Abstract
Background:
Better adjuvants for epidural analgesia are still evolving. Dexmedetomidine that is alpha-2 agonist can be used as an adjuvant in epidural analgesia and anesthesia.
Aims:
The aim of this study was to compare the effect of dexmedetomidine versus clonidine in combination with ropivacaine in epidural anesthesia on intraoperative and postoperative analgesia, to find out the better adjuvant for regional anesthesia.
Settings and Design:
Randomized control trial.
Materials and Methods:
Sixty adult patients (18–60 years) with American Society of Anesthesiologists (ASA) 1/ASA 2 grade and undergoing lower abdominal and lower limbs surgeries were included and randomized into three groups of 20 patients each. Group 1 - received ropivacaine with normal saline. Group 2 - received ropivacaine with dexmedetomidine. Group 3 - received ropivacaine with clonidine.
Statistical Analysis:
Mean and Standard deviation were calculated. All the data were analyzed using analysis of variance and Chi-square test. The value of P< 0.05 was considered significant.
Results:
All the three groups were comparable with respect to age, sex, and ASA grade. There was statistically significant mean time to reach T10 sensory block level (15.8, 5.7, 9.6 min in Groups 1, 2, and 3, respectively). The maximum duration of analgesia was statistically higher in Group 2 patients (383.7 vs. 365.3 and 280.5 min in Group 3 and Group 1, respectively). The mean time to reach motor block was significantly shorter in Group 2. Side effects were comparable in all groups with statistically insignificant fall in mean arterial pressure and hypotension was noted with Group 2.
Conclusion:
We concluded that the patients receiving the addition of dexmedetomidine to ropivacaine in epidural anesthesia had a faster onset and longer duration of sensory and motor blockade. Dexmedetomidine in comparison to clonidine had acceptable sedation and hemodynamic stability and minimal dose requirement make very effective adjuvant in epidural anesthesia with comparable side effects.
Keywords: American Society of Anesthesiologists, clonidine, dexmedetomidine, epidural anesthesia, ropivacaine
INTRODUCTION
Many techniques and drug regimens, with variable success, have been tried to produce effective analgesia during regional anesthesia.[1] Effective pain control is essential for the optimum care of patients in the intraoperative and postoperative period. Epidural anesthesia is a safe and inexpensive technique with the advantage of providing surgical anesthesia and prolonged postoperative pain relief. It has become a common practice to use multidrug approach for the treatment of intra and postoperative pain because no drug has yet been identified that specifically inhibits nociception without associated side effects.[2] Alpha-2 adrenergic agonists have both analgesic and sedative properties when used as an adjuvant in epidural anesthesia.[3] The anesthetic and the analgesic requirement get reduced to a huge extent by the use of this adjuvants.[4]
The aim of the study was to compare the effect of dexmedetomidine versus clonidine in combination with ropivacaine during epidural anesthesia on intraoperative and postoperative analgesia, to find out the better adjuvant for regional anesthesia.
MATERIALS AND METHODS
This was a randomized control trial conducted from May 2012 to December 2012. Sixty patients were included in this study with age ranging from 18 to 60 years. Patients with American Society of Anesthesiologists (ASA) 1 or ASA 2 grade and undergoing lower abdominal and lower limbs surgeries were included. Patients with a history of cardiac diseases, respiratory diseases, significant hepatorenal dysfunction, central nervous system (CNS) diseases, any contraindications to regional anesthesia (local infections, bleeding disorders), drugs that affect CNS and known allergic or hypersensitivity to any drug, uncooperative patients were excluded from this study.
They were randomized into three groups of 20 patients each in a double-blinded fashion. Group 1 - Patients received 0.75% 19 ml ropivacaine with 1 ml N.S. to make 20 ml. Group 2 - patients received 0.75% ropivacaine 19 ml with 1.5 µg/kg dexmedetomidine (1 ml) to make total volume 20 ml. Group 3 - patients received 0.75% ropivacaine 19 ml with 2 µg/kg clonidine (1 ml) to make total volume 20 ml.
The onset of analgesia and complete establishment of motor blockade was observed and recorded.
The bilateral pin-prick method was used to evaluate and check the sensory level. The visual analog scale was used for the sensory level. Modified Bromage scale (0 = no block, 1 = inability to raise extended leg, 2 = inability to flex knee and 3 = inability to flex ankle and foot) was used to measure the motor blockade effect at 5, 10, 15, 20, 25 and 30 min intervals after the epidural administration of the drugs. Grading of sedation was evaluated by a five-point scale (1 - alert and wide awake, 2 - arousable to verbal command, 3 - arousable with gentle tactile stimulation, 4 - arousable with vigorous shaking and 5 - unarousable). Sedation scores were recorded just before the initiation of surgery and thereafter every 20 min during the surgical procedure. Cardio-respiratory parameters were monitored continuously, and recordings were made every 5 min until 30 min and at 10 min interval thereafter up to 60 min and then at 15 min interval for next hour and finally at 30 min until complete recovery. During the surgical procedure, adverse events such as anxiety, nausea, vomiting, pruritus, shivering, etc., were recorded.
Statistical analysis
All the data were analyzed using “analysis of variance and Chi-square test.” Statistical Package for Social Science (SPSS) version 16.0 for Windows, Chicago III was used to compare the continuous variables between the groups. The value of P < 0.05 was considered significant.
RESULTS
All the three groups were comparable with respect to age, sex, and ASA grade. Maximum patients were from ASA Grade I in all the three groups (100% in Group 1, 95% and 90% in Groups 2 and 3, respectively) while 5% patients in Group 2 and 10% patients in Group 3 were from ASA Grade II [Table 1].
Table 1.
Patients demographics
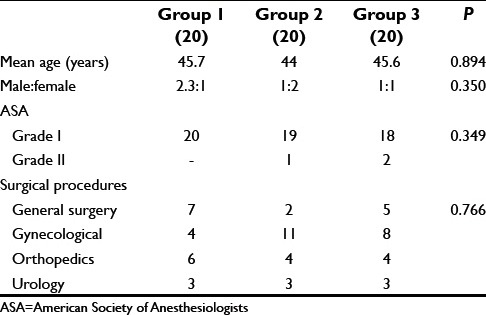
Maximum number of patients underwent gynecological surgery 38.3% (20%, 55%, 40% in Groups 1, 2, and 3 respectively), followed by general surgery 23.3% (35%, 10%, 25% in Groups 1, 2, and 3, respectively) and orthopedic 23.3% (30% in Group 1, 20% in Groups 2 and 3 each).
Table 2 shows maximum patients took 5–10 min to reach T10 sensory block level in Groups 2 and 3 compared to >10 min in Group 1 patients. The mean time to reach T10 sensory block level was 15.6 ± 4.0, 5.7 ± 2.0, 9.6 ± 2.9 min in Groups 1, 2, and 3, respectively. The difference among all three groups was statistically significant. Fastest onset of the block was observed with Group 2 (mean time 11.3 ± 1.6, 65% in 10–20 min range) followed by Group 3 (mean time 14.1 ± 2.2 min, 100% in 10–20 min range). The difference between onset times was highly significant among all three groups. The maximum duration of analgesia was observed with Group 2 (383.7 ± 68.9 min) followed by Group 3 (365.3 ± 45.6 min) and Group 1 (280.5 ± 39.1 min). The intergroup comparison showed that the differences were significant among all the three groups. All the patients (100%) in Group 1 were from sedation score 1, in Group 2, 45% patients each were from sedation score 2 and 3, while in Group 3, 55% patients were from sedation score 1, 35% patients had sedation score 2 while remaining 10% patients had sedation score 3.
Table 2.
Comparison of block characteristics
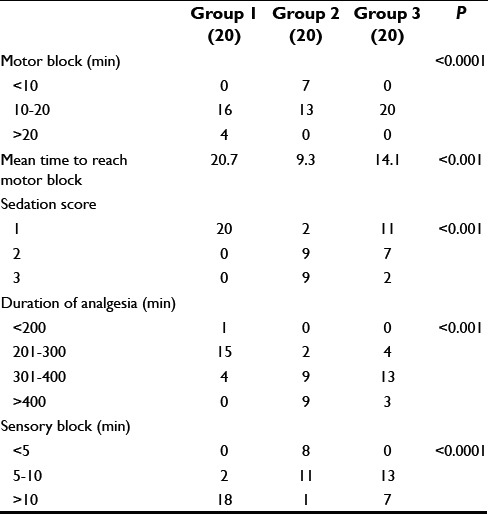
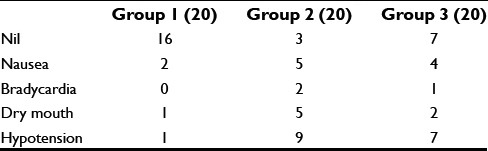
de effects such as hypotension and bradycardia were observed maximum in Group 2 (9 and 2 patients, respectively) followed by Group 3 (7 and 1 patients, respectively) and Group 1 (1 patient of hypotension only). However, these findings are statistically insignificant [Table 3].
Table 3.
Side effects among the three groups
Graphs 1 and 2 shows mean arterial pressure (MAP) and pulse rate at various intervals in all the three groups. Maximum fall in MAP was noted with Group 2, but the intergroup comparison was statistically insignificant among all three groups.
Graph 1.
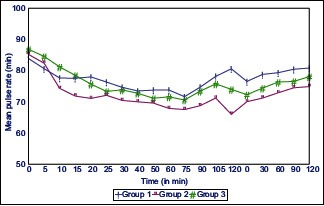
Pulse rate at different time interval in all the three groups
Graph 2.
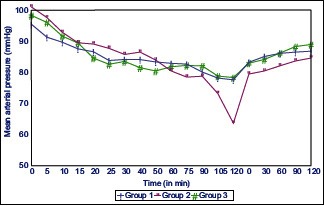
Mean arterial pressure at different time interval in all the three groups
DISCUSSION
The use of neuraxial opioids is associated with quite a few side effects, so various options including alpha-2 agonists are being extensively evaluated as an alternative with an emphasis on opioid-related side effects such as respiratory depression, nausea, urinary retention, and pruritus.[5,6,7] Alpha-2 receptor agonists have been found to have an antinociceptive action for both somatic and visceral pain.[3]
Dexmedetomidine shows high specificity for the alpha-2 receptors compared with Clonidine.[8,11] Alpha-2 adrenergic agonists have both analgesic and sedative properties when used as an adjuvant in epidural anesthesia.[3,8,9,10,11,12] The anesthetic and the analgesic requirement get reduced to a huge extent by the use of these two adjuvants because of their analgesic properties and augmentation of local anesthetic effects.[4,13,14,15,16]
We have included patients from both sex and over a wide range of age undergoing various types of surgeries. In this study, we found that all the groups were comparable in the distribution of patients regarding age, sex, and ASA grade and types of surgeries. Various other studies included an only selective group of patients. Bajwa et al. in their study, included only female patients of age group 44–65 years undergoing an only vaginal hysterectomy.[17]
Although patients in all three groups remained hemodynamically stable perioperatively but fall in pulse rate, and MAP was maximum in Group 2 patients followed by Group 3 and 1 respectively, but it was statistically insignificant. These findings correlate well with a study by Bajwa et al.[17]
Our study showed mean onset of sensory block was 15.75 ± 4.02 min for Group 1, 5.7 ± 2.0 min for Group 2 and 9.6 ± 2.9 min for Group 3. The statistical analysis showed a significant difference between all the three groups which suggest that the addition of dexmedetomidine leads to faster onset of sensory blockade in comparison to clonidine.
The onset of motor block was also hastened by the addition of dexmedetomidine and clonidine, but it was faster with dexmedetomidine than clonidine. Mean time for onset of motor block was 20.6 ± 3.8, 11.3 ± 1.6, and 14.1 ± 2.2 min for Groups 1, 2, and 3, respectively which is also statistically significant among all the three groups. In a study by Bajwa et al. showed that addition of dexmedetomidine to ropivacaine as an adjuvant resulted in statistically significant earlier onset (8.5 ± 2.4 min) of sensory analgesia at T10 as compared to the addition of clonidine (9.7 ± 3.4 min) and also early complete motor block.[17]
However, in contrast, Salgado et al.[18] observed no changes in the onset of sensory and motor blockade when used epidural dexmedetomidine with ropivacaine and ropivacaine alone.
The mean duration of analgesia was maximum with Group 2 (383.7 ± 68.9 min) followed by Group 3 (365.3 ± 45.6 min) and Group 1 (280.5 ± 39.1 min). Hence, both dexmedetomidine and clonidine caused a significant prolongation of the duration of analgesia when compared to ropivacaine alone but it was more with dexmedetomidine in comparison to clonidine.
Others studies by Bajwa et al. and Salgado et al. also showed similar results.[17,18]
Anand et al.[19] concluded that addition of dexmedetomidine to caudal ropivacaine results in improved perioperative anesthesia, significant postoperative pain relief, prolonged duration of arousable sedation, and less incidence of emergence agitation without increasing side effects. El-Hennawy et al.[20] in their study, concluded that caudal dexmedetomidine and clonidine with 0.25% bupivacaine significantly promoted analgesia with no significant advantage of dexmedetomidine over clonidine.
Neogi et al.[21] also found that addition of both clonidine and dexmedetomidine with ropivacaine administered caudally significantly increase the duration of analgesia.
Our current study correlates well with all the above authors, with a significant increase in duration of analgesia with the addition of dexmedetomidine and clonidine.
The results of our study clearly indicate the effectiveness of epidural dexmedetomidine as it produced profound sedation in 45% of the patients who were arousable by gentle tactile stimulation compared to just 10% of the patients in clonidine group. The sedation scores were highly statistically significant with the administration of dexmedetomidine.
The important side effects reported about the use of alpha-2 adrenoreceptor agonists are bradycardia and hypotension, nausea, pruritus, and dry mouth. In our current study, we observed the maximum incidence of bradycardia and hypotension was observed with Group 2 (2 and 9 patients respectively), followed by Group 3 (1 and 7 for bradycardia and hypotension, respectively) and Group 1 (1 case of hypotension). Not any patient was observed having respiratory depression in any group in our study. Although the incidence of side effects is higher in Group 2 patients compared to other groups but its not statistically significant.
Bajwa et al.[17] and El-Hennawy et al.[20] in their study also showed the comparable incidence of side effects in both dexmedetomidine and clonidine group and did not observe the respiratory depression in any patient from either group.
However, Salgado et al. in their study showed there was the insignificant difference in the incidence of hypotension and bradycardia (P > 0.05) between ropivacaine group and ropivacaine with dexmedetomidine group.[18]
CONCLUSION
This study concluded that the patients receiving the addition of dexmedetomidine to ropivacaine during epidural anesthesia had a faster onset and longer duration of sensory and motor blockade with acceptable sedation and hemodynamic stability in comparison to clonidine and make dexmedetomidine very effective adjuvant in epidural anesthesia with comparable side effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Höhener D, Blumenthal S, Borgeat A. Sedation and regional anaesthesia in the adult patient. Br J Anaesth. 2008;100:8–16. doi: 10.1093/bja/aem342. [DOI] [PubMed] [Google Scholar]
- 2.Sirvinskas E, Laurinaitis R. Use of magnesium sulfate in anesthesiology. Medicina (Kaunas) 2002;38:695–8. [PubMed] [Google Scholar]
- 3.Kamibayashi T, Maze M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology. 2000;93:1345–9. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 4.Fukushima K, Nishimi Y, Mori K. The effect of epidural administered dexmeditomidine on central and peripheral nervous system in man. Anesth Analg. 1997;84:S292. [Google Scholar]
- 5.Filos KS, Goudas LC, Patroni O, Polyzou V. Hemodynamic and analgesic profile after intrathecal clonidine in humans. A dose-response study. Anesthesiology. 1994;81:591–601. doi: 10.1097/00000542-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Chiari A, Lorber C, Eisenach JC, Wildling E, Krenn C, Zavrsky A, et al. Analgesic and hemodynamic effects of intrathecal clonidine as the sole analgesic agent during first stage of labor: a dose-response study. Anesthesiology. 1999;91:388–96. doi: 10.1097/00000542-199908000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98:153–8. doi: 10.1213/01.ANE.0000093225.39866.75. [DOI] [PubMed] [Google Scholar]
- 8.Scafati A. Analgesia and alpha agonists 2. Medens Rev. 2004:4–7. [Google Scholar]
- 9.Mauro VA, Brandão ST. Clonidine and dexmedetomidine through epidural route for post-operative analgesia and sedation in a colecistectomy. Rev Bras Anestesiol. 2004;4:1–10. [Google Scholar]
- 10.Gabriel JS, Gordin V. Alpha 2 agonists in regional anesthesia and analgesia. Curr Opin Anaesthesiol. 2001;14:751–3. doi: 10.1097/00001503-200112000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Hall JE, Uhrich TD, Ebert TJ. Sedative, analgesic and cognitive effects of clonidine infusions in humans. Br J Anaesth. 2001;86:5–11. doi: 10.1093/bja/86.1.5. [DOI] [PubMed] [Google Scholar]
- 12.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 13.Milligan KR, Convery PN, Weir P, Quinn P, Connolly D. The efficacy and safety of epidural infusions of levobupivacaine with and without clonidine for postoperative pain relief in patients undergoing total hip replacement. Anesth Analg. 2000;91:393–7. doi: 10.1097/00000539-200008000-00030. [DOI] [PubMed] [Google Scholar]
- 14.Klimscha W, Chiari A, Krafft P, Plattner O, Taslimi R, Mayer N, et al. Hemodynamic and analgesic effects of clonidine added repetitively to continuous epidural and spinal blocks. Anesth Analg. 1995;80:322–7. doi: 10.1097/00000539-199502000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Scheinin M, Pihlavisto M. Molecular pharmacology of alpha2- adrenoceptor agonists. Baillières Clin Anaesth. 2000;14:247–60. [Google Scholar]
- 16.Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76:948–52. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth. 2011;55:116–21. doi: 10.4103/0019-5049.79883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salgado PF, Sabbag AT, Silva PC, Brienze SL, Dalto HP, Módolo NS, et al. Synergistic effect between dexmedetomidine and 0.75% ropivacaine in epidural anesthesia. Rev Assoc Med Bras. 2008;54:110–5. doi: 10.1590/s0104-42302008000200011. [DOI] [PubMed] [Google Scholar]
- 19.Anand VG, Kannan M, Thavamani A, Bridgit MJ. Effects of dexmedetomidine added to caudal ropivacaine in paediatric lower abdominal surgeries. Indian J Anaesth. 2011;55:340–6. doi: 10.4103/0019-5049.84835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103:268–74. doi: 10.1093/bja/aep159. [DOI] [PubMed] [Google Scholar]
- 21.Neogi M, Bhattacharjee DP, Dawn S, Chatterjee N. A comparative study between clonidine and dexmedetomidine used as adjuncts to ropivacaine for caudal analgesia in paediatric patients. J Anaesth Pharmacol. 2010;26:149–53. [Google Scholar]


