Abstract
Delayed awakening from anesthesia remains one of the biggest challenges that involve an anesthesiologist. With the general use of fast-acting anesthetic agents, patients usually awaken quickly in the postoperative period. The time to emerge from anesthesia is affected by patient factors, anesthetic factors, duration of surgery, and painful stimulation. The principal factors responsible for delayed awakening following anesthesia are anesthetic agents and medications used in the perioperative period. Nonpharmacological causes may have a serious sequel, hence recognizing these organic conditions is important. Certain underlying metabolic disorders such as hypoglycemia, severe hyperglycemia, and electrolyte imbalance, especially hypernatremia, hypoxia, hypercapnia, central anticholinergic syndrome, chronic hypertension, liver disease, hypoalbuminemia, uremia, and severe hypothyroidism may also be responsible for delayed recovery following anesthesia. Unexpected delayed emergence after general anesthesia may also be due to intraoperative cerebral hypoxia, hemorrhage, embolism, or thrombosis. Accurate diagnosis of the underlying cause is the key for the institution of appropriate therapy, but primary management is to maintain airway, breathing, and circulation. This comprehensive review discusses the risk factors, causes, evaluation and management of delayed recovery based on our clinical experience, and literature search on the internet, supported by the standard textbooks of anesthesiology.
Keywords: Anesthetic agents, delayed awakening, delayed emergence, delayed recovery, drug effects, general anesthesia, overdose, prolonged neuromuscular block, regional anesthesia, risk factors
INTRODUCTION
“Recovery from anesthesia may be defined as a state of consciousness of an individual when he is awake or easily arousable and aware of his surroundings and identity.”[1,2] Awakening results from elimination of anesthetic agents from the brain. Patients usually respond to verbal stimuli when alveolar anesthetic concentration is decreased to about 30% of minimum alveolar concentration (MAC) (MAC awake) if unimpeded by other factors.[2] Recovery from intravenous (IV) opioids and hypnotics may be more variable and difficult to quantify than recovery from inhalational and neuromuscular blocking agents. Patients should not leave the operating room unless they have stable hemodynamic parameters, a patent airway, have adequate ventilation, and oxygenation. Higher incidence of early postoperative respiratory complications is noted when the patient returns unresponsive in recovery room irrespective of age or American Society of Anesthesiologist category.[3] The most important monitor is a well-informed and skilled person, with immediate access to anesthetic assistance. Since day-care surgery is gaining a great popularity day by day, adequate recovery time and a high vigilance to rare side effects becomes more and more important. This comprehensive review discusses the risk factors, causes, evaluation and management of delayed recovery based on our clinical experience, and literature search on the internet, supported by the standard textbooks of anesthesiology.
PHASES OF RECOVERY FROM ANESTHESIA
The entire process of recovery after anesthesia is divided into three phases.
Immediate recovery
This consists of return of consciousness, recovery of protective airway reflexes, and resumption of motor activity. This stage usually lasts for a short time.
Intermediate recovery
During this stage, the patient regains his power of coordination and the feeling of dizziness disappears. This stage usually lasts for 1 h after short anesthetic. Outpatient may be considered fit for discharge with a responsible escort.
Long-term recovery
There is a full recovery of coordination and higher intellectual function. It may last for hours or even days.[3,4]
MONITORING RECOVERY FROM ANESTHESIA
Glasgow Coma Scale Table 1 originally developed to assess prognosis after head trauma and can also be used to ascertain the level of consciousness after anesthesia. A score of >12 indicates return of consciousness in most patients and <8 indicates coma.[1,2,5]
Table 1.
Glasgow Coma Scale used to trend the level of consciousness

Table 2 shows the most commonly used Aldrete score for assessing recovery from anesthesia, maximum total score is 10; a score of at least 9 is required for discharge from the postanesthesia care unit (PACU).[6,7]
Table 2.
Aldrete score for assessing recovery and discharge from the postanesthesia care unit
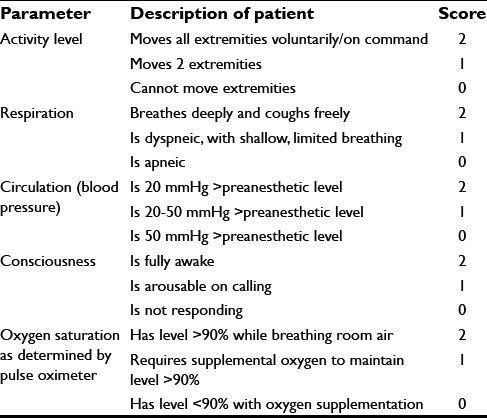
The postoperative quality recovery scale tracks 6 domains of recovery from immediate to long-term periods in patients of different ages, languages, and cultures, namely physiologic, nociceptive, emotive, activities of daily living, cognitive, and patient perspective.[6] Complete physiologic recovery takes place by 40 min in 40% of the patients. The functional quality of recovery in all domains occurs in only 11% of the patients by day 3.[1] Thus, the concept of awakening is involved with far greater dimensions than judging the anesthetic effect as terminated and assessing a patient as being “recovered” or “awakened.” Patients cannot be considered fully recovered until they have returned to their preoperative physiological state.[1]
DELAYED AWAKENING OR EMERGENCE FROM GENERAL ANESTHESIA
Abnormally slow pace of regaining consciousness after general anesthesia is characterized by persistent somnolence. It is medically defined as a state of unresponsiveness from which the patient cannot be aroused. In otherwise healthy patients who have undergone short operative procedures, the occurrence of delayed recovery usually relates to some underlying undiagnosed condition or medical error.[1] Even after prolonged anesthesia, a response to stimulation should occur in 60–90 min.[1,8] Zelcer and Wells found a 9.46% incidence of unresponsiveness between 15 and 90 min after general anesthesia among 443 mixed surgical patients.[9]
RISK FACTORS
Risk factors responsible for slower than normal return to consciousness are roughly divided into four categories Table 3.
Table 3.
Risk factors responsible for delayed emergence from anesthesia
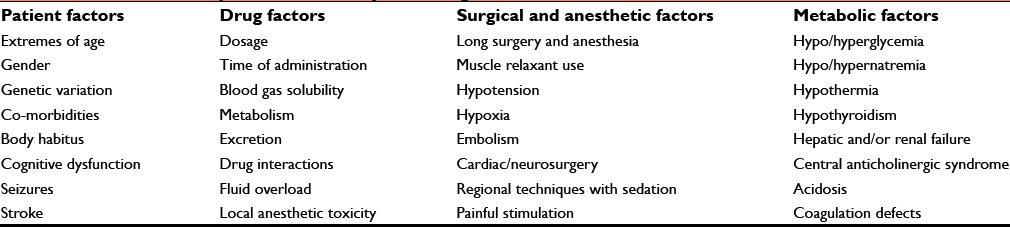
Patient factors
Extremes of age
Geriatric patient
Elderly patients have increased sensitivity toward general anesthetics, opioids and benzodiazepines, and slow return of consciousness due to progressive decline in central nervous system (CNS) function. Studies have shown that demand for opioids is reduced by almost 50% in geriatric patients.[1,10,11] The decrease in volume of distribution, clearance rate, and plasma protein binding results in high free plasma concentration of drugs. Fredman et al. assessed the effect of different doses of midazolam in geriatric patients undergoing brief urologic procedures and found that irrespective of the dose, midazolam significantly depresses mental function and delays PACU discharge.[1,12] Furthermore, muscle relaxants if given on weight basis delay onset of action and prolong drug effect.
Pediatric patients
Because of larger body surface area, heat loss is greater in children resulting in hypothermia, slow drug metabolism, and delayed return of consciousness.[1]
Genetic factors
It is becoming increasingly apparent that genetically controlled variations in drug disposition and response are important determinants of adverse effects of drug therapy. Unexpected responses and prolonged somnolence after specific anesthetic are commonly associated with a genetic defect of the metabolic pathway of the agent or its receptor. Polymorphic changes in gamma-aminobutyric acid 2 receptor can adversely affect the rapid reversal of propofol anesthesia. Also, variable prolongation of suxamethonium apnea is due to abnormal or absent plasma cholinesterase enzyme.[1]
Gender
Apfelbaum et al. reported that men are 1.4 times more likely to have delayed recovery than women.[13] Lower sensitivity to the hypnotic effect of anesthetics in women may account for their faster recovery.[14] The female sex hormone was postulated to play a role in the gender differences in recovery time.
Body habitus
Obesity with increased fat mass requires higher drug doses to attain the same peak plasma concentration than a standard sized person. Drug dose based on lean body weight is considered optimal for most drugs used in anesthesia.[1] Also, underweight patients are having a higher risk of slow recovery after vascular surgery and cardiopulmonary bypass graft surgery.[11]
Comorbidities
Preexisting cardiac and pulmonary disease require adjustments in anesthetic doses to avoid delayed emergence. Significant lung disease decreases the ability to wash out inhalational agents. Similarly, congestive heart failure and decreased cardiac output prolong somnolence.[1] The renal or hepatic disease can prolong action of anesthetic agents dependent on hepatic metabolism or renal excretion.[15] There is reduced elimination of nondepolarizing muscle relaxants such as pancuronium and vecuronium in renal failure. Subclinical hypothyroidism (prevalence 1–10%) may be diagnosed for the first time as hypothermia and delayed emergence.[1,16] Response to l-thyroxin and thyroid profile will confirm the diagnosis. Adrenal insufficiency can also present as delayed recovery.[17]
Cognitive dysfunction
Structural disorders of CNS and psychological disease may all cause postoperative somnolence. For example, patients with Parkinson's disease are more prone to postoperative confusion and hallucinations. Inhaled anesthetic agents have complex effects on brain dopamine concentrations. Patients with Down's syndrome or mental retardation are particularly susceptible to delayed awakening. Risk factor included age <21 years, male gender, and those given >0.032 mg/kg midazolam especially when administered with propofol. IV sedation in cerebral palsy increases the risk of hypoxia, and delayed recovery of >60 min is expected.[1]
Seizures
Seizures are not uncommon after brain injury and after surgical intervention. A postictal state may well mimic unconsciousness. Antiepileptic drugs are known to reduce the responsiveness of neuromuscular blocking agent when given chronically.[1] Sahoo et al. reported a case of long-term seizure history not taking any antiepileptic medication was paralyzed for over 3.5 h following rocuronium and a loading dose of phenytoin.[18]
Stroke
Surgical procedures with increased risk of embolization are coronary artery bypass graft, orthopedic particularly joint replacement surgery, peripheral vascular surgery, and valvular and aortic surgery.[19] Fat embolism from closed chest massage, corticosteroid therapy, or tissue trauma may present as delayed return to cognitive function.[17]
Postoperative pain
Presence of pain speeds up recovery. Recovery may be delayed after minor surgery if potent analgesics or regional anesthesia is given.[15]
Drug factors/pharmacological causes
Delayed awakening after general anesthesia is most commonly caused by anesthetic overdose.[2,8,17,20,21,22] Administration of an ideal dose to one patient may have a very different effect on an apparently similar patient.
Residual drug effects
A heavy premedication or the relative overdose of general anesthetic agents may be the cause of delayed awakening.[23]
Potentiation by other drugs
Prior ingestion of opioids and benzodiazepines or nonanesthetic drugs that affect cognitive function such as tranquilizers, antihypertensives, anticholinergics, clonidine, antihistamines, penicillin-derived antibiotics, amphotericin B, immunosuppressants, lidocaine, and alcohol will potentiate the CNS depressant effects of anesthetic drugs and delay emergence from anesthesia.[8,19,24]
Drug interactions
Patient taking monoamine oxidase inhibitors (MAOIs) or selective serotonin reuptake inhibitors (SSRIs) may experience severe drug interactions with IV agents that can result in hyper/hypotension and postoperative coma or a full-blown serotonergic syndrome. St. John's Wort, ginseng, lithium, ondansetron, metoclopramide, codeine, fentanyl, and oxycodone are among many others.[1] Patients taking bromocriptine or pergolide are prone to excessive vasodilatation exacerbating hypotension. A large number of pharmacological interactions with neuromuscular blocking agents such as aminoglycosides, diuretics, calcium channel antagonists, lithium, polymyxin B, echothiophate, oral contraceptives, local anesthetics etc., will prolong neuromuscular block (NMB).[1,2] Neurotoxic effect of chemotherapeutic drugs such as l-asparaginase and vincristine can also produce CNS depression.[17]
Duration and type of anesthetic used
The selection of anesthetic technique and anesthetic drugs determines the duration of unconsciousness. Time to emergence increases with increasing duration of anesthesia.[15,25] Garg et al. reported a case of delayed recovery after prolonged laparoscopic repair of diaphragmatic hernia under general anesthesia due to tissue handling and fluid replacement responsible for metabolic acidosis, hyperkalemia, and hypocalcemia.[26] Recovery may be delayed if soluble volatile agents are continued until the end of surgery or long-acting drugs are given toward the end of the procedure.[19,23]
Opioids
The sensitivity of the brain stem chemoreceptors to carbon dioxide is reduced by opioids with consequent dose-dependent respiratory depression and hypercapnia. This may affect clearance of volatile agents and carbon dioxide; both can cause unconsciousness.[2] Direct sedation through opioids receptor also prolongs recovery. Active metabolites of morphine and meperidine prolong the duration of action, especially in the presence of renal failure.[2,22] Patient with hepatic insufficiency may develop hepatic coma after morphine and prolong sedation after thiopentone. Neuraxial opioids may cause early respiratory depression and delayed awakening.[27]
Benzodiazepines
They potentiate CNS depressant effects of anesthetic drugs and may delay emergence from anesthesia. Benzodiazepines combined with high-dose opioids can produce respiratory depression, hypercapnia, and coma.[2,22] Midazolam is metabolized by the same cytochrome P450 isoenzymes as alfentanil such that co-administration prolongs the action of both drugs.[2] Midazolam is biotransformed to alpha hydroxyl midazolam, which have clinical potency 20.30% of midazolam and cause profound sedation in patients with renal impairment. Diazepam forms two active metabolites, oxazepam and desmethyl diazepam, that prolong its effect.[28,29]
Intravenous anesthetic agents
The termination of action of IV anesthetic agents given for induction is predominantly determined by redistribution and should not delay recovery.[2,20] The duration of unconsciousness is determined by context-sensitive half-life, total dose of drug and co-administration with other drugs. A reduction of 80% in the effect site concentration is required for emergence.[2] Propofol is rapidly metabolized by liver and other extra hepatic sites, has shorter half-life (10.70 min), and does not accumulate, hence fast recovery is expected. Whereas the elimination of thiopentone is by oxidative metabolism in the liver at the rate of 15%/h. It, therefore, has a long elimination half-life of 3.4.22 h and as much as 30% of the dose may remain in the body for 24 h.[1,22] Cumulative effects may, therefore, become apparent when more than one dose is given.[1] Delayed emergence from thiopentone is also observed in Huntington's chorea.[17] Norketamine (metabolite of ketamine) has 20.30% activity of the parent drug and it does contribute in prolonging the effect of ketamine.[28,30] For most other IV anesthetic agents, the termination of drug action depends on the time required to metabolize or excretion of the drug.
Volatile anesthetic agents
The speed of emergence is directly related to alveolar ventilation and inversely related to blood gas solubility. Hypoventilation lengthens the time taken to exhale the anesthetic agent and delays recovery.[22,24] Prolonged duration of anesthesia causes increased emergence time due to tissue uptake depending upon the concentration used and drug solubility.[1,22,24] If vaporizers are not calibrated correctly, higher than expected dose may be delivered, especially if end tidal drug concentrations are not measured.[1] Release of bromide ions after halothane anesthesia may produce postoperative drowsiness.[17]
Neuromuscular blockers
Residual neuromuscular blockade results in paralysis that is indistinguishable from delayed awakening though the patient is conscious and aware. This occurs secondary to absolute or relative overdose or incomplete reversal of nondepolarizing muscle relaxants or in a patient with suxamethonium apnea. A nerve stimulator will assist the diagnosis. Alternatively, inability to maintain head lift for 5 s in a patient indicates residual NMB of >30% of receptors.[24] The patient may become distressed or agitated, typically twitchy movements of partial reversal may also be seen. A large number of pharmacological interactions with neuromuscular blocking agents prolong NMB. Electrolyte disturbances cause cell-wall hyperpolarization and prolong block. Hypothermia decreases metabolism and acidosis donates protons to tertiary amines, increasing receptor affinity.[2]
Prolonged apnea following suxamethonium is due to abnormal or absent plasma cholinesterase enzyme.[2] Acquired cholinesterase deficiency is seen in pregnancy, liver disease, renal failure, starvation, and thyrotoxicosis. Repeated doses of suxamethonium (>6-8 mg/kg total dose) may produce a dual block that is prolonged and slow to recover.[1] The newer muscle relaxant mivacurium is also metabolized by plasma cholinesterase, and mivacurium apnea may occur rarely.[1] Patients with myasthenia gravis are very sensitive to nondepolarizing muscle relaxants, only 10.50% of the usual dose is required, and long-acting agents like pancuronium should be avoided. Increased sensitivity to muscle relaxants is also seen in patients with muscle dystrophies.[22,24]
Local anesthetic systemic toxicity
Repeated doses of local anesthetics in highly vascular area, intracranial spread of local anesthetics after spinal anesthesia, or accidental subarachnoid injection during epidural or interscalene brachial plexus block may cause prolonged somnolence, seizures, coma, and cardiorespiratory arrest.[1,15]
Metabolic causes
Hyper/hypoglycemia
Both hyper and hypoglycemia may contribute to delayed return of consciousness [Table 4]. An anesthetized patient may not display all clinical signs of metabolic abnormality.
Table 4.
Causes of hyperglycemia and hypoglycemia

Hyperglycemia occurs in known diabetics as a result of inadequate provision of insulin or injudicious glucose supplementation.[15] Hyperglycemia in hyperosmolar hyperglycemic nonketotic acidosis causes an osmotic diuresis and intracellular dehydration.[2,17,22,31] The effects of dehydration range from drowsiness to acidosis. Intraoperative cerebrovascular accident may occur as a result of cerebral vascular occlusion, especially in patients with micro..or macrovascular disease. Diabetic ketoacidosis, stress response of prolonged surgery, and dexamethasone therapy can also lead to severe hyperglycemia.[1]
Hypoglycemia can occur in children with prolonged fasting. It may also occur in starvation, liver failure, end-stage renal disease, alcohol intoxication, septicemia, and malaria.[2,17,24] As the brain is totally dependent on glucose for energy, hypoglycemia may manifests as restlessness, sweating, confusion, blurring of vision or diplopia, seizures, and coma.[2,22] Diabetic patients on insulin and/or oral hypoglycemic may sometimes present with hypoglycemia and delayed emergence.[15,17] Salicylates and ethanol given preoperatively may exacerbate hypoglycemia in starved patients. Hypoglycemia can occur during manipulation of insulin-producing tumor of pancreas or retroperitoneal carcinoma.[17]
Electrolyte imbalance
The acid-base and electrolyte changes observed in the perioperative period could be secondary to the underlying illness or surgical procedure, for example, hyponatremia occurring with transurethral resection of prostate where glycine or other hypotonic fluid is used for irrigation.[2,15,17,19,24] Serum sodium concentration <120 mmol/L will cause confusion and irritability, whereas <110 mmol/L may cause seizures and coma.[2] Syndrome of inappropriate anti-diuretic hormone secretion can result from brain trauma, subarachnoid hemorrhage, and administration of drugs such as opioids, haloperidol, and vasopressin. Sodium loss from kidneys in cerebral salt-wasting syndrome is thought to be mediated by atrial natriuretic peptide secretion. Infusion of mannitol can also result in hyponatremia.[2,21]
Hypernatremia (plasma Na+ >145 mEq/L) during hepatic hydatid cyst removal may also hinder the process of recovery from anesthesia due to cerebral dehydration, vascular rupture, and intracerebral hemorrhage.[2,21,32] Symptoms include thirst, drowsiness, confusion, and coma.
Delayed recovery due to inadvertent hypokalemia after laparoscopic cholecystectomy is reported in a medically optimized case of hypertension, bronchial asthma, and hypothyroidism.[33] Hypokalemia intensifies the effects of nondepolarizing muscle relaxants. Respiratory alkalosis with PaCO2 <36 mmHg results in reduced intracellular proton concentration and draws K+ into the cells. There is a reduction of 0.5 mEq/L of K+/10 mmHg reduction of PaCO2.[21] Hypokalemia can also occur with perioperative use of diuretics, calcium gluconate, sodium bicarbonate, β-adrenergic agonists, glucose, and/or insulin without K+ supplement.[20,21] Mild preoperative hypokalemia without any clinical features could rapidly deteriorate after iatrogenic hyperventilation or surgical stimulation and delays recovery.[2,21,34]
Hypocalcemia after thyroid or parathyroid surgery, hypermagnesemia after MgSO4 therapy in preeclampsia, and severe hypercalcemia produce CNS depression.[17,19] Excess IV fluids may gravitate to the lungs causing decreased oxygen exchange, hypercarbia, and hypoxia.[1]
Acute or chronic renal disease may result in uremia with increased blood urea nitrogen and other toxins. The intracerebral dehydration, cellular damage, and distortion may produce drowsiness, confusion, and coma.[2,22]
Temperature
Hypothermia is usually observed in extremes of ages and in debilitated patients. A core temperature of <33°C has marked anesthetic effects itself and it also reduces the MAC value of inhalational agents, antagonizes muscle relaxant reversal, and limits drug metabolism.[2,17,22,24] Reduction in cardiac output affects circulation and drug pharmacokinetics, and tissue perfusion and arrhythmias may also occur.[2,22]
Skeletal muscle destruction after malignant hyperthermia can delay recovery from anesthesia.[35] Temperature above 40°C leads to loss of consciousness.[17]
Respiratory causes
Postoperative respiratory failure due to primary muscle problems, metabolic imbalance, obesity, residual NMB, or pulmonary disease results in hypoxemia, hypercapnia, or venous admixture.[2,22,24] Hypoxemia will depress cerebral function, ultimately causing cell death. Delayed awakening can occur due to CO2 narcosis when PaCO2 increases above 90–120 mm Hg.[19]
Neurological complications
Residual anesthesia after neurosurgery may either give the false impression of a neurological deficit or prevent the early diagnosis of intracranial lesion such as hematoma, herniation, and cerebral infarction. Periods of hypoxemia or ischemia may occur as a result of intraoperative arrhythmias, deliberate hypotension, or in patients with abnormal cerebral vasculature particularly in neurosurgery, cardiac, and carotid surgery.[2,15,17,22,24,36] Hypoperfusion may occur due to obstruction of blood flow in the vertebral or carotid circulation when the anesthetized patient is positioned improperly or during operations in a sitting position. Hypertension evoked by laryngoscopy and tracheal intubation and extubation as well as prolonged anticoagulant therapy can result in cerebral hemorrhage during anesthesia.[17] Small hemorrhages into ventricles, subependymal area, and around the ventricular catheters are frequently seen following ventriculoperitoneal shunt surgery.[37] This can result in reduced consciousness and may first present as delayed awakening from anesthesia, especially if the hypoxic insult has occurred during anesthesia.[22,24] Previously unknown intracranial mass lesion may have delayed emergence as presenting sign.[15,38] Delayed recovery from anesthesia due to undiagnosed Dandy–Walker malformation and familial hemiplegic migraine is reported.[39,40] Dystonia for deep brain stimulation has 70% incidence of delayed recovery.[41]
CENTRAL ANTICHOLINERGIC SYNDROME
Central anticholinergic syndrome (CAS) occurs not only with anticholinergic drugs such as atropine, scopolamine, or hyoscine, but also after benzodiazepines, IV, and volatile anesthetic agents and manifests as delayed awakening from anesthesia.[2,24] It is thought to be due to a decrease in inhibitory anticholinergic activity in the brain. Symptoms related to cerebral irritation such as delirium, agitation to CNS depression with stupor, and coma is seen.[42] Peripheral anticholinergic effects such as dry mouth, flushing, tachycardia, blurred vision, etc., may also be present. Overdose of atropine or glycopyrrolate is reported to cause CAS in neonates.[43,44] The incidence is between 1% and 11.2% after general anesthesia. Increasing cholinergic transmission by trial of physostigmine 0.03–0.04 mg/kg repeated at 10–30 min intervals, if needed, can attenuate or even reverse delayed emergence.[17,45,46,47]
POSTOPERATIVE NEUROEXCITATORY SYMPTOMS
Neuroexcitatory symptoms such as twitching, myoclonic movements, opisthotonus, and seizures can present during induction, maintenance, as well as recovery from anesthesia. The Swedish Adverse Drug Reaction Advisory Committee reported 34–44 patients and the British Committee on Safety of Medicine in 1989 reported 8 patients who had delay in regaining consciousness after anesthesia. Many of these patients have been anesthetized with propofol, enflurane, isoflurane, or etomidate. The median age was 27 years, male–female ratio 1:2, and the incidence was <1%. The symptoms are caused by imbalance between excitatory and inhibitory pathways in the brain or reduced inhibitory output from the formation reticularis.[48]
RARE CAUSE OF DELAYED RETURN OF CONSCIOUSNESS
Dissociative coma, myxedema coma, thyroid failure, hunter syndrome (mucopolysaccharide storage disease), valproate toxicity, drug abuse, and lidocaine infusion for arrhythmias are some rare causes of delayed recovery.[1,2] Anesthesia practitioners should also be aware of unexplained delayed recovery due to hysteria. Its diagnosis is based on the exclusion of other organic causes.[49,50]
REGIONAL ANESTHESIA
Knowledge of the recovery profile from a spinal anesthesia is helpful in predicting time to meeting discharge criteria from ambulatory surgery center. Previously used end points to analyze recovery from sensory and motor blockade are not used to determine eligibility for discharge after day-care surgery. Information about the time it takes a patient to void, ambulate, and completely resolve sacral anesthesia after spinal anesthesia is important.[25,51] Delayed recovery due to spinal-epidural hematoma following spinal anesthesia is reported. The incidence of hematoma is <1 in 150,000 epidurals and 1 in 220,000 following spinal anesthesia.[52,53] Vascular malformations and anticoagulant therapy with increased pressure in the vertebral venous plexus are some of the common causative factors. The diagnosis is established by magnetic resonance imaging (MRI) followed by surgical decompression. Rao et al. observed an unusual delay in the recovery of sensory and motor blockade following combined spinal-epidural anesthesia in a patient with ankylosing spondylitis. Narrowed epidural spaces, migration of epidural catheter, intrathecal migration of the drug, faulty infusion pump, potentiation by fentanyl, and clonidine are possible mechanisms for prolongation of block.[54]
Delayed recovery after peripheral nerve block is rare. Patients with underlying nerve pathology such as diabetic neuropathy, exposure to neurotoxic chemotherapy, or disruption of neural blood supply are more susceptible to peripheral nerve complications. Neurological deficits may persist for days after high-pressure intraneural injection of local anesthetic. Therefore, neurological follow-up until resolution or stabilization of the condition is mandatory.[55]
Brainstem paralysis due to bupivacaine wound infiltration after foramina magnum decompression and field block is also reported.[56]
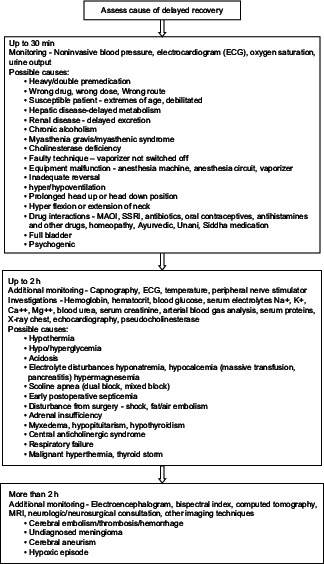
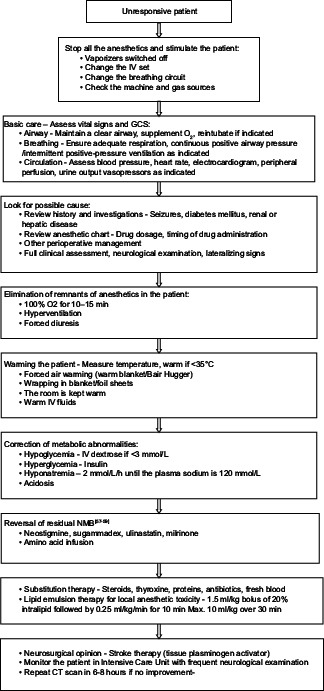
EVALUATION AND MANAGEMENT
A STEPWISE APPROACH TO THE PATIENT WITH PROLONGED UNCONSCIOUSNESS
CONCLUSION
Delayed recovery from anesthesia is often multifactorial, and anesthetic agents may not always be the culprit. When other causes are excluded, the possibility of acute intracranial event should be strongly considered. While the specific cause is being sought, primary management is always support of airway, breathing, and circulation. Good intraoperative care ensures the patient safety. A calm, comprehensive, and timely management with a systematic approach is highly rewarding. We, the anesthesiologists, make the patient sleep, so the recovery from anesthesia is our responsibility.59
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Frost EA. Differential diagnosis of delayed awakening from general anesthesia: A review. Middle East J Anaesthesiol. 2014;22:537–48. [PubMed] [Google Scholar]
- 2.Sinclair R, Faleiro RJ. Delayed recovery of consciousness after anaesthesia. Contin Educ Anaesth Crit Care Pain. 2006;6:124–8. [Google Scholar]
- 3.Parr SM, Robinson BJ, Glover PW, Galletly DC. Level of consciousness on arrival in the recovery room and the development of early respiratory morbidity. Anaesth Intensive Care. 1991;19:369–72. doi: 10.1177/0310057X9101900310. [DOI] [PubMed] [Google Scholar]
- 4.Steward DJ, Volgyesi G. Stabilometry: A new tool for the measurement of recovery following general anaesthesia for out-patients. Can Anaesth Soc J. 1978;25:4–6. doi: 10.1007/BF03006775. [DOI] [PubMed] [Google Scholar]
- 5.Fischer J, Mathieson C. The history of the Glasgow Coma Scale: Implications for practice. Crit Care Nurs Q. 2001;23:52–8. doi: 10.1097/00002727-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg. 1970;49:924–34. [PubMed] [Google Scholar]
- 7.Aldrete JA. The post-anesthetic recovery score revisited. J Clin Anesth. 1995;7:89–91. doi: 10.1016/0952-8180(94)00001-k. [DOI] [PubMed] [Google Scholar]
- 8.Miller RD, editor. Miller's Anesthesia. 7th ed. United States of America: Elsevier, Churchill; 2010. pp. 2722–3. [Google Scholar]
- 9.Zelcer J, Wells DG. Anaesthetic-related recovery room complications. Anaesth Intensive Care. 1987;15:168–74. doi: 10.1177/0310057X8701500209. [DOI] [PubMed] [Google Scholar]
- 10.Bowie MW, Slattum PW. Pharmacodynamics in older adults: A review. Am J Geriatr Pharmacother. 2007;5:263–303. doi: 10.1016/j.amjopharm.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Tsai HJ, Chen CC, Chang KY. Patients and surgery-related factors that affect time to recovery of consciousness in adult patients undergoing elective cardiac surgery. J Chin Med Assoc. 2011;74:345–9. doi: 10.1016/j.jcma.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Fredman B, Lahav M, Zohar E, Golod M, Paruta I, Jedeikin R. The effect of midazolam premedication on mental and psychomotor recovery in geriatric patients undergoing brief surgical procedures. Anesth Analg. 1999;89:1161–6. [PubMed] [Google Scholar]
- 13.Apfelbaum JL, Grasela TH, Hug CC, Jr, McLeskey CH, Nahrwold ML, Roizen MF, et al. The initial clinical experience of 1819 physicians in maintaining anesthesia with propofol: Characteristics associated with prolonged time to awakening. Anesth Analg. 1993;77(4 Suppl):S10–4. [PubMed] [Google Scholar]
- 14.Buchanan FF, Myles PS, Leslie K, Forbes A, Cicuttini F. Gender and recovery after general anesthesia combined with neuromuscular blocking drugs. Anesth Analg. 2006;102:291–7. doi: 10.1213/01.ANE.0000181321.55422.C6. [DOI] [PubMed] [Google Scholar]
- 15.Aitkenhead AR, Rowbotham DJ, Smith G, editors. Textbook of Anaesthesia. 4th ed. London, England: Churchill Livingston; 2001. [Google Scholar]
- 16.Kumar VV, Kaimar P. Subclinical hypothyroidism: A cause for delayed recovery from anaesthesia? Indian J Anaesth. 2011;55:433–4. doi: 10.4103/0019-5049.84836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denlinger JK. Prolonged emergence and failure to regain consciousness. In: Orkin FK, Cooperman LH, editors. Complications in Anesthesiology. Philadelphia, Unites States of America: JB Lippincott; 1983. pp. 368–78. [Google Scholar]
- 18.Sahoo S, Kaur M, Sawhney C, Mishra A. An unusual cause of delayed recovery from anesthesia. J Anaesthesiol Clin Pharmacol. 2012;28:415–6. doi: 10.4103/0970-9185.98380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell CE. Delayed awakening or delirium. In: Bready LL, Dillman D, Noorily SH, editors. Decision Making in Anesthesia. 4th ed. Philadelphia, United States of America: Mosby, Elsevier; 2007. pp. 582–5. [Google Scholar]
- 20.Sarangi S. Delayed awakening from anaesthesia. Internet J Anesthesiol. 2008;19 [Google Scholar]
- 21.Razvi M, Bameshki A, Gilani MT. Delayed awakening from anesthesia following electrolyte and acid-base disorders, two cases. Patient Saf Qual Improv. 2014;2:65–8. [Google Scholar]
- 22.Shaikh SI, Lakshmi RR. Delayed awakening after anaesthesia – A challenge for an anaesthesiologist. [Last accessed on 2015 Jun 17];Int J Biomed Adv Res. 2014 5:252–4. Available from: http://www.ssjournals.com . [Google Scholar]
- 23.Nichoiau D. Postanesthesia recovery. In: Stoelting RK, Miller RD, editors. Basics of Anesthesia. 5th ed. Philadelphia, United States of America: Churchill Livingston, Elsevier; 2007. pp. 577–8. [Google Scholar]
- 24.Radhakrishnan R, Jesudasan S, Jacob R. Delayed awakening or emergence from anaesthesia. Update Anaesth. 2001;13:4–6. [Google Scholar]
- 25.Pavlin DJ, Rapp SE, Polissar NL, Malmgren JA, Koerschgen M, Keyes H. Factors affecting discharge time in adult outpatients. Anesth Analg. 1998;87:816–26. doi: 10.1097/00000539-199810000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Garg R, Punj J, Pandey R, Darlong V. Delayed recovery due to exaggerated acid, base and electrolyte imbalance in prolonged laparoscopic repair of diaphragmatic hernia. Saudi J Anaesth. 2011;5:79–81. doi: 10.4103/1658-354X.76477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61:276–310. [PubMed] [Google Scholar]
- 28.Reves JG, Glass P, Lubarsky DA, McEvoy MD, Ruiz RM. Intravenous anesthetics. In: Miller RD, editor. Miller's Anesthesia. 7th ed. United States of America: Elsevier, Churchill; 2010. pp. 719–68. [Google Scholar]
- 29.Stoelting RK, Hiller SC, editors. Pharmacology and Physiology in Anesthetic Practice. 4th ed. United States of America: Lippincott Williams and Wilkins; 2006. pp. 140–54. [Google Scholar]
- 30.Stoelting RK, Hiller SC, editors. Pharmacology and Physiology in Anesthetic Practice. 4th ed. United States of America: Lippincott Williams and Wilkins; 2006. pp. 155–78. [Google Scholar]
- 31.Feeney JE. Delayed recovery from general anesthesia due to hyperosmolar hyperglycemic non-ketotic acidosis. Anesth Prog. 1978;25:45–6. [PMC free article] [PubMed] [Google Scholar]
- 32.Grati L, Toumi S, Gahbiche M. Failure to recover after anaesthesia for surgery of a liver hydatic cyst assigned to hypernatraemia. Ann Fr Anesth Reanim. 2009;28:261–2. doi: 10.1016/j.annfar.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 33.More P, Laheri VV, Waigankar T, Wagh C. Delayed recovery from anaesthesia in a patient with optimised hypothyroidism and incidental hypokalemia. J Clin Diagn Res. 2015;9:UD06–7. doi: 10.7860/JCDR/2015/10088.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moon HS, Lee SK, Chung JH, In CB. Hypocalcemia and hypokalemia due to hyperventilation syndrome in spinal anesthesia – A case report. Korean J Anesthesiol. 2011;61:519–23. doi: 10.4097/kjae.2011.61.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeda H, Iranami H, Hatano Y. Delayed recovery from muscle weakness due to malignant hyperthermia during sevoflurane anesthesia. Anesthesiology. 1997;87:425–6. doi: 10.1097/00000542-199708000-00031. [DOI] [PubMed] [Google Scholar]
- 36.Bruder N, Ravussin P. Recovery from anesthesia and postoperative extubation of neurosurgical patients: A review. J Neurosurg Anesthesiol. 1999;11:282–93. doi: 10.1097/00008506-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Deuri A, Goswami D, Samplay M, Das J. Nonawakening following general anaesthesia after ventriculo-peritoneal shunt surgery: An acute presentation of intracerebral haemorrhage. Indian J Anaesth. 2010;54:569–71. doi: 10.4103/0019-5049.72650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gercek G, Konya D, Babayev R, Ozgen S. Delayed recovery from general anesthesia from intracranial tumour. Int Anesth Res Soc. 2007;104:235–6. doi: 10.1213/01.ane.0000248176.95402.57. [DOI] [PubMed] [Google Scholar]
- 39.De Santis V, Vitale D, Di Bonaventura C, Tritapepe L. An unusual cause of delayed awakening following coronary artery surgery. Minerva Anestesiol. 2011;77:1228–31. [PubMed] [Google Scholar]
- 40.Greaney D, Vaughan P. Delayed recovery post anesthesia: An atypical presentation of familial hemiplegic migraine. Can J Anaesth. 2014;61:278–9. doi: 10.1007/s12630-013-0090-9. [DOI] [PubMed] [Google Scholar]
- 41.Trombetta C, Deogaonkar A, Deogaonkar M, Ebrahim Z, Rezai A, Machado A, et al. Delayed awakening in dystonia patients undergoing deep brain stimulation surgery. J Clin Neurosci. 2010;17:865–8. doi: 10.1016/j.jocn.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 42.Link J, Papadopoulos G, Dopjans D, Guggenmoos-Holzmann I, Eyrich K. Distinct central anticholinergic syndrome following general anaesthesia. Eur J Anaesthesiol. 1997;14:15–23. doi: 10.1046/j.1365-2346.1997.00004.x. [DOI] [PubMed] [Google Scholar]
- 43.Kumar SS, Jain N, Prakash S, Pawar M. Central anticholinergic syndrome in a neonate operated for tracheoesophageal fistula. Indian J Anaesth. 2015;59:330–1. doi: 10.4103/0019-5049.156901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kulka PJ, Toker H, Heim J, Joist A, Jakschik J. Suspected central anticholinergic syndrome in a 6-week-old infant. Anesth Analg. 2004;99:1376–8. doi: 10.1213/01.ANE.0000134796.83697.CD. [DOI] [PubMed] [Google Scholar]
- 45.Meuret P, Backman SB, Bonhomme V, Plourde G, Fiset P. Physostigmine reverses propofol-induced unconsciousness and attenuation of the auditory steady state response and bispectral index in human volunteers. Anesthesiology. 2000;93:708–17. doi: 10.1097/00000542-200009000-00020. [DOI] [PubMed] [Google Scholar]
- 46.Hill GE, Stanley TH, Sentker CR. Physostigmine reversal of postoperative somnolence. Can Anaesth Soc J. 1977;24:707–11. doi: 10.1007/BF03006714. [DOI] [PubMed] [Google Scholar]
- 47.Plourde G, Chartrand D, Fiset P, Font S, Backman SB. Antagonism of sevoflurane anaesthesia by physostigmine: Effects on the auditory steady-state response and bispectral index. Br J Anaesth. 2003;91:583–6. doi: 10.1093/bja/aeg209. [DOI] [PubMed] [Google Scholar]
- 48.Islander G, Vinge E. Severe neuroexcitatory symptoms after anaesthesia – with focus on propofol anaesthesia. Acta Anaesthesiol Scand. 2000;44:144–9. doi: 10.1034/j.1399-6576.2000.440203.x. [DOI] [PubMed] [Google Scholar]
- 49.Adams AP, Goroszeniuk T. Hysteria. A cause of failure to recover after anaesthesia. Anaesthesia. 1991;46:932–4. doi: 10.1111/j.1365-2044.1991.tb09850.x. [DOI] [PubMed] [Google Scholar]
- 50.Maddock H, Carley S, McCluskey A. An unusual case of hysterical postoperative coma. Anaesthesia. 1999;54:717–8. doi: 10.1046/j.1365-2044.1999.1013v.x. [DOI] [PubMed] [Google Scholar]
- 51.Frey K, Holman S, Mikat-Stevens M, Vazquez J, White L, Pedicini E, et al. The recovery profile of hyperbaric spinal anesthesia with lidocaine, tetracaine, and bupivacaine. Reg Anesth Pain Med. 1998;23:159–63. doi: 10.1097/00115550-199823020-00008. [DOI] [PubMed] [Google Scholar]
- 52.Makris A, Gkliatis E, Diakomi M, Karmaniolou I, Mela A. Delayed spinal epidural hematoma following spinal anesthesia, far from needle puncture site. Spinal Cord. 2014;52(Suppl 1):S14–6. doi: 10.1038/sc.2013.174. [DOI] [PubMed] [Google Scholar]
- 53.Syal K, Sood A, Bhatt R, Gupta H. Prolonged post spinal anaesthesia paralysis. Indian J Anaesth. 2015;59:376–8. doi: 10.4103/0019-5049.158757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao S, Shetty A, Pandya S. Prolonged sensory and motor blockade following combined spinal-epidural anaesthesia in a patient with ankylosing spondylitis. [Last accessed on 2015 Jun 17];Internet J Anesthesiol. 2007 17 Available from: http://www.ISPUB.com/IJA/17/2/6604 . [Google Scholar]
- 55.Jeng CL, Torrillo TM, Rosenblatt MA. Complications of peripheral nerve blocks. Br J Anaesth. 2010;105(Suppl 1):i97–107. doi: 10.1093/bja/aeq273. [DOI] [PubMed] [Google Scholar]
- 56.Joannides AJ, Santarius T, Fernandes HM, Laing RJ, Trivedi RA. Transient perioperative brainstem paralysis secondary to a local anesthetic. J Neurosurg Pediatr. 2012;10:60–1. doi: 10.3171/2012.3.PEDS11394. [DOI] [PubMed] [Google Scholar]
- 57.Gurunathan U, Duncan G. The successful use of sugammadex and uneventful recovery from general anaesthesia in a patient with myotonic dystrophy. Indian J Anaesth. 2015;59:325–6. doi: 10.4103/0019-5049.156894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakurai S, Fukunaga A, Fukuda K, Kasahara M, Ichinohe T, Kaneko Y. Aminophylline reversal of prolonged postoperative sedation induced by propofol. J Anesth. 2008;22:86–8. doi: 10.1007/s00540-007-0587-x. [DOI] [PubMed] [Google Scholar]
- 59.Kalra S, Wadhwa R. Role of amino acid infusion in delayed recovery from neuromuscular blockers. Indian J Anaesth. 2010;54:166–8. doi: 10.4103/0019-5049.63649. [DOI] [PMC free article] [PubMed] [Google Scholar]


