Abstract
Background:
Lignocaine + adrenaline; a local anesthetic agent; frequently used for perilesional infiltration, maintains the stable hemodynamics and decreases the postoperative pain after maxillofacial surgery. α2 agonists have peripheral analgesic effects. This prospective study was to evaluate the effectiveness of perilesional dexmedetomidine administered preincisionally in addition to conventional lignocaine adrenaline combinations for reconstructive maxillofacial surgery in an ambulatory care setting.
Materials and Methods:
76, American Society of Anesthesiologists I-II patients scheduled for unilateral traumatic maxillofacial surgeries were randomly allocated into group DL (n = 38) receiving 15 cc of 2% lignocaine + adrenaline (1:200,000) mixed with 1 μg/kg dexmedetomidine and group PL receiving 15 cc of 2% lignocaine + adrenaline with normal saline (placebo) via local wound infiltration 5 min prior to skin incision. Perioperative hemodynamics, time to first analgesic use, total analgesic need, bleeding, and side effects were recorded for each patient.
Results:
Dosage of supplemental propofol; total perioperative, postoperative, and postanesthesia care unit (PACU) fentanyl consumption was significantly lower (P = 0.0001, P= 0.0001, P= 0.0001, P= 0.004, respectively) in dexmedetomine treated group than placebo. Rescue analgesic requirement was significantly earlier in group PL than group DL. Group DL patients suffered from significantly less (P = 0.02) bleeding and surgeon's satisfaction score was also high in this group. Discharge from PACU was significantly earlier in group DL. Intraoperative hemodynamic parameters were significantly lower in group DL (P < 0.05) without any appreciable side effects.
Conclusion:
Thus, prior dexmedetomidine local infiltration at the site of maxillofacial trauma has significantly reduced bleeding from wound site; perioperative fentanyl, propofol consumption, and subsequently ensured earlier discharge from PACU, better surgeon's satisfaction score with better hemodynamic control and lesser side effects.
Keywords: Dexmedetomidine, lignocaine with adrenaline, perilesional infiltration (infiltration of drug 1 cm outside the planned line of incision), postanesthesia care unit, reconstructive maxillofacial surgery
INTRODUCTION
The face represents the most prominent position in the human body and is often involved in trauma injuries. In hurried modern life high-speed road traffic accidents and physical violence has made facial area is one of the most frequently injured areas of the body, accounting for 23–97% of all fractures.[1] According to reports of developing nations, traffic accidents are the main cause of maxillofacial fractures,[2,3] while data from developed countries pointed to assaults being considered the most frequent etiology of such fractures.[4]
There are changes in patterns of facial injuries, extent, clinical features, and so forth resulting in severe morbidity, loss of function, mild-to-massive disfigurement of maxillofacial skeleton along with functional and financial loss.[5,6]
During reconstructive maxillofacial surgery, impaired visibility due to excessive intraoperative bleeding may be reduced most effectively by maintaining stable hemodynamics throughout the operative duration.[7] There are several important advantages of using controlled hypotensive anesthesia during various maxillofacial surgeries, like reduction in blood loss hence reduction in blood transfusion rate, improvement in the surgical field and reduction of in the duration of surgery.[8,9]
However, reconstructive operations frequently been performed as an outpatient procedure; is often associated with persistent postoperative discomfort and pain.[10,11] Adequate pain relief reduces surgical stress response, so reduces patient's morbidity and improves postoperative recovery. In maxillofacial surgeries various preincisional local anesthetic (LA) infiltration in surgical wound and/or nerve blocks have been used for producing preemptive analgesia.[12,13,14]
During maxillofacial surgeries, perilesional LA infiltration when combined with general anesthesia (GA) provides good operative condition but has relatively shorter duration of postoperative analgesia. So various adjuvants like opioids,[15] clonidine,[16] dexamethasone,[17] ketamine,[18] etc., were added to LA solution for infiltration in surgical wound to achieve dense and prolonged block, but the results are often associated with side effects.
Dexmedetomidine is highly selective (8 times more than clonidine),[19] specific and potent α2-adrenergic agonist having analgesic, sedative, antihypertensive, and anesthetic sparing effects when used in systemic route.[20] Prior administration in nerve block and in GA dexmedetomidine can also provide a hypotensive anesthesia and a better surgical field and finally an abbreviated operative duration.[21,22]
The aim and objective of this study was primarily to compare preincisionally (perilesional) infiltrated dexmedetomidine when added to lignocaine-adrenaline (1:200,000) mixture for producing analgesia and reducing rescue analgesic requirement after maxillofacial surgery in adults in an ambulatory care setting. The secondary goal was to compare it with placebo in the regard of visibility of surgical field, satisfaction of the surgeons, recovery profile, adverse effects, and perioperative hemodynamics.
MATERIALS AND METHODS
After obtaining permission from Institutional Ethics Committee, written informed consent was taken. Total 76 adult patients were randomly allocated to two equal groups (n = 38 in each group) using computer generated random number list. Between 2010 June and 2013 January, patients having American Society of Anesthesiologists (ASA) physical status I and II, aged 20–40 years of both sexes undergoing unilateral reconstructive maxillofacial surgery under GA were enrolled in the study.
Patients in group DL received 15 cc of 2% lignocaine + adrenaline (1:200,000) solution (xylocaine 2% adrenaline [1:200,000], AstraZeneca Pharma India Limited, Bengaluru, Karnataka, India) mixed with 1 µg/kg. Dexmedetomidine, as perilesional infiltration 5 min prior to skin incision but after administration of GA [Figure 1: Schematically]. Similarly patients in group PL received 15 cc of 2% lignocaine + adrenaline (1:200,000) solution mixed with normal saline (placebo), as perilesional infiltration 5 min prior to skin incision but after administration of GA. The drug mixture was infiltrated by same resident maxillofacial surgeon after confirming the lesion clinically and from orthopantomogram in both the groups in all the cases for blinding purpose. The study participants, operation nurse, and the chief maxillofacial surgeon constituted the “blind” study group. The anesthesiologist performing the GA was unaware of the infiltration drug and allotment of the group and similarly resident doctors keeping records of different parameters were also unaware of group allotment. Thus, blinding was properly maintained.
Figure 1.
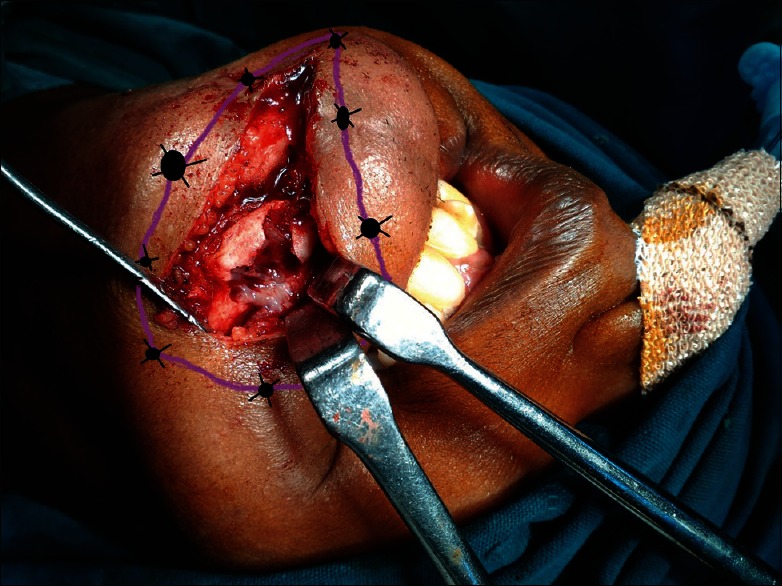
Schematic diagram showing perilesional infiltration line for local anesthetic infiltration
Exclusion criteria
Patient refusal, any known hypersensitivity or contraindication to lignocaine, dexmedetomidine, fentanyl; pregnancy, lactating mothers, hepatic, renal or cardiopulmonary abnormality, alcoholism, diabetes, patients on calcium channel blockers, and bleeding diathesis were excluded from the study.
Patients having a history of significant head injury, intracranial hematoma, neurological, psychiatric, or neuromuscular disorders were also excluded. As we were dealing with day care surgery patients having no assistance in home and dwelling at more than 10 km from our institution were also excluded from this study.
In preoperative assessment the patients were enquired about any history of drug allergy, previous operations or prolonged drug treatment. General examination, systemic examinations, and assessment of the airway (particularly mouth opening) were done. Preoperative fasting for minimum 6 h was ensured before operation in all day care cases. All patients received premedication of tablet alprazolam 0.5 mg orally the night before surgery as per preanesthetic check-up direction to allay anxiety, and for sound sleep. The patients also received tablet ranitidine 150 mg in the previous night and in the morning of operation with sips of water. All patients were investigated for total hemogram blood sugar, urea, creatinine, and liver function tests. A 12 lead electrocardiogram and chest X-ray were also taken. Perioperatively standard monitors were attached for monitoring vital parameters.
Philips IntelliVue (MP20) monitor used for this purpose. Intravenous (IV) infusion of Ringers' lactate started and xylometazoline nasal drop was given in each nasal aperture to reduce bleeding during nasal intubation just 2 h before operation. After transfer, patients were preoxygenated with 100% oxygen for a period of 5 min. Injection fentanyl (2 μg/kg) and glycopyrrolate (0.01 mg/kg) were given intravenously 3 min before induction of anesthesia. All the patients were induced with injection propofol (2 mg/kg). After that, atracurium (0.5 mg/kg) was given to facilitate laryngoscopy and intubation. Nasal intubation was done to ensure proper dental occlusion after open reduction and mini plate fixation in each case. Laryngoscopy, intubation, and cuff inflation were completed within 45 s in all cases. ET tube was fixed at a point where bilateral air entry is ensured. Muscle relaxation was maintained with intermittent IV atracurium (0.2 mg/kg) as and when required. Controlled ventilation was maintained manually with 33% oxygen in 66% nitrous oxide and isoflurane 1.5 MAC using Boyle's apparatus to maintain end tidal CO2 pressure between 32 and 40 mm Hg. Intermittently 1 cc propofol was administered to achieve a target bispectral index (BIS) between 40 and 60.
At the completion of surgery, the residual neuromuscular blockade was antagonized at a train-of-four ratio more than 0.7 with neostigmine 0.05 mg/kg and glycopyrrolate 0.01 mg/kg intravenously and the patient was extubated when BIS ≥ 70. After extubation, all patients were transferred to postanesthesia care unit (PACU).
When preoperative mean arterial pressure (MAP) was ≥70 mm of Hg, 50 µg nitroglycerine was applied. When heart rate was ≤50, 0.6 mg IV atropine was applied to combat bradycardia. The presence of hypertension or tachycardia during anesthesia, while BIS was 40–60, was attributed to insufficient analgesia and a bolus dose of fentanyl 1 µg/kg was given.
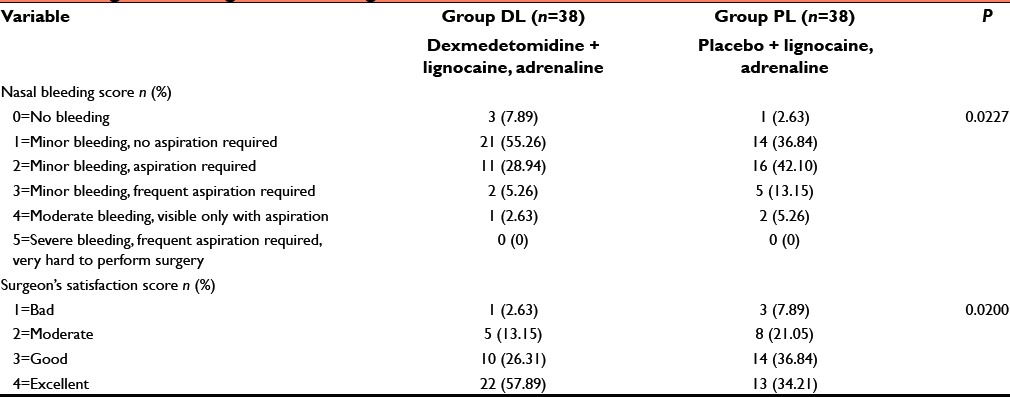
The time for PACU stay (admission discharge from PACU) and hospital discharge (eye opening discharge from hospital), and the incidence of adverse events (nausea, vomiting, dizziness, drowsiness, urinary retention, and bradycardia [HR <60 bpm]) were also recorded. Patients were considered ready for discharge from the PACU when the modified aldrete postanesthesia score was ≥9. Patients were transferred to ward after being discharged from PACU. For nausea and vomiting, ondansetron 0.15 mg/kg IV was administered. All the patients were operated by the same surgeon, and surgical site was rated according to a 6-point scale every 5 min by him in terms of bleeding and dryness [Table 1]. Surgeon's satisfaction was scored by the same surgeon with a 4-point scale [Table 1].
Table 1.
Surgical bleeding score and surgeon satisfaction score
Statistical analysis
Sample size was estimated using first rescue analgesic requirement (after primary preoperative fentanyl administration) among two groups as the main primary variable. The average duration in each group was 110 min and to detect a difference of 10% (i.e., 11 min), at the P < 0.05 level, (with a probability of detecting a difference of 80%) (1−beta = 0.80). On the basis of previous study assuming that within group standard deviation was 20 min and we needed to study at least 33 patients per group to be able to reject the null hypothesis which will be increased to 38 patients per group for possible dropouts. Raw data were entered into a MS Excel spreadsheet and analyzed using standard statistical software SPSS® statistical package version 18.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were analyzed using the Pearson's Chi-square test. Normally distributed continuous variables were analyzed using the independent sample t-test and P < 0.05 was considered to be statistically significant.
RESULTS AND ANALYSIS
We recruited 38 subjects per group, more than the calculated sample size. There were no dropouts. Thirty-eight patients in the dexmedetomidine group (DL) and 38 in the placebo (normal saline) group (PL) were eligible for effectiveness analysis.
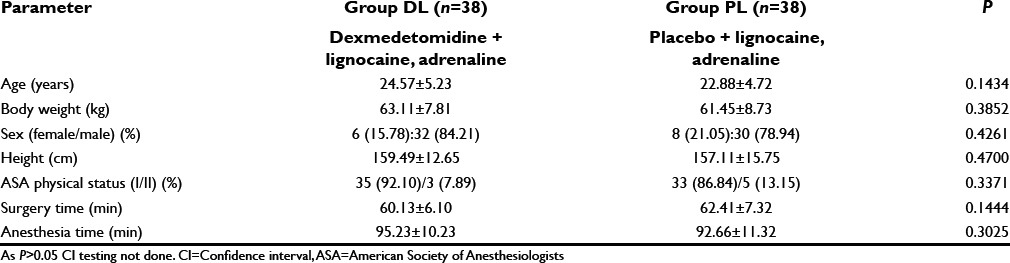
The age, sex distribution, body weight, height, and ASA status were found to be comparable [Table 2]. Duration of surgery and anesthesia time in the two groups were also similar and has no clinical significance (P > 0.05) [Table 2].
Table 2.
Comparison of demographic data between the two study groups
Total requirement of fentanyl (just before induction + intraoperative dose + as postoperative rescue analgesic dose both inside and outside PACU) and supplemental propofol (intraoperative 10 mg aliquot given several times to keep BIS 40–60) was significantly lower in group DL than PL (P < 0.05) [Table 3]. Placebo group required early rescue analgesic and significantly higher dose of fentanyl in the postoperative period (P < 0.05) [Table 3]. In the PACU, number of patients treated with fentanyl and their dose was significantly (P < 0.05) lower in the dexmedetomidine treated group than placebo [Table 4]. Duration of PACU stay was significantly longer in the placebo group (P < 0.05) but the hospital stay was quite comparable among two group (P > 0.05) [Table 4].
Table 3.
Propofol and analgesic requirement in perioperative period (time and amount of IV fentanyl citrate injections)
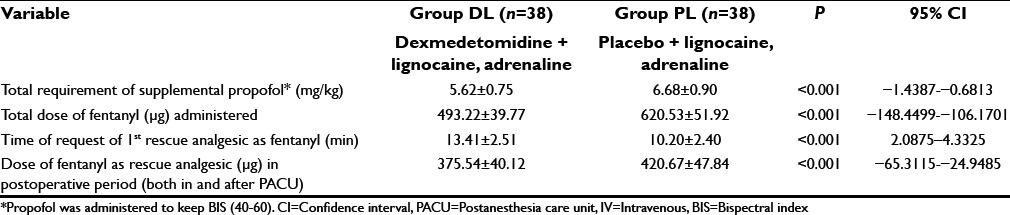
Table 4.
Comparison of PACU management parameters and discharge from hospital
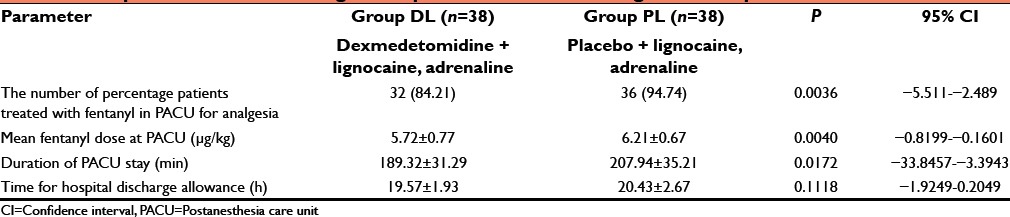
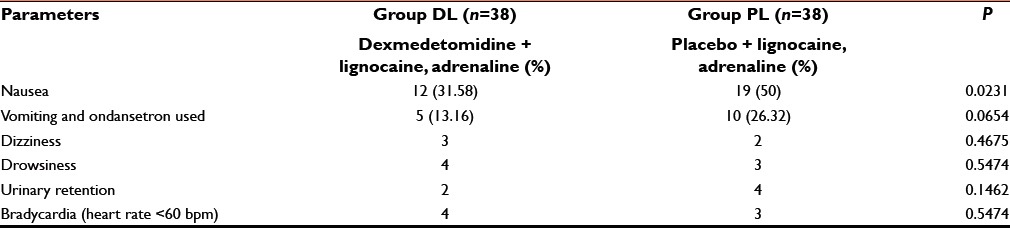
Table 1 shows that perioperative surgical site bleeding score was significantly (P < 0.05) higher in group PL than group DL [Table 1]. Again due to less bleeding and excellent operative condition, surgeon's satisfaction score was significantly better in dexmedetomidine group than placebo infiltrated group [Table 1]. Side effects such as vomiting, shivering, dizziness, drowsiness, urinary retention, and bradycardia were all comparable (P > 0.05) among two groups but nausea was significantly higher in group PL than group DL (P < 0.05) [Table 5].
Table 5.
Comparison of side effects
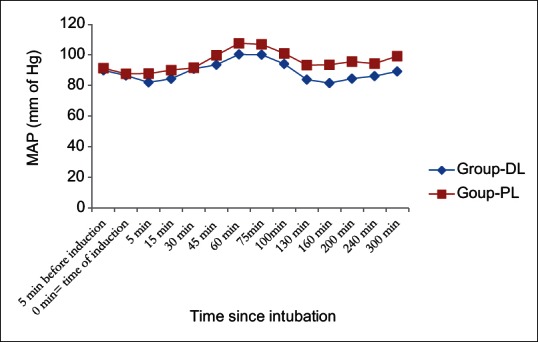
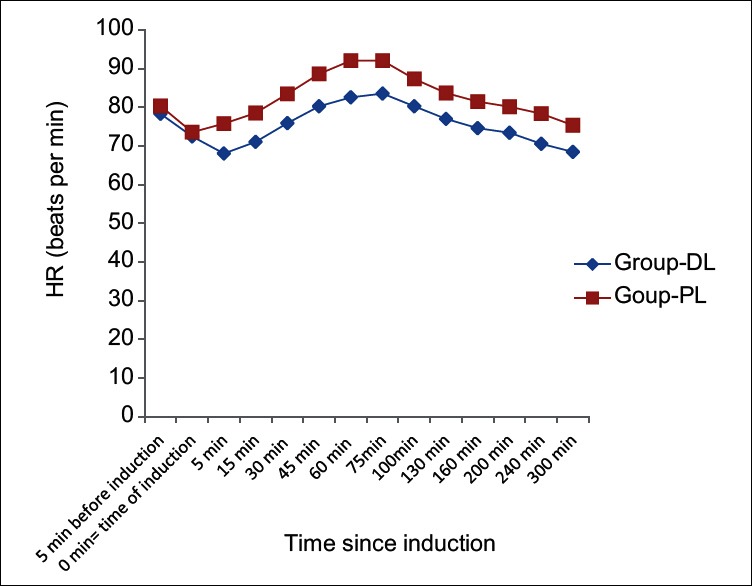
MAP and heart rate among two groups were found to be quite comparable among two groups (P > 0.05) [Figures 2 and 3]. [Consort flow diagram] shows the patient enrollment, allocation, follow up, analysis of all the patients in the study.
Figure 2.
Perioperative mean arterial pressure among two groups
Figure 3.
Perioperative heart rate among two groups
Consort Flow Diagram
DISCUSSION
Day care surgery has proven over the years as the best method to reduce the burden on the health care resources, as well as achievement of extreme patient satisfaction.[23] In developing countries, most of the patients avoid bearing expenses of prolonged hospital stay. In the day care scenario, pain in the immediate postoperative period is the most common cause of delayed recovery, discharge, and bleeding is most frequent (even extending up to 2.68%) cause of unplanned readmission and subsequently delayed return to work and often delayed wound healing.[24,25,26,27]
LA like lignocaine containing adrenaline alone for preoperative surgical field infiltration provide good operative conditions but have very short lived duration of postoperative analgesia. So various drugs have been tried as adjuvant to achieve quick, dense, as well as prolonged block, but the results are either inconclusive or associated with side effects.[15,16,17,18]
Dexmedetomidine, is a more highly specific α2 adrenoreceptor agonist (α2/α1=1620/1) than clonidine (α2α1=220/1), has been approved by Food And Drug Administration as a short-term sedative for mechanically ventilated intensive care unit patients.[20] Kang in his study on inguinal herniorrhaphy found that perilesional dexmedetomidine infiltration along with ropivacaine provides better postoperative analgesia and reduces rescue analgesic (fentanyl) consumption without any side effects.[28] Cheung et al.[29] in their study demonstrated an antihyperalgesic effect by administering local dexmedetomidine for bilateral third molar surgery.
Unfortunately, no clinical trial studied the effectiveness of perilesional infiltration of dexmedetomidine with lignocaine containing adrenaline for alteration in intraoperative surgical bleeding and visibility of surgical field, satisfaction of the surgeons, perioperative hemodynamics, and recovery profile for the patients undergoing maxillofacial surgery.
In this prospective, randomized, double-blinded trial we had compared the efficacy of preincisional wound infiltration with dexmedetomidine and placebo both added to lignocaine adrenaline (1:200,000) as 15 cc mixture for producing analgesia, as well as on visibility of surgical field, satisfaction of the surgeon, and recovery profile after maxillofacial surgery in adults in an ambulatory care setting.
The demographic profile (age, weight, sex, and height); durations of surgery and anesthesia between two groups were statistically insignificant (P > 0.05) which is quite similar with other research investigators and provided us the uniform platform to evenly compare the results obtained.[28]
Le Guen et al.[30] in their placebo-controlled study found that dexmedetomidine pretreated group consumed less propofol and remifentanil for anesthetic induction. They also found that peroperatively propofol dosage for anesthetic maintenance was significantly lower in dexmedetomidine group but remifentanil dosage was quite comparable among two groups. Similarly in our study total dose of propofol required for maintenance of BIS value 40–60 was significantly less in dexmedetomidine infiltrated group than placebo. Regarding analgesic requirement total dose of (perioperative, postoperative [as rescue analgesic], PACU) fentanyl was significantly less (P < 0.0001, 0.0001, 0.004, respectively) in group DL than group PL. This result was quite similar to Le Guen et al.[30] except the mode of administration of our test drug is different from previous researchers.
Abdallah and Brull in a meta-analysis consisting of nine randomized controlled trials found that first rescue analgesic requirement in all dexmedetomidine treated groups (both spinal as well as brachial block) was significantly delayed. In our study we have similarly found that in group DL rescue analgesic request was significantly delayed (P < 0.0001) than group PL.[31]
Ghaffar and Haleem in a study on posttonsillectomy pain found that IV and peritonsillar infiltration of dexmedetomidine reduced the number of patients to be treated with rescue analgesic (but not the no of doses) significantly while compared with placebo.[32] Similarly we have found that in group DL lesser number of patients demanded for fentanyl as rescue analgesic than group PL. This difference was also statistically significant.
Cho et al.[33] in their placebo-controlled study found that intraoperative infusion of dexmedetomidine helped the study group patients to be discharged earlier than control group by inducing the opioid sparing and sympatholytic effect for the patients undergoing laparoscopic gastrectomy. Similarly dexmedetomidine infiltrated group in our study was discharged significantly earlier (P < 0.05) from PACU than placebo group. But overall hospital stay was quite comparable among two groups.
Guven et al. in a placebo-controlled clinical trial found that dexmedetomidine continuous infusion had significantly reduced the bleeding score and improved the visibility in functional endoscopic sinus surgery.[34] Here in our study we have also found that group DL patients suffered from less bleeding when compared with group PL. This is probably due to the hypotensive property of dexmedetomidine as evidenced from [Figure 2].
Parikh et al.[35] in a comparative study found that dexmedetomidine produces better surgical satisfaction score than the midazolam-fentanyl combination. We also found that dexmedetomidine infiltrated group provided significantly better satisfaction (P < 0.05) for the surgeons than placebo. This is probably due to lesser bleeding and clearer surgical field.
In our study; vomiting, ondansetron used for controlling vomiting, dizziness, drowsiness, urinary retention, and bradycardia all were comparable among two groups. But the nausea was more in the placebo group in a significant manner than dexmedetomidine group and this is probably due to higher opioid consumption in the placebo group. Similar results were observed by Guven et al. while doing a study between dexmedetomidine versus magnesium sulfate for producing controlled hypotension for the patients undergoing FESS.[34] Again in our study, dexmedetomidine infiltrated group maintains stable hemodynamics throughout the operative and postoperative period whereas the placebo group frequently suffers from tachycardia and hypertension which were controlled with fentanyl, propofol, nitroglycerine infusion for smooth maintenance of operation. These findings were quite similar to that of Bekker et al. who conducted 56 intracranial surgeries and found that addition of dexmedetomine to sevoflurane opioid anesthesia rendered a much stable hemodynamics than placebo group and dexmedetomidine did not increase the incidence of hypotension or bradycardia, common side effects of the drug.[36]
One of the limitations of the current study is the absence of a complete placebo-controlled group. Secondly, we did not use scores for postoperative pain assessment other than visual analogue scale. We had not measured sedation and plasma catecholamine or stress hormone concentrations which may reveal relations between sympatholytic properties of α2 agonists and earlier discharge after their use. Another limitation is that we used dexmedetomidine based on their known optimal dose without the knowledge of its true optimal doses. However, a larger study with large sample size needs to be conducted to establish the author's point of view with solidarity.
We do conclude that during reconstructive maxillofacial surgery, preincisional perilesional infiltration of dexmedetomidine (1 µg/kg) when co-administered with lignocaine-adrenaline (1:200,000) mixture is more effective in producing perioperative analgesia and amnesia measured by reduced consumption of fentanyl, propofol, and subsequently earlier discharge from PACU. This α2-adrenergic agonist had also reduced surgical site bleeding and rendering an excellent surgical field with higher surgeon's satisfaction score with better hemodynamic control and lesser side effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Edwards TJ, David DJ, Simpson DA, Abbott AA. Patterns of mandibular fractures in Adelaide, South Australia. Aust N Z J Surg. 1994;64:307–11. doi: 10.1111/j.1445-2197.1994.tb02216.x. [DOI] [PubMed] [Google Scholar]
- 2.Ansari MH. Maxillofacial fractures in Hamedan province, Iran: A retrospective study (1987-2001) J Craniomaxillofac Surg. 2004;32:28–34. doi: 10.1016/j.jcms.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Fasola AO, Nyako EA, Obiechina AE, Arotiba JT. Trends in the characteristics of maxillofacial fractures in Nigeria. J Oral Maxillofac Surg. 2003;61:1140–3. doi: 10.1016/s0278-2391(03)00671-2. [DOI] [PubMed] [Google Scholar]
- 4.Iida S, Kogo M, Sugiura T, Mima T, Matsuya T. Retrospective analysis of 1502 patients with facial fractures. Int J Oral Maxillofac Surg. 2001;30:286–90. doi: 10.1054/ijom.2001.0056. [DOI] [PubMed] [Google Scholar]
- 5.Down KE, Boot DA, Gorman DF. Maxillofacial and associated injuries in severely traumatized patients: Implications of a regional survey. Int J Oral Maxillofac Surg. 1995;24:409–12. doi: 10.1016/s0901-5027(05)80469-2. [DOI] [PubMed] [Google Scholar]
- 6.Kieser J, Stephenson S, Liston PN, Tong DC, Langley JD. Serious facial fractures in New Zealand from 1979 to 1998. Int J Oral Maxillofac Surg. 2002;31:206–9. doi: 10.1054/ijom.2002.0208. [DOI] [PubMed] [Google Scholar]
- 7.Ardekian L, Samet N, Shoshani Y, Taicher S. Life-threatening bleeding following maxillofacial trauma. J Craniomaxillofac Surg. 1993;21:336–8. doi: 10.1016/s1010-5182(05)80493-7. [DOI] [PubMed] [Google Scholar]
- 8.Prasant MC, Kar S, Rastogi S, Hada P, Ali FM, Mudhol A. Comparative study of blood loss, quality of surgical field and duration of surgery in maxillofacial cases with and without hypotensive anesthesia. J Int Oral Health. 2014;6:18–21. [PMC free article] [PubMed] [Google Scholar]
- 9.Baek RM, Yun BH, Kim MC. Comparative study of the effectiveness among the four types of induced hypotensive anesthetic methods in maxillofacial reconstructive surgery. J Korean Cleft Palate Craniofac Assoc. 2001;2:30–4. [Google Scholar]
- 10.Krishnan R, Shivananda S, Raman U. Pre-emptive analgesia for elective maxillofacial surgery using 0.25% bupivacaine. Indian J Anaesth. 2008;52:556–61. [Google Scholar]
- 11.Fortier J, Chung F, Su J. Predictive factors of unanticipated admission in ambulatory surgery: A prospective study. Anesthesiology. 1996;85:A27. [Google Scholar]
- 12.Eriksson-Mjöberg M, Kristiansson M, Carlström K, Eklund J, Gustafsson LL, Olund A. Preoperative infiltration of bupivacaine – Effects on pain relief and trauma response (cortisol and interleukin-6) Acta Anaesthesiol Scand. 1997;41:466–72. doi: 10.1111/j.1399-6576.1997.tb04725.x. [DOI] [PubMed] [Google Scholar]
- 13.Reader Al. Ropivacaine is equivalent to bupivacaine in maxillary infiltrations. Evid Based Dent. 2002;3:67–8. [Google Scholar]
- 14.Mesgarzadeh AH, Aghamohamadi D, Asl DM, Yazdani J, Amani M, Purlak T. Effect of long-acting local anesthetic infiltration in surgical wound for post-operative pain control in zygomaticomaxillary complex fracture. Int J Curr Res Acad Rev. 2015;3:405–14. [Google Scholar]
- 15.Mane RS, Sanikop CS, Dhulkhed VK, Gupta T. Comparison of bupivacaine alone and in combination with fentanyl or pethidine for bilateral infraorbital nerve block for postoperative analgesia in paediatric patients for cleft lip repair: A prospective randomized double blind study. J Anaesthesiol Clin Pharmacol. 2011;27:23–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Jindal P, Khurana G, Dvivedi S, Sharma JP. Intra and postoperative outcome of adding clonidine to bupivacaine in infraorbital nerve block for young children undergoing cleft lip surgery. Saudi J Anaesth. 2011;5:289–94. doi: 10.4103/1658-354X.84104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ju NY, Cui GX, Gao W. Ropivacaine plus dexamethasone infiltration reduces postoperative pain after tonsillectomy and adenoidectomy. Int J Pediatr Otorhinolaryngol. 2013;77:1881–5. doi: 10.1016/j.ijporl.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 18.Jha AK, Bhardwaj N, Yaddanapudi S, Sharma RK, Mahajan JK. A randomized study of surgical site infiltration with bupivacaine or ketamine for pain relief in children following cleft palate repair. Paediatr Anaesth. 2013;23:401–6. doi: 10.1111/pan.12124. [DOI] [PubMed] [Google Scholar]
- 19.Gerlach AT, Dasta JF. Dexmedetomidine: An updated review. Ann Pharmacother. 2007;41:245–52. doi: 10.1345/aph.1H314. [DOI] [PubMed] [Google Scholar]
- 20.Huang R, Hertz L. Receptor subtype and dose dependence of dexmedetomidine-induced accumulation of [14C] glutamine in astrocytes suggests glial involvement in its hypnotic-sedative and anesthetic-sparing effects. Brain Res. 2000;873:297–301. doi: 10.1016/s0006-8993(00)02525-7. [DOI] [PubMed] [Google Scholar]
- 21.Shams T, El Bahnasawe NS, Abu-Samra M, El-Masry R. Induced hypotension for functional endoscopic sinus surgery: A comparative study of dexmedetomidine versus esmolol. Saudi J Anaesth. 2013;7:175–80. doi: 10.4103/1658-354X.114073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marhofer D, Kettner SC, Marhofer P, Pils S, Weber M, Zeitlinger M. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: A volunteer study. Br J Anaesth. 2013;110:438–42. doi: 10.1093/bja/aes400. [DOI] [PubMed] [Google Scholar]
- 23.Boothe P, Finegan BA. Changing the admission process for elective surgery: An economic analysis. Can J Anaesth. 1995;42(5 Pt 1):391–4. doi: 10.1007/BF03015483. [DOI] [PubMed] [Google Scholar]
- 24.Georgalas C, Obholzer R, Martinez-Devesa P, Sandhu G. Day-case septoplasty and unexpected re-admissions at a dedicated day-case unit: A 4-year audit. Ann R Coll Surg Engl. 2006;88:202–6. doi: 10.1308/003588406X95039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wirz S, Wartenberg HC, Nadstawek J. Pain management procedures used by dental and maxillofacial surgeons: An investigation with special regard to odontalgia. Head Face Med. 2005;1:14. doi: 10.1186/1746-160X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arole G. Day-case oral and maxillofacial surgery in a Nigerian district general hospital: Scope and limitations. Ann R Coll Surg Engl. 1998;80:108–10. [PMC free article] [PubMed] [Google Scholar]
- 27.Eftekharian H, Zamiri B, Ahzan S, Talebi M, Zarei K. Orthognathic surgery patients (Maxillary Impaction and Setback plus Mandibular Advancement plus Genioplasty) need more intensive care unit (ICU) admission after surgery. J Dent (Shiraz) 2015;16(1 Suppl):43–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Kang H. The effect of dexmedetomidine added to preemptive ropivacaine infiltartion on post-operative pain after inguinal herniorrhaphy: A prospective, randomized, double-blind, placebo-controlled study. Eur Surg. 2012;44:274–80. [Google Scholar]
- 29.Cheung CW, Ng KF, Choi WS, Chiu WK, Ying CL, Irwin MG. Evaluation of the analgesic efficacy of local dexmedetomidine application. Clin J Pain. 2011;27:377–82. doi: 10.1097/AJP.0b013e318208c8c5. [DOI] [PubMed] [Google Scholar]
- 30.Le Guen M, Liu N, Tounou F, Augé M, Tuil O, Chazot T, et al. Dexmedetomidine reduces propofol and remifentanil requirements during bispectral index-guided closed-loop anesthesia: A double-blind, placebo-controlled trial. Anesth Analg. 2014;118:946–55. doi: 10.1213/ANE.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 31.Abdallah FW, Brull R. Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: A systematic review and meta-analysis. Br J Anaesth. 2013;110:915–25. doi: 10.1093/bja/aet066. [DOI] [PubMed] [Google Scholar]
- 32.Ghaffar HS, Haleem AK. Efficacy and safety of intraoperative dexmedetomidine in pediatric posttonsillectomy pain: Peritonsillar versus intravenous administration. Egypt J Anaesth. 2011;27:219–25. [Google Scholar]
- 33.Cho JS, Kim HI, Lee KY, An JY, Bai SJ, Cho JY, et al. Effect of intraoperative dexmedetomidine infusion on postoperative bowel movements in patients undergoing laparoscopic gastrectomy: A prospective, randomized, placebo-controlled study. Medicine (Baltimore) 2015;94:e959. doi: 10.1097/MD.0000000000000959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guven DG, Demiraran Y, Sezen G, Kepek O, Iskender A. Evaluation of outcomes in patients given dexmedetomidine in functional endoscopic sinus surgery. Ann Otol Rhinol Laryngol. 2011;120:586–92. doi: 10.1177/000348941112000906. [DOI] [PubMed] [Google Scholar]
- 35.Parikh DA, Kolli SN, Karnik HS, Lele SS, Tendolkar BA. A prospective randomized double-blind study comparing dexmedetomidine vs. combination of midazolam-fentanyl for tympanoplasty surgery under monitored anesthesia care. J Anaesthesiol Clin Pharmacol. 2013;29:173–8. doi: 10.4103/0970-9185.111671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bekker A, Sturaitis M, Bloom M, Moric M, Golfinos J, Parker E, et al. The effect of dexmedetomidine on perioperative hemodynamics in patients undergoing craniotomy. Anesth Analg. 2008;107:1340–7. doi: 10.1213/ane.0b013e3181804298. [DOI] [PubMed] [Google Scholar]


