Abstract
Background:
Dexmedetomidine, an α2 agonist, when used as an adjuvant in general anesthesia attenuates stress response to various noxious stimuli, maintains perioperative hemodynamic stability and provides sedation without adversely affecting recovery in postoperative period.
Materials and Methods:
Sixty patients were randomly divided into two groups of 30 each. In Group A, dexmedetomidine was given intravenously as loading dose of 1 μg/kg over 10 min, and normal saline was given in Group B patients. After induction with propofol, in Group A, dexmedetomidine was given as infusion at a dose of 0.2–0.8 μg/kg/h. Sevoflurane was used as inhalation agent in both groups. Perioperative monitoring parameters were recorded. Postoperative sedation and recovery were assessed.
Statistical Analysis Used:
Demographic data were analyzed using Pearson's Chi-square test. Changes in the heart rate (HR), systolic blood pressure (BP) and diastolic BP were analyzed using unpaired t-test and Mann–Whitney rank sum test was used to calculate “P” value wherever (Shapiro–Wilk)/normality test gave ambiguous results.
Results:
Dexmedetomidine significantly attenuates stress response at intubation with lesser increase in HR (86.00 ± 5.16 vs. 102.97 ± 7.07/min.), mean BP (95.78 ± 5.35 vs. 110.18 ± 5.35) as compared to the control group (P < 0.05). After pneumoperitoneum, HR was 85.07 ± 6.23 versus 107.10 ± 4.98, mean BP was 98.98 ± 10.16 versus 118.54 ± 6.27 (P < 0.05). Thus maintains intraoperative hemodynamic stability. Postoperatively, the test group showed no statistically significant difference in the extubation time (7.00 ± 0.58 vs. 6.74 ± 0.73) and response to oral commands (8.78 ± 0.72 vs. 8.66 ± 0.73) (P > 0.05).
Conclusion:
Dexmedetomidine attenuates various stress responses during surgery and maintains the hemodynamic stability when used as an adjuvant in general anesthesia and dexmedetomidine does not delay recovery.
Keywords: Clinical recovery score, dexmedetomidine, fentanyl, pneumoperitoneum
INTRODUCTION
Dexmedetomidine is a highly selective α2 adrenergic agonist.[1] It possesses hypnotic, sedative, anxiolytic, analgesic, sympatholytic properties without producing significant respiratory depression. Dexmedetomidine, by its central sympatholytic action, promotes hemodynamic stability when used as an adjuvant during general anesthesia.[2] It has analgesic and anesthetic sparing property. However, because of its sedative property, it is unknown if the recovery from anesthesia would be delayed when used as continuous infusion.
Aims
This placebo controlled, double-blind, prospective comparative study is designed to evaluate the efficacy of dexmedetomidine in providing hemodynamic stability during perioperative period and recovery from anesthesia in patients undergoing laparoscopic cholecystectomy.
MATERIALS AND METHODS
Study period
Six months.
Study design
Prospective, randomized, double-blind, clinical study.
Study approval
The study was approved by the Institutional Medical Ethics Committee and written informed consent was obtained from all included patients.
Study population
Sixty patients scheduled to undergo elective laparoscopic cholecystectomy under general anesthesia were enrolled in the study.
Patient selection
American Society of Anesthesiologists (ASA) Grades I and II with age 30–50 years having weight 40–80 kg were included in the study. Patients unwilling for consent, with ASA III and above, morbid obese, pregnant patients, breastfeeding mothers, allergy to α2 adrenergic agonist/sulfa drugs were excluded from the study.
Study groups
The study was carried out in 60 patients of either sex (20–50 years) allocated in one of two parallel groups containing 30 patients each.
Group A - Dexmedetomidine intravenous (IV) bolus over 10 min and continuous maintenance infusion 0.5 µg/kg/h
Group B - Control group 0.9% normal saline IV bolus and continuous maintenance infusion.
Randomization
A computer generated table of random numbers was prepared allotting equal number of patients in each group.
Study materials
Propofol, fentanyl, dexmedetomidine, circle system (breathing circuit), endotracheal tube.
On the day of surgery on arrival of patient in the operating room monitors were attached. Heart rate (HR), systolic blood pressure (SBP), diastolic BP (DBP), mean arterial pressure (MAP), SpO2 recorded. All patients received 6 L of oxygen by Hudson's mask for 5 min. All patients received injection glycopyrrolate 0.004 mg/kg, injection ondansetron 0.08 mg/kg and injection midazolam 0.02 mg/kg intravenously.
The study medication [Dexmedetomidine 200 µg (2 ml) in 48 ml of normal saline means 4 µg/ml] was prepared. Normal saline was similarly prepared in similar prescribed format for standardization. Appropriate labels were allotted and attached beforehand.
An infusion of the allotted labeled drug for that particular serial number as per the randomization chart was administered by investigator and was started 10 min prior to induction. Initial bolus infusion of study medication (dexmedetomidine 1 µg/kg) over 10 min. Patients were preoxygenated with 100% oxygen with facemask. Induction was carried with injection fentanyl 2 µg/kg and injection propofol 2–2.5 mg/kg in graded doses until loss of consciousness. After confirming adequacy of ventilation, injection succinylcholine 1.5 mg/kg was administered and intubated with adequate size cuffed endotracheal tube. Anesthesia was maintained with O2:N2O in 40:60 proportion with sevoflurane to maintain the HR and BP within 20% of the baseline value. Muscle relaxation was maintained with injection vecuronium bromide, loading dose of 0.08 mg/kg and intermittent top-ups of 0.02 mg/kg as and when required. Patients in Group A, received dexmedetomidine infusion at the dose of 0.5 µg/kg/h intraoperatively, while patients in Group B, were given normal saline at the respectively comparable rate. Patients were ventilated with an initial tidal volume of 6–8 ml/kg and a respiratory rate of 14 breaths/min, which was later adjusted to keep the EtCO2 within 35–40 mm of Hg. Intraabdominal pressure was maintained below 14 mmHg. Fentanyl (0.5 µg/kg) top-ups were given to keep the MAP within 20% of baseline. All patients received injection paracetamol 1 gm IV infusion as analgesia intraoperatively. At the end of pneumoperitoneum, infusion in both the groups and sevoflurane was stopped.
At the end of surgery, 0.125% bupivacaine was injected at the port incisions. Complete reversal of neuromuscular blockade was achieved with injection glycopyrrolate 0.008 mg/kg and injection neostigmine 0.04 mg/kg and patients were extubated after establishment of spontaneous, regular and adequate respiration and good muscle power with appropriate response to verbal commands. Patients having fluctuations in HR and BP >20% of baseline value were recorded and treated accordingly.
Immediately after extubation, recovery was assessed by modified Aldrete's and sedation score as follows: Score 1, awake; 2, sleepy but arousable; score 3, sleepy difficult to arouse. HR, BP, SpO2 were recorded continuously at predetermined time intervals as per the protocol. Number of patients requiring total fentanyl top-ups were recorded. Time interval of time to tracheal extubation, time to respond to verbal command was recorded after the stoppage of infusion.
Postoperatively patients were shifted to the postanesthesia care unit, received oxygen under Hudson's mask and IV fluids. The HR, SBP, DBP, MAP, SpO2, postoperative nausea and vomiting were noted every 15 min thereafter for 2 h and treated accordingly.
Statistical analysis used
Demographic data were analyzed using Pearson's Chi-square test. Changes in the HR, SBP and DBP were analyzed using unpaired t-test and Mann–Whitney rank sum test was used to calculate “P” value wherever (Shapiro–Wilk)/normality test gave ambiguous results. P < 0.05 was considered significant in all statistical tests applied.
RESULTS
Prospective, randomized, double-blind, clinical study designed to evaluate the efficacy of dexmedetomidine to provide intraoperative and postoperative hemodynamic stability and its overall effects on intraoperative monitoring parameters in patients undergoing laparoscopic cholecystectomy.
The comparative study of the two groups is as follows: [Tables 1–10].
Table 1.
Demography of patients
Table 10.
Perioperative complications

Table 2.
Mean duration of surgery and anesthesia in minutes
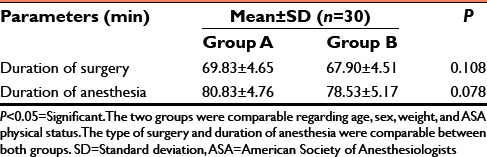
Table 3.
Perioperative mean HR
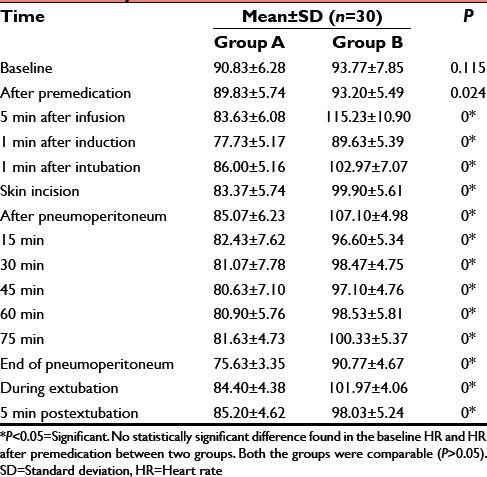
Table 4.
Perioperative MAP
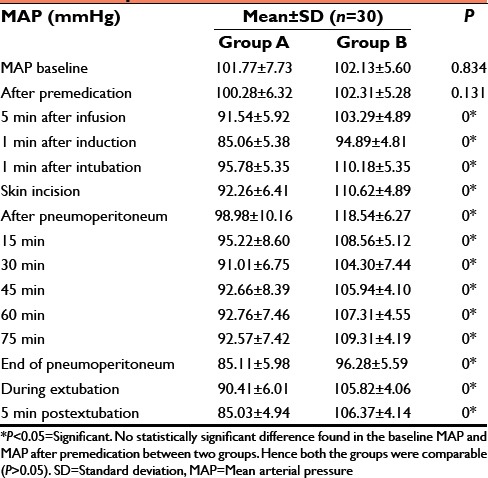
Table 5.
Comparison of recovery time

Table 6.
Number of fentanyl top-ups (0.5 µg/kg) required

Table 7.
Postoperative HR
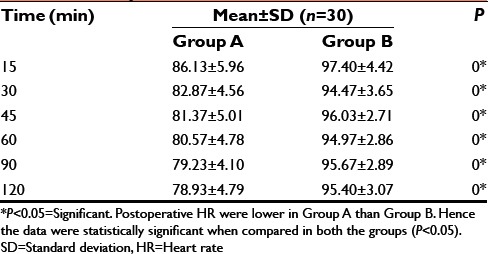
Table 8.
Postoperative MAP
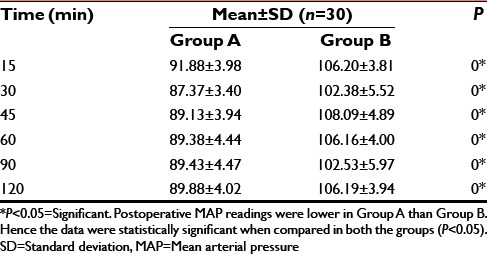
Table 9.
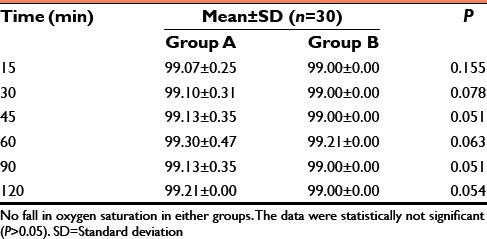
Postoperative oxygen saturation (SpO2)
DISCUSSION
In our study, we observed the effects of dexmedetomidine on hemodynamics during perioperative period in patients undergoing laparoscopic cholecystectomy.[3,4] Dexmedetomidine is a highly selective α2 adrenergic agonist with sedative, anxiolytic, and analgesic, sympatholytic and antihypertensive effects.[5] It also produces sedation and diminishes the intraoperative requirement of analgesics.[6]
Hemodynamic parameters
In another studies, dexmedetomidine infusion rates ranging from 0.1 to 10 µg/kg/h have been used. The studies with higher infusion rates had more incidences of adverse effects like hypotension and bradycardia.[7]
Bakhamees et al.[8] studied the effects of dexmedetomidine in morbidly obese patients undergoing laparoscopic gastric bypass. They gave dexmedetomidine infusion (0.4 µg/kg/h) in one group and normal saline infusion (0.4 µg/kg/h) in other group throughout the surgery and compared the hemodynamics. They found that patients who received dexmedetomidine showed significant decrease of intraoperative and postoperative mean BP and HR. The observations made in both these studies are similar to our study findings in providing perioperative hemodynamic stability.[9,10]
Emergence from anesthetic effects and extubation are equally crucial as is laryngoscopy, intubation, and surgical period. Dexmedetomidine enables a smooth transition from the time of administration of reversal to the postextubation phase by suppressing the central nervous system sympathetic activity, leading to high quality of extubation with minimum hemodynamic changes, as we observed in majority of our patients in dexmedetomidine group. Similar results have also been observed in other studies.[11]
In our study, in Group A during extubation the mean HR (84.40 ± 4.38/min), MAP (90.41 ± 6.01 mmHg) were noted and compared with control group which were: The mean HR (101.97 ± 4.06/min), MAP (105.82 ± 4.06 mmHg). Above data suggest that hemodynamics were much more stable in Group A than control group during extubation.[11] Statistically there was significant difference found when compared in both the groups (P < 0.05).
Recovery from anesthesia
We also observed that the time for extubation and time to respond to oral commands were was similar in both the groups. There was no statistically significant difference in both the groups (P > 0.05) as dexmedetomidine does not seem to have significant respiratory depression property. There was no fall in oxygen saturation parameter noticed in both the groups in our study finding.[12]
Postextubation period
It was observed that, those patients who received dexmedetomidine infusion in the intraoperative period had HR, MAP on the lower side as compared to that of control group which received normal saline infusion in immediate postoperative period. The difference was statistically significant (P < 0.05).
In our study, fentanyl top-ups of 0.5 µg/kg were given intraoperatively whenever required to keep mean BP within 20% of baseline value. Thus those patients who received dexmedetomidine infusion has lesser requirement of fentanyl and the hemodynamics were much more stable than control group.
The findings of our study are in agreement with the findings of various investigators in that dexmedetomidine infusion intraoperatively is an effective agent to reduce the hemodynamic fluctuations associated with laparoscopic surgery.
We have observed that there is more requirement of fentanyl top-ups in control group, which could have led to postoperative nausea and vomiting incidences in control group (13.3%). There was not a single patient with postoperative nausea and vomiting in Group A.[13]
CONCLUSION
Pneumoperitoneum required for laparoscopic surgery results in multiple hemodynamic changes due to raised intraabdominal pressure. Pneumoperitoneum leads to increase in arterial pressure and systemic and pulmonary vascular resistances and a decrease in cardiac output. Both mechanical (increased intraabdominal pressure) and humoral factors contribute to these adverse cardiovascular changes.
We have used injection dexmedetomidine to evaluate its effect on hemodynamic parameters in laparoscopic cholecystectomy patients during perioperative period.
We observed that there is not significant rise in HR, mean BP.[14] There is no difference from emergence of anesthesia in both the groups and the incidence of nausea-vomiting in postoperative period was less in the Group A compared to Group B.
In another studies, an infusion of dexmedetomidine at the rate of 0.5 µg/kg/h 30 min before induction and 0.6 µg/kg/h thereafter until the end of surgery leads to better recovery of patients undergoing laparoscopic cholecystectomy.[15]
We conclude from this study that, dexmedetomidine IV infusion in the loading dose of 1.0 µg/kg over 10 min. and subsequent maintenance infusion over the range of 0.4–0.7 µg/kg/h provides perioperative hemodynamic stability in ASA I/II Class patients during laparoscopic cholecystectomy because of their sedative, hypnotic, anxiolytic, and sympatholytic properties.
It also affords added advantage of reduction in postoperative complications such as nausea-vomiting. However further study is required to evaluate its effect on hemodynamic parameters in high risk group patients with compromised cardio-respiratory function undergoing laparoscopic surgical procedures.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Acknowledgment
Dr. H. K. Shah, Professor, General Surgery.
REFERENCES
- 1.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–8. doi: 10.2165/00003495-200059020-00012. [DOI] [PubMed] [Google Scholar]
- 2.Joris JL, Noirot DP, Legrand MJ, Jacquet NJ, Lamy ML. Hemodynamic changes during laparoscopic cholecystectomy. Anesth Analg. 1993;76:1067–71. doi: 10.1213/00000539-199305000-00027. [DOI] [PubMed] [Google Scholar]
- 3.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–42. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Scheinin B, Lindgren L, Randell T, Scheinin H, Scheinin M. Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need for thiopentone and peroperative fentanyl. Br J Anaesth. 1992;68:126–31. doi: 10.1093/bja/68.2.126. [DOI] [PubMed] [Google Scholar]
- 5.Guler G, Akin A, Tosun Z, Eskitascoglu E, Mizrak A, Boyaci A. Single-dose dexmedetomidine attenuates airway and circulatory reflexes during extubation. Acta Anaesthesiol Scand. 2005;49:1088–91. doi: 10.1111/j.1399-6576.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- 6.Yildiz M, Tavlan A, Tuncer S, Reisli R, Yosunkaya A, Otelcioglu S. Effect of dexmedetomidine on haemodynamic responses to laryngoscopy and intubation: Perioperative haemodynamics and anaesthetic requirements. Drugs R D. 2006;7:43–52. doi: 10.2165/00126839-200607010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharjee DP, Nayek SK, Dawn S, Bandopadhyay G, Gupta K. Effects of dexmedetomidine on haemodynamics in patients undergoing laparoscopic cholecystectomy – A comparative study. J Anaesthesiol Clin Pharmacol. 2010;26:45. [Google Scholar]
- 8.Bakhamees HS, El-Halafawy YM, El-Kerdawy HM, Gouda NM, Altemyatt S. Effects of dexmedetomidine in morbidly obese patients undergoing laparoscopic gastric bypass. Middle East J Anaesthesiol. 2007;19:537–51. [PubMed] [Google Scholar]
- 9.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 10.Keniya VM, Ladi S, Naphade R. Dexmedetomidine attenuates sympathoadrenal response to tracheal intubation and reduces perioperative anaesthetic requirement. Indian J Anaesth. 2011;55:352–7. doi: 10.4103/0019-5049.84846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tufanogullari B, White PF, Peixoto MP, Kianpour D, Lacour T, Griffin J, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: The effect on recovery outcome variables. Anesth Analg. 2008;106:1741–8. doi: 10.1213/ane.0b013e318172c47c. [DOI] [PubMed] [Google Scholar]
- 12.Ohtani N, Kida K, Shoji K, Yasui Y, Masaki E. Recovery profiles from dexmedetomidine as a general anesthetic adjuvant in patients undergoing lower abdominal surgery. Anesth Analg. 2008;107:1871–4. doi: 10.1213/ane.0b013e3181887fcc. [DOI] [PubMed] [Google Scholar]
- 13.Massad IM, Mohsen WA, Basha AS, Al-Zaben KR, Al-Mustafa MM, Alghanem SM. A balanced anaesthesia with dexmedetomidine decreases postoperative nausea and vomiting after laparoscopic surgery. Saudi Med J. 2009;30:1537–41. [PubMed] [Google Scholar]
- 14.Patel CR, Engineer SR, Shah BJ, Madhu S. Effect of intravenous infusion of dexmedetomidine on perioperative haemodynamic changes and postoperative recovery: A study with entropy analysis. Indian J Anaesth. 2012;56:542–6. doi: 10.4103/0019-5049.104571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khanduja S, Ohri A, Panwar M. Dexmedetomidine decreases requirement of thiopentone sodium and pentazocine followed with improved recovery in patients undergoing laparoscopic cholecystectomy. J Anaesthesiol Clin Pharmacol. 2014;30:208–11. doi: 10.4103/0970-9185.130022. [DOI] [PMC free article] [PubMed] [Google Scholar]


