Abstract
The amplitude of low-frequency fluctuations (ALFF) in the blood oxygenation level-dependent (BOLD) signal during resting-state fMRI reflects the magnitude of local low-frequency BOLD oscillations, rather than interregional connectivity. ALFF is of interest to studies of cognition because fluctuations in spontaneous intrinsic brain activity relate to, and possibly even constrain, task-evoked brain responses in healthy people. Lower ALFF has been reported in schizophrenia, but the cognitive correlates of these reductions remain unknown. Here, we assess relationships between ALFF and attention and working memory in order to establish the functional relevance of intrinsic BOLD oscillatory power alterations with respect to specific cognitive impairments in schizophrenia. As part of the multisite FBIRN study, resting-state fMRI data were collected from schizophrenia subjects (SZ; n=168) and healthy controls (HC; n=166). Voxelwise fractional ALFF (fALFF), a normalized ALFF measure, was regressed on neuropsychological measures of sustained attention and working memory in SZ and HC to identify regions showing either common slopes across groups or slope differences between groups (all findings p<0.01 height, p<0.05 family-wise error cluster corrected). Poorer sustained attention was associated with smaller fALFF in the left superior frontal cortex and bilateral temporoparietal junction in both groups, with additional relationships in bilateral posterior parietal, posterior cingulate, dorsal anterior cingulate (ACC), and right dorsolateral prefrontal cortex (DLPFC) evident only in SZ. Poorer working memory was associated with smaller fALFF in bilateral ACC/mPFC, DLPFC, and posterior parietal cortex in both groups. Our findings indicate that smaller amplitudes of low-frequency BOLD oscillations during rest, measured by fALFF, were significantly associated with poorer cognitive performance, sometimes similarly in both groups and sometimes only in SZ, in regions known to subserve sustained attention and working memory. Taken together, these data suggest that the magnitude of resting-state BOLD oscillations shows promise as a biomarker of cognitive function in health and disease.
INTRODUCTION
Functional magnetic resonance imaging (fMRI) can measure blood oxygenation level-dependent (BOLD) signal oscillations during resting (ie, task-free) states. These BOLD signals are believed to reflect intrinsic neural oscillations important for modulating cortical excitability (Raichle, 2011). Though hemodynamically generated, spontaneous low-frequency BOLD signal fluctuations show strong covariation with intracranial electrocorticographic and local field potential recordings, both for slow oscillations in the raw electrophysiological signals and for slow fluctuations in high-frequency (ie, gamma-band) power (He et al, 2008; Leopold et al, 2003). Correlations of spontaneous fluctuations of infraslow range (0.01–0.1 Hz) scalp potentials with resting BOLD signal fluctuations are spatially constrained within functional connectivity networks (Hiltunen et al, 2014), suggesting a role for infraslow fluctuations in functional brain organization (Raichle, 2011).
To date, resting-state BOLD low-frequency oscillations (LFO) have primarily been studied via network-level analyses that examine intrinsic functional connectivity between distal regions via intercorrelations of time series data (Biswal et al, 1995; Raichle, 2011). However, the amplitude of these same BOLD fluctuations (referred to as the amplitude of low-frequency fluctuations (ALFF)) putatively measures variation in BOLD signal strength at a local level, providing an index of regional intrinsic power (Zang et al, 2007). ALFF measures the magnitude of local LFO BOLD activity rather than interregional connectivity. LFO measures are likely important for understanding cognitive performance variation in light of growing evidence that spontaneous intrinsic brain activity relates to, and possibly even constrains, stimulus-evoked brain responses. In healthy adults, LFO amplitude measured during resting-state fMRI relates to cognitive task-evoked brain activation as well as behavioral performance (Fox et al, 2007; Mennes et al, 2011; Zou et al, 2013). Further underscoring the link between resting LFO and cognition, the relationship between intrinsic brain activity and task-evoked brain activity depends on cognitive load, as stronger relationships between ALFF and task-related activity emerge with increased working memory load (Zou et al, 2013).
Measures of LFO amplitude have received relatively little attention in the schizophrenia literature, despite demonstration of regionally specific LFO differences between schizophrenia patients and healthy controls (He et al, 2013; Hoptman et al, 2010; Huang et al, 2010; Lui et al, 2010; Turner et al, 2013; Yu et al, 2013, 2014). A large sample study from the multi-site fMRI Biomedical Informatics Network (FBIRN) (Turner et al, 2013) reported widespread regions of decreased fractional ALFF (fALFF) in schizophrenia, including posterior cortex. These results converge with reports of decreased fALFF in schizophrenia in posterior cortical regions including occipital and precuneus/posterior cingulate cortices (PCC) (Hoptman et al, 2010; Yu et al, 2014). There is, however, considerable variation in the regional pattern and direction of LFO amplitude alterations reported in schizophrenia, possibly owing to clinical and methodological differences among the small number of extant studies. Regardless, LFO amplitude alterations appear to be clinically meaningful, as fALFF values relate to symptom severity (He et al, 2013) and reductions in ALFF normalize with antipsychotic treatment and symptomatic improvement (Lui et al, 2010). Moreover, positive relationships between LFO amplitude and functional connectivity present in healthy controls are attenuated in schizophrenia, suggesting that normal relationships between LFO activity and functional connectivity are disrupted in schizophrenia (Yu et al, 2013).
Despite compelling findings identifying between-group LFO alterations in schizophrenia (Calhoun et al, 2008; Hoptman et al, 2010; Huang et al, 2010; Turner et al, 2013; Yu et al, 2013, 2014) and relating them to pharmacological treatment response (Lui et al, 2010) and clinical symptoms (He et al, 2008), the cognitive relevance of LFO magnitude changes in this population remains largely unexamined. This question is particularly important because though neurocognitive deficits in domains such as attention and working memory are commonly observed in schizophrenia (Heinrichs and Zakzanis, 1998; Mesholam-Gately et al, 2009), and strongly influence functional outcomes (Green et al, 2004), the pathophysiological basis of cognitive deficits is poorly elucidated. A prior study of treatment-naive first-episode patients with schizophrenia reported that lower fALFF related to poorer processing speed in patients (He et al, 2013). The generalizability of these findings to treatment-seeking patients and to other cognitive domains remains unclear. Accordingly, this study extends the prior report from the FBIRN consortium (Turner et al., 2013) to examine relationships between resting-state LFO amplitude and neurocognitive function in patients with schizophrenia and healthy controls. More specifically, this study examines resting-state fALFF and neuropsychological performance on tests of sustained attention (ie, vigilance) and working memory in order to determine whether the magnitude of slow fluctuations in regional BOLD activity correlates with cognitive function and, further, to determine whether these relationships differ between schizophrenia patients and healthy controls. Based on prior work demonstrating positive relationships between cognitive performance and LFO amplitude in task-relevant brain regions (He et al, 2013; Mennes et al, 2011; Zou et al, 2013), we predicted that better attention and working memory performance would relate to higher fALFF. Specifically, we expected LFO–cognition relationships to be detected within frontoparietal circuitry directly implicated in serving attention and working memory functions (eg, dorsolateral prefrontal cortex, dorsal anterior cingulate, posterior parietal cortex) (Cabeza and Nyberg, 2000; Wager and Smith, 2003) and/or circuitry thought to be reciprocally related to these networks (eg, midline structures including medial prefrontal cortex, PCC, precuneus, as well as lateral parietal cortices) (Buckner et al. 2008).
MATERIALS AND METHODS
Participants
Subjects with schizophrenia (SZ; n=168) and healthy controls (HC; n=166) were recruited from seven FBIRN consortium sites (University of California Irvine, University of California Los Angeles, University of California San Francisco, Duke University/University of North Carolina, University of New Mexico, University of Iowa, and University of Minnesota). Inclusion criteria required all participants to be adults between the ages of 18 and 65 years. Patients were included in the SZ group if they met DSM-IV-TR criteria for schizophrenia and were on a stable dose of antipsychotic medication for at least 2 months. Diagnosis of schizophrenia was confirmed by trained raters using the Structured Clinical Interview for DSM-IV (SCID) (First et al, 2002). Current symptom severity was rated using the Positive and Negative Syndrome Scale (PANSS) (Kay et al, 1987). Exclusionary criteria for all participants were contraindication for MRI scanning (eg, metallic implants in the body), current or past major medical illnesses affecting the central nervous system, noncorrectable vision impairments, substance dependence within 5 years of study participation, current substance abuse, or IQ estimate <75 (Blair and Spreen, 1989). In addition, HC participants were excluded for current or past psychiatric illness based on SCID assessment or for having a first-degree relative with a diagnosis of an Axis-I psychotic disorder. Participant socioeconomic status (SES) and parental SES were measured by the Hollingshead 2-Factor Index (Hollingshead and Redlich, 1958). Written informed consent was obtained from all study participants under protocols approved by the Institutional Review Boards at each study site.
Neuropsychological Assessment
Cognitive measures were obtained from testing with the Computerized Multiphasic Interactive Neurocognitive Diagnostic System (CMINDS) neuropsychological battery (NeuroComp Systems) (O'Halloran et al, 2008). In order to address study hypotheses regarding the relationships between intrinsic brain activity and core domains of cognitive impairment in schizophrenia, CMINDS tests of sustained attention, ie, vigilance (Continuous Performance Test-Identical Pairs (CPT-IP)) and working memory (Spatial Memory Span (SMS)), were selected.
Continuous Performance Test-Identical Pairs
For the CPT-IP, participants were serially presented with spans of digit sequences in blocks of 150 stimuli, with span complexity increasing from 2 to 3 to 4 digit sequences over three successive blocks. Participants were told to respond if and only if the digit sequence was identical to the preceding one. Performance was quantified using the sensitivity index d′, a ratio of target hits to false alarms, with higher scores indicating better performance.
Spatial Memory Span
For the SMS, participants were presented on each trial with an array of spatially distributed boxes that lit up in a pseudorandom nonrepeating sequence. Upon verbal prompt, the participant was asked to use an electronic pen to tap the boxes in the same sequence for the Forward condition, or in the reverse sequence for the Reverse condition. The spatial arrays spanned from 2 to 8 boxes, and two trials were presented at each span before increasing to the next higher span, until participants incorrectly recalled both trials at a given span. The Reverse condition was analyzed in this study. Performance was quantified using the total number of correct responses, with higher scores indicating better performance.
Resting-State Data Acquisition and Processing
Resting fMRI data were acquired at 7 sites (six 3T Siemens TIM Trio scanners and one 3T GE MR750 scanner) using the following T2*-weighted AC-PC aligned echo planar imaging (EPI) sequence: TR/TE 2 s/30 ms, flip angle 77°, 32 slices collected sequentially from superior to inferior, 3.4 × 3.4 × 4 mm with 1 mm gap, 162 frames, 5:38 min acquisition time).
Imaging preprocessing was performed with Statistical Parametric Mapping (SPM5) http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). Image preprocessing entailed motion correction via affine registration of all functional images to the first image using INRIAlign (http://www-sop.inria.fr/epidaure/Collaborations/IRMf/INRIAlign.html). Images were slice-time corrected to adjust for timing differences of individual slice acquisitions within each TR. ACompCor, a principal components analysis-based approach to denoising BOLD data, was applied (Behzadi et al, 2007). ACompCor derives principal components from the time series of voxels within noise regions of interest defined on white matter and cerebrospinal fluid (CSF) parcels from participants' segmented high-resolution T1-weighted anatomical images coregistered to their functional data. White matter noise regions were derived via FreeSurfer (http://surfer.nmr.mgh.harvard.edu/) segmentation, whereas CSF noise regions were derived via segmentation in spm5. These principal components are then used as nuisance regressors in voxelwise first-level regression models to remove noise-related variance and improve signal-to-noise ratio.
To further ensure that the data were optimally cleaned of noise, the influence of head motion quantified by six realignment parameters, and their derivatives, were included along with aCompCor noise regressors in the first-level regression model for each subject. Residual time series from this regression model were saved, resulting in denoised resting-state BOLD images. Normalization of the mean functional image from the realignment step to the Montreal Neurological Institute EPI template was done using a 12-parameter affine transformation and 4 × 5 × 4 nonlinear basis functions, including image reslicing to 3 mm3. Data were then spatially smoothed with an 8 mm full width half max (FWHM) Gaussian filter.
In order to calculate voxelwise ALFF maps, each participant's preprocessed and denoised resting-state time series images were subjected to fast Fourier transformation. The square root of the power for each frequency was calculated, yielding amplitude values. To calculate fALFF, the sum of the amplitude values in the 0.01 to 0.08 Hz low-frequency power range was divided by the sum of the amplitudes over the entire detectable power spectrum (range: 0–0.25 Hz). Relative to ALFF, fALFF is a normalized measure (normalized by the square root of the total power) that has been shown to reduce the influence of physiological noise (Zou et al, 2008). Group-level analyses were then conducted on voxelwise fALFF maps, as described in the Data Analysis section.
Data Analysis
In order to address study hypotheses, the relationship between fALFF and cognitive test performance was examined using multiple regression to test for differences in the regression line slopes between the HC and SZ groups, and when the slopes did not significantly differ, to test the pooled estimate of the common slope of the regression line for significance. In the first model, voxelwise fALFF was regressed on Cognitive Test performance (CPT-IP or SMS), Group (dummy coded: HC=0, SZ=1), and the Group × Cognitive Test performance interaction. Whole-brain maps of the interaction effect, which represent the regional distribution of slope differences between the groups, were then tested for significance.
In a second model, in order to identify brain regions showing significant fALFF–Cognitive Test relationships with regression line slopes common to both groups, a voxelwise regression of fALFF on Cognitive Test performance and Group (dummy coded) was run, omitting the interaction term. This model used an explicit mask that excluded voxels showing Group × Cognitive Test interaction effects with p-values <0.05 (uncorrected) in the first model. This exclusive mask was based on a liberal probability threshold in order to exclude voxels showing a trend toward slope differences between the groups. In the remaining voxels, the Cognitive Test effect from the second model, representing the common slope of the regression line fit to the data within each group, was then tested for significance. Both models were run separately for attention (CPT-IP) and working memory (SMS) test scores. Statistical significance was based on an initial voxel-level cluster-defining height threshold of p<0.01 (extent threshold=5 voxels) followed by retaining clusters that met a family-wise error (FWE) cluster-level correction, with a cluster p-value <0.05.
Dummy-coded Site variables were included in all regression models in order to account for variance attributable to the data collection sites. Because there was no a priori basis for expecting that fALFF–Cognition relationships should vary by site, other than due to random sampling error, we did not model site variation in the slopes of the fALFF–Cognition relationships or their interactions with Group. Instead, we adopted the more conservative and parsimonious approach of testing the significance of the best fitting models relating fALFF to Diagnosis and Cognition controlling for Site, with the constraint that the best fitting model be the same across sites (ie, we omitted interactions involving Site from our model).
RESULTS
Participant Group Characteristics
The groups did not significantly differ on age, gender, and handedness. Participant SES and parental SES were significantly lower, whereas scan head motion was significantly greater, in the SZ patients than in the HC participants (all p<0.001). In addition, the SZ group performed more poorly than the HC group on the CPT-IP d' measure of attention and the SMS measure of working memory (all p<0.001). See Table 1 for group means on demographic and neuropsychological performance measures, statistical tests of group differences, and clinical characteristics of the SZ group.
Table 1. Demographic, Neuropsychological, and Clinical Data for Participants in the Healthy Control (HC) and Schizophrenia (SZ) Groups.
| HC | SZ | |
|---|---|---|
| Na | 166 | 168 |
| Gender (% male) | 72.29 | 75.00 |
| Age (years) | 37.80±11.30 | 39.04±11.32 |
| Participant SESb,c | 33.45±12.86 | 50.16±13.19 |
| Parental SESb,c | 30.22±14.85 | 36.29±14.86 |
| Handedness (% right-handed) | 94.58 | 90.48 |
| Average motion displacement (mm)c,d | 0.068±0.047 | 0.086±0.047 |
| CMINDS attention score: CPT-IP d'c | 3.26±0.60 | 2.36±0.88 |
| CMINDS Working Memory Score: Spatial Memory Spanc | 7.97±1.82 | 6.21±2.15 |
| PANSS positive sum | — | 15.55±5.01 |
| PANSS negative sum | — | 14.49±5.32 |
| PANSS general sum | — | 28.73±7.46 |
| PANSS total sum | — | 58.78±14.35 |
| Chlorpromazine equivalents (mg) | — | 377.76±469.99 |
| Antipsychotic medication type (% typical; % atypical; % both; % unknown) | — | 10.71; 81.55; 0.06; 7.14 |
SES scores not available for 3 SZ participants; CPT-IP scores not available for 2 HC and 8 SZ participants; clinical ratings not available for 2 SZ participants; chlorpromazine equivalents not available for 30 SZ participants; medication type not available for 12 SZ participants.
SES (socioeconomic status) measured by the Hollingshead 2-Factor Index. Higher Hollingshead scores indicate lower SES. All other assessment measures are scaled such that higher scores reflect greater levels of the measured variable, and for cognitive tests higher scores reflect better performance.
Significant between-group comparison, p<0.05: participant SES: t(329)=−11.67, p<0.001; parental SES: t(329)=−3.71, p<0.001; average motion displacement: t(332)=3.54, p<0.001; CPT-IP d': t(322)=10.78, p<0.001; Spatial Memory Span: t(332)=8.08, p<0.001.
Average motion displacement in mm as defined by Van Dijk et al (2012) as the root mean square (RMS) of the scan to scan change in the x–y–z translation parameters.
FALFF–Cognition Slope Differences between Groups
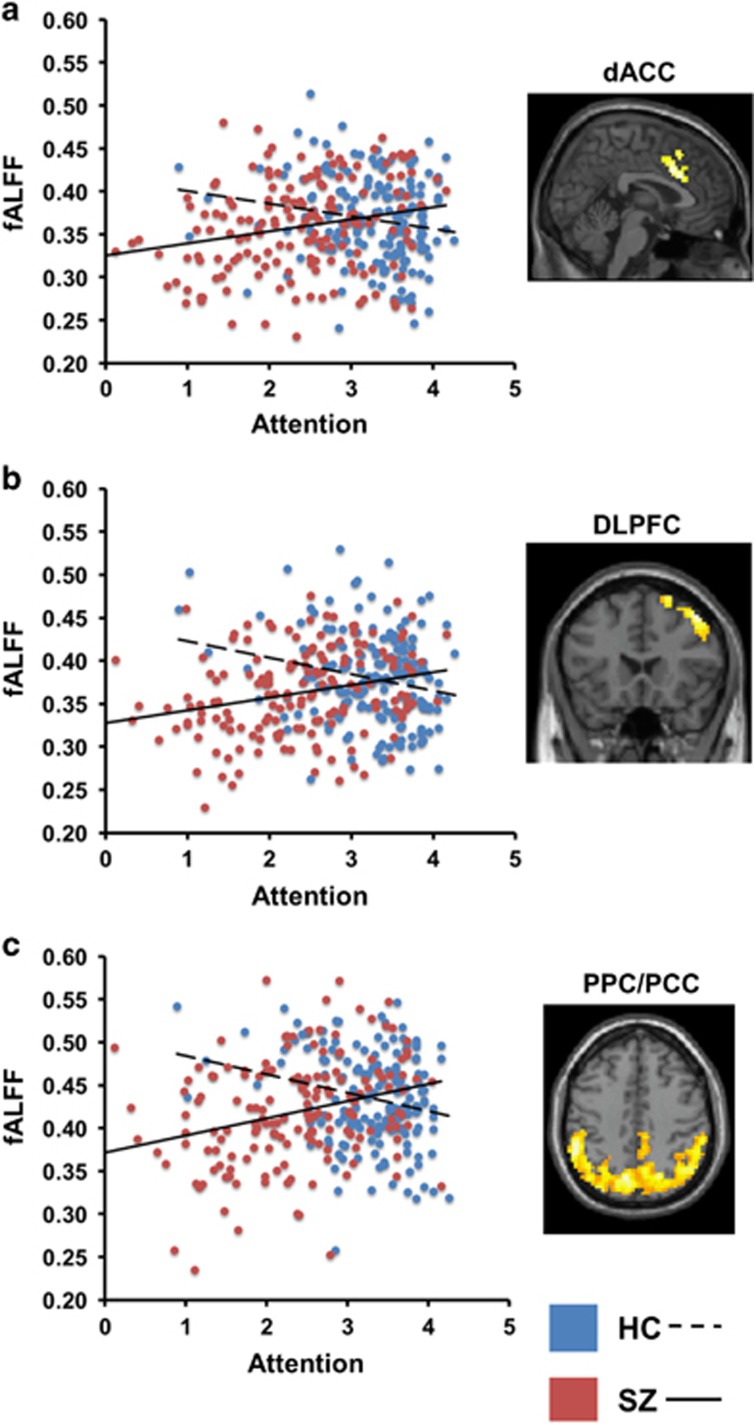
Group differences in the slopes of the regression lines relating fALFF to cognitive test performance were observed for the CPT-IP attention measure (see Table 2). FWE-corrected clusters were detected in (1) dorsal anterior cingulate cortex (ACC)/supplementary motor area, (2) right dorsolateral and superior prefrontal cortex, and (3) a large region of medial and lateral posterior cortex, including precuneus as well as bilateral posterolateral parietal and PCC, extending into temporal and occipital cortex. All regions showing slope differences between the groups emerged because of a positive relationship between fALFF and CPT-IP test performance in the SZ but a slightly negative relationship in the HC (see Figure 1). Thus, in SZ, but not HC, lower fALFF was associated with poorer attention.
Table 2. Neuroanatomical Regions Showing Significant fALFF–Cognition Slopes across Healthy Control and Schizophrenia Groups.
| CMINDS test | Cluster neuroanatomy | FWE cluster-corrected p-value | Number of 3 mm3 voxels | Peak MNI coordinate (x, y, z) |
|---|---|---|---|---|
| Regions showing fALFF–cognition slope differences between groups | ||||
| CPT-IP d' | Dorsal anterior cingulate cortex/supplementary motor area (BA 8, 24, 32) | P=0.04 | 124 | 3x 15y, 33z |
| CPT-IP d' | Right dorsolateral/superior frontal cortex (BA 6, 8, 9, 46) | P<0.001 | 322 | 45x, 0y, 57z |
| CPT-IP d' | Bilateral posterior lateral parietal, medial parietal, and posterior cingulate cortex, extending into temporal and occipital cortex (BA 5, 7, 19, 30, 31, 39, 40) | P<0.001 | 3710 | 6x, −69y, 51z |
| Regions showing fALFF–cognition slopes common to both groups | ||||
| CPT-IP d' | Left superior frontal cortex (BA 6, 8) | P=0.02 | 134 | −30x, 15y, 60z |
| CPT-IP d' | Left temporal cortex | P=0.02 | 136 | −33x, −39y, 3z |
| CPT-IP d' | Right temporal cortex | P=0.03 | 128 | 21x, −42y, 24z |
| Spatial Memory Span | Medial prefrontal cortex, extending to bilateral dorsolateral prefrontal cortex (BA 6, 8, 9, 10, 32) | P<0.001 | 1811 | 12x, 42y, 51z |
| Spatial Memory Span | Left temporoparietal junction/inferior parietal lobule (BA 39, 40) | P=0.002 | 214 | −48x, −66y, 42z |
| Spatial Memory Span | Right temporoparietal junction/inferior parietal lobule (BA 39, 40) | P<0.001 | 298 | 51x, −42y, 30z |
Abbreviation: BA, Brodmann area. Common slopes and slope differences between groups are listed above. Cluster-corrected p-values are family-wise error (FWE) corrected, at a height threshold of p<0.01.
Figure 1.
Scatterplots and neuroanatomical regions showing significant fALFF–cognition slope differences between healthy control (HC) and schizophrenia (SZ) groups for the test of attention (CPT-IP d'). All findings are family-wise error cluster-level corrected at a p<0.01 height threshold. Regions shown: (a) dorsal anterior cingulate/supplementary motor area (dACC), x=3 mm; (b) right dorsolateral prefrontal cortex (DLPFC), y=21 mm; (c) bilateral medial and posterior lateral parietal cortex and posterior cingulate cortex (PPC/PCC), z=42 mm.
For the SMS working memory test, there were no clusters of fALFF–cognitive performance slope differences between the groups that achieved FWE-corrected significance.
Common fALFF–Cognition Slopes across Groups
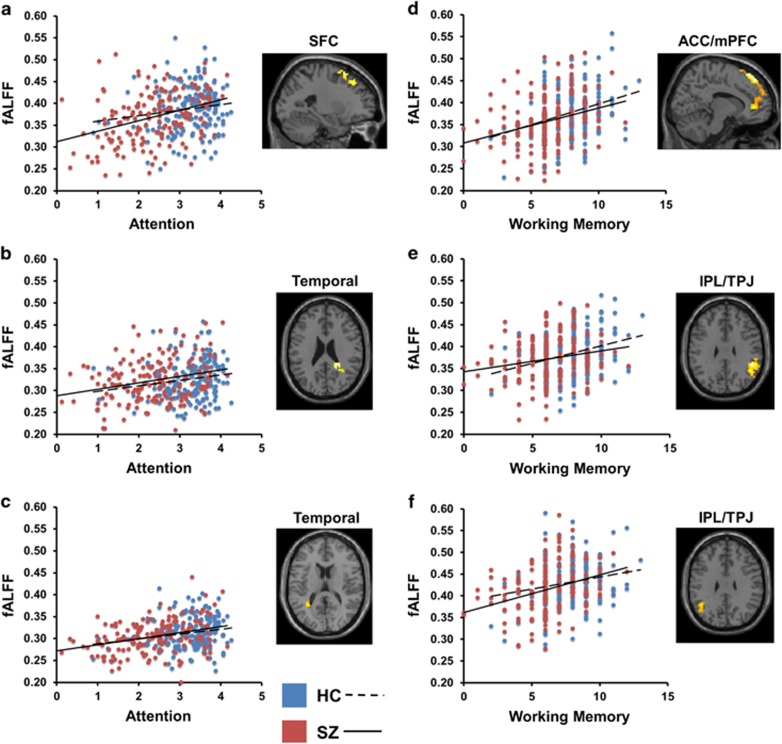
After masking out regions showing slope differences between the groups and dropping the Group × Cognitive Test performance interaction term from the regression model, the remaining regions were interrogated for significant fALFF–cognition regression line slopes common to both groups. Several clusters surpassed our FWE-corrected threshold, and all were indicative of positive relationships between fALFF and cognitive test performance, such that lower fALFF values were related to poorer performance (see Table 2 and Figure 2). For the CPT-IP attention measure, significant common slopes were observed in left superior frontal cortex (Brodmann Area (BA) 6/8) and right and left temporoparietal junction.
Figure 2.
Scatterplots and neuroanatomical regions showing significant common fALFF–cognition slopes across healthy control and schizophrenia groups for tests of attention (CPT-IP d') and working memory (Spatial Memory Span). All findings are family-wise error cluster-level corrected at a p<0.01 height threshold. Regions shown: (a) left superior frontal cortex (SFC) x=−23 mm; (b) right temporal cortex (Temporal), z=24 mm; (c) left temporal cortex (Temporal), z=16 mm; (d) anterior cingulate/medial prefrontal cortex, extending to dorsolateral prefrontal cortex (ACC/mPFC), x=12 mm; (e) right temporoparietal junction/inferior parietal lobule (IPL/TPJ), z=30 mm; (f) left temporoparietal junction/inferior parietal lobule (IPL/TPJ), z=30 mm.
For the SMS measure of working memory, regional clusters showing significant common slopes emerged in (1) a large region of bilateral ACC/medial prefrontal cortex (mPFC), including BA 9/10 and 32, that extended to dorsolateral prefrontal cortex (DLPFC) in both left and right hemispheres, (2) a right posterolateral parietal cortex region including supramarginal and angular gyri that extended inferiorly into temporal lobe, and (3) a left posterolateral parietal region extending inferiorly into temporal lobe that also included supramarginal and angular gyri. For full neuroanatomical extents of findings and regional overlap across cognitive domains see Supplementary Figures 1–5.
Consideration of Potential Confounds on fALFF–Cognition Slopes across Groups
We next undertook post hoc analyses to evaluate potentially confounding factors because our groups differed on parental SES, antipsychotic medication usage, scan motion, and because data were collected from multiple sites.
Socioeconomic status
Regional fALFF clusters showing either significant group differences in slopes or significant common slopes when regressed on cognitive scores were extracted for all subjects and analyses were repeated on the cluster means (average fALFF values over all voxels in the cluster) in subgroups of SZ (n=153) and HC (n=154) matched on parental SES (F(1, 305)=2.19, p=0.14; Cohen's d=0.17). All common slopes and slope differences reported in the full sample remained significant (all p<0.001) when tested in the parental SES-matched subsample, indicating that full-sample effects were not explained by group differences in parental SES.
Antipsychotic medication
Next, we examined the effects of antipsychotic medication dosage on the cognitive and LFO variables under examination within our patient sample. Within the SZ group, Chlorpromazine equivalent dosage did not correlate with the attention or working memory variables under investigation nor with mean fALFF regions showing either slopes difference or common slope effects (all Pearson's r<|0.17| all p>0.05).
Head motion
Regional fALFF clusters showing either significant group differences in slopes or significant common slopes when regressed on cognitive scores were extracted for all subjects and analyses were repeated on the cluster means in subgroups of SZ (n=158) and HC (n=156) matched on mean motion displacement measured in mm (F(1, 313)=2.81, p=0.10; Cohen's d=0.19). All common slopes and slope differences reported in the full sample remained significant (p-values <0.001 to 0.002) when tested in the motion-matched subsample, indicating that full-sample effects were not explained by group differences in motion.
Site
Although the main effect of data acquisition site was controlled for in our voxelwise regression models, we further examined the influence of Site on the mean fALFF values extracted from all FWE-corrected clusters that showed either significant slope differences or common slopes. Significant Site main effects were observed among all 9 regional fALFF clusters (p-values <0.001 to 0.002). Significant effects of Site are consistent with what we previously reported on FBIRN fALFF data (Turner et al, 2013), where we noted that Site effects were largely driven by scanner manufacturer differences. However, when we reran models to include a Group × Site interaction term we did not find any significant Group × Site interaction effects on these fALFF values (0.09<p<0.82). The lack of significant Group × Site interactions indicated that the magnitude of group differences in fALFF did not significantly depend on data acquisition site.
Group Differences in fALFF
Whole-brain between-group comparisons of fALFF from an overlapping FBIRN study sample are reported elsewhere (Turner et al, 2013). We therefore restricted our current analysis of group differences in fALFF to the regions showing significant relationships with cognitive performance. Accordingly, mean fALFF values from FWE-corrected clusters showing either significant slope differences or common slopes were extracted for all participants and subjected to independent t-tests. For all regions, the SZ group had significantly lower fALFF than the HC group (p-values <0.001 to 0.002; see Table 3 for mean cluster fALFF values, t-statistics and p-values, and effect sizes).
Table 3. Between-Group Comparisons of fALFF for Neuroanatomical Regions Showing Significant fALFF–Cognition Slopes.
| CMINDS test | Cluster neuroanatomy | Mean cluster fALFF (HC, M±SD; SZ, M±SD) | T-test, p-value | Cohen's d |
|---|---|---|---|---|
| Regions showing fALFF–cognition slope differences between groups | ||||
| CPT-IP d' | Dorsal anterior cingulate cortex/supplementary motor area | HC: 0.37±0.03 SZ: 0.36±0.04 | t(322)=3.60 p<0.001 | 0.40 |
| CPT-IP d' | Right dorsolateral/superior frontal cortex | HC: 0.38±0.03 SZ: 0.37±0.03 | t(322)=4.07 p<0.001 | 0.45 |
| CPT-IP d' | Bilateral posterior lateral parietal, medial parietal, and posterior cingulate cortex, extending into temporal and occipital cortex | HC: 0.40±0.03 SZ: 0.39±0.03 | t(322)=4.36, p<0.001 | 0.48 |
| Regions showing fALFF–cognition slopes common to both groups | ||||
| CPT-IP d' | Right temporal cortex | HC: 0.33±0.03 SZ: 0.32±0.03 | t(322)=4.21 p<0.001 | 0.35 |
| CPT-IP d' | Left temporal cortex | HC: 0.32±0.02 SZ: 0.31±0.02 | t(322)=2.87 p=0.004 | 0.32 |
| CPT-IP d' | Left superior frontal cortex | HC: 0.36±0.03 SZ: 0.35±0.03 | t(322)=3.19 p=0.002 | 0.47 |
| Spatial Memory Span | Medial prefrontal cortex, extending to bilateral dorsolateral prefrontal cortex | HC: 0.38±0.03 SZ: 0.37±0.03 | t(332)= 4.01, p<0.001 | 0.45 |
| Spatial Memory Span | Left temporoparietal junction/inferior parietal lobule | HC: 0.42±0.04 SZ: 0.40±0.04 | t(332)=3.81, p<0.001 | 0.42 |
| Spatial Memory Span | Right temporoparietal junction/inferior parietal lobule | HC: 0.41±0.03 SZ: 0.39±0.03 | t(332)=3.68, p<0.001 | 0.40 |
DISCUSSION
This study identifies novel associations between regional magnitudes of low-frequency oscillations in BOLD signal activity, measured by fALFF, and attention and working memory in a large, multisite sample of patients with schizophrenia and healthy control subjects. Analyses identifying common fALFF–cognition regression line slopes across the groups revealed that sustained attention performance was positively associated with fALFF in left superior frontal cortex and the right and left temporoparietal junction. Working memory performance was positively associated with fALFF in bilateral ACC, mPFC, right DLPFC, and inferior parietal lobule/temporoparietal cortex in both groups. These regions of fALFF–cognition relationships overlap well with the frontoparietal neuroanatomy implicated in attention and working memory functions (Cabeza and Nyberg, 2000; Wager and Smith, 2003). In contrast, several additional regions were identified where reduced fALFF was associated with poorer sustained attention in the schizophrenia group, but not in the healthy control group, that instead showed a modest inverse relationship between fALFF and cognition. These regions of group slope differences included dorsal ACC, right DLPFC, and a large area of bilateral medial and lateral posterior parietal cortex (including both inferior and superior parietal lobules and PCC), extending into occipital and temporal cortices. The set of regions that differentiated schizophrenia patients from healthy controls showed considerable overlap with posterior nodes of the default mode network (DMN) (shown in Supplementary Figure 6), suggesting that schizophrenia-related alterations of the relationship between fALFF and attention may particularly implicate DMN functioning. Schizophrenia patients also had significantly poorer cognitive performance on the attention and working memory measures as well as lower fALFF in all regions with either significant common slopes or slope differences. The identification of regions in which fALFF and cognitive performance covary contextualizes prior findings of LFO amplitude alterations in schizophrenia by demonstrating that regional amplitude reductions in schizophrenia patients are associated with their deficits in attention and working memory.
Although prior studies comparing BOLD LFO values between schizophrenia patients and healthy controls have yielded mixed results in terms of the regional pattern and direction of schizophrenia-related alterations, there has been some convergence of findings showing posterior cortical decreases in patients (Hoptman et al, 2010; Turner et al, 2013; Yu et al, 2014). Within healthy individuals, resting-state LFO amplitudes show considerable regional variation, with the highest magnitudes occurring in midline and visual regions that substantially overlap with DMN regions (Fransson, 2005; Zuo et al, 2010). Though the functional significance of this regional variation in LFO activity has yet to be elucidated, many of the regions with large LFOs (eg, precuneus, PCC, lateral parietal cortices) overlap with regions previously shown to have reduced LFOs in schizophrenia (Hoptman et al, 2010; Turner et al, 2013; Yu et al, 2014) as well as with regions showing positive correlations with cognition in patients in the current study. Anomalous activity and connectivity of the DMN has been previously reported in schizophrenia (Anticevic et al, 2012; Whitfield-Gabrieli and Ford, 2012). Indeed, insufficient suppression of DMN activity during task engagement may independently contribute to cognitive deficits in schizophrenia beyond dysfunction of task-specific substrates (Anticevic et al, 2012). Given that the posterior cortical slope difference findings extend well into extrastriatal regions of the occipital cortices, an alternative or additional explanation to DMN dysfunction may be that the altered LFO–cognition relationships observed relate to early sensory processing deficits that have been described in schizophrenia and shown to contribute to higher-order cognitive impairments (Dias et al, 2011).
It is interesting to note that regional LFO–cognition slope differences between the groups were limited to the CPT measure of vigilance. One possible explanation for this is attenuated CPT score range and variance in the control group relative to the schizophrenia group. Nonetheless, some regional LFO measures showed relationships with CPT scores with a common slope across both groups, suggesting that these relationships were detectable in both groups despite the restricted healthy control group variance. Furthermore, when considering the regions showing slope differences between the groups, significant correlations were observed in healthy controls, although in the opposite direction to those observed in patients with schizophrenia. Although it is possible that some compensatory mechanism drove the positive correlation between attention and LFO amplitudes in the schizophrenia group, and that in the absence of schizophrenia, larger LFO amplitudes do not promote sustained attention, it should be noted that the control scatterplots (Figure 1) are not strongly supportive of an inverse relationship over the full range of attention scores. Therefore, the modest inverse relationship we observed in healthy controls must be interpreted cautiously. Moreover, despite a similar restriction of range in working memory scores in the control group relative to schizophrenia group, all regional LFO amplitude measures that correlated with working memory performance did so with a common slope across both groups. This suggests that the relatively larger variance in cognitive scores in the schizophrenia group did not necessarily lead to slope differences relative to the control group.
In contrast to the tests of slope differences, all regions identified by tests of common slopes indicated a positive relationship between fALFF and cognition, such that lower resting fALFF was consistently associated with poorer performance on tests of attention and working memory. These commonly identified regions associating fALFF and cognition across groups support our hypothesis that better attention and working memory performance would relate to higher fALFF, consistent with the positive relationships between cognitive performance and LFO amplitude previously observed in healthy controls in task-relevant regions (He et al, 2013; Mennes et al, 2011; Zou et al, 2013). Our demonstration that poorer cognition in schizophrenia patients was correlated with lower resting fALFF is consistent with the direction of the relationships reported by He et al (2013), who showed fALFF decreases in prefrontal gyri to be associated with reduced processing speed in treatment-naive first-episode schizophrenia patients. The current study observed fALFF–cognition relationships over prefrontal areas, but also showed such relationships in lateral parietal and posterior cingulate cortices. The broader regional distribution of our findings of fALFF–cognition relationships, relative to those reported by He et al (2013), may stem from differences in the cognitive domains examined: whereas we focused on working memory and sustained attention, He et al (2013) focused more narrowly on speed of processing. Differences regarding the anatomy and extent of findings between the two studies may also relate to illness progression, as He et al (2013) studied only first-episode patients, whereas we focused on older chronic patients.
Although the LFO signals examined in this study are hemodynamically generated, consensus is emerging that BOLD and electrophysiological signals within the infraslow frequency range may reflect the same underlying mechanism (He et al, 2008; Hiltunen et al, 2014; Palva and Palva, 2012). This study's focus on the cognitive relevance of intrinsic power in the infraslow range raises questions regarding the mechanistic source of these LFOs, as well as how they may interact with higher frequency oscillating brain signals more commonly studied with electrophysiological methods. Because of the inverse relationship between frequency and power observable in neural brain oscillations (ie, power-law frequency distributions), the slow fluctuations characteristic of LFO activity are poised to entrain faster oscillatory signals (Aladjalova, 1957; Buzsaki and Draguhn, 2004). Thus, the well-described modulation of higher frequency power by slower frequency phase is one way in which LFOs may exert a role in cognitive output (Canolty and Knight, 2010). Though the exact generator(s) of LFOs are unknown, it is possible that the slow activity fluctuations characteristic of LFOs reflect self-organizing emergent properties of the networks that generate faster oscillations, or they may arise directly from cellular-level mechanisms that induce slow modulation of the amplitudes of high-frequency neuro-oscillatory networks (Palva and Palva, 2012).
In contrast to interregional-phase synchronization, in this study we focus on amplitude measures of LFO activity that can be construed as a measure of local activity synchronization (Roach and Mathalon, 2008; Uhlhaas et al, 2008). It has been shown in a primate model that modulations of local field potential-recorded band-limited power in higher frequencies (particularly gamma-band) fluctuate with the same low-frequency periodicity as BOLD signals (Leopold et al, 2003). Therefore, fMRI-based LFO amplitude measures, like the amplitude of band-limited power of electrophysiological recordings, may also reflect synchronization of activity in local neuronal assemblies. Such local synchronization strength is a necessary precursor to network-level connectivity. Indeed, in healthy adults, fMRI-based LFO amplitude measures within intrinsic functional connectivity network nodes correlate with interregional network connectivity strength, indicating that within network connectivity is related to the magnitude of local low-frequency activities of network components (Di et al, 2013). In addition, higher LFO magnitudes in the precuneus node of the DMN are associated with stronger negative connectivity between DMN and task-positive networks (Di et al, 2013). Schizophrenia-related LFO alterations reported in the literature (He et al, 2013; Hoptman et al, 2010; Huang et al, 2010; Lui et al, 2010; Turner et al, 2013; Yu et al, 2013, 2014), as well as findings from our study linking lower LFO regional magnitudes to poorer cognitive task performance, may therefore reflect dysfunction of an important mechanism for gating functional neural connectivity. This speculation is consistent with conceptual accounts of schizophrenia as a disease predominantly of network dysconnectivity (Andreasen, 1999; Friston, 1998). The extent to which variations in the magnitudes of local LFO activity levels play a role in maintaining or gating correlated and anticorrelated functional network dynamics has been relatively unexplored in the schizophrenia literature and remains an important direction for future research.
Our study is limited by several factors. Antipsychotic medication usage differed between the groups, as did SES and scan head motion, although analysis of these potential confounds was not consistent with their accounting for observed effects. In addition, further parsing of the infraslow frequency band may be informative in future research examining the association between LFOs and cognition. LFO activity alterations in schizophrenia may depend, in part, on the frequency of LFO examined, as one study has demonstrated group-by-frequency interactions by examining subranges within the 0.01 to 0.1 band (Yu et al, 2014). Our work also did not examine group differences in the relationship between regional brain volume or task-based activity and LFO amplitude, which are worthwhile avenues for further investigation. Despite these limitations, our findings, relating decreased regional fALFF to worse attention and working memory, help establish the functional relevance of previously reported LFO amplitude reductions with respect to specific cognitive impairments frequently present in schizophrenia. Our findings support a growing literature relating features of slow intrinsic brain activity oscillations with cognitive abilities in both health and disease. Furthermore, the relationships between lower fALFF and poorer attention and working memory demonstrated by this study suggests that intrinsic infraslow-range brain activity alterations likely have implications for understanding the pathophysiology of cognitive deficits in schizophrenia.
FUNDING AND DISCLOSURE
Over the past 3 years, Dr Mathalon has received compensation as a consultant from Bristol-Myers Squibb, Amgen, and Hoffmann-La Roche. Dr Van Erp has consulted for Roche Pharmaceuticals and has a contract with Otsuka Pharmaceuticals. Dr Bustillo has consulted and conducted nonpromotional talks for Otsuka Pharmaceuticals. Dr Preda serves on the Boehringer Ingelheim Advisory Board. Over the past 3 years, Dr. Steven Potkin has received compensation from the following companies that conducted scientific or medical research and/or marketed medications related to psychiatric and neurodegenerative disorders: Alkermes, Eli Lilly, Forest, FORUM, Genentech, Janssen Pharmaceutical, Lundbeck, Merck, Novartis, Otsuka, Sunovion, Roche, Vanda, Concert Pharmaceuticals, and Takeda Pharmaceuticals International. The authors declare no conflict of interest.
Acknowledgments
We thank Michael Brandel and Vanessa Palzes for assistance with data analyses and manuscript preparation. This research was supported by NIH (U24 RR021992) and the VA (CX001028).
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Aladjalova NA (1957). Infra-slow rhythmic oscillations of the steady potential of the cerebral cortex. Nature 179: 957–959. [DOI] [PubMed] [Google Scholar]
- Andreasen NC (1999). A unitary model of schizophrenia: Bleuler's "fragmented phrene" as schizencephaly. Arch Gen Psychiatry 56: 781–787. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH (2012). The role of default network deactivation in cognition and disease. Trends Cogn Sci 16: 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37: 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34: 537–541. [DOI] [PubMed] [Google Scholar]
- Blair JR, Spreen O (1989). Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychol 3: 129–136. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL (2008). The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A (2004). Neuronal oscillations in cortical networks. Science 304: 1926–1929. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L (2000). Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD (2008). Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp 29: 828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Knight RT (2010). The functional role of cross-frequency coupling. Trends Cogn Sci 14: 506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Kim EH, Huang CC, Tsai SJ, Lin CP, Biswal BB (2013). The influence of the amplitude of low-frequency fluctuations on resting-state functional connectivity. Front Hum Neurosci 7: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias EC, Butler PD, Hoptman MJ, Javitt DC (2011). Early sensory contributions to contextual encoding deficits in schizophrenia. Arch Gen Psychiatry 68: 654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB (2002) Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). Biometrics Research, New York State Psychiatric Institute: New York. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME (2007). Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 56: 171–184. [DOI] [PubMed] [Google Scholar]
- Fransson P (2005). Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp 26: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ (1998). The disconnection hypothesis. Schizophr Res 30: 115–125. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK (2004). Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res 72: 41–51. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME (2008). Electrophysiological correlates of the brain's intrinsic large-scale functional architecture. Proc Natl Acad Sci USA 105: 16039–16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Deng W, Li M, Chen Z, Jiang L, Wang Q et al (2013). Aberrant intrinsic brain activity and cognitive deficit in first-episode treatment-naive patients with schizophrenia. Psychol Med 43: 769–780. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK (1998). Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 12: 426–445. [DOI] [PubMed] [Google Scholar]
- Hiltunen T, Kantola J, Abou Elseoud A, Lepola P, Suominen K, Starck T et al (2014). Infra-slow EEG fluctuations are correlated with resting-state network dynamics in fMRI. J Neurosci 34: 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A, Redlich F (1958) Social Class and Mental Illness. John Wiley and Sons: New York. [Google Scholar]
- Hoptman MJ, Zuo XN, Butler PD, Javitt DC, D'Angelo D, Mauro CJ et al (2010). Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr Res 117: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XQ, Lui S, Deng W, Chan RC, Wu QZ, Jiang LJ et al (2010). Localization of cerebral functional deficits in treatment-naive, first-episode schizophrenia using resting-state fMRI. Neuroimage 49: 2901–2906. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Murayama Y, Logothetis NK (2003). Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex 13: 422–433. [DOI] [PubMed] [Google Scholar]
- Lui S, Li T, Deng W, Jiang L, Wu Q, Tang H et al (2010). Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by "resting state" functional magnetic resonance imaging. Arch Gen Psychiatry 67: 783–792. [DOI] [PubMed] [Google Scholar]
- Mennes M, Zuo XN, Kelly C, Di Martino A, Zang YF, Biswal B et al (2011). Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage 54: 2950–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ (2009). Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology 23: 315–336. [DOI] [PubMed] [Google Scholar]
- O'Halloran JP, Kemp AS, Gooch KN, Harvey PD, Palmer BW, Reist C et al (2008). Psychometric comparison of computerized and standard administration of the neurocognitive assessment instruments selected by the CATIE and MATRICS consortia among patients with schizophrenia. Schizophr Res 106: 33–41. [DOI] [PubMed] [Google Scholar]
- Palva JM, Palva S (2012). Infra-slow fluctuations in electrophysiological recordings, blood-oxygenation-level-dependent signals, and psychophysical time series. Neuroimage 62: 2201–2211. [DOI] [PubMed] [Google Scholar]
- Raichle ME (2011). The restless brain. Brain Connect 1: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH (2008). Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull 34: 907–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JA, Damaraju E, van Erp TG, Mathalon DH, Ford JM, Voyvodic J et al (2013). A multi-site resting state fMRI study on the amplitude of low frequency fluctuations in schizophrenia. Front Neurosci 7: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolic D, Singer W (2008). The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull 34: 927–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL (2012). The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE (2003). Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci 3: 255–274. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM (2012). Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8: 49–76. [DOI] [PubMed] [Google Scholar]
- Yu Q, Sui J, Liu J, Plis SM, Kiehl KA, Pearlson G et al (2013). Disrupted correlation between low frequency power and connectivity strength of resting state brain networks in schizophrenia. Schizophr Res 143: 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Chien YL, Wang HL, Liu CM, Liu CC, Hwang TJ et al (2014). Frequency-specific alternations in the amplitude of low-frequency fluctuations in schizophrenia. Hum Brain Mapp 35: 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M et al (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 29: 83–91. [DOI] [PubMed] [Google Scholar]
- Zou Q, Ross TJ, Gu H, Geng X, Zuo XN, Hong LE et al (2013). Intrinsic resting-state activity predicts working memory brain activation and behavioral performance. Hum Brain Mapp 34: 3204–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ et al (2008). An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 172: 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF et al (2010). The oscillating brain: complex and reliable. Neuroimage 49: 1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.