Abstract
Background:
Esmolol has an established role in attenuation of hemodynamic response to laryngoscopy and endotracheal intubation. We studied the effect of dexmedetomidine compared to that of esmolol in this study.
Aim:
To study the role of dexmedetomidine in attenuation of hemodynamic response to laryngoscopy and oral endotracheal intubation compared to that of esmolol hydrochloride in patients posted for elective surgery under general anesthesia.
Study Design:
Prospective randomized study double-dummy blinding method.
Materials and Methods:
A total of 60 American Society of Anesthesiologists I patients, aged 18–60 years randomly divided into two groups; Group A patients received dexmedetomidine 1 mcg/kg diluted in 50 ml with normal saline and infused over 10 min before induction and also 20 ml of normal saline intravenous (IV) 2 min before endotracheal intubation. Group B patients received 50 ml IV infusion of normal saline over 10 min before induction and IV bolus of esmolol 0.5 mg/kg diluted in 20 ml with normal saline given 2 min before intubation. Standard induction technique followed. Heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) were recorded just before induction and after intubation at 1 min, 3 min, and 5 min after intubation.
Statistical Analysis:
Independent samples t-test and repeated measures of analysis of variance.
Results:
Dexmedetomidine group showed statistically significant reduction in all the study parameters at all study time intervals following intubation. While esmolol group showed significant attenuation of HR, SBP, and MAP following intubation but failed to produce significant reduction in DBP.
Conclusion:
Dexmedetomidine is more effective in attenuating the hemodynamic response to oral endotracheal intubation compared to that of esmolol hydrochloride.
Keywords: Blood pressure, dexmedetomidine, esmolol, heart rate, intubation
INTRODUCTION
Laryngoscopy and endotracheal intubation, both of which are noxious stimuli's that produce marked sympathetic response manifesting as tachycardia and hypertension.[1] There have been published studies establishing the role of esmolol[2] in attenuation of hemodynamic response to intubation, but the role of dexmedetomidine has still to be defined. Dexmedetomidine has unique pharmacokinetics making it difficult to compare with other routinely used drugs such as esmolol and lignocaine. We adopted a unique blinding technique double-dummy method to make this comparison between dexmedetomidine and esmolol possible in an ideal manner. We hypothesized that intravenous (IV) dexmedetomidine 1 mcg/kg administered over 10 min before endotracheal intubation would attenuate the hemodynamic response to laryngoscopy and endotracheal intubation more effectively than IV esmolol 0.5 mg/kg given as a bolus dose 2 min before intubation. This prospective, randomized, double-blinded study was attempted to study and compare the effect of dexmedetomidine and esmolol in attenuation of hemodynamic response to oral endotracheal intubation.
MATERIALS AND METHODS
After Institutional Ethics Committee approval and informed consent, 60 patients of American Society of Anesthesiologists (ASA) I physical status of 18–60 years scheduled for general surgical procedures under general anesthesia were included in this prospective randomized double-blinded parallel group study undergone in our Department of Anesthesiology over a period of 2 years. This study was designed in accordance to the ethical standards of the Helsinki Declaration of 1975, as revised in 2000. We hypothesized that IV dexmedetomidine at 1 mcg/kg infused over 10 min before endotracheal intubation will be more effective in attenuating the hemodynamic response to endotracheal intubation compared to IV esmolol at 0.5 mg/kg dosage given 2 min before intubation. The sample size of 30 in each group was arrived based on statistical power analysis from the previous study.[3] The following patients are excluded: ASA II and above, pregnancy, emergency surgery, airway abnormalities, and anticipated difficult airway (Mallampatti Class III and IV, thyromental distance <6 cm, interincisor distance <3 cm, cervical instability). Patients with duration of laryngoscopy more than 20 s and need for more than one intubation attempt are expected dropouts. The patients were randomly allocated into two groups A and B, of 30 each. Randomization is performed by computer generated random numbers and concealed by sealed envelope technique. This was done by a separate anesthesiologist who is not aware of the study protocol and not involved in administering drug or data collection during the study. The patient is not aware of the group allocated to them.
Group A - Received dexmedetomidine 1 mcg/kg made into 50 ml with normal saline infused intravenously over 10 min before induction in a 50 ml syringe and a bolus of 20 ml of normal saline loaded in a 20 ml syringe given slowly (over 30 s) IV 2 min before intubation.
Group B - Received 50 ml of normal saline infused intravenously over 10 min before induction in a 50 ml syringe and a bolus of esmolol 0.5 mg/kg diluted into total volume of 20 ml with normal saline in a 20 ml syringe given slowly (over 30 s) IV 2 min before intubation.
Syringe infusion pump was used for infusion of drugs. The infusion and the bolus syringes were loaded by anesthesiologist who was not aware of the study protocol and not involved in the recording of study parameters or performance of laryngoscopy. IV access was secured with 20-gauge size cannula for all patients in the holding area. Ringer lactate was started at maintenance infusion rate. The patients were shifted to the operating room. Multichannel monitor (Nihon Kohden Life Scope A, BSM-5100) with electrocardiogram – II and V5 lead, noninvasive blood pressure, and pulse oximetry were connected to the patients. The study parameters monitored are heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP). The values of the study parameters recorded before the 50 ml infusion of study drug is taken as baseline value in the operating room. The study parameters were recorded before infusion of 50 ml study drug (taken as baseline) and at 1, 3, and 5 min (marked as time interval BL, T1, T3, and T5, respectively) after endotracheal intubation. After preoxygenation for 3 min with 100% oxygen, the patient was induced with fentanyl 2 mcg/kg IV, thiopentone IV 5 mg/kg. sevoflurane 2% in 100% oxygen and checked for mask ventilation following which vecuronium 0.1 mg/kg IV was given. Just 2 min before intubation, both groups received 20 ml of preloaded bolus drug as stated above. EtCO2 maintained below 40 mmHg to avoid the effects of hypercarbia on the hemodynamic variables during the study period. Laryngoscopy and oral intubation were performed in all patients by a senior anesthesiologist who is blinded for the group allocation. Macintosh laryngoscope blade of size 3 used in all patients. Cuffed endotracheal tubes of size 8.5 mm ID in males, 7.5 mm ID in females were used appropriately. The standard intubating pillow is used during intubation in all patients. Bilateral air entry was checked, and endotracheal tube placement is confirmed with EtCO2. Anesthesia maintained with sevoflurane in oxygen 35% and nitrous oxide 65%. All the study parameters collected and documented by a single anesthesiologist in all the cases, who is blinded for the content in the syringes and study protocol. No other medications were administered or procedures performed during the 5 min of data collection period after tracheal intubation.
Duration of laryngoscopy was defined in this study as time starting from the introduction of laryngoscopic blade into the oropharynx up to the appearance of the EtCO2 curve on the monitor. Duration of laryngoscopy was monitored with stop clock by a separate anesthesiologist who is blinded for the study protocol.
We had the following terminologies used in our study: Significant hypotension was defined in this study as SBP <25% of baseline value. Significant bradycardia was defined as HR <60 beats/min. Significant hypotension is treated with 6 mg IV boluses of ephedrine, significant bradycardia is treated with atropine 0.6 mg IV. Patients in whom significant hypotension or bradycardia occurred during the study period were treated and dropped from the study. The other terminologies used are: One intubation attempt defined as an act of introducing laryngoscope blade between the incisors into the oropharynx to achieve endotracheal intubation. If it is removed from the oropharynx for any reason without achieving endotracheal intubation and reinserted it is considered as another intubation attempt.
The hemodynamic variables noted for T1, T3, and T5 min after intubation were compared with the baseline values. The incidence of hypertension, hypotension, tachycardia, and bradycardia between the two groups are recorded.
The sample size of 30 in each group based on statistical power analysis is arrived from previous studies.[3] A sample of at least 25 in each group is needed to detect a MAP effect size of 10.4 mmHg and standard deviation (SD) of 12.7 with a power of 80% and an alpha error of 0.05. Hence, we have included 30 patients in each group with an expected drop rate of 15%.
The collected data were analyzed with the help of SPSS Inc. Released 2007 (SPSS for Windows, Version 16.0. Chicago, SPSS Inc). The results were tabulated and analyzed using appropriate statistical techniques. Intergroup- and intragroup-comparisons of the study parameters were made. To describe about the data descriptive statistics mean and SD were used. The normality of the data was verified with Shapiro–Wilk's test. Hemodynamic parameters and demographic data had a normal distribution. To find the significant difference between the bivariate samples in independent groups the independent sample t-test was used for normal data. For within-group comparison, the repeated measures of analysis of variance (ANOVA) with adjustment for multiple comparisons to control the type I error, with Bonferroni test was used for normal data. In all the above statistical tools, the probability value of P < 0.05 is considered significant.
No changes in the study design were done after the commencement of the study.
RESULTS
The distribution of age, height, and weight between the two groups are statistically comparable as shown in Table 1. The sex distribution is also similar male: female 65%:35% among both the two groups.
Table 1.
Distribution of study population by age, height, and weight

There was no statistically significant difference in baseline values of all study parameters between the two groups and hence, they are comparable.
Both dexmedetomidine and esmolol produced a significant reduction in the values of the study parameters after intubation as shown in Table 2. In dexmedetomidine group, there was a statistically highly significant decrease in all the study parameters after intubation. However, in esmolol group, there was no statistically significant decrease in DBP at T1 min and T3 min after intubation. All other parameters SBP, MAP, and HR showed statistically significant decrease in all time intervals. Repeated measure ANOVA was used for intragroup comparison of hemodynamic variables at various time intervals to the baseline value.
Table 2.
Comparison of study parameters to baseline parameters within the group
In terms of the percentage change in mean of SBP, HR, and DBP from the baseline in both groups, the dexmedetomidine group had about 20% change from baseline while the esmolol group had 10% change from baseline at all-time intervals. However, the percentage change in mean MAP in both dexmedetomidine and esmolol groups were similar at all-time intervals. Thus, dexmedetomidine significantly reduced HR, SBP, DBP, and MAP, the following intubation while esmolol only significantly reduced HR, SBP, and MAP but failed to attenuate DBP.
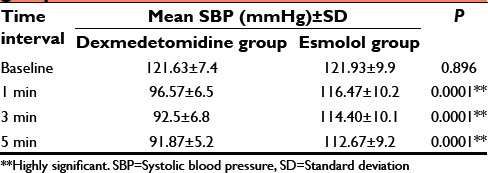
On comparing the changes at various time intervals between the two groups by independent sample t-test, we found that there is a significant difference in HR, SBP, and DBP at all-time intervals as shown in Tables 3–5. The dexmedetomidine group showed more decrease in HR, SBP, and DBP compared to esmolol group. However, there was no statistically significant difference in MAP at all-time intervals between the two groups as shown in Table 6.
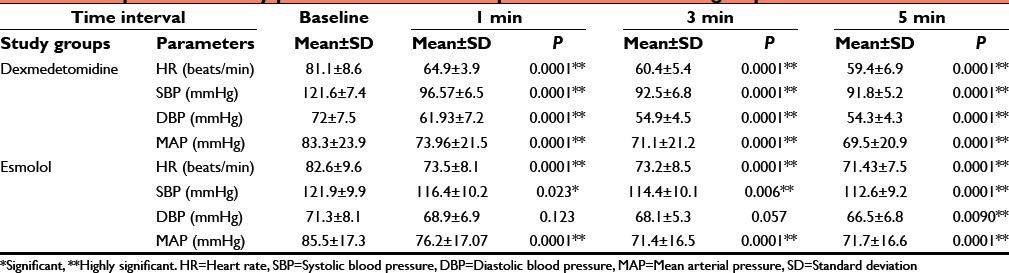
Table 3.
Comparison of mean HR between the groups
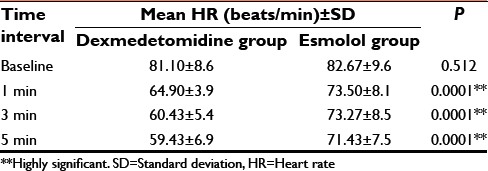
Table 5.
Comparison of mean DBP between the groups
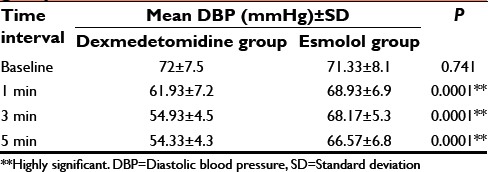
Table 6.
Comparison of mean MAP in both groups
Table 4.
Comparison of mean SBP between the groups
No incidence of bradycardia and hypertension in both the groups. Significant hypotension was defined in this study as SBP <25% of baseline value. Significant bradycardia was defined as HR <60 beats/min. None of the patients met the above-said definition and needed intervention.
No changes in the study design were carried out after the commencement of the study. No dropouts from the study population occurred as shown in Figure 1.
Figure 1.

Flow Chart
DISCUSSION
We compared the effect of IV dexmedetomidine at 1 mcg/kg and IV esmolol 0.5 mg/kg on the hemodynamic response to laryngoscopy and oral endotracheal intubation in ASA I patients posted for surgical procedures under general anesthesia. We found that dexmedetomidine is more effective in attenuating the hemodynamic response to intubation than esmolol. Esmolol was effective in attenuating the HR, SBP, and MAP but did not produce a statistically significant reduction in the DBP. While, dexmedetomidine produced statistically significant reduction in HR, SBP, DBP, and MAP after laryngoscopy and oral endotracheal intubation.
Esmolol is an ultra-short-acting cardio selective beta-blocker which is commonly used for attenuation of intubation response in clinical practice. Dexmedetomidine, apart from attenuation of the hemodynamic response to endotracheal intubation, also reduces the intraoperative anesthetic and opioid requirements.[4] Hence, an adjuvant like dexmedetomidine can give multiple benefits to the anesthesiologist such as attenuation of hemodynamic response to intubation, opioid and anesthetic sparing effect, and smooth emergence from anesthesia. Hence, we compared dexmedetomidine with the commonly used esmolol for attenuation of hemodynamic response to endotracheal intubation in this study. Previous study[4] investigating the effect of dexmedetomidine on perioperative hemodynamics have shown that the hemodynamic parameters returned to baseline values within 5 min after intubation. Hence, we monitored the study parameters for 5 min after intubation.
Many previous studies[5,6,7] have compared the hemodynamic response to intubation between dexmedetomidine and esmolol. In these studies, details of the blinding method adopted while administering the study drugs have not been furnished. We have two drugs with entirely different pharmacokinetic profile which are to be compared. Dexmedetomidine is an alpha-2 agonist with an onset of action at about 5 min at a dosage of 1 µg/kg and has a peak effect which occurs within 15 min. Its elimination half is about 2–3 h.[8,9] While esmolol has an onset of action within few seconds and achieves a peak effect at 2 min after a bolus injection.[10] It has an elimination half-life of 9 min.[11] The other peculiarities of this drugs which challenges the study design and methodology are: Dexmedetomidine has to be given as infusion over 10 min[12] otherwise if given rapidly may itself produce hypertensive response. Esmolol is best when given before 2 min[13,14] as slow IV bolus over 30 s before intubation for effective attenuation of hemodynamic response to oral endotracheal intubation. Hence, the timing of administration and the speed with which these drugs should be administered is also different. Hence, we used the double-dummy method[15] in which Group A receives study drug (dexmedetomidine) as 50 ml infusion over 10 min while the Group B receives placebo (normal saline) as 50 ml infusion over 10 min before intubation. At the same time, Group B received the second study drug (calculated esmolol dose made into 20 ml with normal saline) as 20 ml slow iv bolus over 30 s just 2 min before intubation and the Group A received placebo (normal saline 20 ml) as slow iv bolus over 30 s just 2 min before intubation. We used this method to overcome the challenges of comparing these two drugs in our study design. While some studies[5,6] infused esmolol over 10 min and another study[7] have infused dexmedetomidine over 5 min which may influence the effect on the hemodynamic response for the above said reasons. In our study design, the study drug administration and intubation were timed in such a way that intubation is done at about the peak effect of the two drugs.
Hence, the double-dummy method with two study drugs and two placebos helped to overcome this pharmacokinetic challenge in designing the study methodology. We strongly felt that timing and method of administration are very important when comparing these two drugs for attenuation of intubation response.
We arrived at the dosage based on a previous study[16] in which they have shown that IV dexmedetomidine at 1 mcg/kg is needed for effective attenuation of hemodynamic response to intubation. Another study[3] showed that IV esmolol at 0.5 mg/kg is effective when given 2 min before intubation for attenuation of hemodynamic response to intubation without adverse effects. Other studies used dosages which have shown to be ineffective in attenuation of hemodynamic response to intubation.
There has been a dose-dependent risk of bradycardia and hypotension reported in studies[17,18,19] concerning the effect of dexmedetomidine administration. In contrast to the previously mentioned studies, we did not detect any excessive reduction in HR or blood pressure values in both groups. This can be attributed to the difference in inclusion criteria.
The limitations of our study are:First, the lack of a placebo group. However, the authors considered that tracheal intubation has been proven to produce an excessive sympathoadrenal response, and furthermore, would cause detrimental results even in normotensive patients. Second, we have included only normotensives and the outcomes may not reflect the effectiveness and safety in hypertensives in whom attenuation of intubation response is more crucial. We sincerely, think that attenuation of hemodynamic response to endotracheal intubation is also important in normotensives. Another reason, why we included only ASA I patients is to avoid the bias that would happen when a wide variable group such as ASA II patients were included. Such patients may have conditions such as hypertension, diabetes autonomic neuropathy, hypothyroidism. which can affect the hemodynamic response to intubation. Furthermore, conducting the entire study in controlled hypertensives will be technically difficult to recruit patients as well to standardize the confounding factors such as drug therapy. For example, angiotensin converting enzyme (ACE) inhibitors are one of the first line drug in hypertensive management. Profound hypotension following anesthetic induction in these patients with ACE inhibitors has been noted in previous studies.[20,21] The inclusion of such patients will confound the hemodynamic response to intubation either with or without stopping the ACE inhibitor in the morning of surgery. Hence, we included ASA I patients alone. Unlike other earlier studies where ASA II patients are also included.
Thus, a study to compare dexmedetomidine and esmolol for attenuation of hemodynamic response to laryngoscopy and endotracheal intubation in hypertensive patients is awaited. Our study has shown the peculiarities of comparing this two compounds in terms of their pharmacokinetics and the effective way of blinding in the study design. Our study has shown the effectiveness of dexmedetomidine over esmolol for attenuation of hemodynamic response to laryngoscopy and endotracheal intubation in normotensives and should lead the way for further research in hypertensive individuals.
CONCLUSION
Dexmedetomidine at 1 mcg/kg is more effective than esmolol given at 0.5 mg/kg in attenuation of hemodynamic response to laryngoscopy and oral endotracheal intubation in normotensive patients under general anesthesia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Chraemmer-Jørgensen B, Hertel S, Strøm J, Høilund-Carlsen PF, Bjerre-Jepsen K. Catecholamine response to laryngoscopy and intubation. The influence of three different drug combinations commonly used for induction of anaesthesia. Anaesthesia. 1992;47:750–6. doi: 10.1111/j.1365-2044.1992.tb03252.x. [DOI] [PubMed] [Google Scholar]
- 2.Miller DR, Martineau RJ, Wynands JE, Hill J. Bolus administration of esmolol for controlling the haemodynamic response to tracheal intubation: The Canadian Multicentre Trial. Can J Anaesth. 1991;38:849–58. doi: 10.1007/BF03036959. [DOI] [PubMed] [Google Scholar]
- 3.Yavascaoglu B, Kaya FN, Baykara M, Bozkurt M, Korkmaz S. A comparison of esmolol and dexmedetomidine for attenuation of intraocular pressure and haemodynamic responses to laryngoscopy and tracheal intubation. Eur J Anaesthesiol. 2008;25:517–9. doi: 10.1017/S0265021508003529. [DOI] [PubMed] [Google Scholar]
- 4.Keniya VM, Ladi S, Naphade R. Dexmedetomidine attenuates sympathoadrenal response to tracheal intubation and reduces perioperative anaesthetic requirement. Indian J Anaesth. 2011;55:352–7. doi: 10.4103/0019-5049.84846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy SV, Balaji D, Ahmed SN. Dexmedetomidine versus esmolol to attenuate the hemodynamic response to laryngoscopy and tracheal intubation: A randomized double-blind clinical study. Int J Appl Basic Med Res. 2014;4:95–100. doi: 10.4103/2229-516X.136788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srivastava VK, Agrawal S, Gautam SK, Ahmed M, Sharma S, Kumar R. Comparative evaluation of esmolol and dexmedetomidine for attenuation of sympathomimetic response to laryngoscopy and intubation in neurosurgical patients. J Anaesthesiol Clin Pharmacol. 2015;31:186–90. doi: 10.4103/0970-9185.155146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gogus N, Akan B, Serger N, Baydar M. The comparison of the effects of dexmedetomidine, fentanyl and esmolol on prevention of hemodynamic response to intubation. Braz J Anesthesiol. 2014;64:314–9. doi: 10.1016/j.bjan.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Kaur M, Singh PM. Current role of dexmedetomidine in clinical anesthesia and intensive care. Anesth Essays Res. 2011;5:128–33. doi: 10.4103/0259-1162.94750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudheesh K, Harsoor S. Dexmedetomidine in anaesthesia practice: A wonder drug? Indian J Anaesth. 2011;55:323–4. doi: 10.4103/0019-5049.84824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellenbogen KA, McCarthy EA, Pritchett EL. Effects of bolus injection of esmolol in healthy, exercising subjects. Clin Pharmacol Ther. 1987;41:455–9. doi: 10.1038/clpt.1987.56. [DOI] [PubMed] [Google Scholar]
- 11.Wiest D. Esmolol. A review of its therapeutic efficacy and pharmacokinetic characteristics. Clin Pharmacokinet. 1995;28:190–202. doi: 10.2165/00003088-199528030-00002. [DOI] [PubMed] [Google Scholar]
- 12.Vuyk J, Sitsen E, Reeke M. Intravenous anesthetics. In: Miller RD, editor. Miller's Anesthesia. 8th ed. Philadelphia: Elsevier, Churchill, Livingstone; 2015. p. 858. [Google Scholar]
- 13.Singhal SK, Malhotra N, Kaur K, Dhaiya D. Efficacy of esmolol administration at different time intervals in attenuating hemodynamic response to tracheal intubation. Indian J Med Sci. 2010;64:468–75. [PubMed] [Google Scholar]
- 14.Figueredo E, Garcia-Fuentes EM. Assessment of the efficacy of esmolol on the haemodynamic changes induced by laryngoscopy and tracheal intubation: A meta-analysis. Acta Anaesthesiol Scand. 2001;45:1011–22. doi: 10.1034/j.1399-6576.2001.450815.x. [DOI] [PubMed] [Google Scholar]
- 15.Day SJ, Altman DG. Statistics notes: Blinding in clinical trials and other studies. BMJ. 2000;321:504. doi: 10.1136/bmj.321.7259.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saðýroðlu AE, Celik M, Orhon Z, Yüzer S, Sen B. Different doses of dexmedetomidine on controlling hemodynamic responses to tracheal intubation. Int J Anesthesiol. 2010;27:2. [Google Scholar]
- 17.Yildiz M, Tavlan A, Tuncer S, Reisli R, Yosunkaya A, Otelcioglu S. Effect of dexmedetomidine on haemodynamic responses to laryngoscopy and intubation: Perioperative haemodynamics and anaesthetic requirements. Drugs R D. 2006;7:43–52. doi: 10.2165/00126839-200607010-00004. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence CJ, De Lange S. Effects of a single pre-operative dexmedetomidine dose on isoflurane requirements and peri-operative haemodynamic stability. Anaesthesia. 1997;52:736–44. doi: 10.1111/j.1365-2044.1997.169-az0303.x. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Abraham R, Ogorek D, Weinbroum AA. Dexmedetomidine: A promising agent for anesthesia and perioperative care. Isr Med Assoc J. 2000;2:793–6. [PubMed] [Google Scholar]
- 20.Rajgopal R, Rajan S, Sapru K, Paul J. Effect of pre-operative discontinuation of angiotensin-converting enzyme inhibitors or angiotensin II receptor antagonists on intra-operative arterial pressures after induction of general anesthesia. Anesth Essays Res. 2014;8:32–5. doi: 10.4103/0259-1162.128903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertrand M, Godet G, Meersschaert K, Brun L, Salcedo E, Coriat P. Should the angiotensin II antagonists be discontinued before surgery? Anesth Analg. 2001;92:26–30. doi: 10.1097/00000539-200101000-00006. [DOI] [PubMed] [Google Scholar]


