Abstract
Exposure to early-life stress increases vulnerability to psychiatric disorders, including depression, schizophrenia, and anxiety. Growing evidence implicates aberrant development of the prefrontal cortex (PFC) in the effects of early-life stress, which often emerge in adolescence or young adulthood. Specifically, early-life stress in the form of maternal separation (MS) in rodents has been shown to decrease parvalbumin (PVB)-positive interneurons in the adolescent PFC; however, the mechanism underpinning behavioral dysfunction and PVB loss is not yet known. We recently reported that MS causes overexpression of the NMDA subunit NR2A in the PFC of adolescent rats. Elevated PFC NR2A is also found in developmental models of schizophrenia and is correlated with behavioral deficits, acting largely through its association with the postsynaptic protein PSD-95. In addition, adolescent maturation of PVB-positive interneurons relies on NR2A-driven NMDA activity. Therefore, it is possible that the NR2A/PSD-95 signaling complex has a role in adolescent MS effects. Here, we aimed to determine whether a discrete manipulation of PFC NR2A could prevent MS effects on PFC-controlled behaviors, including cognition, anxiety, and novelty-induced hyperlocomotion, as well as PVB loss in adolescence. We intracranially infused the NR2A-specific blocking peptide TAT2A in order to uncouple NR2A from PSD-95 in the early-adolescent PFC, without antagonizing the NMDA receptor. We demonstrated that MS rats treated with TAT2A during early adolescence were protected from MS-induced PVB loss and exhibited less anxious behavior than those infused with control peptide. These data implicate NR2A-related N-methyl-D-aspartate receptor development in adolescent behavioral and neural consequences of early-life stress.
INTRODUCTION
Exposure to stress during early development is implicated in increased vulnerability to later psychopathologies and emotional maladaptations (Agid et al, 1999; Brake et al, 2004; Kessler et al, 1997; Kohut et al, 2009; Teicher et al, 2006). Delayed appearance of several disorders after early-life stress has been attributed to aberrant maturation of developing microcircuitries (Brenhouse and Andersen, 2011; Leussis et al, 2012). Of particular interest are changes during the transition through adolescence, as internalizing symptoms such as depression and anxiety do not typically emerge immediately following early-postnatal stress, but rather during the adolescent period (Herringa et al, 2013).
The delayed emergence of disorders after early-life adversity makes it difficult to determine mechanistic cause due to intervening variables found in clinical studies. Animal studies help clarify causality through the use of experimental postnatal stress exposure. Daily removal of rat pups from their mothers (eg, maternal separation, MS) during the neonatal period is an ethologically relevant rodent model of early-life stress (Lehmann and Feldon, 2000). MS reportedly leads to several neuronal alterations in the prefrontal cortex (PFC) that first manifest in adolescence (Brenhouse and Andersen, 2011; Chocyk et al, 2013; Jahng et al, 2010; Macri et al, 2009), which is consistent with the delayed appearance of several disorders after early-life adversity (Teicher et al, 2009). Specifically, we found that MS led to decreases in parvalbumin (PVB)-expressing GABAergic interneurons in the adolescent PFC, an effect that co-occurred with cognitive and affective behavioral changes. However, the mechanism underlying these changes is not known.
N-methyl-D-aspartate receptor (NMDAR) channels contribute strongly to PFC activity (Rotaru et al, 2012). Accordingly, NMDAR alteration in the PFC (subunit expression, activity, and localization) has been implicated in many neuropsychiatric disorders, including schizophrenia, depression, and anxiety disorders (Balu and Coyle, 2014; Belforte et al, 2010; Beneyto et al, 2007; Bitanihirwe et al, 2010; Davis, 2011; Laruelle, 2014). NMDARs are heteromeric complexes that typically contain two NR1 subunits and at least two NR2 subunits. Each type of NR2 subunit (eg, NR2A and NR2B) has an intrinsic role in overall receptor and ion channel function (Cull-Candy et al, 2001) largely through its association with the postsynaptic density protein PSD-95 (Zhao et al, 2014). NR2A subunits associate with the PSD-95/discs large/zona occludens-1 domains of PSD-95 proteins in postsynaptic sites (Niethammer et al, 1996). This association with PSD-95 is responsible for the postsynaptic membrane localization of NR2A-containing NMDARs (Lin et al, 2004). In addition, PSD-95-mediated tyrosine phosphorylation of NR2A is an important mechanism for the modulation of NMDAR activity and channel currents (Salter and Kalia, 2004). Through its interaction with PSD-95, NR2A has been shown to induce shorter channel open time that affects NMDAR kinetics with consequent deficits in working memory, reversal learning, and sensory gating (Turnock-Jones et al, 2009). Importantly, NR2A function during adolescence has also been proposed to have a unique role in the development of PVB+ interneurons (Zhang and Sun, 2011).
We have recently reported that MS leads to overexpression of the NR2A subunit within the prelimbic region of the PFC during adolescence, which co-occurs with loss of PFC PVB+ interneurons (Wieck et al, 2013) and increased PSD-95 expression in the medial PFC (Chocyk et al, 2013). MS also reportedly yields behavioral changes such as increased anxiety (Girardi et al, 2014; Li et al, 2013) and increased novelty exploration (Colorado et al, 2006), each of which are largely mediated through the excitability of the infralimbic region of the PFC (ilPFC; Bi et al, 2013; Wall et al, 2004). However, to date, the possibility that MS-induced NR2A changes have a role in observed behavioral and cellular effects has not been investigated. Therefore, here we describe a subunit-specific and developmentally targeted intervention to investigate whether increased influence of NR2A on PFC NMDAR during adolescence is responsible for MS-induced changes in anxiety and ilPFC PVB expression.
Transduction with transactivated transcriptional (Tat) peptides has recently been utilized to dissociate NMDAR subunits from PSD-95 in order to block downstream neurotoxic signaling without blocking synaptic activity or calcium influx (Aarts et al, 2002). TAT2A is one such Tat peptide that uncouples the NR2A/PSD-95 complex, eliminating the functional influence of NR2A on the NMDAR (Gardoni et al, 2012). We hypothesized that if adolescent NR2A activity in the medial PFC is a causal factor in PVB loss and behavioral dysfunction after MS, then functional uncoupling of NR2A during early adolescence with TAT2A would protect against PVB loss and aberrant behavior.
MATERIALS AND METHODS
All experiments were performed in accordance with the 1996 Guide for the Care and Use of Laboratory Animals (NIH) with approval from the Institutional Animal Care and Use Committee at Northeastern University.
Subjects
Pregnant female multiparous Sprague–Dawley rats from Charles River Laboratories (Wilmington, MA) were obtained on day 15 of gestation. Dams were housed under constant temperature and humidity conditions within a 12-h light/dark cycle (light period 0700–1900 hours), with water and food available ad libitum.
Maternal Separation
Postnatal day 0 (P0) was designated as the day when the litters were born. On P1, litters were culled to 10 pups, maintaining an equal ratio of males and females. The litters were then randomly assigned to either a MS or control (CON) group. In order to avoid litter effects, one rat/litter was assigned to each group. Pups in the MS group were isolated in individual cups with home cage shavings, and placed in a circulating water bath with a thermoneutral environment maintained at 36 °C for 4 h/day between P2 and P20. The CON group pups were undisturbed, except for weekly bedding changes and when being weighed, which occurred on P9, P11, P15, and P20 (Supplementary Figure 1), and consisted of a <10-min separation from the dam. Rats were weaned at P21 and housed with same-sex littermates (2–4 rats/cage) until further experimentation. Only male rats were used in this study.
Cannula Implantation
At P27 or P28, MS and CON subjects (n=7–8/group) were pre-weighed and anaesthetized with isoflurane via inhalation (dose: 1–4% (mg/kg)) continuously for the duration of the stereotaxic surgery (~30 min). Intraperitoneal injection of analgesic buprenorphine (0.05 mg/kg) was administered before making the first skull incision. Rats were implanted with bilateral microinjection guide cannulae (Plastics One, Roanoke, VA) above the medial PFC (stereotaxic coordinates: anteroposterior=+2.9, mediolateral=+0.5, and dorsoventral=−1.6; Figure 1; Sherwood and Timeras, 1970). Animals were allowed to recover for 2–3 full days before commencing microinjections.
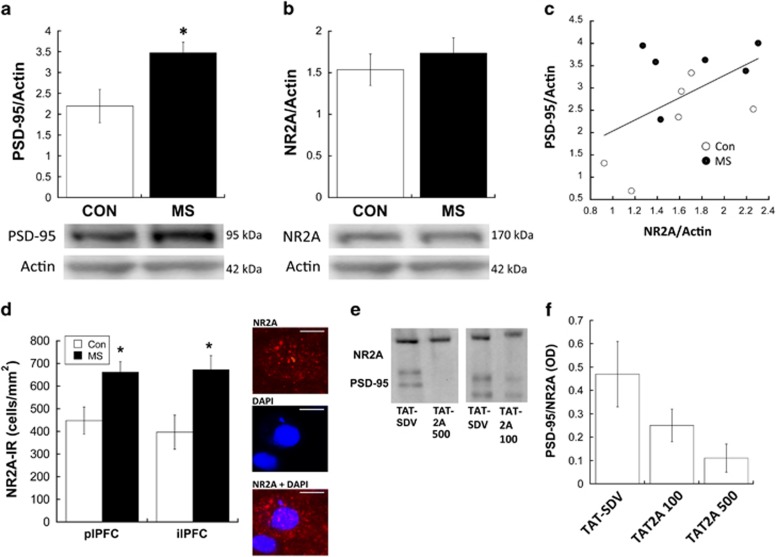
Figure 1.
Western blots show that maternal separation (MS) results in overexpression of PSD-95 (a) with a slight but not significant overexpression of NR2A (b) in the medial prefrontal cortex (PFC) during adolescence. *p<0.05 difference between groups; n=6. (c) NR2A optical density (OD) was positively correlated with PSD-95 expression in the medial PFC (R2=0.471; p=0.020). Subjects from control (CON) and MS are represented as different symbols. (d) Immunohistochemistry reveals that NR2A+ cells are increased in MS-exposed animals; *p<0.05 difference between groups; n=7. Representative photomicrographs are shown of an individual NR2A+ cell with NR2A alone, a DAPI-stained nucleus, and an overlay; scale bars, 10 μm. All photomicrographs are taken from the plPFC. (e) Treatment with TAT2A successfully uncoupled NR2A from PSD-95, as illustrated by representative co-immunoprecipitation blots from subjects treated with either 500 μM TAT2A, 100 μM TAT2A, or TAT-SDV CON peptide). Of note, TAT2A 500 and TAT2A 100 were run on separate gels, therefore CON peptides are shown from each respective gel. (f) Relative levels of co-immunoprecipitated PSD-95 (relative to NR2A) from TAT2A (100 and 500 μM)-treated subjects and TAT-SDV subjects; n=3. NR2A-IR, NR2A-immunoreactive.
TAT2A Microinjections
We microinjected into the PFC a cell-permeable Tat peptide fused to the last C-terminal nine amino acids of the NR2A subunit of NMDAR (TAT2A; Aarts et al, 2002; Gardoni et al, 2012; Paille et al, 2010) to obtain a chronic uncoupling of the PSD-MAGUKs/NR2A complex between P31–P39 (early adolescence). We chose the regimen of every-other-day administration in order to follow our previous early-adolescent microinjection paradigm that reduced NR2A expression and protected PVB+ interneurons (Wieck et al, 2013). Although PSD-95 shows turnover with NMDAR within hours (Gray et al, 2006), here we attempted to prevent the effects of sustained overexpression of NR2A during adolescence by administering TAT2A with intermittent administration. As shown in Supplementary Figure 2, verification with toluidine blue injected through the cannula of one subject revealed that microinjections diffused through the ilPFC and partially into the prelimbic region of the PFC. Treatment with TAT-SDV peptide, lacking the relevant interaction domain with PSD-95, was performed as CON. Both peptides were formulated and purchased from Bachem Inc. (Torrance, CA). The sequence of each peptide was as follows:
TAT2A: H-Tyr-Gly-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg-Lys-Met-Pro-Ser-Ile-Glu-Ser-Asp-Val-OH.
TAT-SDV: H-Tyr-Gly-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg-Lys-Met-Pro-Ser-Ile-Glu-OH.
Once every other day from P31–P39, TAT2A (100 and 500 μM), or TAT-SDV (500 μM) was administered to the MS and CON subjects (awake and gently held) over 1 min through the guide cannula using a microinjector (0.5 μl/side). The high dose of 500 μM was chosen based on previous reports of its in vivo effectiveness using repeated microinjections (Gardoni et al, 2012), whereas the lower 100 μM dose was chosen in the attempt to determine a dose-dependent effect. The injector remained in the brain for an additional minute to ensure peptide diffusion into the surrounding tissue.
Co-Immunoprecipitation and Western Blot
In order to confirm that TAT2A successfully uncoupled NR2A from PSD-95, brains from a subset of 500 μM TAT2A-, 100 μM TAT2A-, and TAT2A-SDV-treated CON animals (n=3/group) were collected following their last infusion and the medial PFC was homogenized in 1% SDS for co-immunoprecipitation of NR2A and PSD-95. An amount of 50 μg of total protein extract from medial PFC was immunoprecipitated with anti-NR2A antibody (50 μg, rabbit; Millipore) using a Dynabeads co-immunoprecipitation kit (Life Technologies). The eluted samples were subjected to SDS–PAGE and western blot analysis with anti-NR2A (rabbit, 1 : 500) and anti-PSD-95 antibodies (mouse, 1 : 2000; Millipore), followed by horseradish peroxidase-conjugated anti-mouse secondary antibodies (1 : 2000). Visualization was done using the Western Lightning Plus ECL Kit (PerkinElmer). Optical density (OD) of NR2A and PSD-95 bands was imaged and analyzed using ChemiDoc XRS (Bio-Rad, Waltham, MA) with Image Lab software. In order to normalize the amount of NR2A that was eluted from the beads within each sample, the ratio of PSD-95 OD to NR2A OD was calculated for each subject. Samples were each run twice, and values between both runs were averaged.
A separate cohort of untreated MS and CON animals (n=6) were killed at P40 for western blot analysis of overall NR2A and PSD-95 levels in the medial PFC. Brains were extracted on ice and the medial PFC was prepared as described previously (Holland et al, 2014; Wieck et al, 2013) for western blotting. Membranes were incubated in primary antibodies to NR2A (1 : 500), PSD-95 (1 : 2000), and actin (1 : 30 000, mouse) followed by secondary antibodies. Membranes were imaged as described above, and ODs of NR2A and PSD-95 were normalized to actin. Samples were each run twice, and values between both runs were averaged. Groups were compared using Student's t-test. A regression analysis was run to assess the relationship between NR2A and PSD-95 expression.
Elevated-Plus Maze
Behavioral testing began 24 h following the last microinjection of TAT2A (on P40). The Plexiglas elevated-plus maze (EPM) had four 50 cm × 10 cm arms, two that were enclosed with 40-cm-high walls and two that were open, connected by a 10 cm × 10 cm center square. The plus maze was cleaned with 30% EtOH in between subjects to remove any odor cues. Subjects (n=7–8) were acclimated to the testing room for 10 min before testing, then placed in the central region of the maze under red light, facing the open arm. Behavior was recorded for 5 min by an observer who was blinded against group. Scoring was done on the following: four-paw entries into and time spent in open and closed arms, number of arm crosses, head dippings, and falls. All measures were compared using a two-way (rearing group × treatment) ANOVA. Post hoc Bonferroni t-tests were used to determine pair-wise differences following two-way ANOVA.
Open-Field Behavior
Open-field behavior in a novel arena was assessed 24 h following EPM, during the first 3 min of habituation to the novel object recognition (NOR) chamber (P41), in order to measure novelty-induced locomotion. Time spent and visits to the center of the arena were also analyzed as a second measurement of anxiety-like behavior. Noldus EthoVision software was used to analyze total distance traveled, as well as time spent within and number of visits to a zone comprising a 200-cm2 oval center of the arena. On P42 (the morning of NOR testing), subjects were placed into the same open-field arena and distance travelled was measured over 3 min in order to assess general locomotion in the absence of novelty. Measurements were compared between groups using two way (rearing group × treatment) ANOVAs. Post hoc Bonferroni t-tests were used to determine pair-wise differences following two-way ANOVA.
Novel Object Recognition
To assess cognitive function in adolescence, subjects (n=7-8) were first habituated to an opaque open-field arena (20 cm × 15 cm) for 15 min between 0900 and 1200 hours on P41 in a dimly lit room. Thirty hours later (afternoon of P42), subjects were exposed to the same arena with two identical objects (grooved Lego pieces, 12 × 6 × 4 cm each), and recorded for 3 min with a CCTV camera (Panasonic WV- CP500, Secaucus, NJ) suspended directly above the arena. Each animal was placed in the arena facing away from the objects. Following this familiarization phase, animals were placed in their cages for an intersession interval of 1.5 h before the test phase. The test phase comprised a re-introduction to the arena for 3 min, with one familiar object as well as one novel object (color-taped 12-well tissue culture plates; same dimensions as familiar object). The objects were placed equidistant from two corners and were secured to the arena floor so that the subject could not displace them during exploration. The relative positions of the two stimulus objects (familiar or novel) were randomly permuted for each experimental animal. Exploration was defined as directing the nose toward the object at a distance of no >2 cm. The camera was interfaced with EthoVision (v9.0; Noldus Information Technology, Leesburn VA) to monitor exploration with each object. A blinded experimenter manually scored 50% of subject recordings to assure the validity of automated measurements. The time spent exploring the novel object compared with the time spent exploring the familiar object during the test session was calculated into a Discrimination Index ((time spent with novel object−time spent with familiar object)/(total time exploring both objects); Bevins and Besheer, 2006)). Rearing group × treatment ANOVA compared DI between groups.
PVB and NR2A Immunohistochemistry
For immunohistochemical analyses, 24 h following the last behavioral test (P43; n=7–8/group), TAT-SDV- and TAT2A (500 μM)-treated male rats were euthanized and intracardially perfused, and immunohistochemistry was performed as previously described (Wieck et al, 2013). All subjects were analyzed for PVB expression, whereas only TAT-SDV subjects were analyzed for NR2A expression in order to confirm western blot analyses of MS effects on NR2A in the PFC. Briefly, 40-μm frozen sections were incubated with a monoclonal mouse antibody raised against PVB (1 : 10 000; Sigma) or polyclonal rabbit antibody raised against NR2A (1 : 1000; Millipore) and then with secondary serum conjugated with Alexa 563 fluospheres (Molecular Probes, Grand Island, NY). All steps were preceded and followed by washes in phosphate-buffered saline–Triton X-100. Sections were mounted on gelatin-coated slides, and coverslipped with Fluoromount (Thermo Fisher Scientific, Waltham MA). Stereo Investigator Image Analysis System (MBF BioScience, Williston, Vermont) was used to estimate the density of PVB+ and NR2A+ cells. The ilPFC and plPFC in four serial coronal sections (intersection interval 240 μm)/animal were analyzed (Sherwood and Timeras, 1970). In each section, the entire ilPFC and plPFC were outlined at × 4 magnification, and the total number of PVB-immunoreactive (IR) or NR2A-IR cells were measured at × 40 exclusively within the outlined area. Investigators were strictly blinded to the conditions for all analyses. Tracings of the plPFC and ilPFC boundaries were used for calculation of the surface area, a, in each section. The density of NR2A-IR or PVB-IR (cells/mm2) was based on the total number of cells divided by Σa for each subject (the sum of areas obtained from all outlined regions). NR2A+ cell densities were compared between groups within each region using Student's t-test. PVB+ cell densities were compared between groups using a rearing group × treatment ANOVA. Post hoc Bonferroni t-tests were used to determine pair-wise differences following two-way ANOVA.
RESULTS
Effects of MS on PFC NR2A and PSD-95
Figure 1a illustrates that PSD-95 expression was elevated in the medial PFC of MS-exposed adolescents compared with CON (t[10]=2.663; p=0.028). However, western blots did not observe a significant elevation of NR2A in the medial PFC of MS subjects (Figure 1b). Regression analysis revealed that NR2A expression was positively correlated with PSD-95 expression (R2=0.471; p=0.020; Figure 1c). We followed up western blot analysis of NR2A with immunofluorescent analysis to obtain better subregion resolution and sensitivity. MS-exposed adolescents displayed significantly more NR2A+ cells in both the plPFC (t[13]=2.84; p=0.014) and the ilPFC (t[13]=2.88; p=0.013), compared with CON (Figure 1d).
Co-Immunoprecipitation of NR2A and PSD-95 after TAT2A Treatment
As previously characterized (Aarts et al, 2002; Gardoni et al, 2012; Paille et al, 2010), treatment with TAT2A (500 μM) decreased the interaction of NR2A with PSD-95 (Figure 1e and f). Co-immunoprecipitation of PSD-95 with NR2A in the PFC was reduced by 75.8%, with a relative OD of co-immunoprecipitated PSD-95 of 0.47±0.15 in TAT-SDV-treated animals and 0.11±0.06 in 500 μM TAT2A-treated animals (relative to NR2A OD). Comparatively, co-immunoprecipitation was reduced by 65.5% in 100 μM TAT2A-treated animals, with a relative OD of co-immunoprecipitated PSD-95 of 0.25±0.07 (Figure 1e and f).
Behavior
Elevated-plus maze
Treatment with 500 μM TAT2A, but not 100 μM TAT2A, protected MS-exposed adolescents from anxiety-like behaviors as assessed in the EPM (Figure 2a). As revealed by a significant rearing group × treatment interaction (F[2,35)=4.37; p=0.035), time in open arms was significantly decreased in TAT-SDV-administered MS animals compared with CON animals, whereas MS subjects administered 500 μM TAT2A did not differ from CON animals, and spent significantly more time in open arms compared with TAT-SDV (p=0.016) and 100 μM TAT2A subjects (p=0.007). Crosses between arms was not different between groups, indicating comparative general locomotion (Figure 2b).
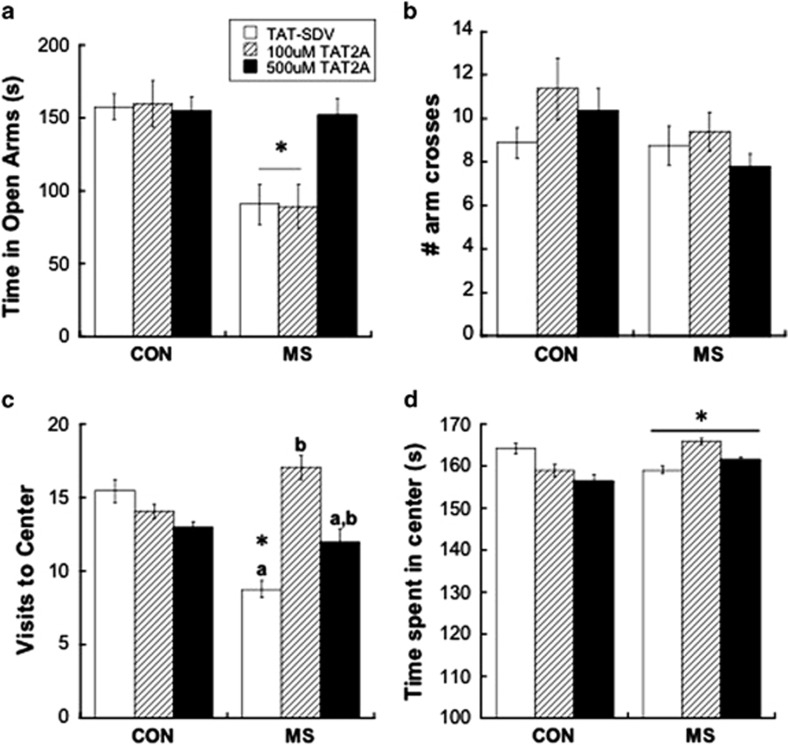
Figure 2.
(a) Treatment with 500 μM TAT2A, but not 100 μM TAT2A, prevented anxiety-like behavior in maternal separation (MS) adolescents, as illustrated by less time spent in the open arms of an elevated plus maze (EPM). *p<0.05 difference from control (CON) group with same treatment; n=7–8. (b) Number of arm crosses over 5 min in the EPM was not affected by rearing group or treatment. (c) Treatment with either 500 μM TAT2A or 100 μM TAT2A prevented anxiety-like behavior in MS adolescents, as illustrated by fewer visits to the center of an open field. *p<0.05 difference from CON group; letters (a,b) represent differences between treatments within the same Rearing group. n=7–8. (d) MS adolescents displayed less time spent in the center of an open field, which was driven by TAT-SDV-treated animals. However, no rearing group × treatment interaction was observed.
Center-time in an open field
As shown in Figure 2c, TAT-SDV-treated MS adolescents made fewer visits to the center of an open field (indicating anxiety-like behavior) compared with CON, which was prevented by administration of either 100 μM TAT2A or 500 μM TAT2A (rearing group × treatment interaction: F[1,40]=3.41; p=0.043). Post hoc t-tests revealed that both low-dose (100 μM) and high-dose (500 μM) TAT2A prevented the significant difference induced by MS rearing compared with CON rearing in TAT-SDV-treated animals (p=0.037). Interestingly, however, low-dose TAT2A administration to MS subjects caused a more robust increase in visits to the center than high-dose TAT2A, illustrated by a significant difference between TAT-SDV treatment and 100 μM TAT2A treatment (p=0.023), but not between TAT-SDV treatment and 500 μM TAT2A treatment. Duration of time spent in the center was also affected by MS rearing (main effect of rearing group: F[1, 23]=6.99; p=0.014; Figure 2d). Although no significant rearing group × treatment interaction was found, the main effect of group was apparently driven by a significant difference between MS and CON in TAT-SDV-administered animals (p=0.035), but not in 100 μM TAT2A- nor 500 μM TAT2A-treated animals (n.s.). Of note, one outlier (Grubb's test p<0.05) was removed from duration analysis in the TAT-SDV MS group.
Novelty-induced locomotion
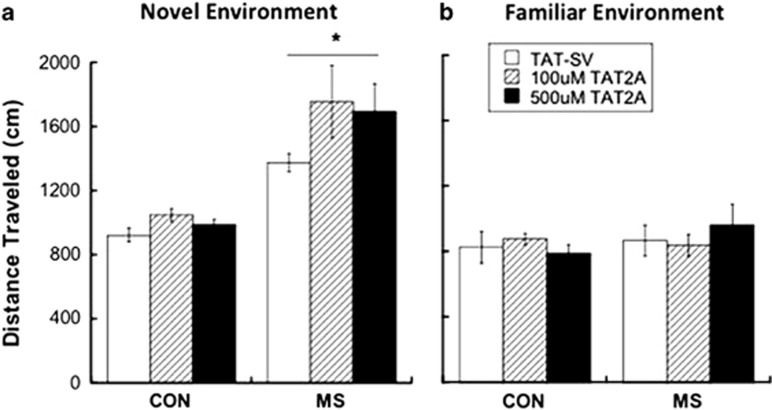
Rearing condition, but not TAT2A administration, affected the locomotor response to a novel environment (Figure 3a). A main effect of rearing condition revealed that MS-exposed adolescents were more active during the first 3 min in a novel arena compared to CON subjects (F[1,26]=6.77; p=0.015). However, a lack of rearing × treatment interaction revealed that treatment with TAT2A did not prevent novelty-induced hyperlocomotion in MS animals. General locomotion was not affected by rearing or by treatment, as there were no group differences in locomotion upon exposure to the same arena the following day (Figure 3b).
Figure 3.
(a) Maternal separation (MS) results in increased novelty-induced locomotion during adolescence, which was not prevented by TAT2A treatment. *p<0.05 difference from control (CON) group. n=7–8 (b) General locomotion in a familiar field was not affected by either rearing condition or treatment.
Novel-object recognition
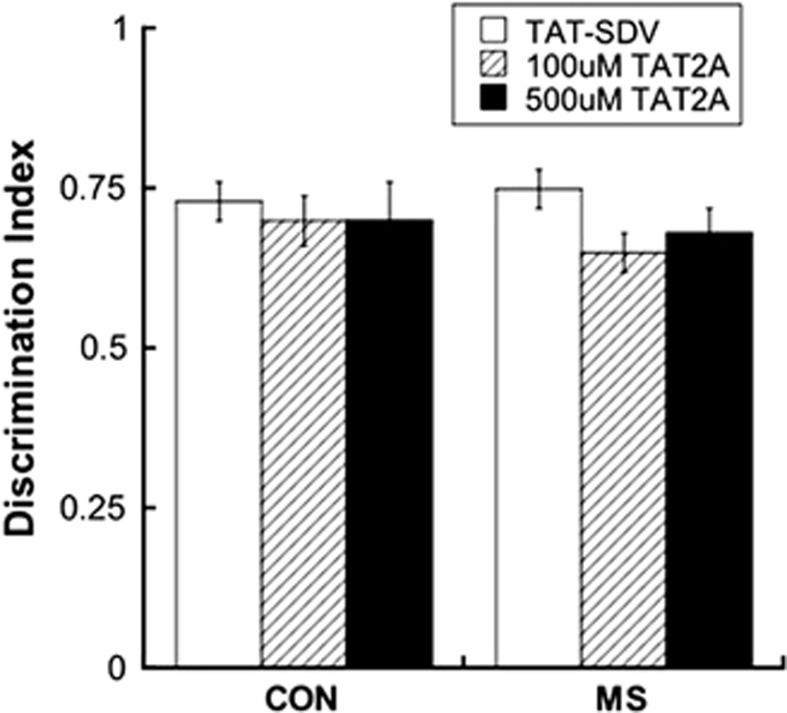
As shown in Figure 4, neither rearing group nor treatment affected adolescent NOR memory as assessed by the NOR.
Figure 4.
Recognition of a novel object was not affected by rearing group or treatment. n=7–8. CON, control; MS, maternal separation.
PFC PVB IR
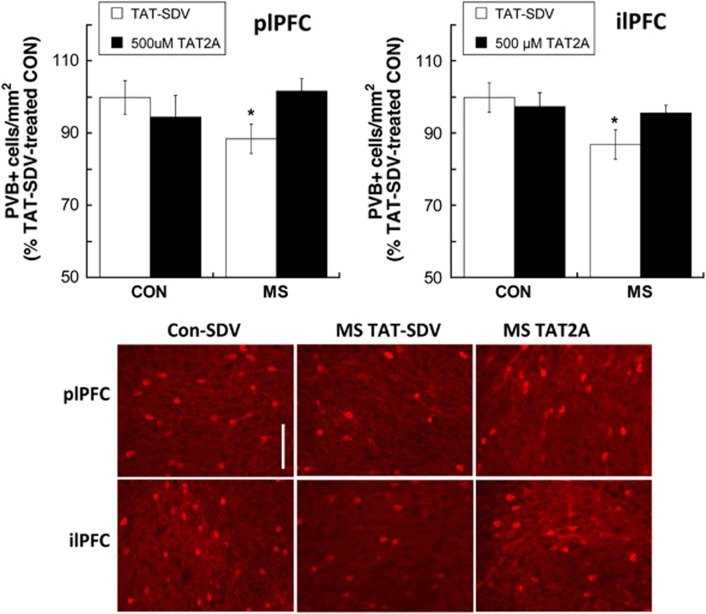
A shown in Figure 5, a significant rearing group × treatment interaction (F[1,24]=4.29; p=0.049) revealed that 500 μM TAT2A prevented an MS-induced reduction in PVB+ interneurons in the ilPFC seen in TAT-SDV-treated animals (p=0.043). TAT2A administration in MS adolescents increased PVB+ interneurons compared with SDV-treated animals (p=0.039). Although no group × treatment interaction was observed in the plPFC, a main effect of group (F[1,26]=4.302; p=0.048) was driven by an MS-induced reduction of PVB+ interneurons in TAT-SDV-treated animals (p=0.03), but not in TAT2A-treated animals. TAT2A administration in MS adolescents increased plPFC PVB+ interneurons compared with SDV-treated animals at a trend level that did not reach statistical significance (p=0.064).
Figure 5.
Treatment with 500 μM TAT2A during early adolescence prevented an maternal separation (MS)-induced decrease in parvalbumin (PVB+) interneurons within the infralimbic prefrontal cortex (PFC) and prelimbic PFC. *p<0.05 difference from control (CON) group with same treatment. Data are presented as % difference from TAT-SDV-treated CON subjects. n=7–8. Bottom: representative photomicrographs of PVB-IR in CON and MS subjects treated with TAT-SDV and an MS subject treated with 500 μM TAT2A. Scale bar, 100 μm.
DISCUSSION
These data are the first to illustrate that increased anxiety-like behaviors in adolescents exposed to MS are mediated by the association of NR2A with PSD-95 within the PFC. In contrast, MS-attributable changes in the locomotor response to novelty do not appear to be regulated by NR2A, as uncoupling of NR2A from PSD-95 did not prevent increased novelty-induced locomotion in an open field. Results also illustrate that MS-induced PVB loss in the medial PFC can be prevented through NR2A manipulation. Together these data implicate NR2A-related NMDAR development in adolescent behavioral and neural consequences of early-life stress.
PSD-95 overexpression in the medial PFC after MS was observed here and previously (Chocyk et al, 2013), which co-occurred with an increase in NR2A. A positive correlation between NR2A and PSD-95 (Figure 2c) further suggests that the elevated PSD-95 was coupled to NR2A. We also observed a decrease in PVB+ interneurons (Wieck et al, 2013) in MS-exposed animals in both the plPFC (as previously described (Brenhouse and Andersen, 2011) and in the ilPFC, as well as increased anxiety-like behaviors (Chocyk et al, 2013; Girardi et al, 2014; Li et al, 2013) and novelty-induced hyperlocomotion (Colorado et al, 2006) in adolescence. Interestingly, postmortem studies have revealed increased phosphorylation of PSD-95 in the frontal cortex of patients with schizophrenia (Beneyto et al, 2007), illustrating the importance of assessing associations between NMDAR subunits with postsynaptic proteins as they relate to disorders.
Our data provides additional evidence that the influence of NR2A on NMDAR function during adolescence has a unique role in interneuron PVB expression. Earlier reports demonstrated that chronic blockade of NR2A-containing NMDAR (but not NR2B-containing NMDAR) during pre-adolescence reduced the density of PVB+ interneurons in the visual cortex, further supporting the importance of proper NR2A function on PVB expression. Here, we demonstrated that uncoupling NR2A from PSD-95 without antagonizing the NMDAR can protect PVB expression in MS animals but not in CON animals. Therefore, targeting NR2A-mediated NMDAR development had discrete protective effects only in MS animals, without gross pharmacological effects.
Notably, the effects we observed from NR2A manipulation may be due to an indirect effect on other NMDAR subunits. Specifically, NR3-containing NMDAR are located in the PFC, and expression of NR2 has been proposed to bidirectionally influence the stoichiometry of NR3-containing NMDAR in the brain (Fukaya et al, 2005). As NR3 changes are implicated in disorders such as schizophrenia and bipolar disorder (Pachernegg et al, 2012), the potential role for NR3 in our observed effects should not be discounted. In general, as adolescence is a period of rapid development of NMDAR, uncoupling NR2A from PSD-95 may also confer compensatory changes that cannot be disentangled from our observed effects. Therefore, future studies will assess consequential changes to other NMDAR subunits in response to NR2A manipulation in adolescence.
If MS yields adolescent changes in PVB and anxiety that can be reversed through early-adolescent NMDAR manipulation, one could hypothesize that MS has early, derailing effects on later NMDA maturation that impacts interneuron development. Although the current study did not target NMDAR on GABAergic interneurons specifically, this hypothesis corroborates with earlier reports that direct, early-postnatal ablation of NR1 in corticolimbic interneurons yields adolescent decreases in PVB, as well as novelty-induced hyperlocomotion and anxiety-like behaviors that first emerge in adolescence (Belforte et al, 2010). Our findings further suggest that derailed NMDAR development after MS can be targeted through the NR2A subunit to prevent PVB loss and anxiety-like behavior. Future studies should aim to determine whether NR2A coupling to PSD-95 is increased on PVB+ interneurons specifically, and whether cell-specific targeting of NR2A is sufficient to prevent MS effects.
An additional unanswered question is whether there is a discrete critical period for NR2A-controlled activity after MS, as all of our subjects received TAT2A during a broad timespan of early adolescence (P31-P39). Although developmental influences of NR2A specifically within the PFC have not been widely examined, NMDAR in the barrel cortex have been shown to shift from NR2B-mediated EPSPs toward more NR2A-mediated EPSP during the early-pre-adolescent period (P20–P30; Wang and Gao, 2009). Therefore, influences of TAT2A may be more robust during the earlier transition from juvenility to adolescence. This hypothesis is currently being tested in our laboratory.
NR2A manipulation during early adolescence prevented heightened anxiety-like behaviors in MS subjects, suggesting that NMDAR dysfunction within the medial PFC during this time period contributes to MS-attributable anxiety. Anxiety-like behaviors coupled with ilPFC PVB loss after MS corroborates previous findings that enhanced excitability via GABA blockade in the ilPFC produces anxiety-like behaviors in adult rodents (Bi et al, 2013). The current report is the first demonstration to our knowledge of a subunit-specific manipulation affecting anxiety-like behavior, which revealed that the association of NR2A with PSD-95 after MS can be targeted to prevent anxiety. Interestingly, different behavioral assessments of anxiety demonstrated differential effects of TAT2A treatment; high-dose TAT2A (500 μM) was required to prevent anxiety-like behavior in the EPM, whereas low-dose TAT2A (100 μM) was more effective at increasing center visits in an open field (Figure 3). This differential dose response between two tests of anxiety highlights important differences in behavioral dimensions that each test weighs differently (Carola et al, 2002). In addition, novelty-induced hyperlocomotion is a behavioral response that requires PFC recruitment and is observed after MS (current report and (Colorado et al, 2006)), but was not prevented by TAT2A administration. Therefore, response to novelty after MS represents a separate behavioral construct with distinct neural CON from anxiety-like behaviors; indeed, novelty-induced hyperlocomotion has been previously shown to occur despite PFC NMDAR deficits (Rompala et al, 2013).
We report here that while anxiety-like behavior and novelty-induced hyperlocomotion are adolescent consequences of MS, recognition memory in adolescence as assessed in the NOR task was not affected by MS. This was the first examination to our knowledge of repeated MS effects on adolescent NOR; however, previous studies have investigated effects of either a single 24-h maternal deprivation on adolescents (Marco et al, 2013) or repeated MS effects in adults (Hill et al, 2014), which have also failed to observe disruption of NOR in males. The lack of adolescent and adult effects of MS on NOR highlight the claim that an intact medial PFC is not necessary for successful NOR (Nelson et al, 2011). In contrast, our group and others have reported that MS results in other cognitive disturbances in adolescence that do rely on medial PFC function, such as long-term working memory (Brenhouse and Andersen, 2011; Wieck et al, 2013) and prepulse inhibition of startle (Li et al, 2013). Therefore, MS affects PVB density and PFC-controlled behaviors during adolescence, while having less of an apparent effect during adolescence on behaviors that do not require medial PFC function, such as NOR and general locomotion in a familiar environment (current report, and Marin and Planeta (2004)). It is widely accepted that MS leads to dysregulation of the HPA axis (eg, Meaney et al (1996)), therefore it follows that a wider array of MS effects are seen in behaviors assessed in response to a subsequent stressor (eg, Aisa et al (2007)).
Taken together, we report here that MS disrupts adolescent PFC PVB expression and facilitates anxiety-like behaviors and response to novelty but not NOR. Transduction of medial PFC neurons with TAT2A during early adolescence uncoupled the NR2A NMDAR subunit from PSD-95, preventing adolescent anxiety and PVB loss, but not adolescent novelty-induced hyperlocomotion. Therefore, the NR2A-PSD-95 complex and its influence on NMDAR during early adolescence can be targeted to prevent discrete adolescent maladaptations after MS. Furthermore, individual differences in NR2A/PSD-95 association may account for psychological resilience in some exposed to early-life stress.
FUNDING AND DISCLOSURE
The work was funded by NIMH 5R21MH097182-02. The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW et al (2002). Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science 298: 846–850. [DOI] [PubMed] [Google Scholar]
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H et al (1999). Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry 4: 163–172. [DOI] [PubMed] [Google Scholar]
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ (2007). Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology 32: 256–266. [DOI] [PubMed] [Google Scholar]
- Balu DT, Coyle JT (2014). The NMDA receptor 'glycine modulatory site' in schizophrenia: d-serine, glycine, and beyond. Curr Opin Pharmacol 20C: 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y et al (2010). Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci 13: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH (2007). Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology 32: 1888–1902. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J (2006). Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory'. Nat Protoc 1: 1306–1311. [DOI] [PubMed] [Google Scholar]
- Bi LL, Wang J, Luo ZY, Chen SP, Geng F, Chen YH et al (2013). Enhanced excitability in the infralimbic cortex produces anxiety-like behaviors. Neuropharmacology 72: 148–156. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Lim MP, Woo TU (2010). N-methyl-D-aspartate receptor expression in parvalbumin-containing inhibitory neurons in the prefrontal cortex in bipolar disorder. Bipolar Disord 12: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A (2004). Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci 19: 1863–1874. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL (2011). Nonsteroidal anti-inflammatory treatment prevents delayed effects of early life stress in rats. Biol Psychiatry 70: 434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carola V, D'Olimpio F, Brunamonti E, Mangia F, Renzi P (2002). Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res 134: 49–57. [DOI] [PubMed] [Google Scholar]
- Chocyk A, Bobula B, Dudys D, Przyborowska A, Majcher-Maslanka I, Hess G et al (2013). Early-life stress affects the structural and functional plasticity of the medial prefrontal cortex in adolescent rats. Eur J Neurosci 38: 2089–2107. [DOI] [PubMed] [Google Scholar]
- Colorado RA, Shumake J, Conejo NM, Gonzalez-Pardo H, Gonzalez-Lima F (2006). Effects of maternal separation, early handling, and standard facility rearing on orienting and impulsive behavior of adolescent rats. Behav Processes 71: 51–58. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M (2001). NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 11: 327–335. [DOI] [PubMed] [Google Scholar]
- Davis M (2011). NMDA receptors and fear extinction: implications for cognitive behavioral therapy. Dialogues Clin Neurosci 13: 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya M, Hayashi Y, Watanabe M (2005). NR2 to NR3B subunit switchover of NMDA receptors in early postnatal motoneurons. Eur J Neurosci 21: 1432–1436. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Sgobio C, Pendolino V, Calabresi P, Di Luca M, Picconi B (2012). Targeting NR2A-containing NMDA receptors reduces L-DOPA-induced dyskinesias. Neurobiol Aging 33: 2138–2144. [DOI] [PubMed] [Google Scholar]
- Girardi CE, Zanta NC, Suchecki D (2014). Neonatal stress-induced affective changes in adolescent Wistar rats: early signs of schizophrenia-like behavior. Front Behav Neurosci 8: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NW, Weimer RM, Bureau I, Svoboda K (2006). Rapid redistribution of synaptic PSD-95 in the neocortex in vivo. PLoS Biol 4: e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ et al (2013). Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci USA 110: 19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Klug M, Kiss Von Soly S, Binder MD, Hannan AJ, van den Buuse M (2014). Sex-specific disruptions in spatial memory and anhedonia in a "two hit" rat model correspond with alterations in hippocampal brain-derived neurotrophic factor expression and signaling. Hippocampus 24: 1197–1211. [DOI] [PubMed] [Google Scholar]
- Holland FH, Ganguly P, Potter DN, Chartoff EH, Brenhouse HC (2014). Early life stress disrupts social behavior and prefrontal cortex parvalbumin interneurons at an earlier time-point in females than in males. Neurosci Lett 566C: 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng JW, Ryu V, Yoo SB, Noh SJ, Kim JY, Lee JH (2010). Mesolimbic dopaminergic activity responding to acute stress is blunted in adolescent rats that experienced neonatal maternal separation. Neuroscience 171: 144–152. [DOI] [PubMed] [Google Scholar]
- Kessler R, Davis C, Kendler K (1997). Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol Med 27: 1101–1119. [DOI] [PubMed] [Google Scholar]
- Kohut S, Roma P, Davis C, Zernig G, Saria A, Dominguez J et al (2009). The impact of early environmental rearing condition on the discriminative stimulus effects and Fos expression induced by cocaine in adult male and female rats. Psychopharmacology 203: 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M (2014). Schizophrenia: from dopaminergic to glutamatergic interventions. Curr Opin Pharmacol 14: 97–102. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Feldon J (2000). Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Rev Neurosci 11: 383–408. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Freund N, Brenhouse HC, Thompson BS, Andersen SL (2012). Depressive-like behavior in adolescents after maternal separation: sex differences, controllability, and GABA. Dev Neurosci 34: 210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Xue X, Shao S, Shao F, Wang W (2013). Cognitive, emotional and neurochemical effects of repeated maternal separation in adolescent rats. Brain Res 1518: 82–90. [DOI] [PubMed] [Google Scholar]
- Lin Y, Skeberdis VA, Francesconi A, Bennett MV, Zukin RS (2004). Postsynaptic density protein-95 regulates NMDA channel gating and surface expression. J Neurosci 24: 10138–10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri S, Laviola G, Leussis MP, Andersen SL (2009). Abnormal behavioral and neurotrophic development in the younger sibling receiving less maternal care in a communal nursing paradigm in rats. Psychoneuroendocrinology 35: 392–402. [DOI] [PubMed] [Google Scholar]
- Marco EM, Valero M, de la Serna O, Aisa B, Borcel E, Ramirez MJ et al (2013). Maternal deprivation effects on brain plasticity and recognition memory in adolescent male and female rats. Neuropharmacology 68: 223–231. [DOI] [PubMed] [Google Scholar]
- Marin MT, Planeta CS (2004). Maternal separation affects cocaine-induced locomotion and response to novelty in adolescent, but not in adult rats. Brain Res 1013: 83–90. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C et al (1996). Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci 18: 49–72. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Cooper MT, Thur KE, Marsden CA, Cassaday HJ (2011). The effect of catecholaminergic depletion within the prelimbic and infralimbic medial prefrontal cortex on recognition memory for recency, location, and objects. Behav Neurosci 125: 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M, Kim E, Sheng M (1996). Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci 16: 2157–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachernegg S, Strutz-Seebohm N, Hollmann M (2012). GluN3 subunit-containing NMDA receptors: not just one-trick ponies. Trends Neurosci 35: 240–249. [DOI] [PubMed] [Google Scholar]
- Paille V, Picconi B, Bagetta V, Ghiglieri V, Sgobio C, Di Filippo M et al (2010). Distinct levels of dopamine denervation differentially alter striatal synaptic plasticity and NMDA receptor subunit composition. J Neurosci 30: 14182–14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, Zsiros V, Zhang S, Kolata SM, Nakazawa K (2013). Contribution of NMDA receptor hypofunction in prefrontal and cortical excitatory neurons to schizophrenia-like phenotypes. PloS One 8: e61278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotaru DC, Lewis DA, Gonzalez-Burgos G (2012). The role of glutamatergic inputs onto parvalbumin-positive interneurons: relevance for schizophrenia. Rev Neurosci 23: 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Kalia LV (2004). Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci 5: 317–328. [DOI] [PubMed] [Google Scholar]
- Sherwood N, Timeras P (1970) A Stereotaxic Atlas of the Developing Rat Brain. University of California Press: Los Angeles, CA, USA: Los Angeles, CA, USA. [Google Scholar]
- Teicher MH, Samson JA, Polcari A, Andersen SL (2009). Length of time between onset of childhood sexual abuse and emergence of depression in a young adult sample: a retrospective clinical report. J Clin Psychiatry 70: 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Tomoda A, Andersen SL (2006). Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann N Y Acad Sci 1071: 313–323. [DOI] [PubMed] [Google Scholar]
- Turnock-Jones JJ, Jennings CA, Robbins MJ, Cluderay JE, Cilia J, Reid JL et al (2009). Increased expression of the NR2A NMDA receptor subunit in the prefrontal cortex of rats reared in isolation. Synapse 63: 836–846. [DOI] [PubMed] [Google Scholar]
- Wall PM, Blanchard RJ, Markham C, Yang M, Blanchard DC (2004). Infralimbic D1 receptor agonist effects on spontaneous novelty exploration and anxiety-like defensive responding in CD-1 mice. Behav Brain Res 152: 67–79. [DOI] [PubMed] [Google Scholar]
- Wang HX, Gao WJ (2009). Cell-type specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology 34: 2028–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieck A, Andersen SL, Brenhouse HC (2013). Evidence for a neuroinflammatory mechanism in delayed effects of early life adversity in rats: relationship to cortical NMDA receptor expression. Brain Behav Immun 28: 218–226. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Sun QQ (2011). Development of NMDA NR2 subunits and their roles in critical period maturation of neocortical GABAergic interneurons. Dev Neurobiol 71: 221–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Du CP, Peng Y, Xu Z, Sun CC, Liu Y et al (2014). The upregulation of NR2A-containing N-methyl-D-aspartate receptor function by tyrosine phosphorylation of postsynaptic density 95 via facilitating Src/Proline-rich tyrosine kinase 2 activation. Mol Neurobiol 51: 500–511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.