Abstract
Drug dependence may be at its core a pathology of choice, defined by continued decisions to use drugs irrespective of negative consequences. Despite evidence of dysregulated decision making in addiction, little is known about the neural processes underlying the most clinically relevant decisions drug users make: decisions to use drugs. Here, we combined functional magnetic resonance imaging (fMRI), machine learning, and human laboratory drug administration to investigate neural activation underlying decisions to smoke cannabis. Nontreatment-seeking daily cannabis smokers completed an fMRI choice task, making repeated decisions to purchase or decline 1–12 placebo or active cannabis ‘puffs' ($0.25–$5/puff). One randomly selected decision was implemented. If the selected choice had been bought, the cost was deducted from study earnings and the purchased cannabis smoked in the laboratory; alternatively, the participant remained in the laboratory without cannabis. Machine learning with leave-one-subject-out cross-validation identified distributed neural activation patterns discriminating decisions to buy cannabis from declined offers. A total of 21 participants were included in behavioral analyses; 17 purchased cannabis and were thus included in fMRI analyses. Purchasing varied lawfully with dose and cost. The classifier discriminated with 100% accuracy between fMRI activation patterns for purchased vs declined cannabis at the level of the individual. Dorsal striatum, insula, posterior parietal regions, anterior and posterior cingulate, and dorsolateral prefrontal cortex all contributed reliably to this neural signature of decisions to smoke cannabis. These findings provide the basis for a brain-based characterization of drug-related decision making in drug abuse, including effects of psychological and pharmacological interventions on these processes.
INTRODUCTION
A defining feature of problematic drug use is continued decisions to use drugs despite adverse consequences. While moral views emphasize the voluntary nature of these decisions and individual responsibility (Hyman, 2007), biomedical explanations highlight compulsivity in problem drug use (Ersche et al, 2011). From this perspective, drug-related neuroadaptations to cortical and subcortical circuitry underpinning decision making are thought to result in suboptimal choices and behavior. Indeed, addiction has been argued to be, at core, a pathology of decision making (Redish et al, 2008).
Consistent with this, altered decision making has been documented in studies of cocaine, alcohol, and heroin abusers relative to nondrug users using monetary reward and gambling paradigms (Kim et al, 2011; Kjome et al, 2010; Lane et al, 2010; Li et al, 2013). These studies suggest that problematic use of different drugs is associated with preferences for immediately rewarding, but ultimately disadvantageous, choices.
Although one study found that cannabis smokers did not show the discounting of delayed rewards characteristic of other substance abusers (Johnson et al, 2010), others have found that frequent cannabis smokers, like other drug-abusing groups, make more choices for disadvantageous high-risk, high-gain monetary options than nonusers (Bolla et al, 2005; Vaidya et al, 2012; Whitlow et al, 2004). Studies of neural substrates indicate that cannabis users have decreased orbitofrontal and dorsolateral prefrontal cortical (dlPFC; Bolla et al, 2005) and increased cerebellum (Bolla et al, 2005; Vaidya et al, 2012) and ventromedial prefrontal cortex (vmPFC; Vaidya et al, 2012) recruitment during monetary decision making relative to controls. Furthermore, blunted responses to negative consequences (ie, loss) in prefrontal and anterior cingulate cortex (ACC) may subserve slower strategy development during monetary decision making in cannabis smokers (Wesley et al, 2011).
Thus, research has examined behavioral and underlying neurocircuit-level alterations to monetary decision making in cannabis smokers and drug-abusing populations more generally. Clearly the most clinically relevant decisions made in problematic drug use, however, are decisions to buy and use drugs. Despite a large literature employing behavioral economic approaches to study drug-related decision making (eg, see Bickel et al, 2014), little is known about the neural processes supporting such decisions. To our knowledge, only two studies have directly examined the neural substrates of drug-related decision making. One examined choices between delayed and immediate cigarette and monetary rewards, finding that choices for delayed relative to immediate reinforcement across commodities were associated with increased medial prefrontal cortex (PFC) and anterior insula cortex activation. Choices for cigarettes were associated with greater activation in left inferior PFC and bilateral posterior parietal cortex and reduced activity in ventral striatum and right inferior PFC relative to choices about money (MacKillop et al, 2012).
A recent study employed a demand economic analysis to assess neural activation underlying decisions to drink alcohol at different prices, finding that decisions to drink were associated with greater activation in bilateral posterior parietal regions, dlPFC, posterior cingulate cortex (PCC), and left anterior insula compared with decisions to decline alcohol. Conversely, decisions to drink that were affected by cost were associated with greater activation in frontostriatal regions and deactivation in default mode (Mackillop et al, 2014).
These findings are broadly consistent with evidence about other types of value-based decision making, which indicate that a distributed network of brain regions underpins decisions of this type, with key nodes in this network including orbitofrontal/vmPFC (Kable and Glimcher, 2009), dlPFC (Hare et al, 2009), ACC (Kennerley et al, 2011), and ventral striatum (Peters and Buchel, 2009). Although there is substantial overlap in the neural systems subserving decision making about different types of rewards (Levy and Glimcher, 2011), there also appear to be distinctions dependent on reward type (Levy and Glimcher, 2011; Sescousse et al, 2010), indicating that domain-specific as well as more generalized choice networks may be involved in decisions about drugs. Characterizing value-based decision making in problem drug use will likely be key to understanding the pathophysiology of drug use disorders (see Volkow et al, 2010). Moreover, investigating the neurobehavioral processes underpinning decision making about drugs may elucidate the ways in which pharmacological and psychological interventions can shift these decisions—the ultimate goal of addiction research.
To date, no research has assessed the neural processes underlying decisions about illegal drugs, and no research has employed multivariate analytic approaches to examine distributed neural activation patterns supporting drug-related decision making. Here, we combined a novel functional magnetic resonance imaging (fMRI) choice task with laboratory cannabis self-administration and multivoxel pattern classification to develop a neural signature, or functional biomarker, of decisions to smoke cannabis. We aimed to contribute to development of a brain-based understanding of drug-related decision making. We studied cannabis smokers because, despite being the most commonly used illegal drug (SAMHSA, 2011), research into problematic cannabis use lags behind research on other drugs (Editorial Board, 2014). Based on recent evidence elucidating the neural basis of value-based decision making (Kable and Glimcher, 2009; Levy et al, 2011) we tested the hypothesis that this approach would allow us to identify a brain-based classifier capable of accurately identifying brain activity underlying decisions to smoke cannabis.
MATERIALS AND METHODS
Participants
Physically and psychiatrically (other than cannabis use disorders) healthy nontreatment-seeking cannabis smokers were recruited. Eligible participants were right-handed, 18–50 years old, and smoked ≥2 cannabis cigarettes/day on ≥4 days/week. Before this study, participants passed screening for, and most (17 of 21) completed, one of three residential laboratory studies, results from two of which are published (Haney et al, 2013a, b). The three residential studies involved recruitment of nontreatment seekers for experimental studies (ie, not for treatment). One of these studies examined interactions between cigarette and cannabis smoking (Haney et al, 2013a), whereas the other two tested the effects of candidate treatment medications for cannabis use disorders (nabilone; Haney et al, 2013b) and comorbid cannabis and cigarette smoking (nabilone, varenicline; data collection ongoing) in a human laboratory model of cannabis withdrawal and relapse (see Supplementary Information). The residential studies involved some days on which participants were administered active (5.6% THC) or placebo (0.0% THC) cannabis, and some days on which they were required to purchase active or placebo cannabis for self-administration using study earnings. A minimum of 10 days passed between completion of the residential study and entry into the present study. Although the candidate treatment medications altered cannabis self-administration while participants were maintained on them, there is no evidence suggesting residual effects on cannabis self-administration after stopping the medication in these nontreatment-seeking participants.
All participants provided written informed consent. After study completion, they were debriefed and paid according to procedures approved by the New York State Psychiatric Institute (NYSPI) IRB.
Experimental Protocol
After screening, participants underwent two cannabis administration sessions in each of which they sampled a different cannabis dose, ‘dose A' (active, 5.5% or 5.6% THC) and ‘dose B' (placebo, 0.0% THC); order of sampling sessions was counterbalanced. Cannabis was administered according to a standardized puffing procedure, in which participants inhaled for 5 s and held the smoke for 10 s before exhaling (Foltin et al, 1987). Participants were informed that the strength of ‘dose A' and ‘dose B' would remain constant throughout the study. Following sampling, most participants completed a residential protocol lasting from 30 to 38 days. In the residential protocols, participants were repeatedly exposed to, and made decisions about, the same doses of cannabis, also referred to in the residential protocols as ‘dose A' and ‘dose B.' The purpose of the initial sampling sessions was thus to expose participants to the strength of the cannabis about which they would subsequently be making decisions in the residential protocols and in the present study. Following the residential studies, participants completed the current study, consisting of a single 10-h outpatient session.
Participants were instructed not to smoke cannabis or cigarettes from midnight the night before the session. At arrival, they underwent urine toxicology and a breathalyzer test to confirm that they were not affected by alcohol and had not recently used drugs other than cannabis. Female participants were screened for pregnancy. Breakfast was provided. Because cigarette abstinence increases self-administration of placebo cannabis in cigarette and cannabis smokers (Haney et al, 2013a), participants could smoke a single tobacco cigarette after breakfast.
Participants were then escorted to the NYSPI MRI Research Center and given $100 in imitation money to be exchanged for study payment at completion. They were trained in the Cannabis Choice Task (CCT; see below). They were informed that: (1) dose A and dose B in the CCT remained the same as in the sampling sessions; (2) they would make repeated decisions to buy or decline 1–12 puffs of dose A or dose B cannabis for varying costs; (3) at scan completion, one decision would be randomly selected and implemented; and (4) because they did not know which item would be selected, their best strategy was to treat each decision as if it were real (see Levy and Glimcher, 2011). The 10 h session was intended to increase the desirability of the cannabis choices; with a shorter session, participants could easily wait until the session finished to smoke their own cannabis.
Cannabis choice task
We used a novel event-based fMRI task similar to Purchase Tasks (Mackillop et al, 2009), in which participants were offered from 1 to 12 puffs of active (dose A) or placebo (dose B) cannabis for prices ranging from USD $0.25 to $5 per puff. Trials consisted of: (1) presentation of the dose (A or B) and amount (in puffs) of cannabis available; (2) a rating screen on which participants rated their desire to smoke that amount of cannabis after the scan on a scale from 1 (not at all) to 4 (very much) before the presentation of prices; (3) a ‘buy' screen that presented the cost per puff and total cost and required subjects to make a yes/no purchasing decision; and a strength of decision screen, on which they rated the strength of their decision on a scale from 1 (strong no) to 4 (strong yes). The task was separated into four sections of approximately 8 min and 36 s length each. The order of trials within each section was randomized (see Supplementary Information).
Cannabis administration
Following scanning, one trial was randomly selected for implementation. If, in the trial selected, the participant had purchased cannabis, the cost was deducted from study payment and the cannabis was administered that afternoon, with up to 3 puffs administered at once and 1.5 h between smoking times. If, in the selected item, the participant had not purchased cannabis, they remained in the laboratory without cannabis and did not forgo any payment. They were provided with lunch and could read or listen to music. During the afternoon, cigarette-smoking participants were allowed to smoke cigarettes ad libitum. At 10 h after arriving, participants were required to pass a sobriety test if they had smoked cannabis. They received compensation (minus any cannabis costs) and were discharged.
MRI acquisition parameters
MRI data were collected on a GE 3 Telsa Signa magnet. Blood oxygenation level-dependent (BOLD) functional images were collected using 30 sequential oblique (oriented ∼15° from the AC–PC line) 3.5 mm thick slices with a 0.5 mm gap using a T2*-sensitive single-shot gradient echo-planar imaging sequence (repetition time=2000 ms; echo=30 ms; flip angle=77° 64 × 64 matrix; FOV=240 mm). Each series started with six dummy volumes to develop signal equilibrium. A high-resolution T1-weighted anatomical scan was also collected.
Analyses: behavioral data
Behavioral analysis was undertaken with SPSS 20 (IBM, Armonk, NY). A repeated measures analysis of variance (ANOVA) with dose (active vs placebo) and puff cost as the two factors assessed the effects of these variables on the proportion of offers purchased. We followed up significant interactions between dose and cost with post hoc pairwise comparisons between doses A and B at each price. The significance level was 0.05 for the overall ANOVA and main effects and 0.01 for pairwise comparisons. A repeated measures t-test assessed the effect of dose on ratings of desire to smoke the cannabis offered.
Analyses: fMRI data
Standard preprocessing was undertaken using Statistical Parametric Mapping 8 (SPM8; Wellcome Trust Center for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/; see Supplementary Information). The fMRI analysis was based on recent work that identified a neural signature of physical pain (Wager et al, 2013). For each participant, we conducted a general linear model (GLM) in SPM8 including regressors for conditions of interest (ie, the ‘buy' decision epoch—the period in which decisions to purchase or decline cannabis were made—for purchased and declined offers convolved with the canonical hemodynamic response function) and conditions of no interest (motion regressions), with a high-pass filter of 128 s. This allowed us to summarize brain activation in each voxel for purchased vs declined offers. A machine learning regression approach, Least Absolute Shrinkage and Selection Operator-Regularized Principal Components Regression (LASSO-PCR; Wager et al, 2011), was applied in MATLAB (MathWorks, Natick, MA) to generate the whole-brain pattern of regression weights (the neural signature) that best differentiated between purchased and declined fMRI activation maps. We employed a leave-one-out cross validation approach, with data from 16 participants used as training data to generate the neural signature that was then applied prospectively to differentiate brain activity associated with purchased from declined cannabis offers in the left-out participant; data from each participant were sequentially excluded from the derivation of the signature to serve as the predicted data set. Prediction involved computation of the cross-product of the out-of-training-sample individual's activation maps and the signature pattern derived from the other 16 participants to estimate the signature response in each individual's activity maps. Predictions were then made using binary forced-choice discrimination, such that the activation map in which the signature was most strongly expressed was classified as purchased, whereas the other activation map was classified as declined. This approach yielded a decision accuracy rate reflecting the number of participants in which the signature could correctly classify the activation maps. To visualize and interpret the neural signature, we employed bootstrap tests to identify voxels contributing reliably to the signature, generating 5000 bootstrap samples and running LASSO-PCR on each sample. We calculated uncorrected p-values for each voxel and subsequently applied false discovery rate (FDR) correction (q<0.1). Whereas family-wise error rate (FWER) procedures are designed to control the probability of obtaining one or more false positives, FDR procedures are instead designed to provide control over the expected proportion of false positives. Hence, FDR controlling procedures provide more power at the cost of somewhat increased false positive rates. In particular, for highly correlated tests (such as the ones observed in brain imaging), FWER controlling procedures (like the Bonferroni procedure) tend to be overly conservative, and FDR controlling procedures provide an attractive alternative (see Lindquist and Mejia, 2015). This threshold was employed for display and interpretation only; for prediction, weights from all voxels were used.
RESULTS
Behavioral Data
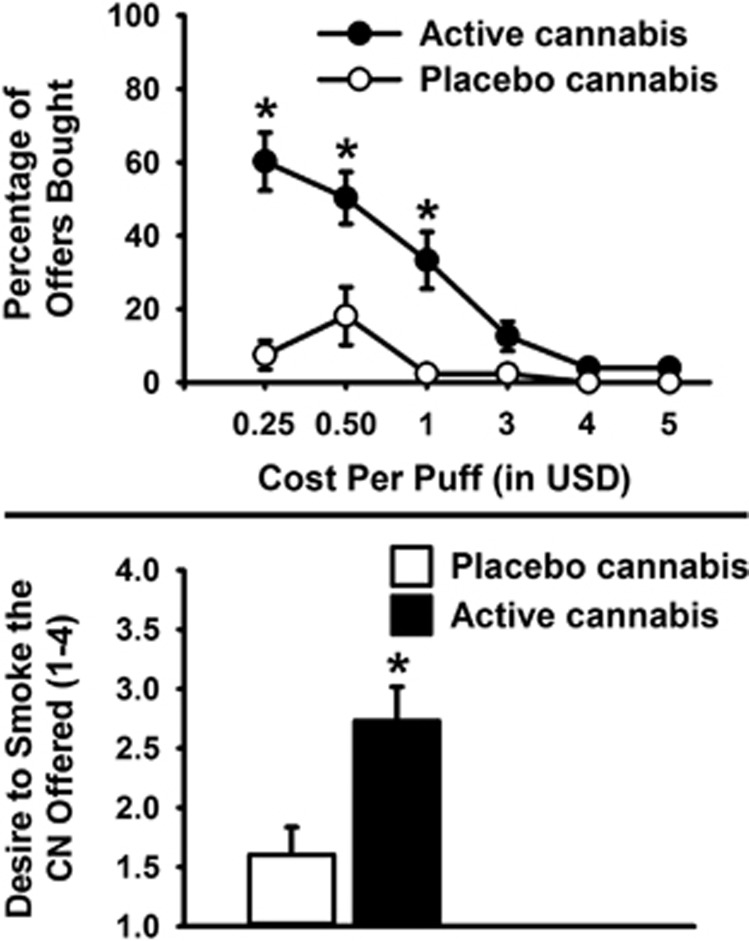
A total of 21 participants (5 female and 16 male) who reported smoking cannabis daily completed the study (see Table 1). As expected, participants bought more active than placebo cannabis (F(1, 20)=19.9, p<0.001, partial η2=0.50). There was a main effect of cost, with the proportion of purchased choices decreasing as a function of increasing cost (F(2.1, 42.5)=30.4, p<0.001, partial η2=0.60). There was an interaction between dose and cost (F(2.5, 50.3)=12.7, p<0.001, partial η2=0.39) such that active cannabis was purchased more often than placebo at the lower ($0.25, $0.50, and $1) but not at the higher puff prices ($3, $4, and $5; see Figure 1a). Participants reported that they wanted to smoke offers containing active cannabis more than those containing placebo cannabis, irrespective of price (t(17)=4.2, p<0.001, partial η2=0.51; Figure 1b).
Table 1. Participant Characteristics.
| Number | % | |
|---|---|---|
| Sex, female | 5 | 24 |
| Race, Black | 16 | 76 |
| Ethnicity, Hispanic | 6 | 29 |
| Mean | SD | |
| Age | 31.0 | 8.3 |
| Education (years) | 12.3 | 1.2 |
| Cannabis (days/week)a | 6.9 | 0.3 |
| Cannabis (‘blunts'/day)a | 3.6 | 2.3 |
| Alcohol (days/week)a,b | 1.2 | 1.5 |
| Cigarettes (per day)a,c | 10.3 | 6.0 |
‘Blunts'=cannabis in cigar wraps.
In the past month.
N=13 reporting past month alcohol use.
N=20 reporting past month cigarette smoking.
Figure 1.
(a) Percentage of choices bought for self-administration as a function of cannabis dose (placebo 0.0% THC vs active 5.5–5.6% THC) and cost per puff (in USD). Data are mean percentages (±SEM). Significant differences between doses, *p<0.01. (b) Effects of dose (placebo 0.0% THC vs active 5.5–5.6% THC) on ratings of desire to smoke the cannabis in each offer, as rated before presentation of item cost. Data are mean ratings (±SEM) on a scale from 1 to 4. Significant difference between doses, *p<0.05. CN, cannabis.
Neural Signature of Decisions to Smoke Cannabis
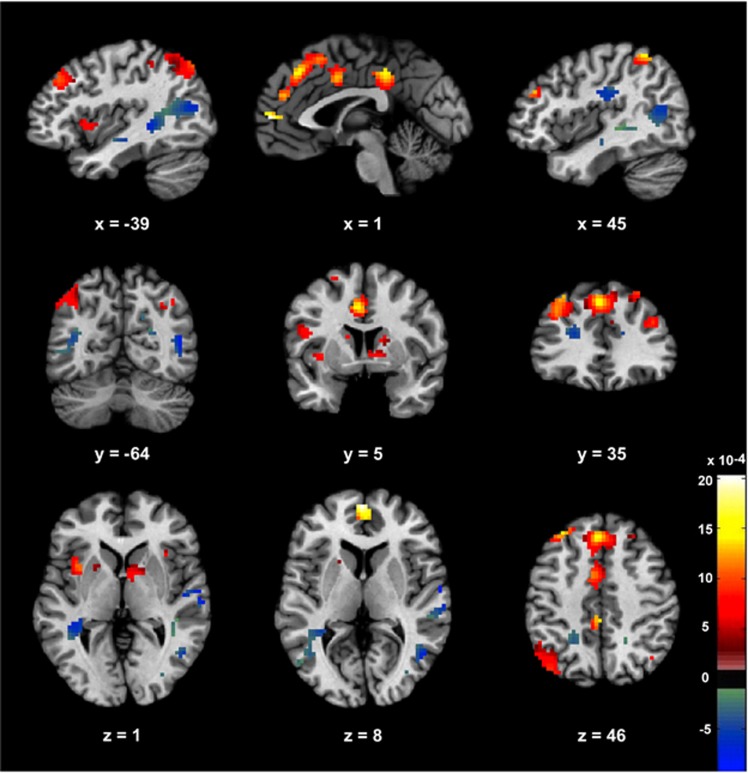
Four participants (1 female and 3 males) did not purchase cannabis; their data were removed from analyses of neural activation during purchased vs declined cannabis choices because the forced-choice classification method employed relied on access to mean activation maps for both purchased and declined cannabis for each individual. The neural signature of bought vs declined cannabis choices, thresholded for display and interpretation only, is presented in Figure 2. The signature comprised a distributed network of regions, with activity in bilateral dorsal striatum, bilateral inferior parietal lobule (IPL), bilateral dlPFC, ACC, PCC, medial superior frontal gyrus, and left anterior insula all predicting decisions to smoke cannabis. Conversely, bilateral posterior parietal cortex including precuneus, bilateral middle and right superior temporal gyrus, bilateral medial frontal gyrus, and right postcentral gyrus extending into IPL all predicted decisions to decline cannabis. A full list of regions reliably contributing to classification is presented in Table 2.
Figure 2.
The signature map consisting of voxels that reliably contributed to prediction of decisions to smoke or to decline cannabis. The signature was subject to FDR correction at q<0.1 for display purposes only; all voxels contributed to prediction. Areas that predicted decisions to buy cannabis are weighted positively, whereas negatively weighted regions predicted decisions to decline cannabis.
Table 2. Clusters Contributing to the Neural Signature (Purchased vs Declined Cannabis).
| Name | Size (mm3) | x | y | z | Name | Size (mm3) | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
|
Purchasing CN: positive predictive weights |
Purchasing CN: negative predictive weights |
||||||||
| SFG, medial | 11 772 | 0 | 29 | 43 | L MTG | 6237 | −39 | −58 | 10 |
| R Medial FG/ACG | 6 | 44 | 28 | L MTG | −42 | −61 | 10 | ||
| MCG | −3 | 5 | 40 | L MTG | −39 | −70 | 13 | ||
| SFG, medial | 0 | 32 | 46 | R STG | 2700 | 54 | −13 | 1 | |
| R SFG | 3 | 23 | 55 | R STG | 51 | −13 | −5 | ||
| L IPL | 4104 | −42 | −64 | 49 | R STG | 63 | −10 | 4 | |
| L IPL | −48 | −67 | 43 | R Postcentral gyrus | 63 | −7 | 16 | ||
| L IPL | −39 | −67 | 46 | R Postcentral gyrus | 2295 | 45 | −19 | 28 | |
| L IPL | −45 | −55 | 52 | R IPL | 48 | −28 | 28 | ||
| L MFG | 3807 | −33 | 35 | 40 | L Precuneus | 2214 | −21 | −46 | 40 |
| PCG | 2646 | 0 | −34 | 40 | L Precuneus | −21 | −49 | 46 | |
| R IPL | 1215 | 45 | −49 | 55 | R Precuneus | 1809 | 24 | −73 | 22 |
| L Caudate | 1053 | −12 | 2 | 16 | R Cuneus | 21 | −79 | 19 | |
| L Putamen | −21 | 11 | 1 | R Cuneus | 18 | −70 | 31 | ||
| L Caudate | −15 | 14 | 13 | R Precuneus | 1674 | 18 | −43 | 55 | |
| L Caudate | −9 | −4 | 16 | R MTG | 1539 | 48 | −61 | 10 | |
| L Insula | 891 | −36 | 11 | 1 | L Fusiform gyrus | 1188 | −21 | −52 | −14 |
| R MFG | 891 | 42 | 38 | 28 | L Cerebellum, culmen | −18 | −52 | −17 | |
| SFG, medial | 702 | 0 | 56 | 7 | R STG | 945 | 63 | −25 | 7 |
| R Caudate | 648 | 9 | 5 | 1 | R STG | 66 | −19 | 4 | |
| L IFG | 648 | −48 | 5 | 19 | R STG | 63 | −28 | 7 | |
| L IPL | 378 | −42 | −37 | 52 | L Medial FG | 783 | −21 | 35 | 19 |
| R Caudate | 351 | 18 | 5 | 16 | L Precentral gyrus | 729 | −15 | −31 | 64 |
| R SPL | 324 | 36 | −67 | 43 | R Medial FG | 594 | 9 | −25 | 64 |
| R MFG | 324 | 27 | 35 | 49 | R Medial FG | 9 | −25 | 61 | |
| R Paracentral lobule | 9 | −28 | 67 | ||||||
Abbreviations: ACG, anterior cingulate gyrus; CN, cannabis; FG, frontal gyrus; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; L, left; MCG, mid cingulate gyrus; MFG, middle frontal gyrus; MTG, middle temporal gyrus; PCG, posterior cingulate gyrus; R, right; SFG, superior frontal gyrus; SPL, superior parietal lobule; STG, superior temporal gyrus.
Clusters contributing significantly to the classification of brain activity associated with purchased vs declined cannabis are presented in order of declining cluster size, with coordinates given in Montreal Neurological Institute (MNI) space. Clusters with positive predictive weights (ie, activation associated with purchasing cannabis) are on the left; those with negative predictive weights (associated with declining cannabis) are on the right. The neural signature was subject to FDR correction at q<0.1 based on bootstrap sampling with a cluster threshold of 10 voxels for display and interpretation purposes only; all voxels were used for prediction.
Prediction Accuracy
In binary forced-choice discrimination, the neural signature classified between bought vs declined activity maps with 100% accuracy (50% accuracy representing chance).
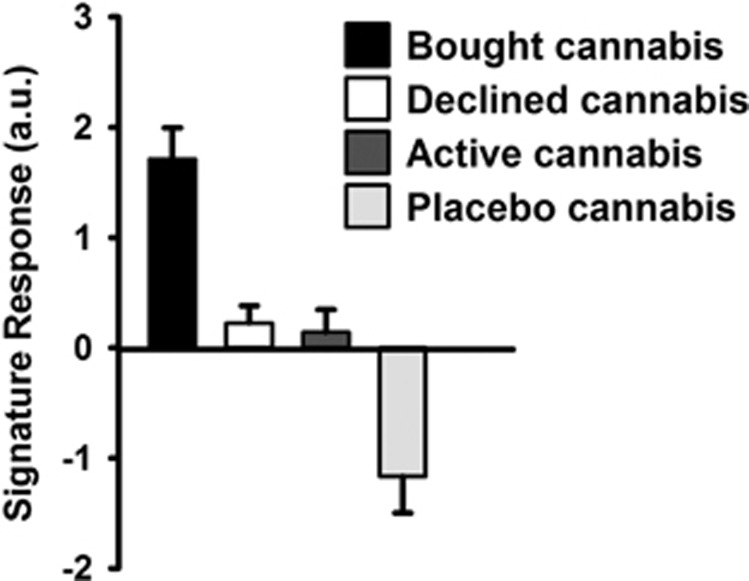
Figure 3 presents the strength of the neural signature expressed in brain activation maps for different conditions, showing that the mean signature response was stronger in the activation maps for bought compared with declined cannabis offers. To assess the possibility that the neural signature was associated with the general salience of the cannabis offers rather than neural processes specifically underlying decision making, we also applied the neural signature to mean activation maps generated from offers (ie, the epoch when participants rated how much they wanted to smoke the cannabis offer before the price had been presented) containing placebo and active cannabis. Importantly, the neural signature was not apparent in activation maps for active cannabis, indicating that it does not represent general salience associated with the drug.
Figure 3.
Signature response in brain maps from bought cannabis offers, declined offers, active cannabis offers, and placebo cannabis offers, calculated by computation of the cross-product of the out-of-training-sample individual's activation maps and the signature pattern derived from the other 16 participants to estimate the signature response in each individual's activity maps (averaged across all voxels). Data are means (±SEM). a.u.=arbitrary units.
DISCUSSION
Using a combination of an fMRI choice task, whole-brain machine learning analyses, and cannabis self-administration, we identified a pattern of regression weights capable of discriminating with 100% accuracy at the level of the individual subject between brain activity underlying decisions to purchase cannabis for self-administration and that associated with decisions to decline the drug. Brain regions contributing to this neural signature included dorsal striatum, frontoparietal (IPL) and posterior parietal (precuneus) regions, ACC, PCC, anterior insula, dlPFC, and middle and superior temporal gyri. Regions associated with buying cannabis were more anteromedial, whereas activation in more posterolateral regions predicted decisions to decline the drug.
Many of the regions identified, including dlPFC, ACC, PCC, anterior insula, and middle and medial frontal gyrus, were also found using univariate analyses to be associated with decision making about cigarettes (MacKillop et al, 2012) or alcohol (Mackillop et al, 2014). Moreover, regions identified in this study include many implicated in other types of goal-directed decision making as well as in responding to drugs or drug cues. Of note, activation of bilateral dorsomedial striatum predicted choices to buy cannabis, whereas recruitment of ventral striatum is more commonly observed in studies of decision making about other reward types, such as money (Levy and Glimcher, 2012). Striatal control over drug use is thought to shift from ventral to dorsal striatum over time, reflecting a movement from voluntary to more compulsive patterns of use (Everitt and Robbins, 2013). Other data, however, suggest a dissociation within the dorsal striatum, with the dorsomedial striatum (caudate nucleus) tracking deliberative value computation during goal-directed decisions, whereas computations associated with extensively trained (ie, more habitual) choices were expressed in dorsolateral striatum (putamen; Wunderlich et al, 2012, see also Murray et al, 2012). Combined with the orderly relationships between cost and purchasing decisions observed (Figure 1), this potentially suggests engagement in more deliberative, goal-directed decisions rather than habit-based responding. Use of higher-level cognitive processes is further supported by the bilateral dlPFC activation observed, given the central role of dlPFC in supporting cognitive control (Lesh et al, 2011), including during decision making (Hare et al, 2009).
The signature comprised other regions involved in decision making for rewards. Insula cortex, implicated in prior studies of decision making (Levy and Glimcher, 2012; Liu et al, 2011; Mackillop et al, 2014), is also thought to play a role in drug abuse, potentially processing interoceptive responses to drug cues (Naqvi and Bechara, 2009). Indeed, anterior insula cortex is particularly implicated in conscious awareness of bodily processes (Craig, 2009), which could be important in decisions about drugs given the centrality of somatic experiences in acute drug effects and craving states (Naqvi et al, 2014). BOLD activity in a region of dorsal PCC overlapping with that observed here correlates reliably with subjective value of rewards during decision making for nondrug rewards (Clithero and Rangel, 2014). The precise function of PCC during value-based decision making is unclear (Clithero and Rangel, 2014). However, the apparent role of dorsal PCC in switching between internally and externally directed attention (Leech et al, 2011) is potentially consistent with the notion that decisions to smoke cannabis involve integration of externally focused, more controlled cognitive processes and internally directed, interoceptive processes.
Many of the regions comprising the signature play a role in other processes, some of which are difficult to dissociate from processes specific to decision making. For instance, lateral parietal regions are strongly implicated in visual attention, which itself is tightly coupled with value-based choice (Louie and Glimcher, 2012). Similarly, precuneus is an important hub in the default mode network that is deactivated during goal-directed focus on external stimuli (Raichle et al, 2001). Thus, the observed association between decisions to decline cannabis and activation in the precuneus may reflect reduced attention during cannabis offers that were less appealing and therefore less salient. A notable feature of the signature was that it did not include vmPFC/orbitofrontal cortex (OFC), a region known to track subjective value in goal-based decision making (Clithero and Rangel, 2014). This may have been because of the BOLD signal dropout that can occur in this region (Du et al, 2007) or because of methodological differences between this study and earlier studies showing vmPFC tracking of value. In particular, other studies have used correlational analysis to search for brain regions parametrically tracking participants' subjective values, calculated from their decisions (Clithero and Rangel, 2014; Levy and Glimcher, 2012), a question not addressed with the current analyses. Of note, vmPFC was also not implicated in decision making for alcohol or nicotine in previous studies that similarly were not designed to identify brain regions parametrically tracking subjective value associated with these drugs (Mackillop et al, 2014; MacKillop et al, 2012). Despite the role that vmPFC/OFC clearly plays in signaling value during decision making (Levy and Glimcher, 2012), the current findings emphasize the contribution of a distributed pattern of regions to goal-directed decisions. An important future direction will be to use neurocomputational approaches to parse out the component processes, including valuation, salience appraisal, and risk processing, contributing to decisions about cannabis and other drugs.
Although further research is needed to disambiguate these component processes, the present data may provide the initial stages of a model of drug-related decision making. Specifically, these data suggest that drug-related decisions may involve integration of value calculations instantiated in the striatum and PCC with representations of visceral states associated with positive drug effects (eg, sensory stimulation of the airways in the case of smoking cannabis) in the insular cortex. Higher-level, reflective cognitive processes recruiting dlPFC potentially contribute to deliberative evaluation of choice options and regulation of bottom-up signals related to the motivational salience of drug choices on offer. Finally, distinct signals associated with internal (ie, interoceptive) vs external information contributing to drug-related decisions may be integrated in the PCC.
A potential limit to the generalizability of these findings is that volunteers for this single-day study were recruited directly from three residential cannabis studies (to maximize efficiency). Because two of these residential studies focused on comorbid cigarette and cannabis use, almost all participants in the present study smoked both cigarettes and cannabis. Although this may limit generalizability, laboratory (Haney et al, 2013a) and clinical (de Dios et al, 2009; Kohn et al, 2003; Peters et al, 2012) studies have shown that cigarette smoking represents a marker for particularly intransigent use of drugs, including cannabis. Thus, although potentially limiting generalizability to the ∼50% of heavy cannabis smokers who also smoke cigarettes (Moore and Budney, 2001; Stinson et al, 2006), this is a particularly clinically relevant group of cannabis smokers to study. A second limitation is that the approach taken, in which participants made several decisions with only one implemented, resulted in partial reinforcement of the decisions. Although it would be preferable to implement all decisions, it was not logistically feasible to do this, and repeated decisions were necessary to ensure adequate power to detect neural correlates of cannabis-related decision making. The approach taken was thus based on standard methodologies within neuroeconomics that commonly employ repeated decisions with only one decision implemented (eg, Hare et al, 2009; Levy and Glimcher, 2011; MacKillop et al, 2014). Participants were explicitly instructed that because they did not know which decision would be implemented, their best strategy was to treat each decision as if it were real. The behavioral data, in which purchasing varied lawfully as a function of cost and dose (see Figure 1), suggest that participants did treat all decisions as if they would be implemented.
One way in which substance use disorders diverge from other psychiatric illnesses is that the key symptom of the disorder, decisions to use drugs, is readily amenable to modeling in the laboratory. Laboratory models may therefore be particularly valuable for studying substance abuse. Indeed, laboratory drug self-administration models, although they differ from naturalistic drug use in several ways, have predictive validity in terms of clinical outcomes (Haney and Spealman, 2008). The current approach, combining laboratory self-administration with fMRI-based decision making, is thus well suited to assess the neural processes underlying the key behavioral pathology in substance abuse, decisions to self-administer drugs.
There are several avenues for future research. The analytic methods used here (multi-voxel pattern classification) can dissociate the neural activity underlying physical and social pain, and show the effect of an analgesic on a neural signature of physical pain (Wager et al, 2013). An obvious question is to what extent the neural signature of decisions to smoke cannabis overlaps with neural activity patterns underlying decision making about other reinforcers. Work in neuroeconomics suggests that both general and reward-specific signals contribute to decision making (eg, Clithero and Rangel, 2014; Levy and Glimcher, 2011). Although brain regions implicated in decision making about nondrug reinforcers contributed to the neural signature we observed, pattern classification approaches may be able to detect subtle differences in neural processes underlying drug- vs nondrug decision making, as was the case for physical and social pain, which also recruit overlapping circuitry (Eisenberger, 2012). Another important direction will be assessment of the effects of pharmacological and psychological interventions on the brain-based classifier. Finally, it remains to be seen whether this neural signature, developed in daily cannabis smokers, would also be observed in other cannabis-using populations. For instance, although participants in this study were daily cannabis smokers, they were nontreatment seekers and were therefore not assessed for cannabis use disorders. Future research could valuably investigate whether cannabis users meeting diagnostic criteria for cannabis use disorders and treatment seekers have different patterns of activation underlying drug-related decision making, and whether these differences are predictive of relapse. Such information could contribute to differentiating clinically and prognostically meaningful subtypes of substance abusers.
Such future possibilities notwithstanding, the present data provide the first evidence of a neural signature of decisions to use cannabis, comprising a distributed whole-brain pattern of regression weights capable of discriminating with a high degree of accuracy between brain activity underlying decisions to smoke vs to decline cannabis. Machine learning approaches applied to fMRI data and combined with human laboratory drug administration appear to provide an important avenue to directly study the neural processes underlying decisions to use drugs. The present results represent a step toward using these methods to inform a brain-based understanding of drug-related decision making in human drug abusers.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
We thank the volunteers for participating, Richard Foltin for helpful input, and Michael Harakas, Sarah Badach, Laura Rolfe, and Christina Hadzitheodorou for assistance in data collection. The NYSPI MRI Center Pilot Scan Program provided scanning time for this study. Thanks to Nicholas Van Dam and Will Lawn for comments on the manuscript. This research was supported by the National Institute on Drug Abuse (DA009236, DA031005, and DA034877) and complies with the laws of the country in which it was performed (USA).
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Bickel WK, Johnson MW, Koffarnus MN, Mackillop J, Murphy JG (2014). The behavioral economics of substance use disorders: reinforcement pathologies and their repair. Annu Rev Clin Psychol 10: 641–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL (2005). Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage 26: 480–492. [DOI] [PubMed] [Google Scholar]
- Clithero JA, Rangel A (2014). Informatic parcellation of the network involved in the computation of subjective value. Soc Cogn Affect Neurosci 9: 1289–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2009). How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70. [DOI] [PubMed] [Google Scholar]
- de Dios MA, Vaughan EL, Stanton CA, Niaura R (2009). Adolescent tobacco use and substance abuse treatment outcomes. J Subst Abuse Treat 37: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du YP, Dalwani M, Wylie K, Claus E, Tregellas JR (2007). Reducing susceptibility artifacts in fMRI using volume-selective z-shim compensation. Magn Reson Med 57: 396–404. [DOI] [PubMed] [Google Scholar]
- Editorial Board (2014). High time for advancing marijuana research. Nat Neurosci 17: 481. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI (2012). The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci 13: 421–434. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET (2011). Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain 134: 2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW (2013). From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev 37: 1946–1954. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pedroso JJ, Pearlson GD (1987). Marijuana and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav 28: 459–464. [DOI] [PubMed] [Google Scholar]
- Haney M, Bedi G, Cooper ZD, Glass A, Vosburg SK, Comer SD et al (2013. a). Predictors of marijuana relapse in the human laboratory: robust impact of tobacco cigarette smoking status. Biol Psychiatry 73: 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW (2013. b). Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology 38: 1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Spealman R (2008). Controversies in translational research: drug self-administration. Psychopharmacology 199: 403–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T, Camerer C, Rangel A (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science 324: 646–648. [DOI] [PubMed] [Google Scholar]
- Hyman SE (2007). The neurobiology of addiction: implications for voluntary control of behavior. Am J Bioeth 7: 8–11. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F, Moore BA, Badger GJ, Budney AJ (2010). Delay discounting in current and former marijuana-dependent individuals. Exp Clin Psychopharmacol 18: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable J, Glimcher P (2009). The neurobiology of decision: consensus and controversy. Neuron 63: 733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Behrens TE, Wallis JD (2011). Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat Neurosci 14: 1581–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YT, Sohn H, Jeong J (2011). Delayed transition from ambiguous to risky decision making in alcohol dependence during Iowa Gambling Task. Psychiatry Res 190: 297–303. [DOI] [PubMed] [Google Scholar]
- Kjome KL, Lane SD, Schmitz JM, Green C, Ma L, Prasla I et al (2010). Relationship between impulsivity and decision making in cocaine dependence. Psychiatry Res 178: 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn CS, Tsoh JY, Weisner CM (2003). Changes in smoking status among substance abusers: baseline characteristics and abstinence from alcohol and drugs at 12-month follow-up. Drug Alcohol Depend 69: 61–71. [DOI] [PubMed] [Google Scholar]
- Lane SD, Steinberg JL, Ma L, Hasan KM, Kramer LA, Zuniga EA et al (2010). Diffusion tensor imaging and decision making in cocaine dependence. PLoS One 5: e11591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ (2011). Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci 31: 3217–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesh TA, Niendam TA, Minzenberg MJ, Carter CS (2011). Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology 36: 316–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Glimcher PW (2011). Comparing apples and oranges: using reward-specific and reward-general subjective value representation in the brain. J Neurosci 31: 14693–14707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Glimcher PW (2012). The root of all value: a neural common currency for choice. Curr Opin Neurobiol 22: 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Lazzaro S, Rutledge R, Glimcher PW (2011). Choice from non-choice: predicting consumer preferences from blood oxygenation level-dependent signals obtained from passive viewing. J Neurosci 31: 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang F, Zhou Y, Zhang M, Wang X, Shen M (2013). Decision-making deficits are still present in heroin abusers after short- to long-term abstinence. Drug Alcohol Depend 130: 61–67. [DOI] [PubMed] [Google Scholar]
- Lindquist MA, Mejia A (2015). Zen and the art of multiple comparisons. Psychosom Med 77: 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J (2011). Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 35: 1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K, Glimcher PW (2012). Efficient coding and the neural representation of value. Ann NY Acad Sci 1251: 13–32. [DOI] [PubMed] [Google Scholar]
- Mackillop J, Amlung MT, Acker J, Gray JC, Brown CL, Murphy JG et al (2014). The neuroeconomics of alcohol demand: an initial investigation of the neural correlates of alcohol cost-benefit decision making in heavy drinking men. Neuropsychopharmacology 39: 1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Wier LM, David SP, Ray LA, Bickel WK et al (2012). The neuroeconomics of nicotine dependence: a preliminary functional magnetic resonance imaging study of delay discounting of monetary and cigarette rewards in smokers. Psychiatry Res 202: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop J, Murphy JG, Tidey JW, Kahler CW, Ray LA, Bickel WK (2009). Latent structure of facets of alcohol reinforcement from a behavioral economic demand curve. Psychopharmacology 203: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Budney AJ (2001). Tobacco smoking in marijuana-dependent outpatients. J Subst Abuse 13: 583–596. [DOI] [PubMed] [Google Scholar]
- Murray JE, Belin D, Everitt BJ (2012). Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology 37: 2456–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A (2009). The hidden island of addiction: the insula. Trends Neurosci 32: 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A (2014). The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann NY Acad Sci 1316: 53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EN, Budney AJ, Carroll KM (2012). Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction 107: 1404–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Buchel C (2009). Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. J Neurosci 29: 15727–15734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001). A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A (2008). A unified framework for addiction: vulnerabilities in the decision process. Behav Brain Sci 31: 415–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA (2011). Results from the 2010 National Survey on Drug Use and Health: Summary of National FindingsIn NSDUH Series H-41. Substance Abuse and Mental Health Services Administration: Rockville, MD. [Google Scholar]
- Sescousse G, Redoute J, Dreher JC (2010). The architecture of reward value coding in the human orbitofrontal cortex. J Neurosci 30: 13095–13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson FS, Ruan WJ, Pickering R, Grant BF (2006). Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychol Med 36: 1447–1460. [DOI] [PubMed] [Google Scholar]
- Vaidya JG, Block RI, O'Leary DS, Ponto LB, Ghoneim MM, Bechara A (2012). Effects of chronic marijuana use on brain activity during monetary decision-making. Neuropsychopharmacology 37: 618–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Goldstein RZ (2010). Addiction: pulling at the neural threads of social behaviors. Neuron 69: 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Leotti LA, Rilling JK (2011). Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci 31: 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E (2013). An fMRI-based neurologic signature of physical pain. N Engl J Med 368: 1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley MJ, Hanlon CA, Porrino LJ (2011). Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res 191: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM et al (2004). Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend 76: 107–111. [DOI] [PubMed] [Google Scholar]
- Wunderlich K, Dayan P, Dolan RJ (2012). Mapping value based planning and extensively trained choice in the human brain. Nat Neurosci 15: 786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.