Abstract
Amphetamine (AMPH) exposure leads to changes in behavior and dopamine receptor function in the prefrontal cortex (PFC). Since dopamine plays an important role in regulating GABAergic transmission in the PFC, we investigated if AMPH exposure induces long-lasting changes in dopamine’s ability to modulate inhibitory transmission in the PFC as well as whether the effects of AMPH differed depending on the age of exposure. Male Sprague–Dawley rats were given saline or 3 mg/kg AMPH (i.p.) repeatedly during adolescence or adulthood and following a withdrawal period of up to 5 weeks (Experiment 1) or up to 14 weeks (Experiment 2), they were sacrificed for in vitro whole-cell recordings in layer V/VI of the medial PFC. We found that in brain slices from either adolescent- or adult-exposed rats, there was an attenuation of dopamine-induced increases in inhibitory synaptic currents in pyramidal cells. These effects did not depend on age of exposure, were mediated at least partially by a reduced sensitivity of D1 receptors in AMPH-treated rats, and were associated with an enhanced behavioral response to the drug in a separate group of rats given an AMPH challenge following the longest withdrawal period. Together, these data reveal a prolonged effect of AMPH exposure on medial PFC function that persisted for up to 14 weeks in adolescent-exposed animals. These long-lasting neurophysiological changes may be a contributing mechanism to the behavioral consequences that have been observed in those with a history of amphetamine abuse.
Keywords: adolescent, plasticity, amphetamine, sIPSC, D1 receptors, whole-cell recordings
INTRODUCTION
Repeated exposure to amphetamine (AMPH) and other psychostimulant drugs is associated with cognitive abnormalities that are contributing factors for the development of drug abuse and dependence (McKetin and Mattick, 1997; Ornstein et al., 2000; Gould, 2010). The precise mechanisms for drug-induced changes in cognition are unknown, but evidence suggests that alterations in dopaminergic system function may play an important role. For example, abstinent methamphetamine abusers have deficits in learning, memory and impulsivity that are associated with decreases in the density of dopamine transporters and dopamine D2 receptors (Volkow et al., 2001a,b; Lee et al., 2009). In animal models, AMPH-induced deficits in an attentional task that requires normal prefrontal cortex (PFC) function are reversed following intra-PFC infusion of a dopamine D1 agonist (Fletcher et al., 2005).
Adolescent users may be especially sensitive to the consequences of repeated drug exposure, especially since the mesolimbic dopamine system undergoes extensive changes throughout adolescence and young adulthood (Wahlstrom et al., 2010; McCutcheon et al., 2012; Gulley and Juraska, 2013). For example, rodent studies of adolescent brain development have shown that there are progressive increases in dopamine fiber density (Kalsbeek et al., 1988; Benes et al., 2000) and changes in dopamine receptor expression (Tarazi and Baldessarini, 2000). In the medial prefrontal cortex (mPFC), changes in D1 receptor expression and function contribute to age-dependent differences in inhibitory tone in this brain region. For example, in early adolescence there is a rapid increase in D1 receptor expression that is followed by a marked reduction, or “pruning”, of receptors that continues into young adulthood (Andersen et al., 2000; Brenhouse et al., 2008). This developmental change is associated with an increase in the excitability of mPFC interneurons following stimulation of D1 or D2 receptors (Tseng and O’Donnell, 2007).
We recently found that AMPH-induced deficits in working memory were greater in rats exposed to AMPH in adolescence compared to those exposed in adulthood (Sherrill et al., 2013). Performance in the task we utilized has been shown to be sensitive to mPFC lesion (Sloan et al., 2006) and disruption of dopamine function (Didriksen, 1995), which suggests that adolescent AMPH exposure may induce greater, or at least specific, changes in PFC dopamine functions that are involved in working memory. One such function of dopamine is the regulation of inhibitory transmission in the deep layer PFC (Seamans and Yang, 2004). Dopamine increases the excitability of GABAergic interneurons (Gonzàlez-Burgos et al., 2005), as well as the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) in PFC pyramidal neurons (Seamans et al., 2001; Paul and Cox, 2013). This heightened inhibitory tone, which is determined by the balance of D1- and D2-like receptor activation (Kroener and Lavin, 2010), is thought to be critical for maintaining a high signal-to-noise ratio while processing mnemonic information (Cohen et al., 2002; Seamans and Yang, 2004). Thus, if repeated AMPH exposure alters prefrontal dopamine receptor function, we hypothesize that dopamine-mediated modulation of inhibitory transmission would also be altered by repeated AMPH exposure. Moreover, to the extent that the effects of AMPH are dependent on age of exposure, this effect might be enhanced in adolescent-compared to adult-exposed animals.
As an initial test of these hypotheses, we used the in vitro slice preparation to investigate if mPFC pyramidal neurons from rats exposed to AMPH during adolescence or adulthood are differentially sensitive to dopamine receptor activation. Non-selective activation was assessed following exposure of slices to dopamine, whereas selective activation of D1 receptors was assessed with the SKF38393. In Experiment 1, recordings were obtained from rats in the adolescent- and adult-exposure groups following a 3–5-week withdrawal period. In Experiment 2, we determined if the effects of adolescent exposure would persist into adulthood by repeating the first set of experiments when rats were all approximately 4.5 months old. Thus, the withdrawal period from the last AMPH injection varied from 3–5 weeks and 11–14 weeks for adult- and adolescent-exposed rats, respectively. Because sensitization to AMPH is known to rely on dopamine in the PFC (Bjijou et al., 2002) and is associated with impaired performance on PFC-sensitive tasks (Fletcher et al., 2005; Hankosky et al., 2013; Sherrill et al., 2013) in a D1-reversible manner (Fletcher et al., 2005; Selemon et al., 2010), we tested a separate group of rats treated using this same injection protocol for evidence of behavioral sensitization following an AMPH challenge.
EXPERIMENTAL PROCEDURES
Subjects
A total of 63 male Sprague–Dawley rats, which were offspring of males and females obtained from Harlan (Indianapolis, IN, USA) and subsequently bred in our facility, were used in these experiments. Animals were weaned on P22 and housed 2–3 per cage with ad libitum access to food and water. They were kept on a 12-h light/dark cycle (lights on at 0800) with experiments performed between 0830 h and 1830 h. Experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois, Urbana-Champaign, and were consistent with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
Pre-treatment
Rats were assigned to one of three treatment groups – control, adolescent-exposed, or adult-exposed – such that all groups were represented in each litter. Injections (i.p.) were given every other day during adolescence and/or adulthood as described in Table 1. Those assigned to the control group were given 0.9% saline (1 ml/kg), whereas those in the treatment groups were given 3 mg/kg AMPH (d-amphetamine hemisulfate salt; Sigma–Aldrich, St. Louis, MO, USA). For the adult-exposed rats in Experiment 1 and both adolescent- and adult-exposed rats in Experiment 2, potential effects of injection experience were controlled by administering injections at both adolescent and adult time periods (Table 1). For all injections, animals were transported to a testing room, given their injection, and placed individually in a clear plastic tub (46 × 25 × 22 cm) lined with hardwood bedding. After 60 min, rats were returned to their home cages in the colony room.
Table 1.
Summary of groups injected (i.p.) with either saline or 3 mg/kg AMPH a total of 10 times (once every other day) during the ages noted. For Experiment 1, rats were sacrificed for slice electrophysiology 3–5 weeks following their last injection. In Experiment 2, rats were sacrificed at about the same age in adulthood such that withdrawal periods were 3–5 weeks and 11–14 weeks for adult and adolescent groups, respectively
| Adolescent | Adult | |||
|---|---|---|---|---|
| P27–P45 | P85–P103 | P27–P45 | P85–P103 | |
| Exp. 1 | ||||
| Control | Saline × 10 | – | Saline × 10 | Saline × 10 |
| Treated | AMPH × 10 | – | Saline × 10 | AMPH × 10 |
| Exp. 2 | ||||
| Control | Saline × 10 | Saline × 10 | Saline × 10 | Saline × 10 |
| Treated | AMPH × 10 | Saline × 10 | Saline × 10 | AMPH × 10 |
Electrophysiology
Rats used in Experiment 1 (n = 24) were sacrificed between P66 and P77 (mean = P70) for those in the adolescent-exposed groups or between P125 and P136 (mean = P130) for those in the adult-exposed groups. This kept the withdrawal period between groups at 3–5 weeks. Rats used in Experiment 2 (n = 19) were sacrificed between P127 and P143 (mean = P133), which corresponded to a withdrawal period in adult-exposed groups of 3–5 weeks and a withdrawal period of 11–14 weeks in adolescent-exposed rats. Animals were deeply anesthetized with pentobarbital sodium (50 mg/kg, i.p.), perfused with cold, oxygenated slicing medium containing (in mM): 2.5 KCl, 10.0 MgCl2, 0.5 CaCl2, 1.25 NaH2PO4, 26.0 NaHCO3, 11.0 glucose, and 234.0 sucrose, and then decapitated. Their brain was quickly removed, sliced into 350- µm-thick coronal sections using a vibrating tissue slicer, and these sections were transferred to a holding chamber where they were incubated for at least 1 h before recording. Individual slices were subsequently transferred to a submersion-type recording chamber on a modified microscope stage (Axioskop 2FS; Zeiss Instruments, Thornwood, NY, USA) and continuously superfused with oxygenated physiological saline at 32 °C. This solution contained (in mM): 126.0 NaCl, 2.5 KCl, 1.25 MgCl2, 2.0 CaCl2, 1.25 NaH2PO4, 26.0 NaHCO3, and 10.0 glucose. It was gassed with 95% O2/5% CO2 to a final pH of 7.4. A 5× objective was used to identify layer V/VI of mPFC and a 63× water-immersion objective equipped with differential interference contrast optics was used to visualize individual neurons.
Using the whole-cell configuration, intracellularly recorded currents were amplified by a Multiclamp 700 amplifier (Molecular Devices, Foster City, CA, USA) with voltage-clamp protocols generated using pClamp software. Recording pipettes (tip resistance = 3–6 MΩ) were filled with a solution containing (in mM): 117.0 Cs-gluconate, 13.0 CsCl, 1.0 MgCl2, 0.07 CaCl2, 0.1 EGTA, 10.0 HEPES, 2.0 Na2-ATP, 0.4 Na-GTP and 0.3% biocytin. The pH and osmolarity were adjusted to 7.3 and 290 ± 1 mosM, respectively. Data were obtained from recordings with the access resistance stabilized between 10 and 20 MΩ. Layer V/VI pyramidal neurons in either the prelimbic or infralimbic regions of the mPFC were identified by their soma shape and apical dendrite oriented toward layer I. A roughly equal number of cells from these subregions of the mPFC were recorded across the groups in Experiments 1 and 2. For recordings of sIPSCs, glutamate receptor antagonists, CPP (10 µM) and DNQX (20 µM) were present in the bath and currents were recorded at a 0 mV holding potential. Neurons were filled with biocytin and subsequent recovery was used to confirm they were pyramidal cells.
A motorized syringe pump was used to apply agonists by injecting a bolus into the input line of the recording chamber. All concentrations reported here are the final bath concentrations that were estimated as previously described (Cox et al., 1995). Dopamine was made with 0.08% ascorbic acid every 2–3 h and was kept on ice and away from light. To minimize light-induced oxidation, lights were off when dopamine was bath-applied. All chemicals were obtained from Tocris (St. Louis, MO, USA) and doses were chosen based on our previous studies (Paul and Cox, 2013).
Behavior
To test for locomotor sensitization in rats who experienced protracted withdrawal from AMPH, separate groups of control, adolescent-exposed and adult-exposed rats (n = 20 rats/group) were injected in the same manner as shown in Table 1 for rats in Experiment 2. They were then challenged with two doses of AMPH when they were between P130 and P146 (mean: P135) and their behavior was assessed in a square open-field arena as described previously (Hankosky et al., 2013). Briefly, rats were placed in the arena for 30 min, momentarily removed and injected (i.p.) with 0.9% saline, and then returned to the arena for an additional 30 min. Lastly, rats were removed, injected with 1 or 3 mg/kg d-AMPH, and returned for an additional 90 min. Two days later, this procedure was repeated except rats received the dose of AMPH they did not get on the first test day. The order of doses was pseudorandomly assigned so that an equal number of rats in each group received both dose orders.
Data analysis
Data are presented as mean ± SEM unless noted otherwise. Analysis of sIPSC time course was done with a two-way, mixed factor ANOVA (time × group). Group differences in the peak response to bath-applied drugs were determined using an adjacent-averaging data smoothing method (Seamans and Yang, 2004; Trantham-Davidson et al., 2004; Paul and Cox, 2013). For each recording, the peak response area was determined as a 5-min window centered around the 1-min bin with the maximal deviation from baseline (the shaded areas in Figs. 2–6). This peak response was analyzed with a two-way ANOVA (group × age) followed by Tukey’s post hoc analyses where appropriate.
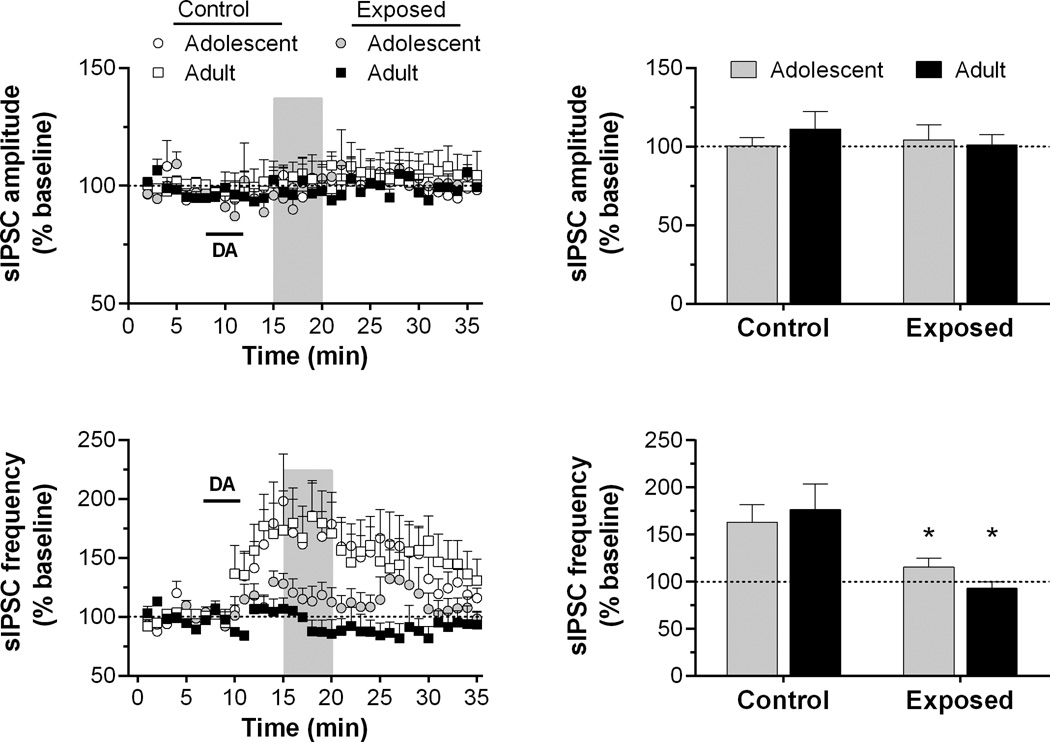
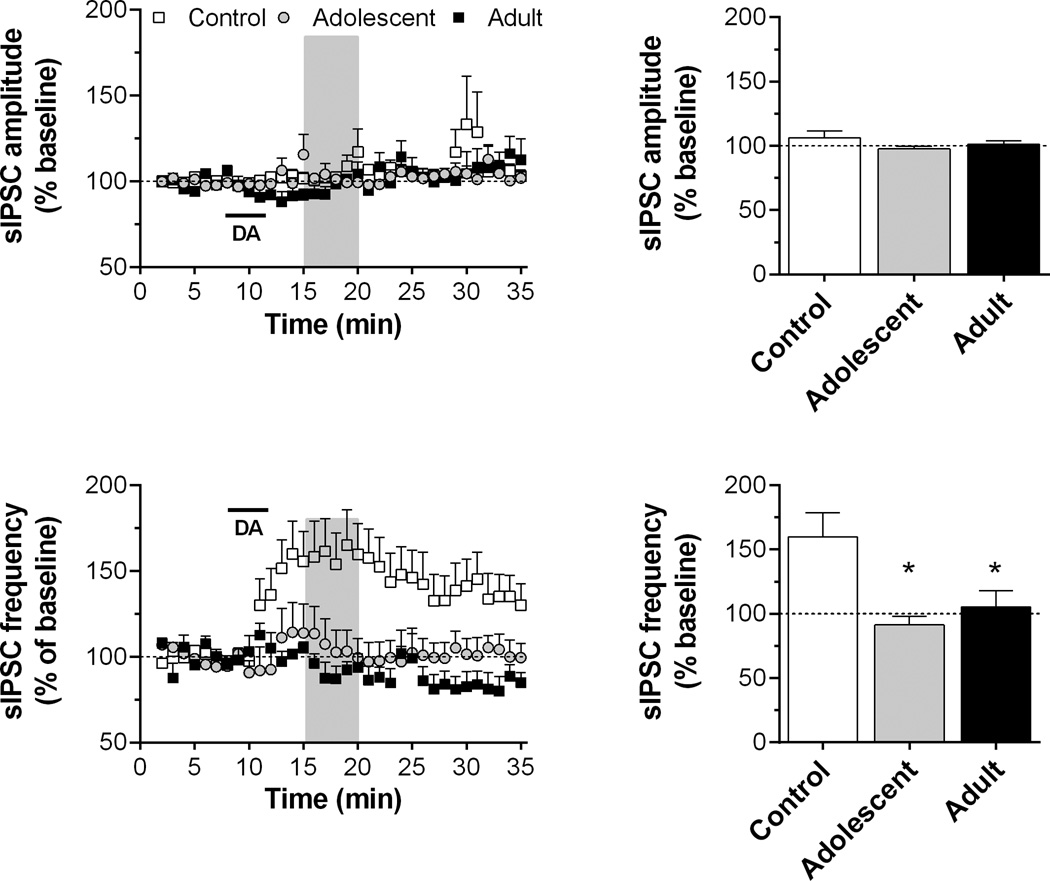
Fig. 2.
Time course and peak response for sIPSC amplitude and frequency in slices exposed to dopamine (DA; 50 µM for 4 min, as indicated by horizontal bar) in Experiment 1. Recordings were obtained from 4 to 6 cells/group in slices from 3 to 4 rats/group. Shaded regions in the time series indicate the areas of peak response (see “Experimental procedures”). The mean responses during these periods are summarized in the bar graphs. *p < 0.05, vs. control.
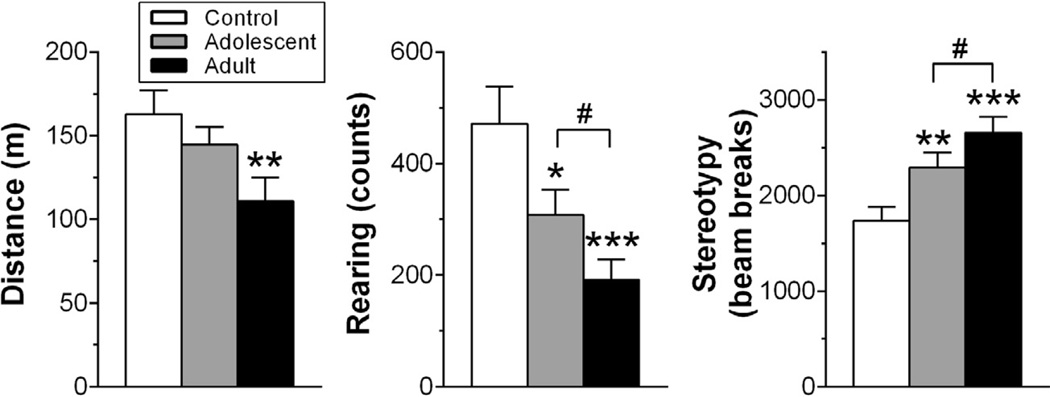
Fig. 6.
Locomotor activity in an open-field arena induced by 3 mg/kg AMPH (n = 20 rats/group). Ambulatory distance (A), number of rears (B) and stereotypy (C) is shown for the entire 90-min period following injection. Rats were pre-exposed to saline or 3 mg/kg AMPH as described for Experiment 2 (see Table 1). *p < 0.05; **p < 0.01; ***p < 0.001, vs. control; #p < 0.05.
For behavior, photobeam breaks were used to measure ambulation, rearing and stereotypy via monitoring software (TruScan v2.01; Coulbourn Instruments). Stereotypy, which is a measure of repetitive behavior such as head and body swaying, head bobbing, and sniffing, was defined as repetitive photobeam breaks in a focused area that did not contribute to large changes in location in the open-field. Cumulative measures following AMPH injection were analyzed with two-way repeated measures ANOVAs, with group and dose as the two factors. Tukey’s post hoc analyses were used to investigate main effects and interactions.
RESULTS
Effects of adolescent or adult AMPH exposure following 3–5 week withdrawal
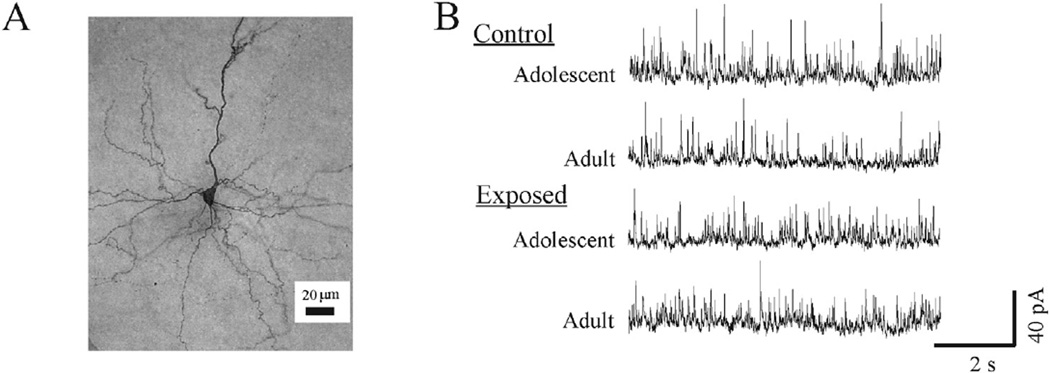
In Experiment 1, we recorded sIPSCs in layer V/VI pyramidal neurons from control (n = 14 and 13 for adolescent and adult, respectively) or AMPH-exposed rats (n = 12 and 13 for adolescent and adult exposed, respectively). A representative example of the neurons recorded in these experiments is shown in Fig. 1A. Although the adolescent control rats had the lowest values of the four groups, the frequency and amplitude of sIPSCs recorded from rats in the control and AMPH-exposed groups were similar during baseline (Fig. 1B). Statistical analysis of group differences revealed that none were significant (Table 2).
Fig. 1.
Representative examples of the neuron type and sIPSCs recorded during baseline. (A) A photomicrograph of a biocytin-filled pyramidal neuron recorded in the prelimbic region of a rat in the adult-exposed control group. (B) Samples of sIPSCs recorded from pyramidal neurons in slices from rats in each of the four groups from Experiment 1.
Table 2.
Summary of postnatal age (P) at sacrifice for slice electrophysiology experiments and sIPSC characteristics during baseline recording. Also shown for AMPH-exposed rats is the mean number of withdrawal days between the last drug injection and sacrifice
| Age at recording (P) | sIPSC frequency (Hz) | sIPSC amplitude (pA) | |
|---|---|---|---|
| Exp. 1 | |||
| Control | |||
| Adolescent | 70 ± 2.2 | 8.9 ± 1.1 | 27.3 ± 1.1 |
| Adult | 133 ± 2.8 | 10.6 ± 1.5 | 29.1 ± 1.3 |
| Exposed (withdrawal duration) | |||
| Adolescent (26 ± 1.2 days) | 71 ± 1.2 | 10.0 ± 1.4 | 30.1 ± 2.1 |
| Adult (25 ± 0.8 days) | 128 ± 0.9 | 9.4 ± 1.3 | 31.0 ± 1.1 |
| Exp. 2 | |||
| Control | 134 ± 1.5 | 7.4 ± 0.9 | 22.6 ± 0.6 |
| Exposed (withdrawal duration) | |||
| Adolescent (88 ± 2.1 days) | 132 ± 2.1 | 9.5 ± 1.1 | 23.5 ± 0.9 |
| Adult (33 ± 2.0 days) | 135 ± 2.0 | 9.1 ± 1.2 | 22.2 ± 0.8 |
In control rats, bath application of dopamine (50 µM, 4 min) produced a significant increase in the sIPSC frequency, but this effect of dopamine was absent in AMPH-exposed rats (Fig. 2). A two-way ANOVA of frequency time course data revealed a significant main effect of time (F34,476 = 5.58, p < 0.001) and a significant group by time interaction (F102,476 = 1.32, p < 0.05). A two-way ANOVA of the peak response showed significant differences in sIPSC frequency between control and AMPH-exposed groups (F3,15 = 3.90, p < 0.05), but no age of exposure-dependent differences. Dopamine application did not change the sIPSC amplitude for any groups.
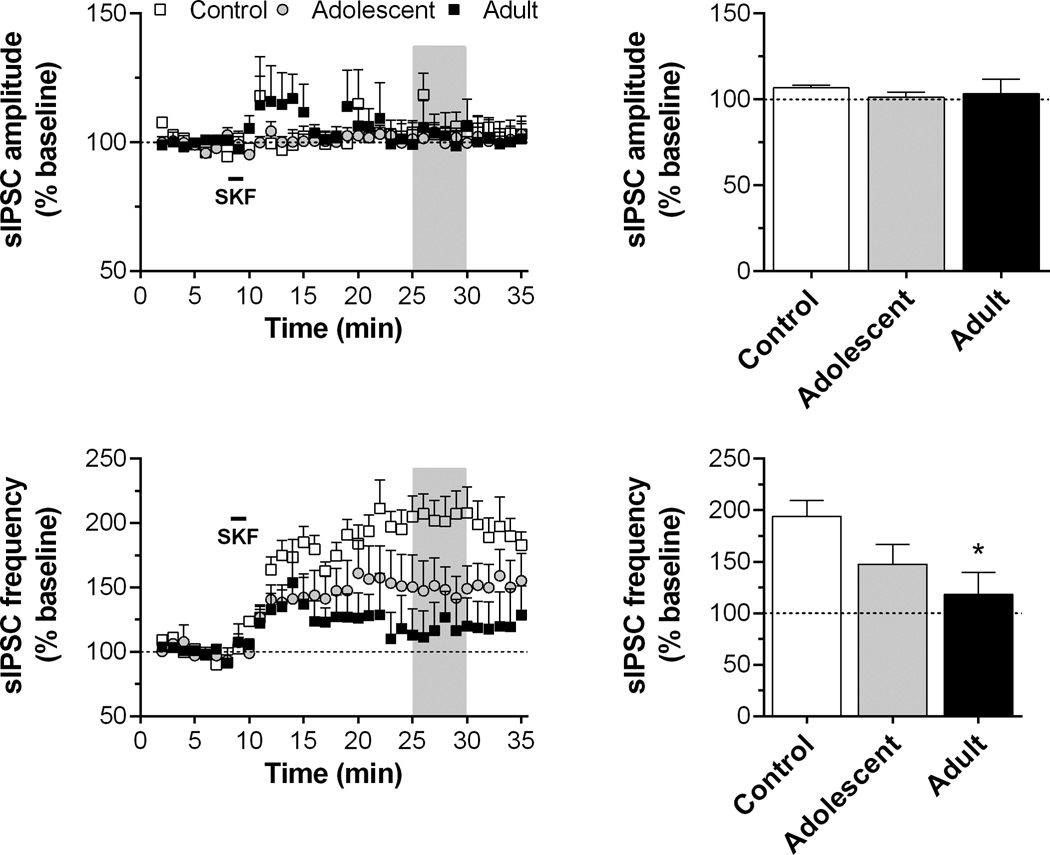
In our previous studies, this DA-mediate increase in inhibitory activity has been linked to the selective activation of D1 receptors (Paul and Cox, 2013). Thus, to determine if D1 receptor function was altered by repeated AMPH exposure, we next tested the ability of the selective D1 agonist SKF38393 to alter sIPSC activity. SKF38393 (10 µM, 90 s) produced a large increase in sIPSC frequency in controls (Fig. 3), but this effect was reduced in both adolescent- and adult-exposed rats. A significant main effect of time (F34,510 = 10.9, p < 0.001), group (F3,510 = 3.59, p < 0.05) and time by group interaction (F102,510 = 1.67, p < 0.001) was observed. Two-way ANOVA of peak response indicated a significant main effect of group (F3,15 = 3.87, p < 0.05). Post hoc comparisons revealed a significant group difference between controls and AMPH-exposed groups, but there were no significant age of exposure-dependent differences. Analysis of sIPSC amplitude revealed no statistically significant differences for either the time course or peak response data (Fig. 3).
Fig. 3.
Time course and peak response for sIPSC amplitude and frequency in slices exposed to SKF38393 (10 µM for 90 s, as indicated by horizontal bar) in Experiment 1. Recordings were obtained from 4 to 7 cells/group in slices from 3 to 4 rats/group. Data are presented as described for Fig. 2. *p < 0.05, vs. control.
Effects of adolescent AMPH exposure following protracted withdrawal (11–14 weeks)
In Experiment 2, we recorded sIPSCs in layer V/VI pyramidal neurons from control (n = 15) or AMPH-exposed rats (n = 8 and 12 for adolescent and adult groups, respectively). In the latter group, the mean withdrawal period was nearly 13 weeks for rats exposed to AMPH during adolescence and nearly 5 weeks for those exposed during adulthood. For this cohort of animals, sIPSC frequency during baseline tended to be relatively lower in controls compared to AMPH-exposed groups. However, as with Exp. 1, there were no statistically significant group differences in this measure or in the amplitude of sIPSCs (Table 2).
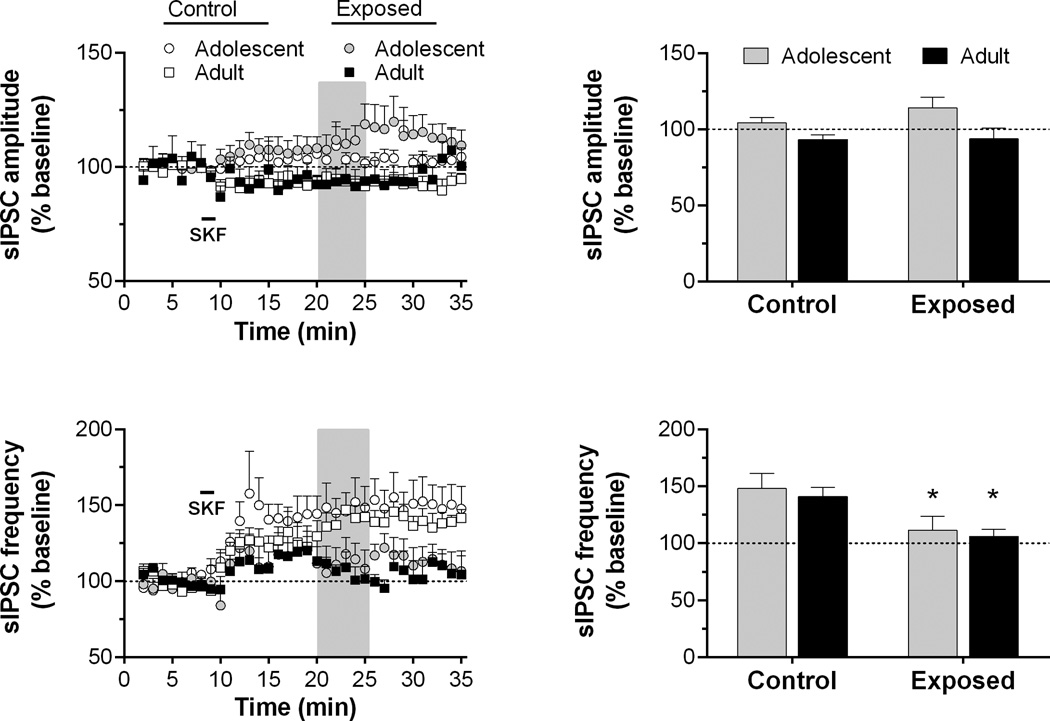
We found an increase in sIPSC frequency, but no change in amplitude, following dopamine application (50 µM, 4 min) in pyramidal neurons from controls (Fig. 4). This dopamine-mediated facilitation was significant reduced or abolished in neurons from adult- and adolescent-exposed rats. Statistical analysis of sIPSC frequency revealed a significant main effect of group (F2,641 = 3.88, p < 0.05), time (F34,641 = 2.53, p < 0.001) and time by group interaction (F68,641 = 3.17, p < 0.001). Analysis of the peak response (Fig. 4) revealed significant differences in sIPSC frequency between control and AMPH-exposed groups, with adolescent- and adult-exposed rats exhibiting a similar lack of sensitivity to dopamine. When D1 receptor function was assessed by bath application of SKF38393 (10 µM, 90 s), we observed an effect similar to what was seen in Experiment 1. Specifically, there was an increase in sIPSC frequency in slices from control rats, but this effect was attenuated in slices from AMPH-exposed rats. A two-way ANOVA revealed a significant main effect of time (F34,374 = 13.2, p < 0.001) and a time by group interaction (F68,374 = 2.12, p < 0.001). Analysis of the peak response revealed that both adolescent and adult-exposed groups exhibited this reduced sensitivity to the D1 agonist (Fig. 5), but the effect was greatest for those exposed in adulthood. This was confirmed statistically through a significant main effect of group (F2,11 = 4.17, p < 0.05) followed by a significant post hoc comparison between controls and the adult-exposed group. The amplitude of sIPSCs did not significantly change over time or between groups.
Fig. 4.
Time course and peak response for sIPSC amplitude and frequency in slices exposed to dopamine (DA; 50 µM for 4 min, as indicated by horizontal bar) in Experiment 2. Recordings were obtained from 4 to 10 cells/group in slices from 4 to 5 rats/group. Data are presented as described for Fig. 2. *p < 0.05, vs. control.
Fig. 5.
Time course and peak response for sIPSC amplitude and frequency in slices exposed to SKF38393 (10 µM for 90 s, as indicated by horizontal bar) in Experiment 2. Recordings were obtained from 4 to 5 cells/group in slices from 4 to 5 rats/group. Data are presented as described for Fig. 2. *p < 0.05, vs. control.
Tests for sensitization following protracted withdrawal (11–14 weeks)
In a separate group of animals, we tested if adolescent-exposed rats exhibited behavioral sensitization following the protracted withdrawal period used in Experiment 2. A two-way ANOVA of post-AMPH ambulation and rearing revealed significant treatment by dose interactions (F2,57 = 3.89; p < 0.05; F2,57 = 7.26; p < 0.01, respectively). Post hoc analysis revealed that following 3 mg/kg AMPH, control rats ambulated significantly farther than adult-exposed rats and reared significantly more than adolescent- and adult-exposed rats (Fig. 6A, B). There were no significant differences in ambulation between AMPH-exposed groups, but adolescent-exposed rats reared significantly more than adult-exposed rats. Analysis of stereotyped behavior revealed that the reduction of locomotor behavior and rearing in AMPH-exposed groups was likely due to a significant increase in this competing behavior (Fig. 1C). A two-way ANOVA revealed a significant treatment by dose interaction (F2,57 = 7.70; p < 0.01). Control rats engaged in significantly less stereotypy than AMPH-exposed rats and adult-exposed rats had the greatest response. For all dependent measures, there were no significant group differences following challenge with 1 mg/kg AMPH (data not shown).
DISCUSSION
The dopamine system, which continues to develop throughout adolescence and young adulthood (Gulley and Juraska, 2013), is a primary target of psychostimulant drugs like AMPH and lasting adaptations in dopamine function in response to repeated drug exposure are hypothesized to contribute significantly to the behavioral consequences that abusers experience (Lüscher and Malenka, 2011). Previously, we used a rat model to show that repeated exposure to AMPH during adolescence induces long-lasting cognitive deficits that, in some cases, are more significant when exposure occurs during adolescence (Hankosky et al., 2013; Sherrill et al., 2013; Hammerslag et al., 2014). Here, we tested if AMPH exposure would induce persistent changes in dopamine-mediated modulation of inhibitory transmission in the mPFC and if the effect of AMPH depends on the timing of exposure. We controlled for withdrawal duration and testing age in Experiments 1 and 2, respectively, and found that layer V/VI pyramidal cells recorded from rats exposed to AMPH during adolescence or adulthood were no longer sensitive to dopamine-induced increases in IPSCs. This effect was at least partially mediated by a reduced sensitivity of D1 receptors in exposed rats. There was no apparent dependency of this effect on age of exposure, but it is notable that the impact of adolescent exposure was evident for as long as three months after the last AMPH injection and was associated with an increased behavioral response to the drug upon re-exposure.
Dopamine-mediated modulation of inhibitory transmission in the mPFC is determined by the balance of D1 and D2 receptor activation (Seamans et al., 2001; Trantham-Davidson et al., 2004). Our data suggest that repeated AMPH exposure, regardless of the age when it occurs, results in long-lasting disruption of this modulatory influence. Previous studies in juvenile (P14–P28) and young adult (P50–P100) rodents have shown that high concentrations of dopamine (> 1 µM) applied in vitro increase sIPSC frequency in mPFC pyramidal cells primarily via D1 activation (Seamans et al., 2001; Gonzalez-Islas and Hablitz, 2001; González-Burgos et al., 2005; Kroener and Lavin, 2010; Paul and Cox, 2013). In line with this, we found a stable increase of sIPSC frequency in control rats following D1 activation by dopamine or SKF 38393. This effect was significantly attenuated in AMPH pre-exposed rats, suggesting a long-lasting impairment of D1-mediated regulation of sIPSC activity. Others have reported that AMPH exposure during adolescence or young adulthood is associated with adaptive changes in mesocortical dopamine circuits (Labonte et al., 2012; Reynolds et al., 2015) and D1 receptor function (Fletcher et al., 2005; Peterson et al., 2006; Tse et al., 2011) following 3 days to 4 weeks of withdrawal. Our results suggest the reduced responsiveness to dopamine in the mPFC can last at least 14 weeks following adolescent exposure (Fig. 4). Interestingly, the D1 receptor insensitivity seemed to diminish after this long withdrawal period (Fig. 5) in adolescent-exposed animals, suggesting mechanisms other than D1 dysfunction contributing to the blunted response to dopamine. However, from the current study, it is not clear if D1 function is still impaired following a prolonged withdrawal in those exposed during adulthood. In addition, the detailed mechanisms underlying the attenuated inhibitory tone following chronic AMPH exposure will require additional study. Previous work suggests that the D1-dependent facilitation of sIPSC activity is due to increased presynaptic GABA release, as dopamine and selective D1 agonists have no significant effect on the frequency or amplitude of miniature IPSC recorded in mPFC pyramidal cells (Seamans et al., 2001; Gorelova et al., 2002; Kroener and Lavin, 2010). D1 activation increases the excitability of interneurons, which results in an enhancement of their output (Gorelova et al., 2002; González-Burgos et al., 2005). Thus, it is possible that repeated AMPH exposure induces changes in interneuron physiology such that they become less sensitive to D1 stimulation and/or have a reduced output capacity (Morshedi and Meredith, 2007). However, the current study does not allow us to rule out the possibility that there are postsynaptic changes that may contribute to the reduced inhibition in the pyramidal cells. Future studies will be necessary to determine the detailed mechanisms for D1 function deficiency and if there are also long-lasting changes in D2 receptor function that might also contribute to the effects of AMPH exposure on inhibitory tone in the PFC.
Following a challenge injection of AMPH (3 mg/kg), adolescent- and adult-exposed rats exhibited sensitization to the locomotor-activating effects of the drug relative to controls. Although both age-of-exposure groups exhibited sensitization, they did so in distinct ways. For instance, adult-exposed rats reared significantly less and engaged in significantly more stereotypy than adolescent-exposed rats. Furthermore, only adult-exposed rats displayed a significant reduction in distance traveled compared to controls. These results replicate and extend previous results from our lab (Hankosky et al., 2013), demonstrating distinct manifestations of behavioral sensitization in adolescent- and adult-exposed rats that only emerge if the challenge dose is high enough (i.e. we found no differences following a 1 mg/kg challenge). As with our electrophysiological findings, these effects are particularly striking for the adolescent-exposed group given that these behavioral changes are evident more than 12 weeks following their last AMPH injection. Although locomotor sensitization is unlikely to be an appropriate substitute for assessment of PFC-sensitive cognition, multiple reports have demonstrated that rats sensitized to AMPH are impaired in tests of PFC-sensitive cognition (Fletcher et al., 2005; Hankosky et al., 2013; Sherrill et al., 2013) and that D1-selective drugs can mitigate these AMPH-induced deficits (Fletcher et al., 2005; Selemon et al., 2010). Additional experiments are necessary to establish a more direct relationship between behavioral sensitization and the electrophysiological changes observed here.
Whereas AMPH-induced adaptations in dopamine receptor expression and/or signaling are a likely candidate mechanism for the effects on mPFC function that we observed, the current studies do not allow us to rule out a potential involvement of 5-HT receptor changes. Previous work has shown that the D1 agonist we used in this study (SKF 38393) has moderate affinity for 5-HT receptors in vitro (Briggs et al., 1991). However, the effect of 5-HT receptor activation on sIPSC frequency is transient, lasting less than 15 min (Tan et al., 2004). In contrast, we found previously (Paul and Cox, 2013) and in the current study that dopamine and SKF 38393 had more enduring effects on sIPSC activity. Thus, AMPH-induced changes in serotonergic signaling are unlikely to play a major role in the loss of sensitivity of prefrontal neurons to D1-induced increases in sIPSCs that we observed in drug-exposed rats.
The PFC’s top-down control of executive functions relies on deep layer output neurons that are tightly controlled by interneurons (González-Burgos et al., 2002). Accordingly, disruption of GABAergic function in the mPFC results in a broad spectrum of cognitive impairments (Gonzalez-Burgos et al., 2011; Enomoto et al., 2011). The current study suggests that a deficit in D1-mediated inhibitory transmission in the mPFC is a candidate mechanism through which repeated AMPH exposure induces lasting deficits in cognition in both humans (Ornstein et al., 2000) and laboratory animals (Gulley and Juraska, 2013). Importantly, the drug exposure paradigm we used in Exp. 2 is identical to the one we used previously to demonstrate impairments in working memory (Sherrill et al., 2013), cognitive flexibility (Hankosky et al., 2013) and impulse control (Hammerslag et al., 2014) after a prolonged abstinence, where certain cognitive changes were specific to adolescent exposure. These diverging behavioral outcomes suggest that there are different neuroadaptations following exposures at the two developmental stages. In this initial study of seeking the drug-induced plasticity specific to adolescent exposure, we did not find evidence for a differential effect of exposure age on dopamine-mediated inhibition in the mPFC. Our results suggest that additional neuroadaptations are likely responsible for the enhanced effect of adolescent AMPH exposure on working memory that we have observed (Sherrill et al., 2013). For example, withdrawal from chronic exposure to psychostimulants during adolescent was recently shown to result in a long-lasting dysregulation of BDNF expression and downstream signaling in the mPFC (Giannotti et al., 2014).
CONCLUSION
In summary, our findings demonstrate that chronic AMPH exposure leads to reduced inhibitory transmission in the mPFC as a result of alterations in dopamine receptor function. Importantly, these effects persist after a protracted withdrawal period. The findings from adolescent-exposed rats reveal that drug-induced changes in mPFC function last throughout the adolescent period and linger well into adulthood, long after drug exposure has ceased. Adolescents may be especially vulnerable to certain aspects of drug-induced plasticity, which in turn may confer a greater risk for developing cognitive dysfunction, addiction and other psychological disorders (Gulley and Juraska, 2013). Previous work in adolescent rats exposed to cocaine (Cass et al., 2013) or cannabinoids (Cass et al., 2014) has also demonstrated lasting changes (up to 35 days) in inhibitory tone in the mPFC. Thus, the ability of drugs of abuse to alter the normal developmental trajectory of prefrontal circuitry may be a principal mechanism through which adolescent drug exposure can lead to longstanding, if not permanent, changes in prefrontal control over behavior. It will be important for future studies to determine the precise mechanisms that underlie drug-induced plasticity leading to vulnerability, as well as understand what factors might lead to resilience to the effects of drugs (Hammerslag and Gulley, 2016).
Acknowledgments
We thank Nikki Kofsky for technical assistance.
FUNDING AND DISCLOSURE
This work is supported by NIH grants DA029815 and EY014024.
Abbreviations
- AMPH
amphetamine
- mPFC
medial prefrontal cortex
- PFC
prefrontal cortex
- sIPSCs
spontaneous inhibitory postsynaptic currents.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex. 2000;10:1014–1027. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- Bjijou Y, De Deurwaerdere P, Spampinato U, Stinus L, Cador M. D-amphetamine-induced behavioral sensitization: effect of lesioning dopaminergic terminals in the medial prefrontal cortex, the amygdala and the entorhinal cortex. Neuroscience. 2002;109:499–516. doi: 10.1016/s0306-4522(01)00508-5. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs CA, Pollock NJ, Frail DE, Paxson CL, Rakowski RF, Kang CH, Kebabian JW. Activation of the 5-HT1C receptor expressed in Xenopus oocytes by the benzazepines SCH 23390 and SKF 38393. Br J Pharmacol. 1991;104:1038–1044. doi: 10.1111/j.1476-5381.1991.tb12546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass DK, Flores-Barrera E, Thomases DR, Vital WF, Caballero A, Tseng KY. CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex. Mol Psychiatry. 2014;19:536–543. doi: 10.1038/mp.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass DK, Thomases DR, Caballero A, Tseng KY. Developmental disruption of gamma-aminobutyric acid function in the medial prefrontal cortex by noncontingent cocaine exposure during early adolescence. Biol Psychiatry. 2013;74:490–501. doi: 10.1016/j.biopsych.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Curr Opin Neurobiol. 2002;12:223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Cox CL, Huguenard JR, Prince DA. Cholecystokinin depolarizes rat thalamic reticular neurons by suppressing a K+ conductance. J Neurophysiol. 1995;74:990–1000. doi: 10.1152/jn.1995.74.3.990. [DOI] [PubMed] [Google Scholar]
- Didriksen M. Effects of antipsychotics on cognitive behaviour in rats using the delayed non-match to position paradigm. Eur J Pharmacol. 1995;281:241–250. doi: 10.1016/0014-2999(95)00242-d. [DOI] [PubMed] [Google Scholar]
- Enomoto T, Tse MT, Floresco SB. Reducing prefrontal gamma-aminobutyric acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol Psychiatry. 2011;69:432–441. doi: 10.1016/j.biopsych.2010.09.038. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tenn CC, Rizos Z, Lovic V, Kapur S. Sensitization to amphetamine, but not PCP, impairs attentional set shifting: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Psychopharmacology. 2005;183:190–200. doi: 10.1007/s00213-005-0157-6. [DOI] [PubMed] [Google Scholar]
- Giannotti G, Caffino L, Calabrese F, Racagni G, Riva MA, Fumagalli F. Prolonged abstinence from developmental cocaine exposure dysregulates BDNF and its signaling network in the medial prefrontal cortex of adult rats. Int J Neuropsychopharmacol. 2014;17:625–634. doi: 10.1017/S1461145713001454. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011;2011:723184. doi: 10.1155/2011/723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Burgos G, Krimer LS, Povysheva NV, Barrionuevo G, Lewis DA. Functional properties of fast spiking interneurons and their synaptic connections with pyramidal cells in primate dorsolateral prefrontal cortex. J Neurophysiol. 2005;93:942–953. doi: 10.1152/jn.00787.2004. [DOI] [PubMed] [Google Scholar]
- González-Burgos G, Kröner S, Krimer LS, Seamans JK, Urban NN, Henze DA, Lewis DA, Barrionuevo G. Dopamine modulation of neuronal function in the monkey prefrontal cortex. Physiol Behav. 2002;77:537–543. doi: 10.1016/s0031-9384(02)00940-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ. Dopamine inhibition of evoked IPSCs in rat prefrontal cortex. J Neurophysiol. 2001;86:2911–2918. doi: 10.1152/jn.2001.86.6.2911. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Addiction and cognition. Addict Sci Clin Pract. 2010;5:4–14. [PMC free article] [PubMed] [Google Scholar]
- Gulley JM, Juraska JM. The effects of abused drugs on adolescent development of corticolimbic circuitry and behavior. Neuroscience. 2013;249:3–20. doi: 10.1016/j.neuroscience.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerslag LR, Waldman AJ, Gulley JM. Effects of amphetamine exposure in adolescence or young adulthood on inhibitory control in adult male and female rats. Behav Brain Res. 2014;263:22–33. doi: 10.1016/j.bbr.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerslag LR, Gulley JM. Sex differences in behavior and neural development and their role in adolescent vulnerability to substance use. Behav Brain Res. 2016;298:15–26. doi: 10.1016/j.bbr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankosky ER, Kofsky NM, Gulley JM. Age of exposure-dependent effects of amphetamine on behavioral flexibility. Behav Brain Res. 2013:1–9. doi: 10.1016/j.bbr.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kroener S, Lavin A. Altered dopamine modulation of inhibition in the prefrontal cortex of cocaine-sensitized rats. Neuropsychopharmacology. 2010;35:2292–2304. doi: 10.1038/npp.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte B, McLaughlin RJ, Dominguez-Lopez S, Bambico FRR, Lucchino I, Ochoa-Sanchez R, Leyton M, Gobbi G. Adolescent amphetamine exposure elicits dose-specific effects on monoaminergic neurotransmission and behaviour in adulthood. Int J Neuropsychopharmacol. 2012;15:1319–1330. doi: 10.1017/S1461145711001544. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Conrad KL, Carr SB, Ford Ka, McGehee DS, Marinelli M. Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J Neurophysiol. 2012;108:1620–1630. doi: 10.1152/jn.00077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetin R, Mattick RP. Attention and memory in illicit amphetamine users. Drug Alcohol Depend. 1997;48:235–242. doi: 10.1016/s0376-8716(97)00132-4. [DOI] [PubMed] [Google Scholar]
- Morshedi MM, Meredith GE. Differential laminar effects of amphetamine on prefrontal parvalbumin interneurons. Neuroscience. 2007;149:617–624. doi: 10.1016/j.neuroscience.2007.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- Paul K, Cox CL. Age-dependent actions of dopamine on inhibitory synaptic transmission in superficial layers of mouse prefrontal cortex. J Neurophysiol. 2013;109:1323–1332. doi: 10.1152/jn.00756.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Repeated amphetamine administration decreases D1 dopamine receptor-mediated inhibition of voltage-gated sodium currents in the prefrontal cortex. J Neurosci. 2006;26:3164–3168. doi: 10.1523/JNEUROSCI.2375-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LM, Makowski CS, Yogendran SV, Kiessling S, Cermakian N, Flores C. Amphetamine in adolescence disrupts the development of medial prefrontal cortex dopamine connectivity in a DCC-dependent manner. Neuropsychopharmacology. 2015;40:1101–1112. doi: 10.1038/npp.2014.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–57. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Begovic A, Williams GV, Castner SA. Reversal of neuronal and cognitive consequences of amphetamine sensitization following chronic treatment with a D1 antagonist. Pharmacol Biochem Behav. 2010;96:325–332. doi: 10.1016/j.pbb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Sherrill LK, Stanis JJ, Gulley JM. Age-dependent effects of repeated amphetamine exposure on working memory in rats. Behav Brain Res. 2013;242:84–94. doi: 10.1016/j.bbr.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav Brain Res. 2006;171:116–126. doi: 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Tan H, Zhong P, Yan Z. Corticotropin-releasing factor and acute stress prolongs serotonergic regulation of GABA transmission in prefrontal cortical pyramidal neurons. J Neurosci. 2004;24:5000–5008. doi: 10.1523/JNEUROSCI.0143-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Neely L, Lavin A, Seamans J. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J Neurosci. 2004;24:10652–10659. doi: 10.1523/JNEUROSCI.3179-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse MTL, Cantor A, Floresco SB. Repeated amphetamine exposure disrupts dopaminergic modulation of amygdala-prefrontal circuitry and cognitive/emotional functioning. J Neurosci. 2011;31:11282–11294. doi: 10.1523/JNEUROSCI.1810-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng K, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang G, Fowler J, Ding Y, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in the methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001b;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn. 2010;72:146–159. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]