Abstract
The lateral habenula (LHb) is viewed as a relay between the limbic system, the basal ganglia (BG), and monoaminergic neurons of the midbrain. If a prominent role has been evidenced in BG-mediated functions such as value-based decision-making, very little is known about the involvement of the LHb in limbic functions such as memory processing. In the present study, we used two pharmacological approaches—LHb reversible inactivation with intra-LHb infusion of muscimol, an agonist of the GABA-A receptor, or blockade of excitatory inputs with intra-LHb infusion of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), an antagonist of the glutamatergic AMPA receptor—to investigate the involvement of the LHb in encoding, consolidation, and retrieval of spatial memory in the water maze (WM) in rats. We found that intra-LHb infusion of muscimol or CNQX prevented encoding and retrieval, but not consolidation of spatial information. In addition, muscimol but not CNQX induced impairments during a cued version of the WM task, and marked anxiety in the elevated plus maze. These results confirm the involvement of the LHb in higher cognitive functions. They further suggest a dichotomy between the role of glutamatergic and other inputs to the LHb in hippocampus-dependent memory processing, as well as in emotional aspects of goal-directed behaviors.
INTRODUCTION
The lateral habenula (LHb) is described as a structure integrating and relaying striatal and limbic information toward midbrain monoaminergic nuclei (Geisler and Trimble, 2008; Hikosaka et al, 2008). A large majority of the studies conducted in the past decade focused on the role of the LHb in basal ganglia-mediated functions. Its fundamental implication has been evidenced in reward and information prediction errors, the coding of negative motivational value, and subjective decision biases(Bromberg-Martin and Hikosaka, 2011; Matsumoto and Hikosaka, 2007, 2009; Stopper and Floresco, 2014). Hikosaka (2010) further proposed that the LHb governs the suppression of motor activity upon the occurrence of negative outcomes.
To date, few studies have investigated the contribution of the LHb to limbic functions such as spatial reference memory, which depends upon hippocampal networks (Winocur et al, 2010). This issue appears relevant, regarding the modulatory role of the LHb upon dopaminergic and serotonergic transmissions (Bernard and Veh, 2012; Ji and Shepard, 2007; Wang and Aghajanian, 1977), as well the cholinergic septo-hippocampal pathway (Nilsson et al, 1990), which are key systems involved in memory processes. It is also relevant because dysfunction of the LHb has been evidenced in disorders such as depression (Aizawa et al, 2013a; Sartorius and Henn, 2007), which encompasses alterations of hippocampal networks, as well as memory impairments (Trivedi and Greer, 2014; Belzung et al, 2015; Lecca et al, 2014). In rats, LHb electric stimulation prevented avoidance learning (Shumake et al, 2010). We have shown that electrolytic habenular lesion altered hippocampus (HPC)-dependent spatial learning in the water maze (WM) (Lecourtier et al, 2004), whereas selective LHb inactivation induced memory deficits in an object-based, HPC-dependent memory task (Goutagny et al, 2013). Moreover, a high synchrony between LHb and dorsal HPC (dHPC) theta oscillations has been evidenced (Aizawa et al, 2013b; Goutagny et al, 2013). On the basis of the well-accepted view that communication between regions involves coherence of their oscillations (Fries, 2005), these data suggest that the LHb and dHPC are functionally connected and are likely to exchange information. With regard to the above-cited literature, one could even further hypothesize that such information relates to memory processing, for which theta oscillations are particularly relevant (Düzel et al, 2010).
If the aforementioned studies pointed to an involvement of the LHb in HPC-dependent spatial memory, it has not yet been investigated at which step of spatial memory processing was the LHb involved: ie, encoding, consolidation, or retrieval. To address this question, we assessed the involvement of the LHb in encoding, consolidations, and retrieval of spatial information in the WM. We used two reversible pharmacological strategies: inactivation of the LHb with muscimol, an agonist of the GABA-A receptor, or selective blockade of fast glutamatergic excitatory transmission to the LHb with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), an antagonist of the glutamatergic AMPA receptor. The second strategy enabled a more precise investigation of the role of glutamatergic inputs to the LHb, which have gained interest recently. They arise from the basal ganglia (Shabel et al, 2012), the ventral tegmental area (Hnasko et al, 2012), and potentially from the prefrontal cortex given the likely glutamatergic projections from several prefrontal regions to the LHb (Kim and Lee, 2012). Complementary experiments controlled for potential drug-induced behavioral alterations, which could have biased WM performance. Those comprised sensorimotor capacities in a cued version of the WM test and in the beam-walking test, anxiety level in the elevated plus maze (EPM), and horizontal locomotor activity in home-cages. We also controlled for potential bias induced by possible diffusion of the injected drugs to surrounding structures.
We found that, although CNQX infusion impaired learning and retrieval in the WM, muscimol infusion also induced deficits in the cued version of the WM, as well as marked anxiety in the EPM. These results reveal a specific involvement of the LHb in spatial reference memory encoding and retrieval, likely through integration of glutamatergic inputs. They further suggest a dichotomy between excitatory glutamatergic and other inputs to the LHb regarding memory, but also emotional processing during goal-directed behaviors.
MATERIALS AND METHODS
Animals and Surgery
Male Long–Evans rats (250–350 g; Centre d'Elevage R. Janvier, France) were used in compliance with the European Committee Council Directive (86/609/EEC; authorization no. 67–215 for J.-C.C.). This project has also been validated by the local ethics committee (CREMEAS, authorization no. AL/92/99/02/13). Rats were housed individually under a 12-h light/dark cycle (light on at 7 : 00 A.M.) with ad libitum access to food and water.
Rats were anesthetized by intraperitoneal infusion of a mixture of ketamine (82.5 mg/kg) and xylazine (11 mg/kg) and placed within a stereotaxic apparatus (flat skull). Lidocaine (0.1 ml, sc) was injected at the incision location. Once the holes were drilled, stainless steel guide cannulae were implanted bilaterally 1 mm above the LHb at the following coordinates (in mm): AP, −3.9 from Bregma; ML, ±0.7; DV, −3.6 from dura. Guides, in which dummy cannulae were inserted, were secured to the skull and 3 screws with dental cement. Rats were administered an antibiotic (amoxicilline, im), a painkiller (meloxicam, sc), and given a 10-day recovery period.
Three batches of rats were used: 75 rats for the study of memory encoding and consolidation (experiment 1); 40 rats for the study of memory retrieval (experiment 2); and 75 rats for the assessment of locomotor activity, and the performance of beam-walking (BW) and EPM tests.
In addition, other batches of rats underwent the same surgical procedure in order to verify whether the observed deficits, and more particularly those of spatial reference memory in the WM, could be owing to a possible spread of the injected drugs in the surrounding regions—ie, the dHPC, the underlying thalamic region, and the third ventricle (Supplementary Information).
Drugs and Infusion Procedure
Muscimol (Sigma; 0.08 μg/μl) was dissolved in artificial cerebrospinal fluid (aCSF) (in mM: NaCl, 145; KCl, 2.7; MgCl2, 1.0; CaCl2, 1.2). CNQX (Sigma; 0.89 μg/μl (3 mM)) was dissolved in 2% DMSO/aCSF. Once the dummy cannulae were taken out, infusion cannulae were bilaterally inserted, protruding 1 mm below the guides; 0.3 μl of either drug—or the same volume of the respective vehicles in controls—was bilaterally administered over 1 min. Infusion cannulae were left in place for an additional 30 sec before being removed, and the dummy cannulae were reinserted. The final amounts per side were 24 ng (muscimol) and 267 ng (CNQX). The amount of muscimol was chosen in accordance with studies conducted in the laboratory comprising its infusion in diencephalic structures (Cholvin et al, 2014). The amount of CNQX was chosen in reference to Bast et al (2005). Behavioral testing took place 15 min after CNQX or 30 min after muscimol infusion, except where otherwise described.
WM Testing
Spatial reference memory was assessed as in Lecourtier et al (2011). Training comprised one block of four consecutive trials per day for 5 days. Rats were allowed 60 s to find the hidden platform, which remained in the same quadrant; they were left on it for 10 s before the next trial was started. In case they did not find the platform within 60 s, they were gently guided to it and left there for 10 s before the next trial was started. One day after completion of training, the platform was removed and a 60-sec retention test was performed.
Experiment 1
Treatments were administered before each training session (‘-pre' groups) to investigate encoding, or after (~2 min) each training session (‘-post' groups) to investigate early consolidation; the retention test was performed drug-free.
Three days after the retention test, only rats tested for encoding (ie, ‘-pre' rats) performed a cued version of the WM—where the cue was a platform—in order to verify whether encoding performance was biased by drug-induced motivational deficits. Each rat received the same treatment than during training. Testing comprised a block of four consecutive trials. A different platform was used. It was placed in another location than during training (opposite quadrant) and was situated 0.5 cm above the water surface. A plastic ball painted with black and white stripes was attached to it. A curtain was placed around the pool to mask all spatial cues.
Experiment 2
Drug treatments were administered before the retention test, following a training comprising vehicle administration before each session: half of the rats received aCSF and the other half received 2%DMSO/aCSF. For the retention test, treatment groups were composed so that there was no statistical difference between their training performance (Supplementary Figure S2).
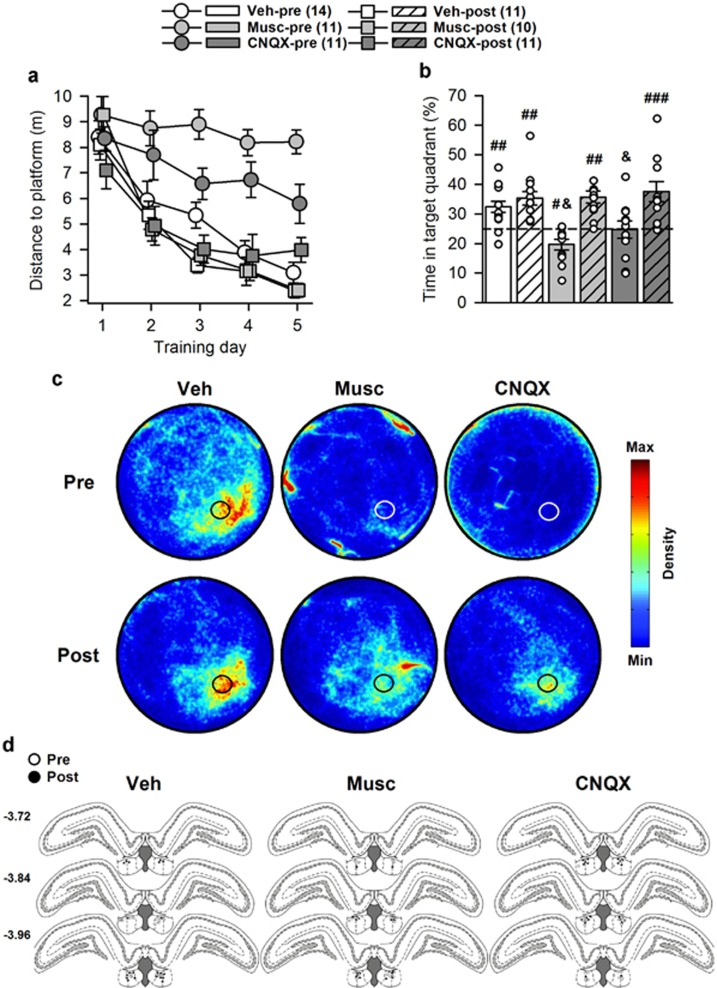
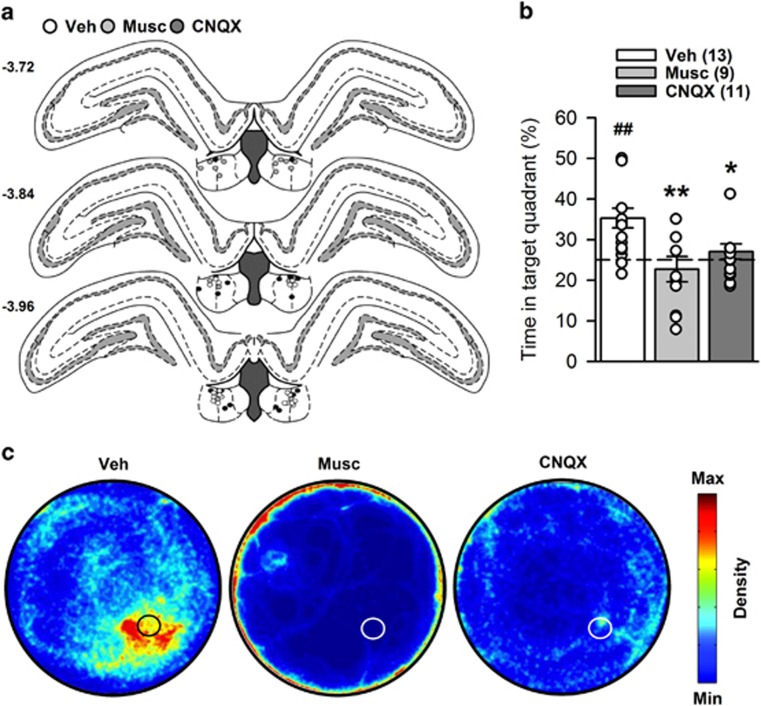
The movements of the rats were followed by a computer-based video tracking system (Ethovision, Noldus, The Netherlands). Training analyses comprised for each trial the distance and latency to reach the platform; the mean swim speed; the resting time, ie, when the rats were floating; and the time in thigmotaxis, ie, when the rats were swimming along the edge of the WM within a 10-cm wide corridor. Retention test analyses comprised the time spent within the target quadrant—ie, the quadrant where the platform was located during training, the number of crossings of the former platform location, the time in thigmotaxis, the mean swim speed, the total distance swum, and the resting time. In addition, to provide a representation of the overall spatial occupancy during the retention tests, all X and Y coordinates (one measure recorded every 0.2 s) of all rats from each group have been concatenated in Matlab and processed using a density plot script. These are shown in Figure 1c and Figure 2c.
Figure 1.
Water maze performance during experiment 1. Numbers between brackets indicate group sizes. (a) Muscimol and CNQX impaired learning when injected before (‘-pre' groups), but not after (‘-post' groups), each training session, as evidenced by longer distance to reach the platform in Musc-pre and CNQX-pre rats. (b) This impaired learning was confirmed during the drug-free retention test, as Musc-pre and CNQX-pre rats performed below and at chance level (25% time spent in the target quadrant), respectively. White dots represent individual performance. Because of partial or complete overlap, the number of dots may be smaller than the total number of rats. (c) Density plots illustrating the water maze occupancy during the 60-sec retention test of all rats in each group. The color scale settings are identical for each group. The black or white circles represent the position of the platform during training. (d) Position of the infusion sites in each group according to treatments received before (Pre; white circle) or after (Post; black circles) each training session. Numbers on the left correspond to anteroposterior coordinates, in mm from Bregma. Statistics: &p<0.05 vs all other groups; #p<0.05, ##p<0.01, ###p<0.001 vs chance level. CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; Musc, muscimol; Veh, vehicle.
Figure 2.
Water maze performance during experiment 2. Numbers between brackets indicate group sizes. (a) Position of the infusion sites in each group according to treatments received before the retention test (Veh, white circles; muscimol, light-gray circles; CNQX, dark-gray circles). Numbers on the left correspond to anteroposterior coordinates, in mm from Bregma. (b) Retention was impaired by both muscimol and CNQX infusion following a training under vehicle treatment. Whereas controls performed above chance level, muscimol- and CNQX-treated rats did not. White dots represent individual performance. Because of partial or complete overlap, the number of dots may be smaller than the total number of rats. (c) Density plots illustrating WM occupancy during the 60-sec retention test of all rats in each group. The color scale settings are identical for each group. The black or white circles represent the position of the platform during training. Statistics: ##p<0.01, vs chance level; *p<0.05, **p<0.01 vs vehicle. CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; Musc, muscimol; Veh, vehicle.
Horizontal Locomotor Activity
It was measured in home-cages by means of two infrared light beams perpendicular to the width of the cage, each 4.5 cm above floor level and 28 cm apart along the length of the cage. The number of longitudinal cage crossings, ie, each time a rat consecutively interrupted the two light beams, was monitored and saved in 15-min bins. After a 1-h baseline recording, treatments were administered and recording was continued for 90 min.
Beam-Walking Test
It was performed on a 4-cm-wide, 200-cm-long wooden beam elevated 80 cm above the floor and virtually divided into four 50-cm segments. Home-cages were placed at one extremity of the beam. Training was as follows: day 1, rats were placed on the beam close to their home-cage for 5 consecutive trials; day 2, rats were placed successively 50, 100, and 150 cm from the home-cage (one trial for each distance); day 3, rats were placed twice 100 cm and twice 200 cm from the home-cage; and day 4, rats were placed 200 cm from the home-cage for 3 consecutive runs. Testing under treatments occurred on day 5 with the same protocol as on day 4. For each 50-cm segment of the beam, a score of 1 was attributed when a rat kept all paws on the upper surface, whereas a score of 0 was attributed when the rat slipped or placed its toes on the side surfaces. For each rat, the scores of the 3 consecutive runs were summed (maximum score =12).
EPM test
The EPM, made of black Plexiglas, was elevated 73 cm above the floor, and it consisted of four arms (50 cm × 10 cm), two comprising 40-cm-high walls (closed) and two comprising 1.5-cm-high borders (open). Light intensity was 10 lux in open arms, 7 lux at the center of the maze, and 4 lux in closed arms. The test lasted 5 min; the number of entries and time spent in each arm were scored. The maze was cleaned with absolute ethanol between each rat.
Histology
Infusion sites were verified by means of Evans blue (0.15 μl, 0.15 μl/min) infusion. Rats were subsequently deeply anesthetized (pentobarbital, 100 mg/kg, ip), and brains were removed and fixed in phosphate-buffered 4% paraformaldehyde (pH 7.4; 4 °C, 48 h). Brains were then transferred into a 0.1 M phosphate-buffered 20% sucrose solution (4 °C, 48 h) and subsequently frozen. Serial 40-μm-thick sections were cut in the coronal plane at −22 °C, collected on gelatin-coated slides, and stained with hematoxylin. Only rats with blue marks, in conjunction with gliosis, restricted to both LHb, were kept. Group sizes are indicated in each figure.
Statistical Analyses
All data are represented as mean±SEM. For all experiments, control groups of each drug, which received different vehicles, have been pooled to simplify iconography, after it was confirmed that their performances were not statistically different (see Supplementary Information). WM training performance and locomotor activity were analyzed with two-way ANOVA, treatment being the between-subject factor, and training day (5) or time (4 or 6 bins) the repeated measure. WM retention tests performance was analyzed with one-way ANOVA (Treatment); average performance was compared with chance level (25%) with a t-test. EPM and beam-walking performance was analyzed with one-way ANOVA (treatment). Post hoc analyses used the Newman–Keuls multiple range test when appropriate. Values of p<0.05 were considered significant.
RESULTS
Intra-LHb Muscimol or CNQX Infusion Prevented Learning
Analysis of distance to platform showed significant effects of group (F(5,62)=23.20; p<0.0001) and training day (F(4,248)=39.88; p<0.0001), and a significant interaction (F(20,244)=1.86; p<0.05). Post hoc analysis revealed an alteration of learning performance in Musc-pre and CNQX-pre rats (p<0.001 and p<0.01 vs the four other groups, respectively) (Figure 1a). This was confirmed during the retention test (F(5,62)=8.37; p<0.0001) (Figure 1b), performance of Musc-pre and CNQX-pre rats being significantly below that of the four other groups (p<0.05 to p<0.01). Musc-pre rats performed below chance level (t(10)=−2.98, p<0.05), CNQX-pre rats performed at chance level (t(10)=−0.10, p>0.1), whereas all other groups performed above chance level (Veh-pre: (t(13)=4.02, p<0.01); Veh-post: (t(10)=4.52, p<0.01); Musc-post: (t(9)=5.67, p<0.001); CNQX-post: (t(10)=3.72, p<0.01)). In addition, both drugs, when infused before training, also increased thigmotaxis and/or decreased swim speed, whereas they did not affect the amount of resting (see Supplementary Figure S1).
Following a Drug-Free Training, Intra-LHb Muscimol or CNQX Infusion Disrupted Memory Retrieval
Analysis of retention performance showed a significant effect of group (F(2,30)=6.83; p<0.01). Post hoc analysis indicated impaired performance in muscimol- and CNQX-treated rats (p<0.01 and p<0.05 vs vehicle, respectively) (Figure 2b). Whereas controls performed above chance level (t(12)=4.25, p<0.01), muscimol- and CNQX-treated rats did not (t(8)=−0.72, p>0.1 and t(10)=1.09, p>0.1, respectively). Both drugs also increased thigmotaxis, whereas muscimol also decreased swim speed, and did not affect the amount of resting (see Supplementary Figure S3).
In summary, these data indicate that the LHb is involved in encoding, but not immediate post-training consolidation, and retrieval of spatial information. As indicated in the Supplementary Information, microinjections of muscimol or CNQX had no effect on encoding or retrieval of spatial memory when infused into the hippocampal region immediately dorsal to the LHb (Supplementary Figure S4), into the thalamic regions immediately ventral to the LHb (Supplementary Figure S7), or into the adjacent ventricular region (Supplementary Figure S10). Therefore, the deficits seen after intra-LHb infusions are unlikely to be the consequences of the possible diffusion of the injected drugs to those surrounding structures.
Intra-LHb Muscimol, but not CNQX, Infusion Altered Performance in a Cued Version of the WM
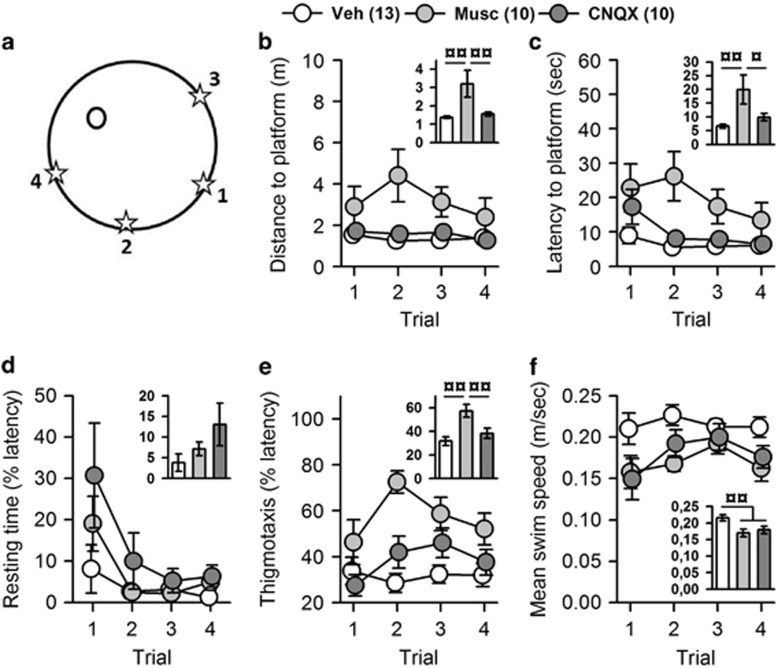
Analysis of distance to platform showed a significant effect of group (F(2,30)=6.29; p<0.01), but no effect of trial (F(3,90)=1.50; p>0.1), and no interaction (F(6,90)=1.38; p>0.1) (Figure 3b). Post hoc analysis indicated that muscimol altered performance (p<0.01 vs vehicle and CNQX), whereas CNQX did not (p>0.1 vs vehicle) (Figure 3b inset). Analysis of the latency yielded identical results (Figure 3c). Analysis of resting time showed no effect of group (F(2,30)=1.74; p>0.1), a significant effect of trial (F(3,90)=10.63; p<0.00001), and no interaction (F(6,90)=1.43; p>0.1), suggesting that muscimol did not alter the motivation to swim (Figure 3d). Analysis of thigmotaxis showed a significant effect of group (F(2,30)=5.47; p<0.01) and trial (F(3,90)=3.65; p<0.05) and a significant interaction (F(6,90)=2.22; p<0.05) (Figure 3e); post hoc analysis indicated that muscimol-treated rats did significantly more thigmotaxis than controls (p<0.01), whereas CNQX did not (p>0.1) (Figure 3e inset). Analysis of swim speed showed a significant effect of group (F(2,30)=6.34; p<0.01), no effect of trial (F(3,90)=2.23; p>0.05), and no interaction (F(6,90)=1.38; p>0.1) (Figure 3f); post hoc analysis indicated that both muscimol- and CNQX-treated rats were slower than controls (p<0.05 for each comparison) (Figure 3f inset). Overall, these analyses suggest that although muscimol did not alter the motivation to swim it induced a greater thigmotaxis and a decreased speed; this could explain the increased distance and latency and suggest a decreased motivation to reach for an escape in the WM.
Figure 3.
Performance during the cued version of the water maze. One vehicle-, one muscimol-, and one CNQX-treated rat were discarded from the analyses because of excessive resting (>20% of latency) throughout the session (mean of 22 s, 41.6 s and 20.5 s, respectively). Numbers between brackets indicate group sizes. (a) Representation of the platform position (black circle), and of the 4 start positions (stars) numbered in chronologic order. Only muscimol-treated rats showed deficits, evidenced by increased distance (b) and latency (c) to reach the visible platform. These increased distance and latency are unilkely to be due to a lack of motivation to swim as there were no differences in the amount of resting before reaching the platform (d). However, muscimol increased thigmotaxis (e) and, as also found with CNQX, decreased swim speed (f). Insets represent the mean performance of the 4 trials. Statistics: ¤p<0.05, ¤¤p<0.01. CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; Musc, muscimol; Veh, vehicle.
Intra-LHb Muscimol or CNQX Infusion Increased Locomotor Activity
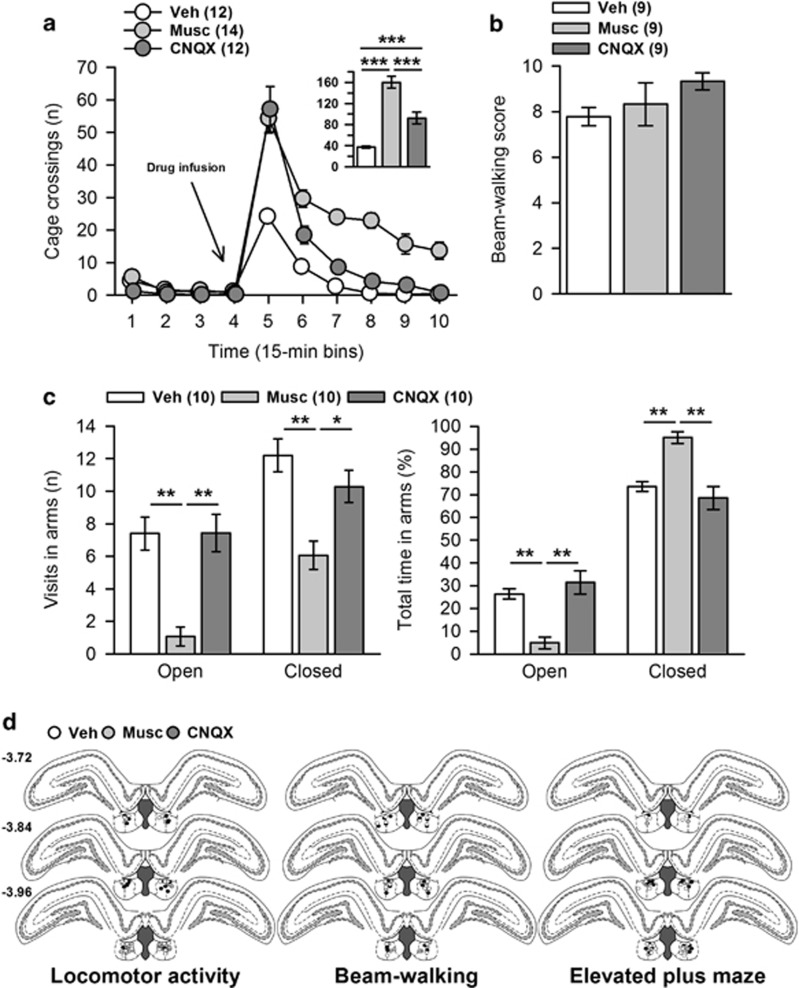
There was no difference between groups during baseline recording (F(2,35)=2.67; p>0.05). Postinfusion analyses showed a significant effect of group (F(2,35)=43.88; p<0.0001) and time (F(5,175)=126.24; p<0.0001) and a significant interaction (F(10,175)=6.90; p<0.0001). Post hoc analysis of the group effect indicated that both CNQX and muscimol increased locomotor activity (p<0.001 and p<0.001 vs vehicle, respectively). Moreover, the activation was greater following muscimol (p<0.001 vs CNQX) (Figure 4a). These results suggest that behavioral alterations seen in the WM experiments in muscimol- and CNQX-treated rats are unlikely to be the consequence of reduced behavioral activity.
Figure 4.
Additional behavioral controls included assessment of horizontal locomotor activity, sensorimotor coordination in the beam-walking test, and anxiety-like behavior in the elevated plus maze. These tests were performed in this order, using a 1-week rest delay between each, in order to progress from the less to the more anxiogenic one, and to limit possible inter-test biases. Each rat performed a maximum of 2 tests, treatment being counterbalanced between tests. Numbers between brackets indicate group sizes. (a) Both muscimol and CNQX induced a marked increase of locomotor activity, which lasted longer following muscimol; CNQX increased locomotion for 30 min (p<0.00001 and p<0.05 vs Veh for Bin5 for Bin6, respectively; p>0.1 vs vehicle for all other Bins), whereas muscimol did so for the entire recording session (p<0.0001 and p<0.01 vs vehicle for Bins5–8 and Bins9–10, respectively). The inset illustrates the total post-infusion locomotor activity. (b) In the beam-walking test, both treatments spared sensorimotor coordination. (c) In the elevated plus-maze test, muscimol induced marked anxiety-like behavior. Whereas CNQX-treated rats behaved like controls, muscimol-treated rats were less active, as they made fewer visits to both arms (left), and spent less time in the open arms and more time in the closed arms (right). (d) Position of the infusion sites in each group according to treatments received before the retention test (vehicle, while circles; muscimol, light gray circles; CNQX, dark-gray circles). Numbers on the left correspond to anteroposterior coordinates, in mm from Bregma. Statistics: *p<0.05, **p<0.01, ***p<0.001. CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; Musc, muscimol; Veh, vehicle.
Intra-LHb Muscimol or CNQX Infusion Spared Sensorimotor Coordination
In the beam-walking test, both drugs spared sensorimotor coordination (F(2,24)=1,57; p>0.1) (Figure 4b), suggesting that impairments seen during WM experiments were unlikely to be the consequence of sensorimotor coordination deficits.
Intra-LHb Muscimol, but not CNQX, Infusion Produced Marked Anxiety in the EPM
In the EPM, there was a significant effect of group according to the number of visits and time in open arms (F(2,27)=8.87; p<0.01 and F(2,27)=7.39; p<0.01, respectively), and in closed arms (F(2,27)=6.55; p<0.01 and F(2,27)=7.15; p<0.01, respectively) (Figure 4c). Post hoc analyses indicated that only muscimol induced anxiety-like behavior, along with an overall decrease of activity (p<0.01 vs vehicle and CNQX for each analysis). It is noteworthy that Gill et al, 2013 found no alteration of rats' behavior in the EPM following inactivation of the LHb. This discrepancy could result from the fact that these authors used a mixture of muscimol and baclofen; the latter might have profoundly affected the medial habenula where GABA-B receptors are densely localized (Charles et al, 2003). Finally, the consequences of muscimol infusion in the thalamic regions below the LHb have not yet been investigated; therefore, it is possible that the muscimol-induced increased anxiety observed is partly owing to a diffusion of the drug in such an area. This issue will require further investigation. In conclusion, our results suggest that during WM experiments an increased anxiety could have participated in the induction of impairments following muscimol, but not CNQX, administration.
DISCUSSION
The present study demonstrates an involvement of the LHb in encoding and retrieval of spatial memory. The deficits observed cannot be the consequence of impaired sensorimotor coordination, as beam-walking scores were not affected by CNQX or muscimol, or of failures to produce movements as both drugs stimulated locomotion in the home-cage. Moreover, those deficits are unlikely to be attributed to the possible diffusion of the drugs to surrounding regions (Supplementary Figures S4).
The involvement of the LHb in spatial memory had previously been suggested by the findings that lesions of the habenular complex in rats altered learning and retrieval in the WM (Lecourtier et al, 2004). More recently, we showed that intra-LHb muscimol infusion in rats impaired the detection of object displacements in a familiar context, suggesting alterations in spatial configuration processing (Goutagny et al, 2013). Memory formation and retrieval depend upon the HPC (D'Hooge and De Deyn, 2001), and more specifically upon the presence of theta oscillations within the dHPC (Düzel et al, 2010). A high degree of synchrony between LHb and dHPC theta oscillations has recently been demonstrated (Aizawa et al, 2013b; Goutagny et al, 2013). Moreover, we have shown that spatial memory performance was positively correlated with theta coherence between the LHb and the dHPC (Goutagny et al, 2013). As two regions are likely to exchange information when their oscillatory activity is synchronous (Fries, 2005), we propose that the LHb and the HPC are specifically communicating during online processing of spatial information, ie, during encoding, when relevant information is selected to be further memorized, and during retrieval, when an ongoing situation is compared with past experience. This proposal is consistent with a view considering theta oscillations as reflecting the online state of the HPC (Buzsáki, 2002). Thus, once spatial information has been selected by a network comprising the HPC and associated cortical and subcortical regions, it is communicated downstream to the LHb, which might act as a relay of topdown information to midbrain monoaminergic and cholinergic systems, to further engage early processing of memory encoding and retrieval.
It is noteworthy that CNQX induced an increase in thigmotaxis. Thigmotaxis is the first strategy engaged by rodents when placed in the WM with no knowledge of the platform location (Devan et al, 1996). The fact that thigmotaxis is maintained to a high level in CNQX-treated rats strengthens the view that CNQX infusion before training prevented the rats from properly learning the platform location. The learning and memory deficits induced by CNQX strongly suggest that excitatory glutamatergic transmission to the LHb participates in encoding and retrieval of spatial information. One possible origin of glutamatergic inputs to the LHb is the prefrontal cortex, which sends projections to the LHb (Kim and Lee, 2012). Therefore, the hippocampocortical system, whose participation in memory processes has been clearly demonstrated (Winocur et al, 2010), could transmit relevant information to the LHb through the prefrontal cortex. Such information would be directly related to memory formation and retrieval (Cholvin et al, 2014) but also to other aspects of a spatial memory task, ie, understanding of the rule, behavioral flexibility, or attention (see reviews Dalley et al, 2004; Kesner and Churchwell, 2011).
If the consequences of intra-LHb CNQX infusion clearly point to learning and memory deficits, the effects of muscimol are more ambiguous. Muscimol also induced deficits in the cued version of the WM, which engages nonlearning mechanisms as the platform is made visible, as well as anxiety in the EPM. Such high level of anxiety in the EPM is of particular interest and might shed light on the deficits observed in the WM experiments following LHb inactivation, and therefore on the role of this structure during the WM paradigm. The WM paradigm engages rats to find a way to escape from a stressful situation. Therefore, our suggestion is that the LHb is not only involved in cognitive aspects but also emotional aspects of goal-directed behaviors and more particularly stress-coping mechanisms. Such a role for the LHb is consistent with the marked cellular response within the LHb during stressful situations (Chastrette et al, 1991; Cullinan et al, 1995; Duncan et al, 1996). Furthermore, the LHb modulates the monoaminergic systems involved in the stress response (Cabib and Puglisi-Allegra, 2012; Puglisi-Allegra and Andolina, 2014), as already shown for serotonin (Amat et al, 2001). One can postulate that the monoaminergic turn-over necessary to face a stressful event might not be efficiently engaged during WM testing upon inactivation of the LHb. The lack of deficits induced by CNQX in the EPM further suggests that the involvement of the LHb in stress-coping does not depend on AMPA-mediated mechanisms. Finally, the deficits induced by muscimol in the visible platform condition might suggest that muscimol-induced learning and memory deficits in the WM could be attributed to a decreased motivation for searching and climbing onto the platform. However, this appears rather unlikely. In our study, muscimol did not increase the amount of resting, suggesting an unaltered motivation to swim. In addition, LHb inactivation in animal models of depression results in an increased motivation to swim in the forced swim paradigm (Li et al, 2011; Nair et al, 2013).
In conclusion, the present study shows that blockade of LHb glutamatergic inputs impaired encoding and retrieval of spatial memory. Muscimol-induced LHb inactivation additionally produced anxiety in the EPM, deficits in the cued version of the WM, as well as a marked increase of thigmotaxis. Our findings suggest that the LHb is likely to participate in online memory processes at both encoding and retrieval steps, and that this participation relies upon glutamatergic mechanisms. In addition, the behavioral phenotype induced by LHb inactivation confirms its implication not only in the cognitive aspects but also in the emotional aspects of goaldirected behaviors. This is likely to occur through the integration of topdown information from several macrosystems and its transmission to midbrain monoaminergic and cholinergic nuclei, such as proposed by Geisler and Trimble (2008). The LHb could then be viewed as a key structure involved in the elaboration of adapted cognitive and motor responses under stressful situations, such as that proposed by Okamoto et al (2012). Finally, our findings add to the view developped by Stopper and Floresco (2014) that the LHb has a key role in the elaboration of the more relevant behavioral strategy through the course of an action. They are in line with the idea developed by Hikosaka (2010) that the LHb is at the crossroad of cognitive and emotional processes.
FUNDING AND DISCLOSURE
This work was financed through the European community (ERA-NET Neuron SuppHab grant), the Centre National de la Recherche Scientifique (CNRS), the University of Strasbourg and the French government (PhD fellowship to VM). The authors declare no conflict of interest.
Acknowledgments
The authors thank Dr Romain Goutagny for his help with the figures and O Bildstein, D Egesi, and G Edomwonyi for their assistance in animal care.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Aizawa H, Cui W, Tanaka K, Okamoto H (2013. a). Hyperactivation of the habenula as a link between depression and sleep disturbance. Front Hum Neurosci 7: 826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa H, Yanagihara S, Kobayashi M, Niisato K, Takekawa T, Harukuni R et al (2013. b). The synchronous activity of lateral habenular neurons is essential for regulating hippocampal theta oscillation. J Neurosci Off J Soc Neurosci 33: 8909–8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF (2001). The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res 917: 118–126. [DOI] [PubMed] [Google Scholar]
- Bast T, Silva BM, da, Morris RGM (2005). Distinct contributions of hippocampal NMDA and AMPA receptors to encoding and retrieval of one-trial place memory. J Neurosci Off J Soc Neurosci 25: 5845–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Willner P, Philippot P (2015). Depression: from psychopathology to pathophysiology. Curr Opin Neurobiol 30: 24–30. [DOI] [PubMed] [Google Scholar]
- Bernard R, Veh RW (2012). Individual neurons in the rat lateral habenular complex project mostly to the dopaminergic ventral tegmental area or to the serotonergic raphe nuclei. J Comp Neurol 520: 2545–2558. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O (2011). Lateral habenula neurons signal errors in the prediction of reward information. Nat Neurosci 14: 1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G (2002). Theta oscillations in the hippocampus. Neuron 33: 325–340. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S (2012). The mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev 36: 79–89. [DOI] [PubMed] [Google Scholar]
- Charles KJ, Calver AR, Jourdain S, Pangalos MN (2003). Distribution of a GABAB-like receptor protein in the rat central nervous system. Brain Res 989: 135–146. [DOI] [PubMed] [Google Scholar]
- Chastrette N, Pfaff DW, Gibbs RB (1991). Effects of daytime and nighttime stress on Fos-like immunoreactivity in the paraventricular nucleus of the hypothalamus, the habenula, and the posterior paraventricular nucleus of the thalamus. Brain Res 563: 339–344. [DOI] [PubMed] [Google Scholar]
- Cholvin T, Loureiro M, Cassel R, Cosquer B, Herbeaux K, de Vasconcelos AP et al (2014). Dorsal hippocampus and medial prefrontal cortex each contribute to the retrieval of a recent spatial memory in rats. Brain Struct Funct (in press). [DOI] [PubMed]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ (1995). Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64: 477–505. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW (2004). Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28: 771–784. [DOI] [PubMed] [Google Scholar]
- Devan BD, Goad EH, Petri HL (1996). Dissociation of hippocampal and striatal contributions to spatial navigation in the water maze. Neurobiol Learn Mem 66: 305–323. [DOI] [PubMed] [Google Scholar]
- D'Hooge R, De Deyn PP (2001). Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36: 60–90. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Knapp DJ, Breese GR (1996). Neuroanatomical characterization of Fos induction in rat behavioral models of anxiety. Brain Res 713: 79–91. [DOI] [PubMed] [Google Scholar]
- Düzel E, Penny WD, Burgess N (2010). Brain oscillations and memory. Curr Opin Neurobiol 20: 143–149. [DOI] [PubMed] [Google Scholar]
- Fries P (2005). A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci 9: 474–480. [DOI] [PubMed] [Google Scholar]
- Geisler S, Trimble M (2008). The lateral habenula: no longer neglected. CNS Spectr 13: 484–489. [DOI] [PubMed] [Google Scholar]
- Gill MJ, Ghee SM, Harper SM, See RE (2013). Inactivation of the lateral habenula reduces anxiogenic behavior and cocaine seeking under conditions of heightened stress. Pharmacol Biochem Behav 111: 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutagny R, Loureiro M, Jackson J, Chaumont J, Williams S, Isope P et al (2013). Interactions between the lateral habenula and the hippocampus: implication for spatial memory processes. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 38: 2418–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O (2010). The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci 11: 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Sesack SR, Lecourtier L, Shepard PD (2008). Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci Off J Soc Neurosci 28: 11825–11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH (2012). Ventral Tegmental Area Glutamate Neurons: Electrophysiological Properties and Projections. J Neurosci 32: 15076–15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD (2007). Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci Off J. Soc Neurosci 27: 6923–6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Churchwell JC (2011). An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem 96: 417–431. [DOI] [PubMed] [Google Scholar]
- Kim U, Lee T (2012). Topography of descending projections from anterior insular and medial prefrontal regions to the lateral habenula of the epithalamus in the rat. Eur J Neurosci 35: 1253–1269. [DOI] [PubMed] [Google Scholar]
- Lecca S, Meye FJ, Mameli M (2014). The lateral habenula in addiction and depression: an anatomical, synaptic and behavioral overview. Eur J Neurosci 39: 1170–1178. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Neijt HC, Kelly PH (2004). Habenula lesions cause impaired cognitive performance in rats: implications for schizophrenia. Eur J Neurosci 19: 2551–2560. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, de Vasconcelos AP, Leroux E, Cosquer B, Geiger K, Lithfous S et al (2011). Septohippocampal pathways contribute to system consolidation of a spatial memory: sequential implication of GABAergic and cholinergic neurons. Hippocampus 21: 1277–1289. [DOI] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D et al (2011). Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature 470: 535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O (2007). Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447: 1111–1115. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O (2009). Representation of negative motivational value in the primate lateral habenula. Nat Neurosci 12: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Strand NS, Neumaier JF (2013). DREADDing the lateral habenula: a review of methodological approaches for studying lateral habenula function. Brain Res 1511: 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson OG, Kalen P, Rosengren E, Björklund A (1990). Acetylcholine release in the rat hippocampus as studied by microdialysis is dependent on axonal impulse flow and increases during behavioural activation. Neuroscience 36: 325–338. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Agetsuma M, Aizawa H (2012). Genetic dissection of the zebrafish habenula, a possible switching board for selection of behavioral strategy to cope with fear and anxiety. Dev Neurobiol 72: 386–394. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Andolina D (2014). Serotonin and stress coping. Behav Brain Res 277: 58–67. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Henn FA (2007). Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med Hypotheses 69: 1305–1308. [DOI] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R (2012). Input to the Lateral Habenula from the Basal Ganglia Is Excitatory, Aversive, and Suppressed by Serotonin. Neuron 74: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, Ilango A, Scheich H, Wetzel W, Ohl FW (2010). Differential neuromodulation of acquisition and retrieval of avoidance learning by the lateral habenula and ventral tegmental area. J Neurosci 30: 5876–5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Floresco SB (2014). What's better for me? Fundamental role for lateral habenula in promoting subjective decision biases. Nat Neurosci 17: 33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL (2014). Cognitive dysfunction in unipolar depression: implications for treatment. J Affect Disord 152-154: 19–27. [DOI] [PubMed] [Google Scholar]
- Wang RY, Aghajanian GK (1977). Physiological evidence for habenula as major link between forebrain and midbrain raphe. Science 197: 89–91. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, Bontempi B (2010). Memory formation and long-term retention in humans and animals: Convergence towards a transformation account of hippocampal–neocortical interactions. Neuropsychologia 48: 2339–2356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.