩽0.002 for each), whereas ABST and PP rats were not different. There were no effects of terazosin on infusion number in any group.
Pellet-only session pellets (Figure 4c) were affected by preference (F2,24=10.01, p=0.001), treatment (F2,48=32.38, p<0.001), and preference × treatment interaction (F4,48=7.25, p<0.001). Propranolol reduced pellet intake in PP and CP rats (p=0.004 and p<0.001, respectively), with significantly greater reduction in CP rats (49±6.3%) compared with PP (12±5.1%, p=0.012) and ABST rats (3±2.9%, p<0.001) (preference effect: H(2)=16.33, p<0.001). Terazosin increased pellet intake in CP rats (p=0.011). CP rats earned fewer pellets than ABST and PP during vehicle (p⩽0.023 for each) and propranolol (p<0.001 for each), and fewer than PP rats during terazosin (p=0.03).
Choice session pellets (Figure 4d) were also affected by preference (VEH: H(2)=19.80, p<0.001; PRO: H(2)=20.01, p<0.001; TER: H(2)=15.11, p=0.001), with significant treatment effects in PP and CP rats (k=3: F=6.87, p=0.032; F=19.60, p<0.001, respectively). Propranolol reduced pellet intake in PP rats (p=0.014), reflecting a 15±6.5% reduction. Terazosin increased pellet intake in CP rats (p=0.002), which was largely due to preference shifts in ~30% of CP individuals. Shifts were apparent on the first day and lasted 1–4 days (4 days: two rats, 3 days: one rat, 1 day: one rat; Supplementary Figure S6). Two additional CP rats showed no preference (NP) for 1 day, such that terazosin significantly shifted preferences compared with vehicle (CP reverting to NP/PP, McNemar's test=4.17, p=0.031). All six shifting rats were female, whereas none of the five CP males shifted preferences. As a group, CP rats earned fewer pellets than ABST and PP rats in choice sessions during all treatments (p⩽0.002 for each).
PR schedule
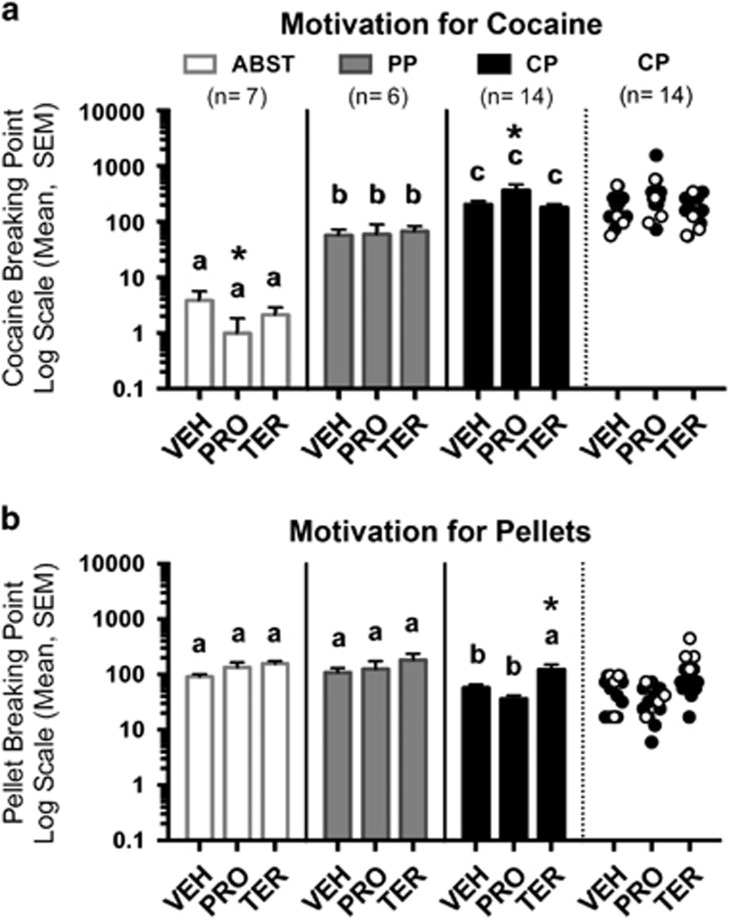
Cocaine BP (Figure 5a) were affected by preference (F2,24=86.38, p<0.001) and preference × treatment interaction (F4,48=7.95, p<0.001). Pellet BP (Figure 5b) were also affected by preference (F2,24=7.81, p=0.002) and treatment (F2,48=8.87, p=0.001). Propranolol increased cocaine motivation in CP rats (p=0.004) and reduced it in ABST rats (p=0.018). Terazosin increased pellet motivation in all groups compared with vehicle (p=0.002). Cocaine motivation was different between all three preferences under all treatments (p⩽0.003 for each), whereas CP rats had reduced pellet motivation compared with ABST and PP rats during vehicle (p=0.048 and p=0.027, respectively) and propranolol (p⩽0.005 for each).
Figure 5.
Effects of propranolol (PRO) and terazosin (TER) on motivation for cocaine and pellets. (a) PRO increased cocaine BP in CP rats and reduced them in ABST rats. (b) TER increases pellet BP in all groups; however, pair-wise comparisons were only significant in CP rats, which most likely reflects a power issue, as effect sizes were all relatively large (Cohen's d=1.84, 0.78 and 0.83 for ABST, PP, and CP, respectively). The far right section of each panel shows the data for the CP rats that shifted to pellet preferences during TER treatment (n=4, white circles), which shows that pellet BP were generally shifted upwards in all CP rats, not just the four most affected individuals. Preference groups without common letters are significantly different from one another within a given treatment (p<0.05). Treatment differs from vehicle (VEH) condition within given preference group (*p<0.05). Vertical lines represent SEM. Comparisons were not made between preference groups with different treatments. ABST, abstaining; CP, cocaine preferring; PP, pellet preferring.
DISCUSSION
Our results demonstrate that even though more female rats than males displayed a cocaine preference, both male and female CP rats exhibited attenuated cocaine-induced DA overflow in the NAc after the cocaine preference developed. Furthermore, we demonstrated differential effects of α1- and β-AR antagonists on intake and motivation for drug and food rewards that suggest altered NE neurotransmission in CP rats.
Our finding that pellet intake and weight, but not cocaine intake, vary with the estrous cycle confirms our previous finding (Perry et al, 2013) and is consistent with results from another choice procedure (Kerstetter et al, 2012). Previous work has found that estradiol enhances acquisition of cocaine self-administration as well as cocaine intake and acute motivation (refer to Becker et al (2012) for review). This phenomena is similar to the effects of estradiol on maternal behavior, where estradiol priming enhances initial acquisition and performance, but the cues from the pups maintain the behavior once established (Numan, 2006).
The repeated PR data extended our previous findings in which motivation was only examined twice during self-administration (Perry et al, 2013). We now demonstrate that pellet motivation increases over time in all groups and is selectively attenuated in CP rats once cocaine preferences develop. In addition, the ‘before' and ‘after' data show that the patterns of CP group means (ie, ‘escalating' cocaine BP, ‘declining' pellet BP) primarily reflect the cumulative transition of CP rats to their final preference designation and not changes within individuals after shifting.
The attenuated cocaine-induced DA overflow in the NAc of CP rats is consistent with human imaging studies that also demonstrate reduced striatal DA neurotransmission in cocaine addicts (Martinez et al, 2009; Volkow et al, 1997). Individuals with the most attenuated amphetamine-induced DA were more likely to choose cocaine over alternative monetary rewards (Martinez et al, 2007), which is strikingly consistent with the neurochemistry and choice behavior of CP rats. While others have reported reduced DA responses following cocaine self-administration (Ferris et al, 2011; Gerrits et al, 2002), the longitudinal nature of our experiment revealed that the attenuation occurred after cocaine preferences developed, which may contribute to the increased cocaine intake of CP rats (Guillem et al, 2013; Lau and Sun, 2002; Wise et al, 1995). Our data from PP rats suggest that cocaine exposure itself does not attenuate cocaine-induced DA in the NAc, which is consistent with other studies linking escalation (but not intake) to changes in DA neurotransmission (Willuhn et al, 2014). Sex differences and hormone effects on cocaine-induced DA in the NAc and striatum (the latter of which has received more attention) are more pronounced in gonadectomized animals (refer to Becker et al (2012) for review). The lack of sex differences on cocaine-induced DA in the NAc of our gonad-intact rats is consistent with another study (Holly et al, 2012). However, the use of additional cocaine doses might have revealed more subtle sex differences and/or estrous cycle effects.
Propranolol reduced infusions on the FR5 schedule and increased cocaine BP on the PR schedule—both consistent with enhanced cocaine-induced DA overflow in the NAc. Increased DA would lead to longer inter-infusion intervals and hence fewer infusions (FR5 schedule), whereas it would lead to longer occupation of the ‘compulsion zone' and increased likelihood of completing the current ratio (PR schedule). (Goldberg and Gonzalez, 1976; Harris et al, 1996; Norman and Tsibulsky, 2006; Willuhn et al, 2014; Wise et al, 1995). Propranolol also reduced pellet intake on the FR5 schedule in PP and CP rats with negligible effects on motivation, which is consistent with another study ((Harris et al, 1996), but see the study by Goldberg and Gonzalez (1976)), suggesting greater satiety/anorexia—potentially via enhanced NE overflow and subsequent α1-AR activation in (hypothalamic) feeding circuits (Levone et al, 2015; Tuross and Patrick, 1986; Wellman et al, 2002). In this regard, the greater propranolol-induced hypophagia in CP rats may reflect more pronounced alterations in NE neurotransmission. Therefore, future studies should examine changes in cocaine-induced NE and α1- and β-AR and NE transporter (NET) expression. Increased NET expression may be particularly relevant to the CP phenotype, as NET is upregulated in monkeys self-administering cocaine (Beveridge et al, 2005) and human cocaine users (Mash et al, 2005).
Terazosin had no direct effects on cocaine self-administration. Others have shown that α1-AR antagonists reduce cocaine-induced hyper-locomotion, hypophagia, and reinstatement in rats (Mitrano et al, 2012; Wellman et al, 2002; Zhang and Kosten, 2005) and subjective effects in humans (Newton et al, 2012), with marginal effects on intake (Wee et al, 2008; Woolverton, 1987). While terazosin has limited blood–brain barrier (BBB) penetration, high peripheral doses recapitulate effects of lower central doses (Stone et al, 1999), suggesting some may enter the brain. Alternatively, terazosin may affect pellet intake/motivation via peripheral actions or through brain regions outside the BBB (Fry et al, 2007). This may explain the lack of direct effects on cocaine measures, which presumably requires central α1-AR blockade (Mitrano et al, 2012). Therefore, additional work is required to determine terazosin's site/mechanism of action, which largely blocked cocaine's acute anorectic effects in CP rats (Supplementary Figure S7), increased motivation for the alternative food reward, and acutely reversed preferences in a small number of (female) CP rats. Thus, α1-AR antagonists may shift the subjective balance between drug and non-drug rewards and serve as effective adjuncts for interventions targeting cocaine's reinforcing effects (Newton et al, 2012).
Despite the fact that women cocaine users are equally likely to become dependent as men (Cotto et al, 2010) and addiction is associated with higher rates of psychopathologies in women (Chen et al, 2011), the bulk of knowledge regarding drug abuse is based on studies in males. Our results are consistent with greater vulnerability in females, as they were more likely to develop cocaine preferences. However, our use of preferences and motivation to identify an addiction severity spectrum (Supplementary Figure S8; Supplementary Table S2) suggests more equivalent rates between the sexes. Nevertheless, similar behavior does not necessarily mean that the underlying neurobiology is identical in males and females. Here we see similar patterns of changes in NAc DA in CP males and females, but potentially different responses to the α1-AR antagonist.
In conclusion, our results indicate roles for both DA and NE in the pathology of addiction. Furthermore, more complex behavioral paradigms are required to capture aspects of addiction beyond reinforcement—particularly those impacting mental and physical health, and quality of life, which are often worse in addicts and can interfere with treatment retention and long-term abstinence (Chen et al, 2011; Degenhardt and Hall, 2012; Ersche et al, 2013; Morales-Manrique et al, 2011). Our results with the α1-AR antagonist directly address the nutritional/metabolic consequences of addiction (Ersche et al, 2013), and provide a framework for examining how derogation of other natural rewards influences addiction and recovery.
FUNDING AND DISCLOSURE
Research was supported by funding from NIDA (DA012677 to JBB, DA032856 to JBB and ANP, and support to ANP from T32 DA007268). Cocaine hydrochloride was provided by the NIDA Drug Supply Program (the Chemistry & Physiological Systems Research Branch, Division of Basic Neuroscience & Behavioral Research, the National Institute on Drug Abuse, NIH). The authors declare no conflict of interest.
Acknowledgments
We would like to thank Brandon Luma for technical assistance, Melissa Luma Dunn for administrative assistance, and Dr Bruce Cushing for editorial comments.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Alonso-Galicia M, Brands MW, Zappe DH, Hall JE (1996). Hypertension in obese Zucker rats. Role of angiotensin II and adrenergic activity. Hypertension 28: 1047–1054. [DOI] [PubMed] [Google Scholar]
- Baik J-H (2013). Dopamine signaling in reward-related behaviors. Front Neural Circuits 7: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB (2005). Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146: 1650–1673. [DOI] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C (2012). Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Diff 3: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJR, Smith HR, Nader MA, Porrino LJ (2005). Effects of chronic cocaine self-administration on norepinephrine transporters in the nonhuman primate brain. Psychopharmacology 180: 781–788. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Jones SR (2014). Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J Neurochem 128: 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KW, Banducci AN, Guller L, Macatee RJ, Lavelle A, Daughters SB et al (2011). An examination of psychiatric comorbidities as a function of gender and substance type within an inpatient substance use treatment program. Drug Alcohol Depend 118: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotto JH, Davis E, Dowling GJ, Elcano JC, Staton AB, Weiss SR (2010). Gender effects on drug use, abuse, and dependence: a special analysis of results from the national survey on drug use and health. Gend Med 7: 402–413. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W (2012). Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet 379: 55–70. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Stochl J, Woodward JM, Fletcher PC (2013). The skinny on cocaine: insights into eating behavior and body weight in cocaine-dependent men. Appetite 71: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Mateo Y, Roberts DCS, Jones SR (2011). Cocaine-insensitive dopamine transporters with intact substrate transport produced by self-administration. Biol Psychiatry 69: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry M, Hoyda TD, Ferguson AV (2007). Making sense of it: roles of the sensory circumventricular organs in feeding and regulation of energy homeostasis. Exp Biol Med (Maywood) 232: 14–26. [PubMed] [Google Scholar]
- Gerrits MAFM, Petromilli P, Westenberg HGM, Di Chiara G, van Ree JM (2002). Decrease in basal dopamine levels in the nucleus accumbens shell during daily drug-seeking behaviour in rats. Brain Res 924: 141–150. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Gonzalez FA (1976). Effects of propranolol on behavior maintained under fixed-ratio schedules of cocaine injection or food presentation in squirrel monkeys. J Pharmacol Exp Ther 198: 626–634. [PubMed] [Google Scholar]
- Guillem K, Ahmed SH, Peoples LL (2013). Escalation of cocaine intake and incubation of cocaine seeking are correlated with dissociable neuronal processes in different accumbens subregions. Biol Psychiatry 76: 31–39. [DOI] [PubMed] [Google Scholar]
- Harris GC, Hedaya MA, Pan WJ, Kalivas P (1996). beta-adrenergic antagonism alters the behavioral and neurochemical responses to cocaine. Neuropsychopharmacology 14: 195–204. [DOI] [PubMed] [Google Scholar]
- Holly EN, Shimamoto A, Debold JF, Miczek KA (2012). Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats. Psychopharmacology 224: 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE (2012). Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology 37: 2605–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyncl JJ (1986). Pharmacology of terazosin. Am J Med 80: 12–19. [DOI] [PubMed] [Google Scholar]
- Lau CE, Sun L (2002). The pharmacokinetic determinants of the frequency and pattern of intravenous cocaine self-administration in rats by pharmacokinetic modeling. Drug Metab Dispos 30: 254–261. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF (1975). Amphetamine: possible site and mode of action for producing anorexia in the rat. Brain Res 84: 160–167. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH (2007). Intense sweetness surpasses cocaine reward. PLoS One 2: e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levone BR, Cella EC, Kochenborger L, da Silva ES, Taschetto APD, Mansur SS et al (2015). Ingestive and locomotor behaviours induced by pharmacological manipulation of α-adrenoceptors into the median raphe nucleus. Neuropharmacology 89: 136–145. [DOI] [PubMed] [Google Scholar]
- Liu C, Grigson PS (2005). Brief access to sweets protect against relapse to cocaine-seeking. Brain Res 1049: 128–131. [DOI] [PubMed] [Google Scholar]
- Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R et al (2009). Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am J Psychiatry 166: 1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang D-R, Broft A et al (2007). Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry 164: 622–629. [DOI] [PubMed] [Google Scholar]
- Mash DC, Ouyang Q, Qin Y, Pablo J (2005). Norepinephrine transporter immunoblotting and radioligand binding in cocaine abusers. J Neurosci Methods 143: 79–85. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Schroeder JP, Smith Y, Cortright JJ, Bubula N, Vezina P et al (2012). Alpha-1 adrenergic receptors are localized on presynaptic elements in the nucleus accumbens and regulate mesolimbic dopamine transmission. Neuropsychopharmacology 37: 2161–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Manrique CC, Palepu A, Castellano-Gomez M, Aleixandre-Benavent R, Comunidad Valenciana CG, Valderrama-Zurián JC (2011). Quality of life, needs, and interest among cocaine users: differences by cocaine use intensity and lifetime severity of addiction to cocaine. Subst Use Misuse 46: 390–397. [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, Brown G, Kosten TR, Mahoney JJ, Haile CN (2012). Noradrenergic α1 receptor antagonist treatment attenuates positive subjective effects of cocaine in humans: a randomized trial. PLoS One 7: e30854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AB, Tsibulsky VL (2006). The compulsion zone: a pharmacological theory of acquired cocaine self-administration. Brain Res 1116: 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M (2006). Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol 49: 12–21. [DOI] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB (2013). The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PLoS One 8: e79465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AB, Hivick DP, Lynch WJ (2014). Dose-dependent effectiveness of wheel running to attenuate cocaine-seeking: impact of sex and estrous cycle in rats. Psychopharmacology 231: 2661–2670. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11. [DOI] [PubMed] [Google Scholar]
- Schmidt KT, Weinshenker D (2014). Adrenaline rush: the role of adrenergic receptors in stimulant-induced behaviors. Mol Pharmacol 85: 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers I, Baskin D, Baskin-Sommers A (2006). Methamphetamine use among young adults: health and social consequences. Addict Behav 31: 1469–1476. [DOI] [PubMed] [Google Scholar]
- Stone EA, Zhang Y, Rosengarten H, Yeretsian J, Quartermain D (1999). Brain alpha 1-adrenergic neurotransmission is necessary for behavioral activation to environmental change in mice. Neuroscience 94: 1245–1252. [DOI] [PubMed] [Google Scholar]
- Tuross N, Patrick RL (1986). Effects of propranolol on catecholamine synthesis and uptake in the central nervous system of the rat. J Pharmacol Exp Ther 237: 739–745. [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F, Baler R (2010). Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. Bioessays 32: 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R et al (1997). Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386: 830–833. [DOI] [PubMed] [Google Scholar]
- Wee S, Mandyam CD, Lekic DM, Koob GF (2008). α1-Noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol 18: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman P, Ho D, Cepeda-Benito A, Bellinger L, Nation J (2002). Cocaine-induced hypophagia and hyperlocomotion in rats are attenuated by prazosin. Eur J Pharmacol 455: 117–126. [DOI] [PubMed] [Google Scholar]
- Wellman PJ (2000). Norepinephrine and the control of food intake. Nutrition 16: 837–842. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Perry AN, Becker JB (2013). Pair housing differentially affects motivation to self-administer cocaine in male and female rats. Behav Brain Res 252: 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Groblewski PA, Phillips PEM (2014). Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nature Neurosci 17: 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB (1995). Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology 120: 10–20. [DOI] [PubMed] [Google Scholar]
- Woolverton WL (1987). Evaluation of the role of norepinephrine in the reinforcing effects of psychomotor stimulants in rhesus monkeys. Pharmacol Biochem Behav 26: 835–839. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA (2005). Prazosin, an α-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biol Psychiatry 57: 1202–1204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.