Abstract
SCO2 mutations cause recessively inherited cytochrome c oxidase deficiency. Recently Tran-Viet et al. proposed that heterozygosity for pathogenic SCO2 variants, including the common E140K variant, causes high-grade myopia. To investigate the association of SCO2 mutations with myopia, ophthalmic examinations were performed on 35 E140K carriers, one homozygous infant, and on a mouse model of Sco2 deficiency. Additionally, a screen for other putative effects of SCO2 heterozygosity was carried out by comparing the prevalence of the common E140K variant in a population of patients with undiagnosed diseases compatible with SCO2-related pathogenesis to that in a general population sample. High-grade myopia was not identified in any of the studied individuals. Of the carriers, 17 were emmetropic, and 18 possessed refractive errors. Additionally, no significant axial elongation indicative of high-grade myopia was found in mice carrying E129K (corresponding to E140K in humans) knock-in mutations. The prevalence of E140K carriers in the symptomatic cohort was evaluated as 1:103 (CI: 0.44–2.09) and did not differ significantly from the population prevalence (1:147, CI: 0.45–1.04).
Our study demonstrates that heterozygosity for pathogenic SCO2 variants is not associated with high-grade myopia in either human patients or in mice.
Keywords: E140K carrier, Myopia, SCO2 gene variant, SMA negative
Introduction
Pathogenic mutations in SCO2 (RefSeq: NM_005138.2; NP_005129.2) are a frequent cause of cytochrome c oxidase deficiency, with symptoms including neonatal encephalocardiomyopathy and mortality by three months of life (OMIM 604377). The typical form of the disease is associated with compound heterozygosity, with at least one allele expressing the common c.418G>A (p.E140K) variant (Pronicka et al. 2013).
When homozygous, E140K presents with delayed onset and death by the age of ~9 months. Hypotonia generally precedes onset, and up to 70% of cases are screened for SMN1/NAIP deletion prior to detection of Sco2 mutations, causing delay in diagnosis (Pronicki et al. 2010).
Recently, Tran-Viet et al. (2013) proposed that heterozygous SCO2 mutations are not phenotypically neutral but cause high-grade myopia. They identified four mutations in SCO2 from unrelated high-myopic individuals. Additionally, using a mouse model of lens-induced myopia, they provided evidence for SCO2 expression in the retina, which is downregulated in myopic mouse eyes.
The aim of our study was to verify the findings of Tran-Viet et al., by investigating Sco2 mutations in both patients and a mouse model. We compared the incidence of ophthalmologic phenotypes in a group of families with the E140K variant and undiagnosed disorders compatible with SCO2 deficiency, to the incidence in the general population. Additionally, we investigated the putative ophthalmologic phenotype in mice carrying a heterozygous Sco2 E129K mutation (the homolog of the human E140K mutation) (KI) and/or a knockout allele (KO) (Yang et al. 2010). Previous characterization of this mouse model of Sco2 deficiency revealed that heterozygous KI/WT, homozygous KI/KI, and compound heterozygous KI/KO mice were viable but had muscle weakness and respiratory chain deficiencies in multiple tissues (most prominently in the KI/KO mice) (Brosel et al. 2010; Yang et al. 2010); however, no ophthalmologic analysis had been performed. High-grade myopia is associated with globe axial elongation sufficient to cause a refractive distortion of at least −6.0 diopters, and the level of myopia in mouse models has previously been assessed by measuring globe axial length (Park et al. 2012).
Materials and Methods
Ophthalmic Examination of Patients
Using our database of 34 SCO2-deficient families (Pronicka et al. 2013), the confirmed/obligate carriers were polled for their results of recent ophthalmic examinations. Thirty-five subjects responded, representing six different pathogenic SCO2 variants. Individual family members (Family 16, Table 1) and one homozygous patient were reexamined in the Children’s Memorial Health Institute (CMHI). The refraction was expressed as spherical equivalent (SE) calculated as sphere plus half of the cylinder. The total refractions in children were measured after cycloplegia.
Table 1.
Refraction in 36 individuals studied
| Individual/family number | Age (years) | Mutation | Right eye refraction | Left eye refraction |
|---|---|---|---|---|
| Confirmed or obligatory carriers | ||||
| f/3 | 43 | [E140K] | E | E |
| m/3 | 42 | [E140K] | +0.75 | +0.5 |
| m/5 | 44 | [E140K] | E | E |
| f/5 | 43 | [E140K] | −1.0 | E |
| f/9 | 36 | [E140K] | E | E |
| m/9 | 35 | E140K | +1.25 | +0.75 |
| m/12 | 26 | [E140K] | E | E |
| f/12 | 28 | [E140K] | E | E |
| m/13 | 41 | E140K | −0.5 | −0.75 |
| f/13 | 42 | E140K | −1.0 | −0.75 |
| f/14 | 34 | E140K | −1.25 | −0.75 |
| m/14 | 30 | E140K | E | E |
| f/16 | 35 | E140K | +0.25 | +0.25 |
| m/16 | 34 | E140K | +0.75 | +0.25 |
| b1/16 | 14 | E140K | +1.0 | +0.75 |
| m/17 | 27 | E140K | −0.25 | −0.25 |
| f/17 | 29 | E140K | E | E |
| f/18 | 54 | E140K | −0.75 | −0.5 |
| m/18 | 53 | E140K | +1.0 | +1.0 |
| f/19 | 50 | [E140K] | E | E |
| m/19 | 48 | E140K | −0.5 | E |
| f/23 | 46 | Q53* | −2.0 | −3.25 |
| gm/23 | 70 | Q53* | +2.5 | +2.5 |
| m/23 | 45 | E140K | −0.5 | −0.25 |
| m/24 | 30 | M177T | E | E |
| f/24 | 30 | E140K | E | E |
| m/27 | 40 | E140K | E | E |
| f/27 | 46 | W75R | −4.5 | −4.5 |
| s/27 | 5 | E140K | E | E |
| m/31 | 33 | E140K | E | E |
| m/32 | 33 | E140K | E | E |
| f/32 | 34 | E140K | E | E |
| m/33 | 28 | E140K | E | E |
| f/33 | 31 | Y241* | −0.5 | −0.1 |
| p/34 | 47 | E140K | E | E |
| Homozygous patient | 1.3 | E140K/E140K | +1.75 | +2.0 |
| Carriership excluded | ||||
| b3/16 | 6 | E140K negative | +2.25 | +2.75 |
| b2/16 | 11.5 | E140K negative | +0.0 | +0.25 |
f father, m mother, b brother, s sister, gm grandmother, p patient with SCO2 unrelated disease, E emmetropic, [E140K] obligatory heterozygote
Screening of the Symptomatic and Population Cohorts
DNA samples from 626 patients with diseases of unknown cause (symptomatic cohort) were next screened for the E140K variant allele, in order to identify alternative symptomatic conditions associated with heterozygosity of this Sco2 mutation. The material consisted of (1) 468 blood samples negative for the SMN1/NAIP homozygous deletion, selected from 932 cases referred to the Institute of Psychiatry and Neurology (Warsaw, Poland) for early SMA screening in the 1998–2010 time period, and (2) 158 dry blood spots of the patients with increased lactate excretion who died undiagnosed. These samples were sent to the CMHI for selective metabolic screening in the 2000–2013 time period. Probing for the E140K variant was part of an ongoing diagnostic study of suspected mitochondrial disorders, which also included the search for mtDNA deletions and common pathogenic SNV in mtDNA, SURF1, SCO2, and POLG. The material for analysis in the general population consisted of 3,080 dried blood spots collected for newborn screening for phenylketonuria in the 2002–2006 time period. Written informed consent was obtained from the parents or guardians of investigated children, and the study was approved by the Bioethical Commission of CMHI.
Genotyping for the E140K variant was performed by allele-specific TaqMan assay (Life Technologies). In all cases, a positive result was confirmed by Sanger sequencing with BigDye terminators (Applied Biosystems). Confidence intervals (CI) were calculated as 95% CI according to Wilson (1927). Whole exome sequencing (WES) was performed using HiSeq 1500 (Illumina) at the Warsaw Medical University as described previously (Płoski et al. 2014).
Generation and Animal Care of Sco2-Deficient Mice
Two mouse models of Sco2 deficiency generated as described previously (Yang et al. 2010), an E129K mutation in Sco2 (KI) and a knockout allele (KO), were used in this study. Experiments were performed by mating KI/KI and KI/+ mice with KO/+ heterozygotes and then comparing KI/+, KI/KI, and KI/KO mice with wild-type littermate control mice. All mouse experiments were performed according to a protocol approved by the Columbia University Medical Center Institutional Animal Care and Use Committee, which is consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were housed and bred according to international standard conditions, with a 12-h light, 12-h dark cycle.
Mouse Globe Axial Length Measurement by Ultrasound Biomicroscopy
Adult mice (approximately 14 weeks of age) were anesthetized with intraperitoneal ketamine (100 mg/kg) and xylazine (8 mg/kg). The axial lengths were followed with an adapted immersion B-scan ultrasound (Quantel Aviso, Bozeman, MT, USA) employing a 50 MHz ultrasound biomicroscopy (UBM) probe attached to a water-filled ClearScan bubble-tip (ESI, Inc., Plymouth, MN, USA) (Pavlin et al. 1991; Pavlin and Foster 1995; Coleman et al. 2006). After placing a drop of GenTeal (hypromellose, 0.3%, Alcon) on the open eye, the ClearScan was placed in contact with the cornea and the range between probe and eye adjusted to place the eye in the focal region. Two cineloop videos were acquired imaging the entire length of the eye in UBM mode, and two individual frames that contained proper reflections from the cornea, lens, iris, and posterior wall were selected from each cineloop for biometric analysis. Axial length was measured along the visual axis from the anterior surface of the cornea to the posterior wall, and the average from the four measurements was recorded.
Results
Ophthalmic Examination
None of the SCO2 carriers examined demonstrated high-grade myopia as defined by Tran-Viet (>−6 diopters). Seventeen carriers were emmetropic. In the homozygous infant, mild physiologic hyperopia (+1.75 diopters in the right eye and +2.0 diopters in the left eye) was identified. Refractive errors occurred in 18 carriers (33 eyes): hyperopia up to +1.0 diopter in 13 eyes and myopia in 20 eyes. Of these 20 myopic eyes, 17 were categorized as mild myopia (16 eyes up to −1.25 diopters) and 3 demonstrated moderate myopia. The highest myopia level of 4.5 diopters occurred in an individual with a c.223T>C (p.W75R) variant, rather than the E140K variant. In a family carrying a c.157C>T (p.Q53*) variant, both moderate myopia (−3.5 diopters) and hyperopia (+2.0 diopters) were found (Family 23, Table 1). An individual bearing a different severe variant, c.723C>G (p.Y241*), had no refractive error. We did not find significant refractive differences among three E140K carriers (6 eyes, mean 0.54; p = 0.35, t-test) vs. two healthy individuals in the same family (4 eyes, mean 1.31; Family 16, Table 1).
Screening of the Symptomatic and Population Cohorts
Screening of a symptomatic patient cohort with previously unidentified disease yielded eleven subjects possessing the E140K allele (1:59). Sequencing of the SCO2 gene in all of these subjects showed five homozygotes (Pronicka et al. 2013), whereas in the six remaining cases no pathogenic variant was found on the other allele. Thus, the apparent prevalence of random carriers was 6:621 (1:103, 0.96%, CI: 0.44–2.09). Thorough investigations led to the identification of spinal muscular atrophy with respiratory distress (SMARD1) (Jędrzejowska et al. 2014) in one case and to the identification of candidate genes responsible for neurological symptoms in two other patients (data not shown). Twenty-one E140K carriers were found in the population cohort (1:147), indicating the carrier frequency of 0.68% (CI: 0.45–1.04%).
Mouse Globe Axial Length Measurement by Ultrasound Biomicroscopy
High-grade myopia, corresponding to a refractive error of at least −6.0 diopters, correlates with an axial length (AL) elongation of over 30–40 micrometers in mouse eyes (Schmucker and Schaeffel 2004); likewise the average distortion of −22 diopters described by Tran-Viet et al. would correspond to a mouse globe AL elongation over control of approximately 120 μm. We measured mouse globe axial length in adult Sco2-deficient mice (KI/+, KI/KI and KI/KO) and wild-type littermate controls, using ultrasound biomicroscopy (Coleman et al. 2006; Pavlin et al. 1991; Pavlin and Foster 1995). For each genotype, the left and right eyes of six mice (three males and three females) were analyzed, with no significant differences found between right vs. left eye or male vs. female. No significant axial length elongation was found in mice carrying Sco2 mutations (Fig. 1), with average lengths of 3,020 ± 70 μm for +/+ control mice, 2,950 ± 40 μm for KI/+ mice, 2,950 ± 40 μm for KI/KI mice, and 2,950 ± 60 μm for KI/KO mice. Surprisingly, all Sco2-variant mice exhibited slightly shorter average axial length, despite no significant difference in animal size/weight (data not shown), although this difference was not statistically significant.
Fig. 1.
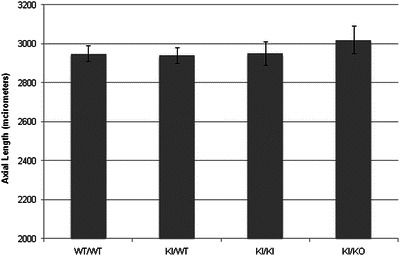
Ultrasound biomicroscopy measurement of Sco2-deficient mouse eye axial lengths. Axial length is given in microns. Each bar with indicated genotype represents the average of the left and right eyes (3–6 independent axial length measurements per eye) for 6 adult mice (3 males, 3 females) of approximately 14 weeks’ age. Bars represent standard deviation
Discussion
Our results do not support the hypothesis that heterozygosity for deleterious SCO2 variants is pathogenic, as none of 35 subjects examined in this study displayed high-grade myopia and mice carrying heterozygous, compound heterozygous, or homozygous pathogenic Sco2 mutations revealed no axial elongation indicative of high-grade myopia. Furthermore, analysis of undiagnosed patients with symptoms related to SCO2 pathology showed a carriage rate with CI largely overlapping the carriage rate for general population (0.96%, CI: 0.44–2.09 vs. 0.68%, CI: 0.45–1.04%, respectively). Noteworthy, this is a first study documenting the presence of E140K mutation in the large cohort of 468 symptomatic infants with a histological pattern resembling SMA and negative for the SMN1/NAIP deletion.
Similar E140K carrier frequency (1:140) was reported by Tran-Viet et al. in high-myopic individuals (Tran-Viet et al. 2013). The nature of visual impairment in the studied patients did not differ from that diagnosed in the general population (Nowak et al. 2008).
The discrepancy between our results and those reported previously (Tran-Viet et al. 2013) may be caused by very low penetrance of heterozygous pathogenic SCO2 variants. However, an unrecognized population heterogeneity seems more probable. The E140K variant is quite rare worldwide, as it was not reported in the 1000 Genomes Database and had a prevalence of only 3/8597 (0.035%) among European alleles in the NHLBI Exome Sequencing Project (ESP). The high prevalence of E140K carriers in Poland is in agreement with a frequent occurrence of SCO2 deficiency in Slavic regions and with an uneven distribution of homozygous patients who, in the majority, were born in Central-Eastern Europe (Böhm et al. 2006; Papadopoulou et al. 1999; Pronicka et al. 2013). Since >20 million Poles live abroad, the high prevalence of E140K indicates that patients and controls used in SCO2 population studies should be matched carefully, not only for broad ethnicity but also specifically for Polish (Central European) admixture.
Acknowledgements
We are grateful to parents of affected infants participating in the survey.
The study was supported by grants from the National Science Centre 1154/B/P01/2011/40, 2012/05/B/NZ2/01627 and the CMHI projects S211/10, S136/13, and S126/12; grants from the US National Institutes of Health (EY013435, EY019007, EY023595, HD032062, and HD080642) and the US Department of Defense (W911NF-12-1-9159 and W911F-15-1-0169); and an unrestricted grant to the Department of Ophthalmology of Columbia University from Research to Prevent Blindness, the Muscular Dystrophy Association, and the J. Willard and Alice S. Marriott Foundation. This publication was also supported by Career Development Awards from Research to Prevent Blindness, the Louis V. Gerstner Jr. Scholars Program, and the AR and JR Peacock Trust.
Take-Home Message
The heterozygosity for pathogenic SCO2 variants is not associated with high-grade myopia in either human patients or in mice.
Compliance with Ethics Guidelines
Conflict of Interest Statement
The authors of this manuscript declare that there are no conflicts of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Animal Rights
All institutional and national guidelines for the care and use of laboratory animals were followed.
Details of the Contributions of Individual Authors
DPA devised study protocol, analyzed the data, conceived the purpose of the manuscript, and wrote the article.
BKK performed ophthalmological assessments analysis and assisted in writing the manuscript.
SŁ and MM performed population studies and analyzed the data.
OS, KC, and PK performed population studies.
KIP, JP, JZ, JZ, MKW, and EC collected previous data and assisted in reviewing and editing the manuscript.
RP conducted whole genome sequencing experiments and assisted in writing the manuscript.
EAS designed experiments, analyzed the data, and assisted in writing the paper.
SS designed experiments, assisted with experiments, and analyzed the data.
HY designed experiments and assisted with experiments.
QW assisted with experiments and analyzed the data.
QH designed experiments, assisted with experiments, analyzed the data, and assisted in writing the manuscript.
RS assisted with experiments.
EP conceived the purpose of the manuscript, analyzed the data, and involved in writing and editing the manuscript.
Footnotes
Competing interests: None declared
Contributor Information
Ewa Pronicka, Email: e.pronicka@czd.pl.
Collaborators: Matthias Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Böhm M, Pronicka E, Karczmarewicz E, et al. Retrospective, multicentric study of 180 children with cytochrome c oxidase deficiency. Pediatr Res. 2006;59:21–26. doi: 10.1203/01.pdr.0000190572.68191.13. [DOI] [PubMed] [Google Scholar]
- Brosel S, Yang H, Tanji K, et al. Unexpected vascular enrichment of SCO1 over SCO2 in mammalian tissues: implications for human mitochondrial disease. Am J Pathol. 2010;177:2541–2548. doi: 10.2353/ajpath.2010.100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DJ, Silverman RH, Lizzi FL, et al. Ultrasonography of the eye and orbit. 2. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- Jędrzejowska M, Madej-Pilarczyk A, Fidziańska A, et al. Severe phenotypes of SMARD1 associated with novel mutations of the IGHMBP2 gene and nuclear degeneration of muscle and Schwann cells. Eur J Paediatr Neurol. 2014;18:183–192. doi: 10.1016/j.ejpn.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Nowak MS, Goś R, Smigielski J. Character of refractive errors in population study performed by the Area Military Medical Commission in Lodz. Klin Oczna. 2008;110:55–59. [PubMed] [Google Scholar]
- Papadopoulou LC, Sue CM, Davidson MM, et al. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat Genet. 1999;23:333–337. doi: 10.1038/15513. [DOI] [PubMed] [Google Scholar]
- Park H, Qazi Y, Tan C, et al. Assessment of axial length measurements in mouse eyes. Optom Vis Sci. 2012;89:296–303. doi: 10.1097/OPX.0b013e31824529e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlin CJ, Foster FS. Ultrasound biomicroscopy of the eye. New York: Springer-Verlag; 1995. [Google Scholar]
- Pavlin CJ, Harasiewicz K, Foster FS. Clinical application of ultrasound biomicroscopy. Ophthalmology. 1991;98:287–295. doi: 10.1016/S0161-6420(91)32298-X. [DOI] [PubMed] [Google Scholar]
- Płoski R, Pollak A, Müller S, et al. Does p.Q247X in TRIM63 cause human hypertrophic cardiomyopathy? Circ Res. 2014;114:e2–e5. doi: 10.1161/CIRCRESAHA.114.302662. [DOI] [PubMed] [Google Scholar]
- Pronicka E, Piekutowska-Abramczuk D, Szymańska-Dębinska T, et al. The natural history of SCO2 deficiency in 36 Polish children confirmed the genotype-phenotype correlation. Mitochondrion. 2013;13:810–816. doi: 10.1016/j.mito.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Pronicki M, Kowalski P, Piekutowska-Abramczuk D, et al. A homozygous mutation in the SCO2 gene causes a spinal muscular atrophy like presentation with stridor and respiratory insufficiency. Eur J Paediatr Neurol. 2010;14:253–260. doi: 10.1016/j.ejpn.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Schmucker C, Schaeffel F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vis Res. 2004;44:1857–1867. doi: 10.1016/j.visres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Tran-Viet KN, Powell C, Barathi VA, et al. Mutations in SCO2 are associated with autosomal-dominant high-grade myopia. Am J Hum Genet. 2013;92:820–825. doi: 10.1016/j.ajhg.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB. Probable interference, the law of succession, and statistical interference. J Am Stat Assoc. 1927;22:209–212. doi: 10.1080/01621459.1927.10502953. [DOI] [Google Scholar]
- Yang H, Brosel S, Acin-Perez R, et al. Analysis of mouse models of cytochrome c oxidase deficiency owing to mutations in Sco2. Hum Mol Genet. 2010;19:170–180. doi: 10.1093/hmg/ddp477. [DOI] [PMC free article] [PubMed] [Google Scholar]


