Abstract
Context:
Postoperative pain management is becoming an integral part of anesthesia care. Various techniques of pediatric pain relief have been designed among which the most commonly practiced is caudal epidural block. Several adjuvants have been used to prolong the duration of caudal analgesia such as clonidine, neostigmine, ketamine, opioids, and ephedrine. We have designed the study using dexmedetomidine as an adjuvant to assess analgesic efficacy, duration of postoperative analgesia, hemodynamic stability, postoperative sedation, and any adverse effects in children.
Aims:
The aim is to study the effects of dexmedetomidine as an adjuvant to bupivacaine in caudal analgesia in pediatric patients posted for infraumbilical surgeries.
Settings and Design:
This is a randomized, double-blind study in which effect of dexmedetomidine is studied when added to bupivacaine in the caudal epidural block. The observations are made intraoperatively for hemodynamic stability and postoperatively for the duration of analgesia.
Subjects and Methods:
This study was conducted in 100 children of American Society of Anesthesiologists physical status I and II, aged 2–10 years, undergoing elective infraumbilical surgeries. They were divided into two groups as follows: Group A: (0.25%) bupivacaine 1 ml/kg + normal saline (NS) 1 ml. Group B: (0.25%) bupivacaine 1 ml/kg + 1 μg/kg dexmedetomidine in 1 ml NS. As this study was double-blind, patients were randomly assigned to receive either (bupivacaine + saline) or (bupivacaine + dexmedetomidine) in each group. The patients were observed for hemodynamic stability, respiratory depression, and postoperative pain using face, legs, activity, cry, consolability (FLACC) pain scale for 24 h postoperatively.
Statistical Analysis Used:
Unpaired Student's t-test.
Results:
The mean duration of effective analgesia in Group A patients was 4.33 ± 0.98 h versus 9.88 ± 0.90 h in Group B patients. Likewise, the difference in mean FLACC score of both the groups was also statistically significant, 7.21 ± 0.76 and 6.49 ± 1.72 in Group A and Group B, respectively.
Conclusion:
Dexmedetomidine as adjuvant to Bupivacaine increases duration of caudal analgesia and improves hemodynamic stability without an increase in adverse effects in children undergoing infraumbilical surgeries.
Keywords: Caudal block, dexmedetomidine, pediatric patients, postoperative analgesia
INTRODUCTION
Pain is one of the most misunderstood, underdiagnosed, and untreated medical problems, particularly in children. New Joint Commission on Accreditation of Health Care Organization regards pain as fifth vital sign and requires caregivers to regularly assess pain. Inadequate pain relief during childhood may have long-term negative effects including harmful neuroendocrine responses disrupted eating and sleep cycles and increased pain perception during subsequent painful experiences. Postoperative pain can result in an uncooperative and restless child. Hence, it is preferable to prevent the onset of pain rather than to relieve its existence.
Various multimodal techniques have been designed for pediatric pain relief. These include both systemic and regional analgesia. The most commonly used regional technique is caudal epidural block. Advantages of the caudal block are early extubation, ambulation, and decreased the risk of chest infections, decreased postoperative analgesic requirements, and early discharge.
As one of the disadvantages of the caudal block is the relatively short duration of analgesia. Therefore, various additives, e.g., ketamine,[1,2] ephedrine,[2] clonidine,[3] and opioids[4,5,6] have been used to prolong the duration of analgesia provided by single injection.
Both clonidine and dexmedetomidine belong to same α2 agonists group, but the difference is that latter has 1600 times' greater affinity for α2a receptors. These drugs interact with local anesthetics by three possible mechanisms. First, by blocking Aδ and C fibers as a consequence of an increase in potassium conductance in isolated neurons, thus intensifying local anesthetic conduction block, second by causing local vasoconstriction, thus decreasing local anesthetic spread, and removal around neural structures. This effect is mediated by drug action on postsynaptic α2 receptors; spinal α2 adrenergic agonists may also induce analgesia by activating spinal cholinergic neurons resulting acetylcholine release. Dexmedetomidine has an 8-fold greater affinity for α2a receptors, responsible for the hypnotic and analgesic effects of such drugs.
Considering the above facts, we designed this study using bupivacaine alone and bupivacaine with dexmedetomidine to assess analgesic efficacy, duration of postoperative analgesia, hemodynamic stability, postoperative sedation, and any adverse effects in children undergoing infraumbilical operations.
SUBJECTS AND METHODS
After obtaining approval from Ethical Committee, a written informed consent was obtained from all the parents of the children who participated in this study. This study was conducted in 100 children of American Society of Anesthesiologists (ASA) physical status I and II, aged 2–10 years, undergoing elective infraumbilical surgeries such as herniotomy, orchidopexy, hypospadias repair, etc. The patients with a history or evidence of infection at back, allergy to drugs, congenital malformations of the back, preexisting neurological or spinal diseases were excluded from this study.
All these patients underwent a preanesthetic check-up the day before surgery, and all the routine and specific investigations were noted. The children were electively kept nil by mouth for 6 h. Before surgery and before operation a written and informed parental consent was taken from the parents. An intravenous line was secured, and injection isolyte P was started. Standard monitors such as an electrocardiogram, pulse oximeter, and noninvasive blood pressure (BP) were applied. All children were premedicated intravenously with injection glycopyrrolate 0.04 mg/kg and Emeset 0.1 mg/kg. General anesthesia was induced with sevoflurane, and orotracheal intubation was done. Anesthesia was maintained on O2+ N2O + sevoflurane/isoflurane + injection atracurium/injection vecuronium bromide.
As this study was double-blind, both groups were given according to calculated weight basis dose with an equal volume of 1 ml of (dexmedetomidine or saline) to the observer. The patients were randomly assigned to receive either (bupivacaine + saline) or (bupivacaine + dexmedetomidine) in each group. All assessments were made by single observer in a double-blind fashion.
After induction, caudal block was performed with full aseptic and antiseptic precautions with the patient in the left lateral position. According to the drug administered the patients were randomly allocated into two groups:
Group A: (0.25%) bupivacaine 1 ml/kg + normal saline (NS) 1 ml
Group B: (0.25%) bupivacaine 1 ml/kg + 1 µg/kg dexmedetomidine in 1 ml NS.
The site of injection was dressed, and the patient was turned supine. Heart rate (HR), BP, respiratory rate (RR), and oxygen saturation were recorded before induction and then immediately after caudal anesthesia, and every 10 min during surgery thereafter. Adequate analgesia was defined as hemodynamic stability as indicated by the absence of an increase in systolic BP or HR of more than 20% compared with baseline value and from the intraoperative requirement of the inhalational agent. A decrease in mean arterial pressure >30% was defined as hypotension and was treated with intravenous fluids/injection ephedrine. A decrease in HR >30% was considered as bradycardia and was treated with injection atropine 0.01 mg/kg.
After completion of surgery, the residual neuromuscular block was reversed with injection neostigmine 0.05 mg/kg and injection glycopyrrolate 0.008 mg/kg. The patients were then extubated after thorough oral and endotracheal suction.
All patients were observed for 2 h, in recovery room before returning to ward. HR, BP, RR were monitored continuously. Postoperative pain was assessed at 30 min 1, 2, 4, 6, 8, 10, 12, 18, and 24 h after recovery from anesthesia using face, legs, activity, cry, consolability (FLACC) scale [Table 1].
Table 1.
Face, legs, activity, cry, consolability pain scale

Duration of analgesia (time from caudal block to the first dose of rescue analgesic) was recorded. Postoperative rescue analgesia was given in the form of paracetamol 10 mg/kg suppository. The time of the first rescue analgesia received, and total number of doses received in 12 h were noted in all the groups postoperatively.
All the observations were recorded and all the results were analyzed. Statistically data were presented as a mean ± standard deviation. A value of P < 0.05 was considered as a statistically significant difference with unpaired Student's t- test.
RESULTS
This study is a randomized, double-blind study of comparison of a bupivacaine–dexmedetomidine mixture with plain bupivacaine for caudal analgesia in children posted for infraumbilical surgeries.
This study was conducted in 100 children of ASA physical status I and II, aged 2–10 years, and divided into two groups, 50 patients in each group.
As per the [Table 2] mean age, weight and sex distribution in all groups are nearly same without any significant differences (P > 0.5) (unpaired Student's t-test).
Table 2.
Demographic data

Table 3 shows that there was no significant difference between the groups with respect to the lowest mean intraoperative and postoperative systolic BP and pulse rate.
Table 3.
Hemodynamic data

The duration of caudal analgesia was defined from the time of caudal injection to the time child first complained of pain or the time of first postoperative rescue analgesic required. The mean duration of postoperative caudal analgesia in Group A patients was 4.33 ± 0.98 while in patients of Group B this duration was 9.88 ± 0.90 in h [Table 4 and Graph 1]. This shows the duration was significantly prolonged by the addition of dexmedetomidine to bupivacaine (P < 0.0001).
Table 4.
Mean duration of caudal analgesia

Graph 1.
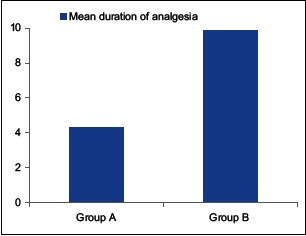
Duration of analgesia
Table 5 shows that Group B required significantly less number of rescue analgesics as compared to Group A. In Group A, all patients required 2 or more than 2 rescue analgesic within 12 h. In Group B 82% patients required single rescue analgesic and 8% required two rescue analgesic, respectively [Graph 2].
Table 5.
Rescue analgesics required

Graph 2.
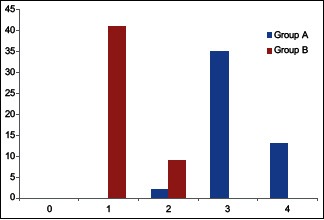
Rescue analgesics
The mean FLACC pain score was less in patients belonging to Group B throughout the initial 12 h of postoperative period. The mean FLACC score of Group A points was 7.21 ± 1.76 while that of Group B was 6.49 ± 1.72. The results are comparable and statistically significant (P < 0.0001) [Table 6 and Graph 3].
Table 6.
Mean face, legs, activity, cry, consolability pain score

Graph 3.
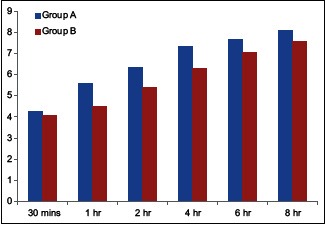
Mean face, legs, activity, cry, consolability score
The incidence of nausea vomiting was higher in Group A compared to Group B [Table 7 and Graph 4].
Table 7.
Postoperative complications

Graph 4.
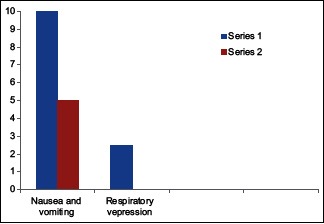
Postoperative complications
DISCUSSION
Caudal epidural analgesia is one of the most popular and commonly performed regional blocks in pediatric anesthesia. It is a reliable and safe technique that can be used with general anesthesia for intra and postoperative analgesia in patients undergoing abdominal and lower limb surgeries.[7]
The main disadvantage of caudal anesthesia is the short duration of action after a single injection of local anesthetic solution. The use of caudal catheters to administer repeated doses or infusions of local anesthetics is not popular because of the risk of infection. “Double-caudal” technique whereby the caudal is “topped up” at the end of the procedure has recently been advocated.[7,8]
Dexmedetomidine is highly selective α2 adrenoreceptor agonist, the analgesic action of intrathecal or epidural dexmedetomidine results from direct stimulation of pre- and post-synaptic α2 adrenoreceptors in the dorsal grey matter of spinal cord thereby inhibiting the release of nociceptive neurotransmitters. This effect correlates with the concentration of dexmedetomidine in the cerebrospinal fluid but not that in the plasma.[9,10]
This study was undertaken to assess the efficacy and safety of dexmedetomidine with bupivacaine in pediatric patients undergoing infraumbilical surgeries under caudal analgesia.
Group A: (0.25%) plain bupivacaine 1 ml/kg + NS 1 ml.
Group B: (0.25%) plain bupivacaine 1 ml/kg + 1 µg/kg dexmedetomidine in 1 ml NS.
Sharpe et al.[11] speculated that small volume of bupivacaine (0.5 ml/kg) may not be enough to deliver clonidine up to the spinal cord leaving only direct action on the nerve routes in the caudal area. These findings suggest that the addition of clonidine 2 µg/kg to the low volume of caudal anesthetics has limited clinical benefit in children undergoing circumcision. Furthermore, Joshi et al.[12] in their study did not recommend the addition of 2 µg/kg clonidine to 0.125% bupivacaine mg/kg. Hence, we chose a standard dose of 1 ml/kg 0.25% bupivacaine in both the groups.[13,14]
We chose a dose of 1 µg/kg of dexmedetomidine in our study as there were similar studies[15] done with clonidine showing that increasing the dose from 1 µg/kg to 2 µg/kg did not enhance the analgesic effect of clonidine but increased the incidence of side effects such as respiratory depression, bradycardia, and hypotension with increasing dose. Data are still insufficient about the effects of different concentrations of dexmedetomidine when used to prolong caudal analgesia. Recently, a study was done by Al-Zaben et al.[16] in which they assessed analgesic efficacy and side effects of two doses (1 µg/kg and 2 µg/kg) of dexmedetomidine administered along with bupivacaine and concluded that increasing the dose increased side effects such as bradycardia, hypotension, and urinary retention whereas duration of postoperative analgesia was comparable.
We chose the FLACC pain scale to evaluate postoperative pain as it is easy to use, is validated and gives an objective evaluation.[17]
Intra- and post-operative pulse rate and blood pressure
In children, dexmedetomidine has been used without clinically important respiratory or hemodynamic effects. Although hemodynamic side effects appears to be less pronounced in children than in adults, they may be dose-dependent as reported by Konakci et al.[10] The side effects of neuraxial dexmedetomidine administration include hypotension and bradycardia. The antihypertensive effect results from stimulation of α2 inhibitory neurons in the medullary vasomotor center of the brainstem, which leads to a reduction in norepinephrine turnover and sympathetic outflow from the central nervous system to the peripheral tissues. Bradycardia is caused by an increase in vagal tone resulting from central stimulation of parasympathetic outflow, as well as reduced sympathetic drive. Dexmedetomidine has an 8-fold greater affinity for α2 receptors as compared to clonidine. It is more selective for α2a receptors that are responsible for sedative and analgesic effects of such drugs. Our study confirms the finding of hemodynamic changes as shown by other workers.[18,19,20] There was no significant decrease in HR and BP from the baseline with the use of clonidine with bupivacaine in caudal anesthesia.
Duration of analgesia
In children, a mixture of 0.25% bupivacaine with 0.5–1 µg/kg dexmedetomidine has shown to improve the duration and quality of analgesia provided by the caudal block. The mean duration of analgesia in our study was found to be 4.33 ± 0.98 h for the plain bupivacaine group versus 9.88 ± 0.90 h for the dexmedetomidine group.
Our study was similar to that conducted by El-Hennawy et al.[20] who studied that addition of clonidine or dexmedetomidine to bupivacaine prolongs the duration of analgesia in the caudal block in pediatric patients. Sixty patients of age group 6 months to 6 years were randomly allocated to three groups. After sevoflurane anesthesia, each patient received 0.25% bupivacaine 1 ml/kg with 2 µg/kg clonidine in 1 ml NS or 2 µg/kg dexmedetomidine in 1 ml NS or a corresponding volume of NS according to group assignment. The addition of clonidine or dexmedetomidine significantly increased the postoperative analgesic time (median 95% confidence interval 12 h [3–21] and 16 h [14–18], respectively) as compared to bupivacaine alone 5 h.[4,5,6]
Parameswari et al.[21] evaluated the efficacy of clonidine added to bupivacaine in prolonging the analgesia produced by caudal bupivacaine in children undergoing subumbilical surgeries and concluded mean duration of analgesia was significantly longer in clonidine group.
Xiang et al.[22] used caudal dexmedetomidine for hernia sac traction and found a significant increase in duration of postoperative analgesia in dexmedetomidine group.
Rescue analgesics in the first 12 h postoperative period
In our study, the dexmedetomidine group required significantly less number of rescue analgesics as compared to plain bupivacaine group. In plain bupivacaine group, all patients required 2 or more than 2 rescue analgesic within 12 h. In dexmedetomidine group, 82% patients required single rescue analgesic and 18% required two rescue analgesic. This is in agreement with a study conducted by Saadawy et al.[19]
Mean face, legs, activity, cry, consolability score
At FLACC score of 7, the patient needs rescue analgesic. This score was reached at 4 h in Group A (mean 7.30 ± 0.70) and at 8 h in Group B (mean 7.58 ± 1.1). Fares et al.[23] also performed a similar study in pediatric abdominal cancer surgeries. They used FLACC scale in their study and came to similar conclusion.
Postoperative complications
The incidence of nausea vomiting was higher in children who received plain bupivacaine. Five children in dexmedetomidine group complained of nausea vomiting compared to 10 in plain bupivacaine group. At no time in this study, there was a decrease in RR and fall in SpO2 requiring oxygen supplementation. Similar findings were demonstrated by Lee and Rubin[24] and Upadhyay et al.[25]
A meta-analysis was done by Tong et al.[26] in which they selected six randomized controlled trials and studied analgesic effects and adverse events of caudal dexmedetomidine. They concluded that increase in duration of analgesia was significant with caudal dexmedetomidine with comparable side effects.
The addition of caudal dexmedetomidine 1 µg/kg to bupivacaine (0.25%) 1 ml/kg prolonged the duration of analgesia in comparison to plain bupivacaine (0.25%) 1 ml/kg without an increase in adverse effects in children undergoing infraumbilical surgeries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Hager H, Marhofer P, Sitzwohl C, Adler L, Kettner S, Semsroth M. Caudal clonidine prolongs analgesia from caudal S(+)-ketamine in children. Anesth Analg. 2002;94:1169–72. doi: 10.1097/00000539-200205000-00021. [DOI] [PubMed] [Google Scholar]
- 2.Cook B, Grubb DJ, Aldridge LA, Doyle E. Comparison of the effects of adrenaline, clonidine and ketamine on the duration of caudal analgesia produced by bupivacaine in children. Br J Anaesth. 1995;75:698–701. doi: 10.1093/bja/75.6.698. [DOI] [PubMed] [Google Scholar]
- 3.Koul A, Pant D, Sood J. Caudal clonidine in day-care paediatric surgery. Indian J Anaesth. 2009;53:450–4. [PMC free article] [PubMed] [Google Scholar]
- 4.Singh R, Kumar N, Singh P. Randomized controlled trial comparing morphine or clonidine with bupivacaine for caudal analgesia in children undergoing upper abdominal surgery. Br J Anaesth. 2011;106:96–100. doi: 10.1093/bja/aeq274. [DOI] [PubMed] [Google Scholar]
- 5.Constant I, Gall O, Gouyet L, Chauvin M, Murat I. Addition of clonidine or fentanyl to local anaesthetics prolongs the duration of surgical analgesia after single shot caudal block in children. Br J Anaesth. 1998;80:294–8. doi: 10.1093/bja/80.3.294. [DOI] [PubMed] [Google Scholar]
- 6.Shukla U, Prabhakar T, Malhotra K. Postoperative analgesia in children when using clonidine or fentanyl with ropivacaine given caudally. J Anaesthesiol Clin Pharmacol. 2011;27:205–10. doi: 10.4103/0970-9185.81842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd-Thomas AR. Pain management in paediatric patients. Br J Anaesth. 1990;64:85–104. doi: 10.1093/bja/64.1.85. [DOI] [PubMed] [Google Scholar]
- 8.De Beer DA, Thomas ML. Caudal additives in children – Solutions or problems? Br J Anaesth. 2003;90:487–98. doi: 10.1093/bja/aeg064. [DOI] [PubMed] [Google Scholar]
- 9.Fukushima K, Nishima Y, Mori K. The effect of epidural administered dexmedetomidine on central and peripheral nervous system in man. Anaesth Analg. 1997;84:s292. [Google Scholar]
- 10.Konakci S, Adanir T, Yilmaz G, Rezanko T. The efficacy and neurotoxicity of dexmedetomidine administered via the epidural route. Eur J Anaesthesiol. 2008;25:403–9. doi: 10.1017/S0265021507003079. [DOI] [PubMed] [Google Scholar]
- 11.Sharpe P, Klein JR, Thompson JP, Rushman SC, Sherwin J, Wandless JG, et al. Analgesia for circumcision in a paediatric population: Comparison of caudal bupivacaine alone with bupivacaine plus two doses of clonidine. Paediatr Anaesth. 2001;11:695–700. doi: 10.1046/j.1460-9592.2001.00748.x. [DOI] [PubMed] [Google Scholar]
- 12.Joshi W, Connelly NR, Freeman K, Reuben SS. Analgesic effect of clonidine added to bupivacaine 0.125% in paediatric caudal blockade. Paediatr Anaesth. 2004;14:483–6. doi: 10.1111/j.1460-9592.2004.01229.x. [DOI] [PubMed] [Google Scholar]
- 13.Coad NR, Hain WR. Caudal anaesthesia for postoperative pain relief in children: A comparative trial of different regimens using plain bupivacaine. Ann R Coll Surg Engl. 1989;71:245–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Malviya S, Fear DW, Roy WL, Lerman J. Adequacy of caudal analgesia in children after penoscrotal and inguinal surgery using 0.5 or 1.0 ml.kg-1 bupivacaine 0.125% Can J Anaesth. 1992;39(5 Pt 1):449–53. doi: 10.1007/BF03008708. [DOI] [PubMed] [Google Scholar]
- 15.Klimscha W, Chiari A, Michalek-Sauberer A, Wildling E, Lerche A, Lorber C, et al. The efficacy and safety of a clonidine/bupivacaine combination in caudal blockade for pediatric hernia repair. Anesth Analg. 1998;86:54–61. doi: 10.1097/00000539-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Al-Zaben KR, Qudaisat IY, Abu-Halaweh SA, Al-Ghanem SM, Al-Mustafa MM, Alja'bari AN, et al. Comparison of caudal bupivacaine alone with bupivacaine plus two doses of dexmedetomidine for postoperative analgesia in pediatric patients undergoing infra-umbilical surgery: A randomized controlled double-blinded study. Pediatr Anaesth. 2015;25:883–90. doi: 10.1111/pan.12686. [DOI] [PubMed] [Google Scholar]
- 17.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–7. [PubMed] [Google Scholar]
- 18.Anand VG, Kannan M, Thavamani A, Bridgit MJ. Effect of dexmedetomidine on caudal ropivacaine in pediatric lower abdominal surgeries. Indian J Anaesth. 2011;55:340–6. doi: 10.4103/0019-5049.84835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saadawy I, Boker A, Elshahawy MA, Almazrooa A, Melibary S, Abdellatif AA, et al. Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anaesthesiol Scand. 2009;53:251–6. doi: 10.1111/j.1399-6576.2008.01818.x. [DOI] [PubMed] [Google Scholar]
- 20.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103:268–74. doi: 10.1093/bja/aep159. [DOI] [PubMed] [Google Scholar]
- 21.Parameswari A, Dhev AM, Vakamudi M. Efficacy of clonidine as an adjuvant to bupivacaine for caudal analgesia in children undergoing sub-umbilical surgery. Indian J Anaesth. 2010;54:458–63. doi: 10.4103/0019-5049.71047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang Q, Huang DY, Zhao YL, Wang GH, Liu YX, Zhong L, et al. Caudal dexmedetomidine combined with bupivacaine inhibit the response to hernial sac traction in children undergoing inguinal hernia repair. Br J Anaesth. 2013;110:420–4. doi: 10.1093/bja/aes385. [DOI] [PubMed] [Google Scholar]
- 23.Fares KM, Othman AH, Alieldin NH. Efficacy and safety of dexmedetomidine added to caudal bupivacaine in pediatric major abdominal cancer surgery. Pain Physician. 2014;17:393–400. [PubMed] [Google Scholar]
- 24.Lee JJ, Rubin AP. Comparison of a bupivacaine-clonidine mixture with plain bupivacaine for caudal analgesia in children. Br J Anaesth. 1994;72:258–62. doi: 10.1093/bja/72.3.258. [DOI] [PubMed] [Google Scholar]
- 25.Upadhyay KK, Prabhakar TB, Handa R, Haridas B. Study of the efficacy and safety of clonidine as an adjuvant to bupivacaine for caudal analgesia in children. Indian J Anaesth. 2005;49:199–201. [Google Scholar]
- 26.Tong Y, Ren H, Ding X, Jin S, Chen Z, Li Q. Analgesic effect and adverse events of dexmedetomidine as additive for pediatric caudal anesthesia: A meta-analysis. Paediatr Anaesth. 2014;24:1224–30. doi: 10.1111/pan.12519. [DOI] [PubMed] [Google Scholar]


