Abstract
Background:
The main stay of treatment in organophophosphorous [OP] poisoning is with atropine, oximes and supportive therapy. Despite the therapy, no improvement in mortality and morbidity. Fresh frozen plasma [FFP] a source of serum cholinesterase act as bio-scavenger to neutralise organophosphate toxins to improve the patients out come.
Methods:
The prospective study was conducted in 80 patients with acute OP poisoning. Patients with moderate to severe grade of OP poisoning with serum cholinesterase level <1000 IU/L were included in the study. Study group received atropine and oximes along with FFP given as 4 units first day, 3units on 2nd day, 2 units on 3rd day. Control group was given atropine and oximes only. Serum cholinesterase enzymes level, consumption of atropine per day, number of days on ventilator, length of ICU stay, and need for tracheostomy were assessed.
Results:
There was a significant increase in the serum cholinesterase levels after FFP infusion in the study group in comparison to the control group. Mean duration of Intensive Care Unit [ICU] stay was 8.35±4.3 in the study group and 12.45±4.13 in the control group. 06 patients in the control group succumbed whereas there were no fatalities in the study group.
Conclusion:
Daily reducing dose of FFP therapy for 3 consecutive days has beneficial effect in acute OP poisoning by increasing serum cholinesterase enzymes in blood with reduction in total dose of atropine consumption per day. It also reduces the ICU stay with zero mortality in OP poisoning.
Keywords: Acetyl cholinesterase, atropine, fresh frozen plasma, organophosphate, oximes
INTRODUCTION
Organophosphorus (OP) poisoning is a major public health problem with mortality range of 4–30%.[1]
OP toxicity is due to carbon, phosphorous acid derivatives that are well absorbed through the skin, lungs, and gastrointestinal tract. Binding to red blood cholinesterase, renders this enzyme nonfunctional leading to excessive accumulation of acetylcholine in central nervous system (CNS).[2]
Atropine and oximes are the main stay of treatment, but search for newer line of treatment continues to reduce mortality and morbidity.
Hence, this study is undertaken to assess the effect of daily reducing dose infusion of fresh frozen plasma (FFP) for 3 consecutive days in OP poisoning, correlation with elevation of serum cholinesterase level, and to evaluate clinical outcome of the patients.
MATERIALS AND METHODS
Prospective randomized control study was conducted on 80 patients admitted to Intensive Care Unit (ICU) of Victoria and Bowring and Lady Curzon Hospital, Bengaluru, during the period of March 2014–June 2015. Patients with history and clinical picture suggestive of acute OP poisoning were studied. Thorough observation of poison container was done and sent for toxicology analysis. Institutional Ethical Committee approval was taken. Informed consent was obtained after explaining to patient/relatives in their language.
Eighty patients who fulfill the inclusion – exclusion criteria are randomized into two equal groups of 40 each.
Inclusion criteria
All orally ingested OP poisoning cases with American Society of Anesthesiologists grades I and II, in the age group of 18–60 years were studied. Moderate to severe grade OP patients as per peradeniya OP poisoning scale were included.[3]
Exclusion criteria
Patients having serum cholinestarase level more than 1000 IU/L, those who had consumed mixed poisons, unknown compound consumption, and suspected aspiration were excluded from the study.
Patients were divided into two groups:
Control group received atropine and oximes only.
Study group received atropine and oximes along with FFP 4 units (800 ml) 1st day, while 3 units (600) were given on 2nd day, and 2 units (400) on 3rd day.
Atropine infusion to maintain a state of atropinization was started with 5 mg/h and titrated accordingly to maintain heart rate of 80–100 bpm and drying of salivary, bronchial secretions. Injection oximes was given 30 mg/kg intravenous (IV) bolus followed by 10 mg/kg/h until 48–72 h, depending up on clinical improvement.
Stomach wash and decontamination was done before shifting to ICU.
Hemodynamic parameters, saturation, muscle power, and CNS status were continuously monitored and recorded regularly. Arterial blood gas (ABG) analysis done once in 24 h and also when required.
Plasma cholinesterase level was measured on 1st day on admission and also on 4th day in both the groups.
If signs of respiratory distress occurred, patients were intubated and put on ventilator. Ventilation done with synchronized intermittent mandatory ventilation or bi-level positive airway pressure mode and settings were managed according to ABG status. Antibiotic and stress ulcer prophylaxis were given to both groups according to institutional protocol.
Patients were weaned from ventilator according to clinical assessment of muscle power and respiratory effort.
Tracheostomy was done if there was no recovery of muscle power and adequate respiration even after 10 days.
Patients were extubated when they achieved extubation criteria and monitored in ICU for respiratory distress for 24 h, and later shifted to ward.
Nosocomial infection, electrolyte imbalance, and organ dysfunction were checked and if any, were recorded.
Patients were analyzed for increase in serum cholinesterase level, consumption of atropine and oximes, length of ICU stay, need of ventilator support, need for tracheostomy and mortality rate.
Sample size was selected on the basis of pervious study, two groups detected a projected difference of 30% between the two groups with type I error (alpha) of 0.05 and power of study 0.3.
The Statistical software namely SAS 9.2, (SAS Institute Inc., Nc, USA), SPSS 15.0 (IBM, USA), Stata 10.1 (Statat Corp. Lp Texas, USA), MedCalc 9.0.1 (Medcalc software buda ostnd Belgium), Systat 12.0 and R environment version 2.11.1 were used for the analysis. Discrete variables were compared by Student's t-test, Chi-square/Fisher exact test, whichever appropriate, *moderately significant P value: 0.01 < P ≤ 0.05, **strongly significant P value: P ≤ 0.01.
RESULTS
In both the study groups, patients were more in 20–30 years age and male gender was more common [Tables 1 and 2]. However, this was not statistically significant.
Table 1.
Age distribution of patients in study group and control group
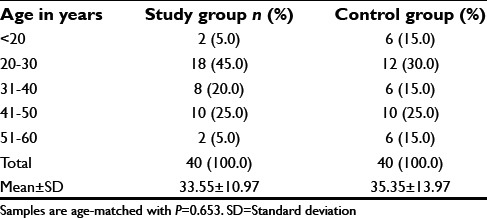
Table 2.
Gender distribution of patients studied

Tables 3 and 4 depict 35–65% increase in serum cholinesterase in the study group. In the control group, only 30–40% increase was seen from day 1 to day 4 and this was statistically significant (P ≤ 0.001).
Table 3.
Serum enzymes in two groups of patients studied
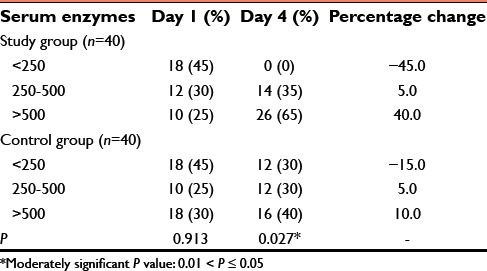
Table 4.
Serum enzymes: An evaluation

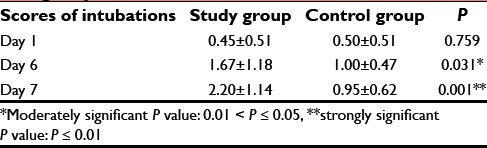
In addition, scores of intubation in both groups on 6th and 7th day were statistically significant with P = 0.001 [Table 5].
Table 5.
Comparison of scores of intubations in two groups studied
Six patients expired in the control group during the study, while there was no mortality in the study group [Table 6].
Table 6.
Outcome in two groups of patients studied

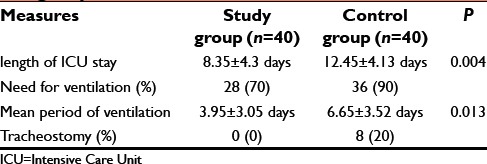
The length of ICU stay, need for ventilation, mean duration of ventilation, and tracheostomy requirement were less in the study group [Table 7].
Table 7.
Length of ICU stay, need for ventilation, need for tracheostomy, and mortality in both the groups
Graphical representation shows per day consumption of atropine 12.78 ± 5.6 in the study group. In the control group, it was 22.13 ± 12.6 [Graph 1].
Graph 1.
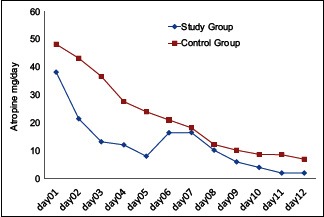
Atropine consumption per day
DISCUSSION
Majority of OP pesticides are liquid formulations and this makes oral poison the most common route of poisoning. Poisoning due to OP compound is classified as mild, moderate, and severe depending on signs and symptoms according to peradeniya organophosphorus poisoning scale.[3]
Basic line of treatment for OP is atropine and oxime. The efficacy of atropine is clinically proven, but clinical experience with oxime is controversial. Along with these, many other drugs have been tried to improve the patients' outcome.[4]
Benzodiazepines are favorable in OP poisoning and to control anxiety, restlessness, muscle fasciculation, and agitation along with atropine and oxime.[5] Sodium bicarbonate IV infusion at the rate of 5 mEq/kg in 60 min followed by 5–6 mEq/kg/day promotes moderate alkalinization (blood pH between 7.45 and 7.55) in OP poisoning, which is beneficial though not promising.[6] Magnesium sulfate is also tried for OP poisoning to improve the outcome of patients.[7]
OP pesticides are fat-soluble macromolecular substances and bind to the proteins easily. A recent study in China found that early and repeated hemoperfusion is more efficient than single hemoperfusion in treating organophosphate poisoning.[8] However, the procedure is cumbersome and chance of infection occurs.
FFP, a bio-scavenger, is being suggested as a useful therapy for clearing of free organophosphates. FFP is produced by rapidly freezing the plasma removed from a whole-blood donation or collected by apheresis. This was performed within 8 h of blood donation. FFP was transfused on the basis of ABO-blood group compatibility. FFP stored in 4°C, cholinesterase enzyme stable for few weeks when dry and freeze, enzyme activity is for 2 years. Cholinesterase enzyme levels in FFP is 90 mg in 11 packs of FFP or every two bags of FFP increases serum cholinesterase by 461.7 ± 142.1 IU/L, approximately.[9] Other uses of FFP are in case of multiple blood transfusion, congenital bleeding diathesis, and coagulation abnormality.
It is hypothesized that the enzyme serum cholinesterase present in FFP will sequester free OP compound present in blood and remove it from circulation, thus preventing toxic effect on neuromuscular junction. Exact requirement of FFP in OP patients is not clearly defined.[10] Direct measurement of serum cholinesterase activity provides a measure of the degree of toxicity.[11]
Study conducted by Yun et al. and Kavya et al. found elevated serum cholinesterase enzyme level in survived patients.[12,13] In our study, we measured the serum cholinesterase enzyme level on day 1 of admission and on 4th day of treatment to see the percentage of increase in the enzyme level. Serum cholinesterase progressively increased from 35% to 65% from 1st to 4th day in the study group compared to the control group and this was statistically significant with P = 0.027 [Graph 2].
Graph 2.
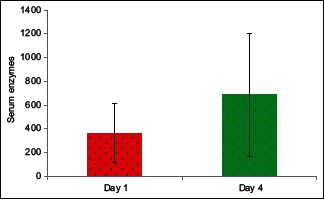
Serum cholinesterase enzymes in two groups of patients studied on day 1 and day 4
Manu et al. reported that serial measurements of serum cholinesterase levels help predict the length of ICU stay, duration of mechanical ventilation, and prognosis of the patient following OP poisoning. The severity of poisoning directly correlates with serum cholinesterase level.[14] Hence, progressive increase in the level of serum cholinesterase dictates better outcome with minimal complication in OP poisoning as observed in our study.
We transfused FFP as main source of serum cholinesterase given as daily reducing dose for 3 consecutive days to maintain high level of serum cholinesterase in blood so as to neutralize the toxin in the blood. Similar beneficial effect was observed by Guven et al. and Eddleston et al. and also it prevents development of intermediate syndrome and related mortality.[10,15] But, Aygun et al. reported no correlation between serum cholinesterase enzyme and severity of OP poisoning. However, he opined that the cholinesterase enzyme level decrease prompts the diagnosis of OP poisoning.
Pazooki et al. treated OP poisoning with four packs stat dose of FFP at the onset of treatment. They observed no significant effect on the outcome in their patients.[16]
In the present study, daily reducing dose of FFP was given for 3 consecutive days. They act by neutralizing toxins released into circulation by redistribution from adipose tissue.[17]
Utilization of atropine in the study group was less than that of the control group (12.78 ± 5.62 vs. 22.13 ± 12.6) and is statistically significant.
In addition, length of ICU stay in the study group was 8.35 ± 4.3 days, which is less when compared to the control group (12.45 ± 4.13 days). Thirty six patients needed ventilation in control group but only twenty eight patients needed ventilation in study group. We also observed that the duration of ventilation was less in the study group [Graph 3]. Hence, the early recovery in our study group was due to high level of serum cholinesterase in blood.[11]
Graph 3.
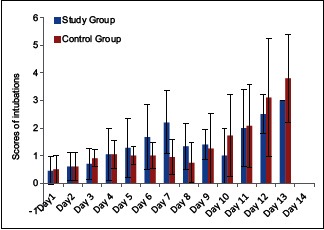
Comparative evaluation of intubation scores in two groups of patients studied
Chiranjeevi et al. concluded that increase in plasma cholinesterase, in 31 patients of OP poisoning, have significant clinical improvement with less patients requiring mechanical ventilation and better survival rate.[18]
FFP prompted early extubation, less ventilation, and nil tracheostomy in our study similar to an Indian study by Chiranjeevi et al.[18] On the contrary patients needed prolonged ventilatory assistance, difficulty in extubation and eight cases required tracheostomy in control group.
In our study, no mortality was observed in the study group, but in the control group six patients died almost similar to Yang and Deng's study.[19]
CONCLUSION
Reducing daily dose of FFP infusion therapy for treating organophosphate poisoning given 3 days consecutively as adjuvant to atropine and oximes promotes progressive elevation in serum cholinesterase enzyme with significant clinical improvement. FFP transfusion reduces ICU stay with lesser period of ventilation and tracheostomy is not required with zero mortality in OP poisoning cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Bairy KL, Vidyasagar S, Sharma A, Sammad V. Controversies in the management of organophosphate pesticide poisoning. Indian J Pharmacol. 2007;39:71–4. [Google Scholar]
- 2.Rotenberg M, Shefi M, Dany S, Dore I, Tirosh M, Almog S. Differentiation between organophosphate and carbamate poisoning. Clin Chim Acta. 1995;234:11–21. doi: 10.1016/0009-8981(94)05969-y. [DOI] [PubMed] [Google Scholar]
- 3.Senanayake N, de Silva HJ, Karalliedde L. A scale to assess severity in organophosphorus intoxication: POP scale. Hum Exp Toxicol. 1993;12:297–9. doi: 10.1177/096032719301200407. [DOI] [PubMed] [Google Scholar]
- 4.Braitberg G. Drugs and antidotes in acute intoxication. In: Ronco C, Bellomo R, Kellum JA, editors. Critical Care of Nephrology. 2nd ed. Canada: Saunders Publications; 2009. pp. 928–30. [Google Scholar]
- 5.Blain PG. Organophosphorus poisoning (acute) Clin Evid. 2011;17:2102. [PMC free article] [PubMed] [Google Scholar]
- 6.Balali-Mood M, Ayati MH, Ali-Akbarian H. Effect of high doses of sodium bicarbonate in acute organophosphorous pesticide poisoning. Clin Toxicol (Phila) 2005;43:571–4. doi: 10.1081/clt-200068845. [DOI] [PubMed] [Google Scholar]
- 7.Pajoumand A, Shadnia S, Rezaie A, Abdi M, Abdollahi M. Benefits of magnesium sulfate in the management of acute human poisoning by organophosphorus insecticides. Hum Exp Toxicol. 2004;23:565–9. doi: 10.1191/0960327104ht489oa. [DOI] [PubMed] [Google Scholar]
- 8.Bo L. Therapeutic efficacies of different hemoperfusion frequencies in patients with organophosphate poisoning. Eur Rev Med Pharmacol Sci. 2014;18:3521–3. [PubMed] [Google Scholar]
- 9.Stovner J, Stadskleiv K. Suxamethonium apnoea terminated with commercial serumcholinesterase. Acta Anaesthesiol Scand. 1976;20:211–5. doi: 10.1111/j.1399-6576.1976.tb05031.x. [DOI] [PubMed] [Google Scholar]
- 10.Guven M, Sungur M, Eser B, Sari I, Altuntas F. The effects of fresh frozen plasma on cholinesterase levels and outcomes in patients with organophosphate poisoning. J ClinToxicol. 2004;42:617–23. doi: 10.1081/clt-200026967. [DOI] [PubMed] [Google Scholar]
- 11.Aygun D, Doganay Z, Altintop L, Guven H, Onar M, Deniz T, et al. Serum acetylcholinesterase and prognosis of acute organophosphate poisoning. J Toxicol Clin Toxicol. 2002;40:903–10. doi: 10.1081/clt-120016962. [DOI] [PubMed] [Google Scholar]
- 12.Yun HW, Lee DH, Lee JH, Cheon YJ, Choi YH. Serial serum Cholinesterase activities as a prognostic factor in organophosphate poisoned patients. Hong Kong J Emerg Med. 2012;19:92–7. [Google Scholar]
- 13.Kavya ST, Srinivas V, Chandana, Madhumati R. Clinical profile of patients with organophosphorus poisoning in an intensive care unit in a tertiary hospital. Int J Clin Cases Investig. 2012;24:31. [Google Scholar]
- 14.Manu MS, Prashant V, Akila P, Suma MN, Basavanagowdappa H. A retrospective analysis of serial measurement of serum cholinesterase in acute poisoning with organophosphate compounds. Toxicol Int. 2012;19:255–9. doi: 10.4103/0971-6580.103662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eddleston M, Buckley NA, Ayer P, Dawson AH. Management of acute organophosphorous poisoning. Lancet. 2008;397:591–607. doi: 10.1016/S0140-6736(07)61202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pazooki S, Solhi H, Vishteh HR, Shadnia S, Beigi MJ. Effectiveness of fresh frozen plasma as supplementary treatment in organophosphate poisoning. Med J Malaysia. 2011;66:342–5. [PubMed] [Google Scholar]
- 17.Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, von Meyer L, et al. Differences between organophosphorus insecticides in human self-poisoning: A prospective cohort study. Lancet. 2005;366:1452–9. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]
- 18.Chiranjeevi UK, Kishan PV, Chandrasekhar E, Usharani P. The utility of serial serum cholinesterase as a prognostic marker in organophosphorus compound poisoning. Int J Basic Clin Pharmacol. 2014;3:529–33. [Google Scholar]
- 19.Yang CC, Deng JF. Intermediate syndrome following organophosphate insecticide poisoning. J Chin Med Assoc. 2007;70:467–72. doi: 10.1016/S1726-4901(08)70043-1. [DOI] [PubMed] [Google Scholar]


