Abstract
Background
Recent studies have shown that C4-like photosynthetic pathways partly reside in photosynthetic cells surrounding the vascular system of C3 dicots. However, it is still unclear whether this is the case in C3 monocots, especially at the molecular level.
Results
In order to fill this gap, we investigated several characteristics required for C4 photosynthesis, including C4 pathway enzymes, cyclic/non-cyclic photophosphorylation rates, the levels and assembly state of photosynthetic machineries, in the mid-veins of C3 monocots rice with leaf laminae used as controls. The signature of photosystem photochemistry was also recorded via non-invasive chlorophyll a fluorescence and reflectance changes at 820 nm in vivo. Our results showed that rice mid-veins were photosynthetically active with higher levels of three C4 decarboxylases. Meanwhile, the linear electron transport chain was blocked in mid-veins due to the selective loss of dysfunctional photosystem II subunits. However, photosystem I was sufficient to support cyclic electron flow in mid-veins, reminiscent of the bundle sheath in C4 plants.
Conclusions
The photosynthetic attributes required for C4 photosynthesis were identified for the first time in the monocotyledon model crop rice, suggesting that this is likely a general innate characteristic of C3 plants which might be preconditioned for the C4 pathway evolution. Understanding these attributes would provide a base for improved strategies for engineering C4 photosynthetic pathways into rice.
Electronic supplementary material
The online version of this article (doi:10.1186/s12284-016-0094-5) contains supplementary material, which is available to authorized users.
Keywords: C3 and C4 photosynthesis, Cyclic/linear electron flow, Dysfunctional PSII, Mid-vein, Rice
Background
C4 plants partition photosynthetic reactions between two distinct cell types: vascular bundle sheath (BS) and mesophyll (M) cells, a structure called ‘Kranz’ anatomy (Majeran and van Wijk 2009). Atmospheric CO2 is firstly fixed as C4 acids by phosphoenolpyruvate carboxylase (PEPC) in M cells, then the C4 acids are transferred into BS cells to be degraded by decarboxylating enzymes. The released CO2 is refixed by ribulose-1, 5-bisphosphate carboxylase/oxygenase (RuBisCO) and incorporated into the C3 cycle (Furbank 2011). This structural specialization of leaf tissue allows optimum operation of the C4 photosynthetic pathway to concentrate CO2.
C4 plants are divided into three biochemical subtypes based on different decarboxylating mechanisms: nicotinamide adenine dinucleotide phosphate-dependent malic enzyme (NADP-ME), nicotinamide adenine dinucleotide-dependent malic enzyme (NAD-ME), and phosphoenolpyruvate carboxykinase (PEPCK) types (Yoshimura et al. 2004). The majority of C4 crop species belong to the NADP-ME group (Furbank 2011) in which BS cells utilize malate as the C4 acid. Since malate decarboxylation results in a donation of reductive power, the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH), substantial production of additional ATP would be required in BS cells (Finazzi et al. 2002). Therefore, NADP-ME C4 monocots such as sorghum (Sorghum bicolor) have completely agranular BS. They lack linear electron flow for NADPH generation due to photosystem II (PSII) deficiency and only function to generate ATP by photosystem I (PSI)-mediated cyclic electron flow (Voznesenskaya et al. 1999). The suppression of PSII activity mainly results from selective losses of subunits PsbP and PsbQ of the oxygen evolving complex (OEC) as well as PsbR, rather than the entire PSII complex (Meierhoff and Westhoff 1993).
It has been reported that a C4-like pathway existed in photosynthetic cells surrounding the vascular system (PCSVS) of C3 dicots tobacco and celery (Hibberd and Quick 2002). PCSVS of C3 plants is a more spatially separated version of the C4 photosynthetic pathway with high activity levels of three C4 photosynthesis decarboxylases, allowing to decarboxylate malate from the transpiration stream. This is also the case in mid-veins of Arabidopsis, a model C3 dicot (Brown et al. 2010). These findings suggest that essential biochemical components and the regulatory elements controlling the cell-specific gene expression required for C4 photosynthesis are already present in C3 plants. Thus, C4 photosynthesis can evolve from C3 plants with some modifications (Hibberd and Quick 2002).
Furthermore, PCSVS internal microenvironment and metabolic demands are similar to BS cells in NADP-ME C4 plants rather than C3 leaves. Because of fewer stomata and intercellular air spaces, but more layers of surrounding cells, the vascular tissue has been previously noted to reduce solubility and diffusivity of oxygen (Raven 1991; Hibberd and Quick 2002). Those further suppress mitochondrial respiration and result in ATP losses (Geigenberger 2003) in conjunction with the NADP+ requirement for high C4-acid decarboxylating activity (Yiotis et al. 2009). Therefore, the compensatory metabolic demands of higher ATP/NADPH ratios in mid-veins are comparable to that found in BS cells of NADP-ME type C4 plants.
These properties may require qualitative and quantitative adjustments of the photosynthetic attributes in such organs for both light and biochemical reactions (Yiotis and Manetas 2010). As observed in NADP-ME type C4 plants, chloroplasts in PCSVS of C3 plants deficient in linear but sufficient in cyclic electron flow would act as electron valves restoring the ATP/NADPH ratio (Yiotis et al. 2009). For example, Kotakis et al. (2006) show that twigs of Eleagnus angustifolius display low dark-adapted PSII photochemical efficiency and linear electron transport rates. Kalachanis and Manetas (2010) further demonstrate that the innately low linear flow is limited in the donor side (OEC) of PSII and the acceptor side of both PSII and PSI.
The origin, function and selective advantages of PCSVS in C3 lineages are critical for the understanding of the environmental, molecular and phylogenetic determinants for C4 evolution (Griffiths et al. 2012). With no doubt, more studies of PCSVS in C3 species are required to fill this fundamental gap (Leegood 2008) with molecular evidence. In particular, the neglected investigation on the monocot model plant rice (Oryza sativa), may have more significant impacts in Asia, because it would potentially simplify the approaches to generate a two-celled C4 shuttle in rice by expressing the classical enzymes of the NADP-ME C4 cycle (Kajala et al. 2011).
Following up the preliminary findings as mentioned above, we took advantage of the O. sativa. cv. Liangyoupeijiu as the plant material, which had large mid-veins. We have elucidated a wealth of photosynthetic traits of leaf laminae in the super hybrid rice (Zhang et al. 2007). In order to examine whether rice mid-veins had innate properties of C4-like photosynthesis, physiological traits, biochemical parameters, and spectral indicators were compared between leaf laminae and mid-veins in O. sativa.
Results
Rice mid-veins had photosynthesizable chloroplasts
The presence of chlorophyll (Chl) in chloroplasts caused greenness and bright red fluorescence under optical and epifluorescence microscopy, respectively (Berveiller and Damesin 2007). To examine the distribution of chlorophyllous cell in rice leaves, transverse sections of leaves were subjected to the microscopy (Fig. 1b and c). Our results demonstrated that both mid-veins and leaf laminae showed green regions under optical microscopy (Fig. 1b). Under epifluorescence microscopy, the red fluorescence signals emitted by the Chl also surrounded the xylem vessels in mid-veins (Fig. 1c).
Fig. 1.
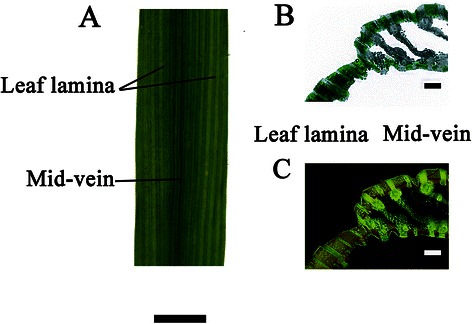
Phenotypic characteristics of rice mid-veins and leaf laminae. a The photograph of the midsection of a rice leaf, scale bar = 3 cm. b The optical micrograph of transverse sections of a rice leaf, and green areas illustrate the distribution of chlorophyllous cells. c The epifluorescence micrograph of transverse sections of a rice leaf, and chlorophyllous cells can be identified by the bright red fluorescence, scale bar =250 μm. Transverse sections of leaves were subjected to the epifluorescence microscopy. However, because of the intrinsic background noise from the epifluorescence microscopy and the low resolution of the Insight camera (CFI60, Nikon), images had to be first treated with Photoshop (CS5, Adobe) to discard the noise, which may have affected their quality (Wittmann et al. 2006; Berveiller and Damesin 2008)
Rice mid-veins accumulated high levels of C4 acid decarboxylases
To test whether rice mid-veins possessed some enzymatic features of C4 BS cells or not, we evaluated various key enzymatic activities in either C3 or C4 cycles. The key enzyme of C4 cycle, PEPC, exhibited a little higher activity in rice mid-veins than in leaf laminae, while the key enzyme of C3 cycle, RuBisCO, had lower activity. It was worth noting that mid-veins showed greatly elevated activities for three C4 acid decarboxylases (NADP-ME, NAD-ME and PEPCK) and pyruvate phosphate dikinase (PPDK) per Chl unit: 6.2 to 7.6-fold greater than that of the leaf laminae (Fig. 2a). Immunoblot results showed that the enzyme protein levels were consistent with the enzymatic data (Fig. 2b). Mid-veins enriched more than 3.5-fold three C4 acid decarboxylases than leaf laminae, while the large subunit of RuBisCO was reduced by 42 % in mid-veins (Fig. 2c).
Fig. 2.
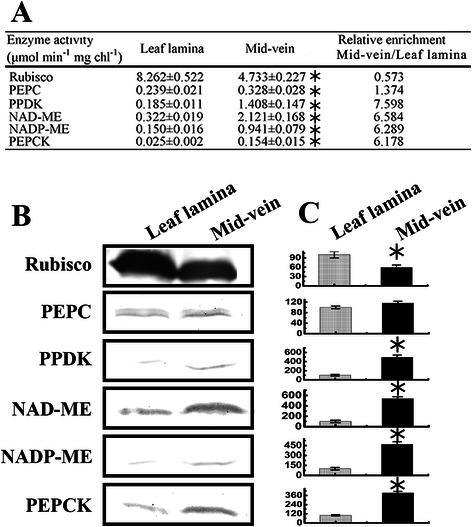
Enzymatic activities and protein levels in C3 or C4 cycle of mid-veins and leaf laminae. a Enzymatic activities are expressed as micromole per minute per mg Chl. b Immunoblot of C3 and C4 cycle enzymes (15 μg protein per spot). c Quantification of immunoblot data. Images were analyzed using Quantity One (Bio-Rad, USA). Data are means ± SE (n = 3), with the asterisk indicating statistically significant differences (P < 0.05) between mid-veins and leaf laminae
Rice mid-veins showed unusual fluorescence signature of photosynthetic machineries
Fast Chl a fluorescence transients (Ft) can provide information for the whole photosynthetic process from water splitting to PSI electron acceptor (feredoxin and NADP+) (Strasser et al. 2010). To study whether mid-veins operated unique photosynthetic machineries, fluorescence analysis was employed.
As shown in Fig. 3, both leaf laminae and mid-veins displayed a typical and distinct polyphasic Ft rise, which meant that they were both photosynthetically active and not interfered by rectangular window fitted on the clip.
Fig. 3.
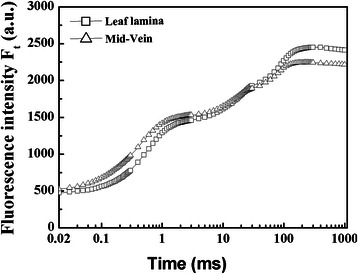
Fast Chl fluorescence kinetics (Ft) of rice mid-veins (△) and leaf laminae (□). Each curve represents the average kinetics recorded from 15 independent measurements of 15 individual plants
Various additional normalizations and difference kinetics were employed to reveal bands that were hidden in Ft (Fig. 4). Ft was firstly normalized between the step O and P and presented as relative variable fluorescence, Vt (Fig. 4a). The O, L, K, J, I and P steps were marked in the plot. Positive ΔL-, ΔK-, ΔJ- and ΔI-bands were displayed in ΔVt (Fig. 4a), and the most distinct peak appeared at K step in mid-veins. Compared to leaf laminae, mid-veins had positive L-bands (Fig. 4b) and K-bands (Fig. 4c), as well as less half time and the maximum amplitude of IP rise (Fig. 4d).
Fig. 4.
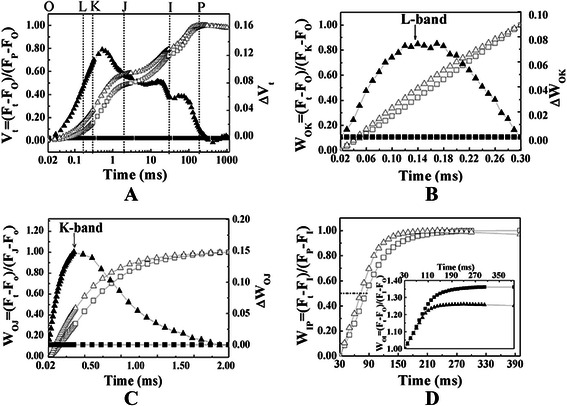
The different expressions of relative variable fluorescence (V or W, left vertical axis) of rice mid-veins (△) and leaf laminae (□). The difference fluorescence kinetics of mid-veins (▲) to leaf laminae (█) was calculated using the equation: ΔV (or ΔW) = V (or W)mid ‐ vein – V (or W)leaf laminae (right vertical axis). Each curve represents the average kinetics derived from 15 independent measurements of Ft. a Normalized between FO and FM: Vt = (Ft – FO)/(FM – FO), and △Vt was marked by the O, L, K, J, I, P steps. The graph was plotted on a logarithmic time scale (0.02 ms to 1 s). b Normalized between FO and FK: Wok = (Ft – Fo)/(FK – Fo), and △WOk reveals L-bands which indicate the degree of energetic dis-connectivity (grouping) of the PSII units. The graph was plotted on a linear time scale (0.02 ms to 0.3 ms). c Normalized between FO and FJ: WoJ = (Ft – FO)/(FJ – FO), and △WOJ reveals K-bands which indicate the degree of inactivation of OEC. The graph was plotted on a linear time scale (0.02 ms to 2 ms). d In the main panel, normalized between FI and FP: WIP = (Ft – FI)/(FP – FI), and horizontal dashed line at 0.5 indicates half time needed to reduce pool of the end electron acceptor with electrons donated by intermediate carriers. In the inserted panel, normalized between FO and FI: WOI (≥1) = (Ft – FO)/(FI – FO), and the maximum amplitude of IP phase illustrates the differences in the pool size of the end electron acceptors. The graph was plotted on a linear time scale (30 ms to 400 ms)
To provide further quantitative and precise information, radar plot graphs were presented in Fig. 5a, b. The behavior of structural and functional parameters was analyzed according to the JIP-test (Strasser and Srivastava 1995; Strasser et al. 2004). The parameter definition and derivation were shown in Additional file 1: Table S1. Although specific fluxes parameters (ABS/RC, TRO/RC, ETO/RC and REO/RC), VK/VJ, VJ, MO and the parameters about heat dissipation (DIO/ABS and DIO/RC) increased in mid-veins, Sm/tFM, γRC, 1/VI, t1/2(I−P), ETO/TRO, ETO/ABS, ECO/ABS, REO/ABS, REO/TRO and PItotal in mid-veins exhibited a smaller value than those in leaf laminae. Other parameters, such as Sm, TRO/ABS and REO/ETO did not differ significantly between two tissues.
Fig. 5.
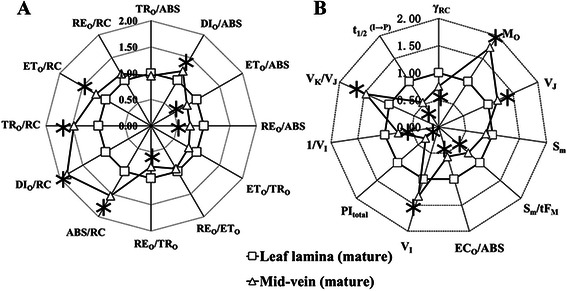
Radar plot representation of the behavior of structural and functional parameters. a Quantum yields, flux ratios, and specific energy fluxes per absorption flux (ABS) and reaction center (RC), Mid-veins (△) and leaf laminae (□). b Performance indexes, density of RCs and other fluorescence parameters. Each parameter was derived from JIP-test of the corresponding Ft, and then normalized to the leaf laminae (with value of 100 % = 1). The significant difference between two tissues (P < 0.05) is indicated by the asterisk. The parameters represent the average kinetics collected from 15 independent measurements
To test PSI activity and the connectivity of the two photosystems, the kinetics of the normalized modulated reflection at 820 nm (MR/MRO) were recorded according to Strasser et al. (2010). As shown in Fig. 6a, the amplitude of MR/MRO diminished in mid-veins as compared to leaf laminae. To further characterize MR/MRO, the derived parameters (△MRfast/MRO and △MRslow/MRO) from MR/MRO were shown in Fig. 6b and c. The fast phase (△MRfast/MRO) had no significant difference in both tissues, whereas the slow phase (△MRslow/MRO) was distinctly higher in leaf laminae than mid-veins.
Fig. 6.
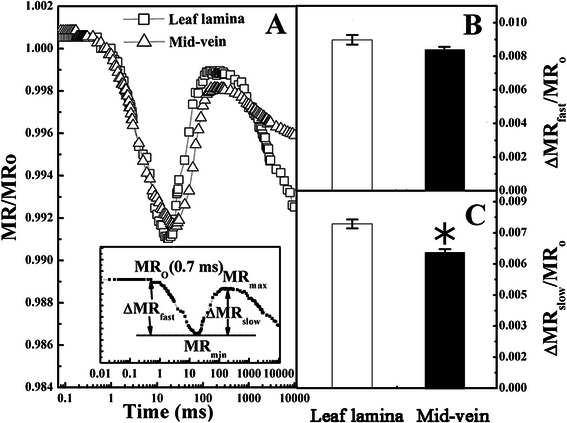
Mean kinetics of modulated reflection at 820 nm (MR). a MR was normalized and expressed by MR/MRO on logarithmic time scale from 0 ms to 10 s, where MRO was the value at 0.7 ms (taken at the onset of the red light illumination, the first reliable MR measurement). Each curve represents the average kinetics recorded from 15 independent measurements. The inserted graph showed the definition of characteristic parameters of the MR/MRO kinetics (MRmin, MRmax) according to Strasser et al. (2010), where MRmin was the minimal MR reached during the fast phase (i.e., between 0.7 ms and 10–200 ms) and MRmax was the maximal MR reached by the end of the slow phase (taken at 1 s). b Amplitudes of the fast phase: ΔMRfast/MRO = (MRO − MRmin)/MRO, which corresponds to the kinetics of the photo-induced changes in P700 redox state and the activated PSI RC (P700+). Mid-veins (△) and leaf laminae (□). c Amplitudes of the slow phase: ΔMRslow/MRO = (MRmax − MRmin)/MRO, which reflects P700+ re-reduction and connectivity of the two photosystems. Data are means ± SE from 15 independent measurements. The significant difference between two tissues (P < 0.05) is indicated by the asterisk
Rice mid-veins possessed a lower linear electron flow
To further confirm that mid-veins had an unbalanced requirement for the electron transport flows, the cyclic and non-cyclic (linear) photophosphorylation rate of the chloroplasts were measured. Non-cyclic phosphorylation rate of mid-veins was lower than that of leaf laminae, while cyclic photophosphorylation exhibited no obvious changes (Fig. 7a and b). Hence, mid-veins were superior in cyclic/non-cyclic photophosphorylation ratio (Fig. 7c).
Fig. 7.
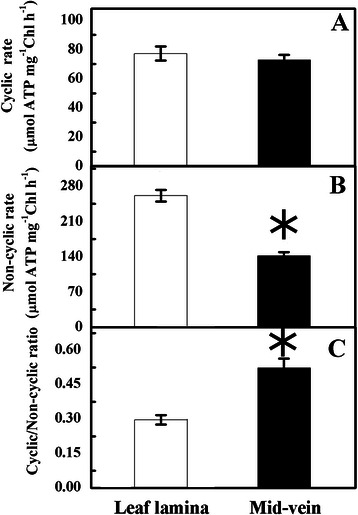
Cyclic (a), non-cyclic/linear (b) photophosphorylation activity, and their ratios (c) of mid-veins and leaf laminae. Data are means ± SE from 5 independent measurements. The significant difference between two tissues (P < 0.05) is indicated by asterisk
Rice mid-veins had a lower accumulation of PSII supercomplexes and selective subunits
Thylakoid multi-subunit complexes can be separated in their native form with high resolution in Blue native polyacrylamide gel electrophoresis (BN-PAGE), whereas specific subunit stoichiometry of these complexes can be detected with immunoblots (Takabayashi et al. 2009). To understand the molecular mechanism for the modified photosystems in mid-veins, the assembly status of thylakoid membrane complexes was determined by BN-PAGE (Fig. 8a). Seven major bands were resolved, apparently corresponding to PSII supercomplexes, PSI-light harvesting complex (LHC) I, PSI core, ATP synthase-Cytb6f-PSII core, CP43 less PSII core, LHCII trimer, and dimer.
Fig. 8.
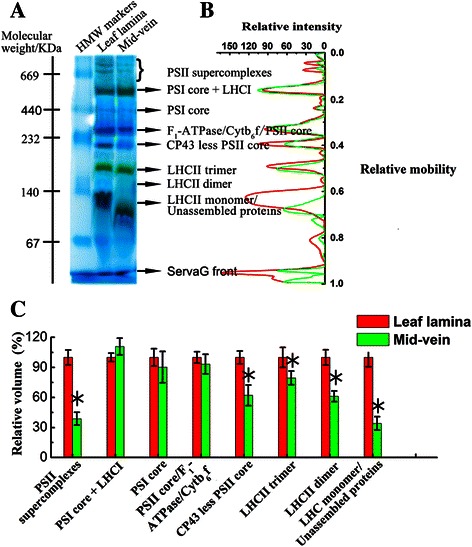
BN-PAGE profile of thylakoid membranes isolated from rice mid-veins (green) and leaf laminae (red). a Protein complexes (10 mg Chl per spot) were subjected to BN-PAGE electrophoresis. Molecular weight of HMW markers was labeled on the left, and the nomenclature of the resolved bands was labeled on the right. b Quantitation of the complexes. The BN-gels were scanned and the optical density along each lane was quantified (Quantity one, Bio-Rad, USA) to determine the relative mobility. c The abundance of each complex band. The abundance of each band was calculated by integrating the peak areas, and expressed as the percentage of that in leaf laminae
As compared to leaf laminae, we did not observe any obvious differences for PSI core + LHCI, PSI core, and ATP synthase-Cytb6f complex-PSII core, whereas PSII supercomplexes and CP43 less PSII core in mid-veins were far below detection in the leaf laminae. LHCII trimer and LHCII dimer were also reduced in the mid-vein (Fig. 8b and c).
In order to fingerprint the composition of these complexes, representative subunits were examined by immunoblots (Fig. 9). In PSII supercomplexes, the level of PsbP, PsbQ of OEC and PsbR were reduced most significantly than other subunits. PsbO of OEC, PsbA (PSII core subunit), Lhcb1 and Lhcb2 (LHCII subunits) were also decreased in mid-veins. Lhcb3 level was not altered significantly. In contrast, the relative level of the Cytb6f subunits, Cyt b6 and Cyt f, and ATP synthase subunits, Atpβ were not vulnerable in rice mid-veins. In respect of PSI supercomplex, PsaA (PSI core subunits), Lhca1 and Lhca2 (LHCI subunits) were also not changed in mid-veins (Fig. 9b).
Fig. 9.
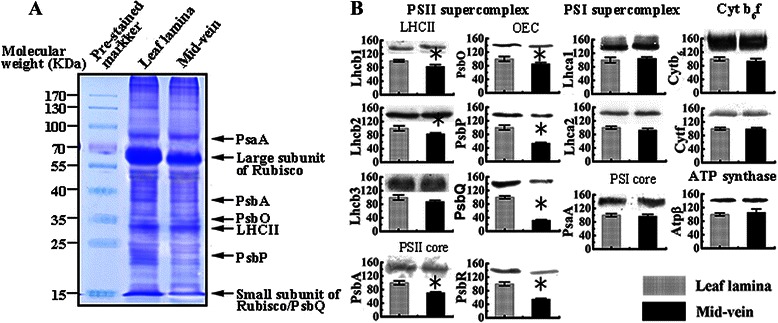
Immunoblot analysis of photosynthetic subunits in rice mid-veins and leaf laminae. a For protein loading (15 μg per spot) verification, SDS–PAGE gels were stained with Coomassie brilliant blue R-250. Pre-stained protein marker was indicated on the left, and bands corresponding to PsaA, large subunit of RuBisCO, PsbA, PsbO, LHCII, PsbP, and small subunit of RuBisCO/PsbQ were shown on the right. b Immunoblot analysis was carried out using the polyclonal antibodies against the thylakoid membrane subunits. Quantitation of band signal was performed using Quantity One (Bio-Rad, Hercules, USA) based on densitometric analysis. The amount of protein was expressed as the percentage of that in leaf laminae (100 %). Data are means ± SE from three independent experiments, with the asterisk indicating statistically significant differences (P < 0.05) between mid-veins and leaf laminae
Discussion
Rice mid-veins are photosynthetically active with high enrichment of C4 acid decarboxylases
With transverse section of rice leaves under epifluorescence or optical microscopy, both mid-veins and leaf laminae were proved to be photosynthesizable with chloroplasts (Fig. 1b, c). The chlorophyllous cells also border the vascular system in dicots such as celery, tobacco (Hibberd and Quick 2002), and woody species (Berveiller and Damesin 2008). Therefore, the cells around the veins in C3 plants (PCSVS) can also be termed ‘BS’ cells as C4 plants (Kinsman and Pyke 1998; Hibberd and Quick 2002).
The stems and petioles of tobacco (Hibberd and Quick 2002) and mid-veins of Arabidopsis thaliana (Brown et al. 2010) possess high activities of decarboxylating enzymes (NADP-ME, NAD-ME and PEPCK) and PPDK. Thus the ability of PCSVS to decarboxylate organic acids is phylogenetically widespread among C3 dicotyledons (Aubry et al. 2011). Our results firstly provided direct molecular evidence to the accumulation of decarboxylating enzymes in monocot rice mid-veins by immunoblot analyses (Fig. 2). The preferential accumulation of decarboxylating enzymes in rice mid-veins is analogous with that in BS cells of C4 plants beyond the typical range observed for C3 leaf laminae (Marshall et al. 2007; Kocurek and Pilarski 2011). Therefore, the location of chlorophyllous cells near PCSVS could be advantageous in terms of the carbon assimilation, which allow the decarboxylation of malate from the xylem and phloem, thus releasing CO2 for C3 cycle.
Indeed, these enzymes recruited into C4 photosynthesis fulfill conserved roles in distantly related C3 plants. During C3 plant defense response, PEPCK provides PEP to the shikimate pathway for the biosynthesis of aromatic compounds (Leegood et al. 1999; Lai et al. 2002). PPDK increases in rice roots during anoxia (Moons et al. 1998). NADP-ME2 also appears to be involved in the generation of reactive oxygen species (Voll et al. 2012). Especially, those members abundant in the PCSVS play an indispensable role in C3 plants. For example, in Arabidopsis, cucumber, and grape, PEPCK is suggested to be localized in phloem companion cells, where it may function in nitrogenous metabolism and pH regulation (Walker et al. 1999; Delgado-Alvarado et al. 2007; Malone et al. 2007); NAD-ME, NADP-ME and PPDK would also be linked with vascular bundles of C3 plants. NADP-ME and NAD-ME operate in the decarboxylation direction to supply CO2 to photosynthesis in mid-veins, and regulate the flux of carbon into soluble sugars, amino acids and glucosamine (Hibberd and Quick 2002; Brown et al. 2010).
Meanwhile, our results also found lower activities (Fig. 2a) and accumulations (Fig. 2b, c) of RuBisCO in rice mid-veins. It is consistent with the observation in Arabidopsis grown under elevated CO2 conditions (Bae and Sicher 2004), and in BS cells of NADP-ME type C4 plants. It is proposed that the elevated CO2 reduces the requirement for low-CO2-affinity RuBisCO in mid-veins.
Rice mid-veins are equipped with C4-like photosystems, with lower linear electron transport
Leaf laminae chloroplasts of C3 plants function primarily in linear electron transport, which produces 3 ATP and 2 NADPH per O2 evolved to meet the requirements for C3 cycle (Finazzi et al. 2002). In contrast, in the NADP-ME type C4 plants, the decarboxylation of malate in BS cells results in a donation of NADPH. Therefore, Chl a fluorescence results from BS cells of C4 maize show the closure of PSII reaction center (RC) and a low PSII activity (Ivanov et al. 2005). BS cells lack PSII-initiated linear electron transport and only function to generate ATP by PSI-mediated cyclic electron flow. The ATP production per NADPH is about 2-fold higher in BS chloroplasts than that in M chloroplasts (Voznesenskaya et al. 1999).
Higher activity of three C4 acid decarboxylases also allows PCSVS in C3 mid-veins to decarboxylate malate for generating NADPH (Hibberd and Quick 2002). Hence, mid-veins similarly encounter the particular metabolic demands of a higher ATP/NADPH ratio as BS cells of C4 plants (Kotakis et al. 2006; Kalachanis and Manetas 2010). Since the demands would further shape the structure and function of photosystems and the associated electron flow (Kalachanis and Manetas 2010), we decided to further evaluate the differences of photosystems between mid-veins and leaf laminae by the non-invasive spectral technology.
Vt from Ft was higher in mid-veins than leaf laminae (Fig. 4a), indicating that the fraction of closed PSII RCs is higher at any time (Kalachanis and Manetas 2010; Yiotis and Manetas 2010) in rice mid-veins. The significantly increased initial slope of the fluorescence transient (MO) and decreased average redox state of QA−/QA in the time span from 0 to tFM (Sm/tFM) (Fig. 5b) in mid-veins also demonstrate that mid-veins have a higher proportion of closed RCs of PSII (Chen and Cheng 2009). The positive value of L-bands indicates a low energetic connectivity/grouping of PSII units (Strasser et al. 2004). Hence, the higher L-bands in mid-veins (Fig. 4b) further suggest that the closed RCs of PSII lose stability and become inefficient to transfer energy.
ΔVt (Fig. 4a) uncovered the main bottleneck of PSII occurring at K step. The appearance of positive K-bands in mid-veins (Fig. 4c) reflects an inactivation of the OEC at the donor side of PSII (Yusuf et al. 2010). The destruction of OEC, also supported by higher VK/VJ quantitation (Fig. 5b) and a decrease in ΔMRslow/MRO (Oukarroum et al. 2012), would allow light to over-energize PSII and deactivate PSII RCs. Meanwhile, the lower γRC (the fraction of PSII Chl a molecules that function as RCs), and higher ABS/RC (a relative measure of antenna size feeding active RCs) (Fig. 5) in mid-veins suggest that the harvesting antenna complexes (LHC) of PSII were relatively larger than active RCs (Kirst et al. 2012). Therefore, higher proportions of absorbed energy in mid-veins needs to be dissipated as heat, which was supported by the higher DIo/RC (dissipated energy flux per RC) and DIO/ABS (quantum yield for energy dissipation, Fig. 5a). An inactivated fraction of RCs also forced each RC to bear more specific fluxes of absorbed, trapped energy, electron transfer and reduction of PSI end electron acceptors (ABS/RC, TRO/RC, ETO/RC and REO/RC, Fig. 5a) in mid-veins.
IP phase depends on electron transfer through PSI and is caused by the transient block at the acceptor side of PSI (NADP+) (Schansker et al. 2005). The maximal amplitude of IP phase, parameterized by 1/VI, represents the relative pool size of the final electron acceptors of PSI (Yusuf et al. 2010). As NADP+ is continuously consumed by C4 acid decarboxylases, mid-veins are supposed to have a smaller pool of PSI end electron acceptors with the lower maximal amplitude of IP (Fig. 4d) and 1/VI (Fig. 5b).
On the other hand, the operation of cyclic electron flow in C4 BS chloroplasts is also studied by the redox kinetics of P700+, which predominantly excites PSI. Since the electron flow solely from PSII is insufficient to reduce the active PSI RCs, the decarboxylation of malate generates NADPH as an electron donor to PSI for a larger cyclic electron flow (Ivanov et al. 2005). Our results similarly showed that the activation status of PSI RC (P700+), as indicated by ΔMRfast/MRO (Fig. 6b), in rice mid-veins was kept as high as in leaf laminae. Consequently, filling of PSI acceptors with electrons proceeded faster in mid-veins (Fig. 4d), resulting in the shorter time needed for half saturation of these pools with electrons donated by intermediate carriers (t1/2(I−P), Fig. 5b). This phenomenon is also observed in pericarps of Nerium oleander where the completion of electron flow toward the acceptor side of PSI is likely facilitated due to the reduced final electron acceptor pools of PSI and their high affinities for electrons (Kalachanis and Manetas 2010). Therefore, the quantum yield for reduction of end electron acceptors at the PSI acceptor side (REO/ABS), and efficiency/probability that a trapped excitation can move an electron into the electron transport chain from QA- to the PSI end electron acceptors (REO/TRO) were lower in mid-veins (Fig. 5a). Better-preserved pools of intermediate carriers (Sm, Fig. 5b) and activation status of PSI RC (P700+) (ΔMRfast/MRO, Fig. 6c) further ensured mid-veins to achieve higher cyclic electron flow (Fig. 7a) by diverting electrons back to intermediate carriers around PSI (Kalachanis and Manetas 2010), while the maximal performance for linear electron transport from water to reduce the end electron acceptor, PItotal (Fig. 5b), was still precluded with lower efficiency/probability that an electron comes into the linear electron transport chain beyond QA− of PSII RCs (ETO/TRO and ETO/ABS accordingly, Fig. 5a).
Rice mid-veins lacking linear electron flow is attributed to a selective loss of PSII-bound subunits
In C4 plants, M thylakoids have a complete linear electron transport chain, containing PSII, Cytb6f, and PSI complexes, similar to C3 leaf laminae. In contrast, BS thylakoids contain fewer functional PSII, but normal number of PSI, Cytb6f and ATP synthase complexes which primarily participate in cyclic electron transport. Subunits of PSI, LHCI, Cyt b6f, and ATP synthase complexes show BS/M accumulation ratios of 1.6, 1.72, 1.0, and 1.33, respectively, whereas ratios for the PSII and LHCII were 0.45 and 0.68, respectively (Wojciech et al. 2008).
As direct molecular evidence for the physiological data, BN-PAGE and immunoblot analysis of the thylakoid photosynthetic apparatus prove an uneven distribution of photosystems between mid-veins and leaf laminae in rice. Either the integrated forms or CP43-less core of PSII supercomplexes was far below detections in leaf laminae (Fig. 8). The loss of PSII complexes explains the dysfunction of PSII and the hindered linear electron transport rates in mid-veins as mention above. Considering the subsets of PSII supercomplexes, the accumulation of LHCII trimers and their subunits (Lhcb1 and Lhcb2) was reduced less than PsbA (PSII core subunit) in rice mid-veins (Fig. 9b). It corresponds well to the fluorescence results that LHCII is larger than active PSII RCs.
Early studies in NADP-ME type C4 species maize (Zea may) and sorghum (Sorghum bicolor) show that limited PSII activities in chloroplasts of BS cells are mainly caused by the depletion in PsbP and PsbQ of OEC and PsbR, which play an important role in water oxidation (Meierhoff and Westhoff 1993). Likewise, the loss of PsbP, PsbQ and PsbR in mid-veins was more than other PSII subunits (Fig. 9b), although not as significant as that in BS cells of C4 plants (Meierhoff and Westhoff 1993). Hence, K-bands (inactivation of OEC, Fig. 4c) in rice mid-veins also perhaps result from the selective loss of distinct OEC polypeptides (PsbP and PsbQ). An RNAi-induced Arabidopsis mutant lacking detectable PsbP proteins exhibits a significant defect in electron transfer from QA− to QB with loss of the J to I transition of Ft, and shows seriously retarded charge recombination between QA− and OEC (Andréasson et al. 1995). Therefore, removals of PsbP and PsbQ of OEC are speculated to be the most important reasons for introducing linear electron transport defects in the PSII.
Furthermore, rice mid-veins assembled intact PSI core complex (Fig. 8), PsaA (PSI core subunit), and Lhca1 and Lhca2 (LHCI subunits, Fig. 9b) into the oligomeric forms of PSI-LHCI (Fig. 8). Cyt b6f and ATP synthase complexes (Fig. 8), as well as their subunits (Cytf, Cytb6 and Atpβ, Fig. 9b) were also well-preserved in rice mid-veins. If most of the excitation energy is utilized by PSI, cyclic electron transport around PSI and Cytb6f may prevail over linear electron flow mediated by both PSII and PSI (Finazzi et al. 2002). Therefore, the particular photosystems in mid-veins ensured the cyclic electron flow to work efficiently at the expense of the linear one (Fig. 7).
C4 photosynthesis is considered as one of the most convergent of the complex evolutionary phenomena on Earth, and the majority of C4 crop species have quite a degree of mechanistic flexibility and circumstantial superiority (Furbank 2011). Introducing their multigenic trait into rice has been recognized as an ambitious and multinational project for increasing rice yields. Without doubt, it still faces enormous challenges, because it is not clear how C4 photosynthesis has evolved independently from the ancestral C3 pathway (Sage et al. 2011), and how the metabolism of the rice leaf will be changed after introduction (Aubry et al. 2011). However, our finding that the existence of C4-like photosynthesis (high enrichment of C4 acid decarboxylases, lower linear electron transport and a selective loss of PSII-bound subunits) in rice mid-veins makes it seem more feasible to introduce components of the C4 pathway into rice and gives some indication that the evolution of C4 photosynthesis may not be as difficult as first appears (Kajala et al. 2011).
Conclusions
In summary, this was the first study showing higher levels of C4 cycle key enzymes and special adjustments in the photosynthetic machinery in rice mid-veins. The linear electron transport chain was mostly blocked by a “traffic jam” occurring on PSII complexes, whereas PSI in mid-veins is sufficient to support a larger cyclic one. The loss-of-function of PSII was primarily attributed to selective subunits, such as PsbP and PsbQ of OEC and PsbR. These attributes of photosynthetic machinery in rice mid-veins partly resemble that in the BS cells of C4 plants, as mentioned in Introduction. Hence, our findings indicate that the photosynthetic cells surrounding mid-veins in the rice, a typical C3 monocot, innately possess C4-like features as do other C3 dicots. Of course, further research is needed to extend these findings to more C3 plants. Such studies would provide a simple explanation for the polyphyletic evolution of C4 photosynthesis (Hibberd and Quick 2002).
Methods
Plant materials and growth conditions
Oryza sativa. cv. Liangyoupeijiu, a typical monocot C3 plant was pot-cultivated, watered and fertilized routinely in a net house under natural conditions at the Institute of Agricultural Sciences of Jiangsu Nanjing, China (32° 03’ N, 118° 47’ E). Air temperature, rainfall and global radiation were available in previous studies (Yu et al. 2012). The flag leaves of the main culm were sampled during mature stage (24 days after leaf emergence). To avoid regional discrepancy due to developmental stages, only midsections of flag leaves were utilized. For in vitro experiments, mid-veins were pulled out from leaf laminae according to a method developed by Brown et al. (2010), and both mid-veins and residual leaf laminae were immediately frozen in liquid nitrogen, and stored at −80 °C. Several plant samples were pooled to obtain sufficient material for further analyses. For fast Chl a fluorescence transients and modulated 820 nm reflection experiments in vivo, leaf laminae and mid-veins were still attached to the rice, and the measurements were operated at ambient temperature and 8–10 a.m.
Determination of chlorophyllous cell distribution
Transverse sections of leaves containing mid-veins and leaf laminae were cut manually using razor blades, and mixed with a glycerol-PBS solution (50 % glycerol, 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4, pH = 7.2) for optical and epifluorescence microscopy (Nikon CFI60, Tokyo, Japan). Chlorophyllous cells were visualized as bright red fluorescence (LP 520) when excited with blue light (BP 450–490) in epifluorescence microscopy.
Enzyme-linked assays
Enzymes were isolated according to Ivanov et al. (2006). Briefly, samples were ground in liquid nitrogen and extracted with 1 ml of a buffer containing 100 mM Tris/HCl (pH 7.6), 5 % (w/v) PVP-40, 0.85 % (w/v) BSA, and 10 mM DTT.
The enzyme-linked assays for detection of PEPCK (EC 4.1.1.32), NAD-ME (EC 1.1.1.39), NADP-ME (EC 1.1.1.40) and PPDK (EC 2.7.9.1) were conducted as described previously (Ashton et al. 1990; Marshall et al. 2007). Enzymatic activities of PEPC (EC 4.1.1.31) and RuBisCO (EC 4.1.1.39) were determined according to Tietz and Wild (1991) and Berveiller and Damesin (2007), respectively. Enzyme activity was expressed as μmol substrate consumed or product generated per second to total Chl content. Experiments were carried out at 25 °C.
Measurement of fast Chl a fluorescence transients (JIP curve, Ft) and modulated 820 nm reflection (MR)
Light intensity actually falling on a cylindrical mid-vein cannot be accurately determined and small sized mid-veins cannot fill the leaf clip space. Therefore, photon exchange between the instrument and mid-veins or leaf laminae was through a 3 mm × 15 mm rectangular window. The window was a non-fluorescing black tape aligned along the mid-vein axes which was made by ourselves according to the method of Manetas (2004). Leaf laminae and mid-veins, from the midsection of the same leaf and still attached to plants, were mounted in a leaf clip fitted by the window and dark adapted for 60 min before measurements.
Ft was captured by a Handy Plant Efficiency Analyzer (Hansatech Instruments Ltd, Norfolk, UK) according to Strasser and Srivastava (1995) after excitation by a band of three red light emitting diodes (650 nm, 3000 μmol photons m−2 s−1). Data were recorded from 20 μs to 1 s, and analyzed according to the JIP test (Strasser et al. 2004; Jiang et al. 2008) for calculation of structural and functional parameters. The corresponding definitions and calculations of the parameters were given in Additional file 1: Table S1. The average values were expressed as ratios to that of leaf laminae in radar plots. Extended analysis of Ft was done by normalization as various relative variable fluorescence (V or W) between different time points, according to previous studies (Strasser et al. 2007; Tsimilli-Michael and Strasser 2008). The difference fluorescence kinetics (ΔV or ΔW) between mid-veins and leaf laminae were calculated through the equation: ΔV(W) = V(W)mid ‐ vein – V(W)leaf lamina. See details below: (A) normalized between FO and FM: Vt = (Ft – FO)/(FM – FO), and △Vt was marked by the O, L, K, J, I, P steps. The graph was plotted on a logarithmic time scale (0.02 ms to 1 s). (B) normalized between FO and FK: Wok = (Ft – Fo)/(FK – Fo), and △WOk revealed L-bands which indicated the degree of energetic dis-connectivity (grouping) of the PSII units. The graph was plotted on a linear time scale (0.02 ms to 0.3 ms). (C) normalized between FO and FJ: WoJ = (Ft – FO)/(FJ – FO), and △WOJ revealed K-bands which indicated the degree of inactivation of OEC. The graph was plotted on a linear time scale (0.02 ms to 2 ms). (D) normalized between FI and FP: WIP = (Ft – FI)/(FP – FI), and horizontal dashed line at 0.5 indicated half time needed to reduce pool of the end electron acceptor with electrons donated by intermediate carriers. (E) normalized between FO and FI: WOI (≥1) = (Ft – FO)/(FI – FO), and the maximum amplitude of IP phase illustrated the differences in the pool size of the end electron acceptors of PSI. The graph was plotted on a linear time scale (30 ms to 400 ms).
Modulated reflection at 820 nm (MR) was recorded on a Multifunctional Plant Efficiency Analyzer M-PEA (Hansatech Instrument Ltd., Norfolk, UK), according to an operating protocol elucidated by Strasser et al. (2010). MR was normalized and expressed by MR/MRO on logarithmic time scale from 0 ms to 10 s, where MRO was the value at 0.7 ms (taken at the onset of the red light illumination, the first reliable MR measurement). The definition of characteristic parameters of MR/MRO kinetics (MRmin and MRmax) was shown on an inserted graph (Fig. 6a), where MRmin represented the minimal MR reached during the fast phase (i.e., between 0.7 ms and 10–200 ms) and MRmax was the maximal MR reached by the end of the slow phase (taken at 1 s). Each curve represented the average kinetics collected from 15 independent measurements of 15 individual plants and was used to calculate relevant parameters.
Isolation of thylakoid membrane and total soluble protein
Thylakoid membrane was isolated from mid-veins and leaf laminae as described earlier (Kang et al. 2012). The Chl content was determined spectrophotometrically in 80 % acetone (Arnon 1949). Total soluble proteins were isolated as described by Ku et al. (1999). PPDK proteins needed to be concentrated before immunoblot (Chastain et al. 2002). Protein concentration was determined using the method of Lowry et al. (1951), with bovine serum albumin as a standard.
Immunodetection of photosynthetic proteins
The isolated thylakoid membrane (for thylakoid membrane subunits) and total soluble protein (for key enzymes in C3 or C4 cycles) were pretreated with the loading buffer (0.5 M Tris–HCl, pH 6.8, 1 % SDS, 24 % glycerol, 4 % β-mercaptoethanol, and 0.001 % w/v bromophenol blue) and denatured for 10 min at 90 °C. Thylakoid membrane polypeptides (2 μg Chl per spot) or total soluble protein (15 μg protein per spot) from mid-veins and leaf laminae were separated by 12 % SDS-PAGE. After electrophoresis, gels were stained with Coomassie brilliant blue R-250 or transferred electrophoretically to PVDF membranes (0.2 μm pore size, Bio-Rad, USA). PVDF membranes were subsequently incubated with primary antibodies raised in rabbits against key enzymes in C3 or C4 cycles [the large subunit of RuBisCO, PEPC, PPDK, PEPCK (Agrisera, Sweden, http://www.agrisera.com/), NAD-ME and NADP-ME (Beijing Protein Innovation, China, http://proteomics.bioon.com.cn/)] and the thylakoid membrane subunits [Lhcb1, Lhcb2, Lhcb3, PsbA, PsbO, PsbP, PsbQ, PsbR, Lhca1, Lhca2, PsaA, Cytb6, Cytf, Atpβ (Agrisera, Sweden, http://www.agrisera.com/)] at recommended dilutions. Afterward, samples were incubated in goat anti-rabbit IgG conjugated with alkaline phosphatase (Bio-Rad, USA). The immunoblot signals were visualized using BCIP/NBT (Roche, Switzerland) as substrate according to Lindahl et al. (1996).
Blue native polyacrylamide gel electrophoresis (BN-PAGE)
For the separation of thylakoid membrane complexes, BN-PAGE was carried out in the procedure described by Chen et al. (2007) with some modifications. Briefly, the thylakoid membranes were washed with 330 mM sorbitol and 50 mM BisTris-HCl (pH 7.0), and then resuspended in buffer containing 20 % glycerol, 25 mM BisTris-HCl (pH 7.0) at 1.0 mg Chl ml−1. The suspension was solubilized with an equal volume of resuspension buffer containing 2 % (w/v) dodecyl-β-D-maltoside. After incubation at 4 °C for 30 min, insoluble material was removed by centrifugation at 40,000 × g for 10 min. The supernatant equal to 10 mg Chl was mixed with one-tenth volume of 1 % Coomassie brilliant blue G-250 in 100 mM BisTris-HCl (pH 7.0), 0.5 M 6-amino-n-caproic acid, and 30 % glycerol. The mixture was then applied to 1-mm-thick 5–12 % polyacrylamide gradient gels for electrophoresis. Electrophoresis was performed at 4 °C, 120 V for 5 h. High molecular weight native protein marker kit (GE Healthcare, Amersham-Pharmacia, 17-0445-01, UK) was used to determine the molecular mass of these complexes.
Quantitation of band signal in BN-PAGE and immunoblot analyses was performed using Quantity One, version: 4.52 (Bio-Rad, Hercules, USA) based on densitometric analysis. The experiments were repeated three times and the representative images were taken. The amount of protein was expressed as the percentage of that in leaf laminae.
Chloroplast isolation and cyclic/non-cyclic (linear) photophosphorylation rate assays
Chloroplast isolation was performed according to Ketcham et al. (1984). The cyclic/non-cyclic (linear) photophosphorylation activity of chloroplasts was assessed by using the luciferin-luciferase method to measure the amount of ATP synthesized within 2 min at a saturating irradiance of about 1,500 μmol quanta m−2 s−1 at 25 °C (Allnutt et al. 1991).
Statistical analyses
Statistical analyses were carried out using SPSS 15.00 statistical package (Chicago, USA). Parametric one-way ANOVA was used to determine statistical significance between leaf laminae and mid-veins. Differences in the measured parameters were considered significant at p < 0.05.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (grant no.31271621/C1302), the Natural Science Foundation of Jiangsu Province (grant no.11KJA180001), the Youth Natural Science Foundation of Jiangsu Province (grant No. BK20140916), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (grant no.14KJB180011), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). We are also very grateful to Dr. Tang. R.H (Eshelman School of Pharmacy, University of North Carolina) who reviewed the manuscript and Dr. Chen S.G. (Weed Research Laboratory, Nanjing Agricultural University) who provided the M-PEA instrument.
Abbreviations
- ABS
absorption flux
- BN-PAGE
blue native polyacrylamide gel electrophoresis
- BS
bundle sheath
- Chl
chlorophyll
- DIO
dissipated energy flux
- ECO
total electron carriers
- ETO
electron transport flux
- Ft
fast chlorophyll fluorescence kinetics
- M
mesophyll
- MR
modulated reflection at 820 nm
- NAD-ME
nicotinamide adenine dinucleotide-dependent malic enzyme
- NADPH
the reduced form of nicotinamide adenine dinucleotide phosphate
- NADP-ME
nicotinamide adenine dinucleotide phosphate-dependent malic enzyme
- OEC
oxygen evolving complex
- PCSVS
photosynthetic cells surrounding the vascular system
- PEPC
phosphoenolpyruvate carboxylase
- PEPCK
phosphoenolpyruvate carboxykinase
- PPDK
pyruvate phosphate dikinase
- PSI
photosystem I
- PSII
photosystem II
- RC
reaction center
- REO
reduction of end acceptors of PSI
- RuBisCO
ribulose-1, 5-bisphosphate carboxylase/oxygenase
- TRO
trapped energy flux
Additional file
Formulae and definitions of the selected JIP-test fluorescence parameters used in this study. (DOCX 38 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
WS and ZG participated in the design of the study, the analysis and interpretation of data, and performed the determination of chlorophyllous cell distribution and pigments. GC carried out the immunoassays. LY carried out chloroplast isolation and cyclic/non-cyclic (linear) photophosphorylation rate assays. ZY performed fast chlorophyll a fluorescence transient assays. CL carried out enzyme-linked assays. ZZ and XC participated in results interpretation and the manuscript revision. All authors read and approved the final manuscript.
Contributor Information
Zhiping Gao, Phone: +862585891079, Email: 1053803594@qq.com, Email: ketty.gao@gmail.com.
Guoxiang Chen, Email: gxchen@njnu.edu.cn.
References
- Allnutt FCT, Ewy R, Renganathan M, Pan RS, Dilley RA. Nigericin and hexylamine effects on localized proton gradients in thylakoids. Biochim Biophys Acta Bioenerg. 1991;1059(1):28–36. doi: 10.1016/S0005-2728(05)80184-7. [DOI] [PubMed] [Google Scholar]
- Andréasson L-E, Vass I, Styring S. Ca2+ depletion modifies the electron transfer on both donor and acceptor sides in photosystem II from spinach. Biochim Biophys Acta Bioenerg. 1995;1230(3):155–164. doi: 10.1016/0005-2728(95)00047-M. [DOI] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton AR, Burnell JN, Furbank RT, Jenkins CLD, Hatch MD. Methods in Plant Biochemistry. London: Academic; 1990. Enzymes of C4 photosynthesis. [Google Scholar]
- Aubry S, Brown NJ, Hibberd JM. The role of proteins in C3 plants prior to their recruitment into the C4 pathway. J Exp Bot. 2011;62(9):3049–3059. doi: 10.1093/jxb/err012. [DOI] [PubMed] [Google Scholar]
- Bae H, Sicher R. Changes of soluble protein expression and leaf metabolite levels in Arabidopsis thaliana grown in elevated atmospheric carbon dioxide. Field Crops Res. 2004;90(1):61–73. doi: 10.1016/j.fcr.2004.07.005. [DOI] [Google Scholar]
- Berveiller D, Damesin C. Carbon assimilation by tree stems: potential involvement of phosphoenolpyruvate carboxylase. Trees. 2007;22(2):149–157. doi: 10.1007/s00468-007-0193-4. [DOI] [Google Scholar]
- Berveiller D, Damesin C. Carbon assimilation by tree stems: potential involvement of phosphoenol pyruvate carboxylase. Trees Struct Funct. 2008;22(2):149–157. doi: 10.1007/s00468-007-0193-4. [DOI] [Google Scholar]
- Brown NJ, Palmer BG, Stanley S, Hajaji H, Janacek SH, Astley HM, Parsley K, Kajala K, Quick WP, Trenkamp S, Fernie AR, Maurino VG, Hibberd JM. C4 acid decarboxylases required for C4 photosynthesis are active in the mid-vein of the C4 species Arabidopsis thaliana, and are important in sugar and amino acid metabolism. Plant J. 2010;61(1):122–133. doi: 10.1111/j.1365-313X.2009.04040.x. [DOI] [PubMed] [Google Scholar]
- Chastain CJ, Fries JP, Vogel JA, Randklev CL, Vossen AP, Dittmer SK, Watkins EE, Fiedler LJ, Wacker SA, Meinhover KC, Sarath G, Chollet R. Pyruvate, orthophosphate dikinase in leaves and chloroplasts of C(3) plants undergoes light-/dark-induced reversible phosphorylation. Plant Physiol. 2002;128(4):1368–1378. doi: 10.1104/pp.010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L-S, Cheng L. The acceptor side of photosystem II is damaged more severely than the donor side of photosystem II in ‘Honeycrisp’ apple leaves with zonal chlorosis. Acta Physiol Plant. 2009;32(2):253–261. doi: 10.1007/s11738-009-0402-4. [DOI] [Google Scholar]
- Chen X, Zhang W, Xie Y, Lu W, Zhang R. Comparative proteomics of thylakoid membrane from a chlorophyll b-less rice mutant and its wild type. Plant Sci. 2007;173(4):397–407. doi: 10.1016/j.plantsci.2007.06.012. [DOI] [Google Scholar]
- Delgado-Alvarado A, Walker RP, Leegood RC. Phosphoenolpyruvate carboxykinase in developing pea seeds is associated with tissues involved in solute transport and is nitrogen-responsive. Plant Cell Environ. 2007;30(2):225–235. doi: 10.1111/j.1365-3040.2006.01622.x. [DOI] [PubMed] [Google Scholar]
- Finazzi G, Rappaport F, Furia A, Fleischmann M, Rochaix J-D, Zito F, Forti G. Involvement of state transitions in the switch between linear and cyclic electron flow in Chlamydomonas reinhardtii. Embo Rep. 2002;3(3):280–285. doi: 10.1093/embo-reports/kvf047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank RT. Evolution of the C4 photosynthetic mechanism: are there really three C4 acid decarboxylation types? J Exp Bot. 2011;62(9):3103–3108. doi: 10.1093/jxb/err080. [DOI] [PubMed] [Google Scholar]
- Geigenberger P. Response of plant metabolism to too little oxygen. Curr Opin Plant Biol. 2003;6(3):247–256. doi: 10.1016/S1369-5266(03)00038-4. [DOI] [PubMed] [Google Scholar]
- Griffiths H, Weller G, Toy LF, Dennis RJ. You're so vein: bundle sheath physiology, phylogeny and evolution in C3 and C4 plants. Plant Cell Environ. 2012 doi: 10.1111/j.1365-3040.2012.02585.x. [DOI] [PubMed] [Google Scholar]
- Hibberd JM, Quick WP. Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature. 2002;415(6870):451–454. doi: 10.1038/415451a. [DOI] [PubMed] [Google Scholar]
- Ivanov AG, Krol M, Sveshnikov D, Malmberg G, Gardestrom P, Hurry V, Oquist G, Huner NP. Characterization of the photosynthetic apparatus in cortical bark chlorenchyma of Scots pine. Planta. 2006;223(6):1165–1177. doi: 10.1007/s00425-005-0164-1. [DOI] [PubMed] [Google Scholar]
- Ivanov B, Asada K, Kramer DM, Edwards G. Characterization of photosynthetic electron transport in bundle sheath cells of maize. I. Ascorbate effectively stimulates cyclic electron flow around PSI. Planta. 2005;220(4):572–581. doi: 10.1007/s00425-004-1367-6. [DOI] [PubMed] [Google Scholar]
- Jiang H-X, Chen L-S, Zheng J-G, Han S, Tang N, Smith BR. Aluminum-induced effects on photosystem II photochemistry in Citrus leaves assessed by the chlorophyll a fluorescence transient. Tree Physiol. 2008;28(12):1863–1871. doi: 10.1093/treephys/28.12.1863. [DOI] [PubMed] [Google Scholar]
- Kajala K, Covshoff S, Karki S, Woodfield H, Tolley BJ, Dionora MJ, Mogul RT, Mabilangan AE, Danila FR, Hibberd JM, Quick WP. Strategies for engineering a two-celled C4 photosynthetic pathway into rice. J Exp Bot. 2011;62(9):3001–3010. doi: 10.1093/jxb/err022. [DOI] [PubMed] [Google Scholar]
- Kalachanis D, Manetas Y. Analysis of fast chlorophyll fluorescence rise (O-K-J-I-P) curves in green fruits indicates electron flow limitations at the donor side of PSII and the acceptor sides of both photosystems. Physiol Plant. 2010;139(3):313–323. doi: 10.1111/j.1399-3054.2010.01362.x. [DOI] [PubMed] [Google Scholar]
- Kang Z, Li G, Huang J, Niu X, Zou H, Zang G, Wenwen Y, Wang G. Photosynthetic and physiological analysis of the rice high-chlorophyll mutant (Gc) Plant Physiol Biochem. 2012;60:81–87. doi: 10.1016/j.plaphy.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Ketcham SR, Davenport JW, Warncke K, McCarty RE. Role of the gamma subunit of chloroplast coupling factor 1 in the light-dependent activation of photophosphorylation and ATPase activity by dithiothreitol. J Biol Chem. 1984;259(11):7286–7293. [PubMed] [Google Scholar]
- Kinsman EA, Pyke KA. Bundle sheath cells and cell-specific plastid development in Arabidopsis leaves. Development. 1998;125(10):1815–1822. doi: 10.1242/dev.125.10.1815. [DOI] [PubMed] [Google Scholar]
- Kirst H, Garcia-Cerdan JG, Zurbriggen A, Ruehle T, Melis A. Truncated photosystem chlorophyll antenna size in the green microalga Chlamydomonas reinhardtii upon deletion of the TLA3-CpSRP43 gene. Plant Physiol. 2012;160(4):2251–2260. doi: 10.1104/pp.112.206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocurek M, Pilarski J. Activity of C4 enzymes in C3-type herbaceous plants. Photosynthetica. 2011;49(3):473–477. doi: 10.1007/s11099-011-0053-8. [DOI] [Google Scholar]
- Kotakis C, Petropoulou Y, Stamatakis K, Yiotis C, Manetas Y. Evidence for active cyclic electron flow in twig chlorenchyma in the presence of an extremely deficient linear electron transport activity. Planta. 2006;225(1):245–253. doi: 10.1007/s00425-006-0327-8. [DOI] [PubMed] [Google Scholar]
- Ku MSB, Agarie S, Nomura M, Fukayama H, Tsuchida H, Ono K, Hirose S, Toki S, Miyao M, Matsuoka M. High-level expression of maize phosphoenolpyruvate carboxylase in transgenic rice plants. Nat Biotech. 1999;17(1):76–80. doi: 10.1038/5256. [DOI] [PubMed] [Google Scholar]
- Lai LB, Tausta SL, Nelson TM. Differential regulation of transcripts encoding cytosolic NADP-malic enzyme in C3 and C4 Flaveria species. Plant Physiol. 2002;128(1):140–149. doi: 10.1104/pp.010449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegood R, Acheson R, Técsi L, Walker R. The many-faceted function of phosphoenolpyruvate carboxykinase in plants. In: Kruger N, Hill S, Ratcliffe RG, editors. Regulation of Primary Metabolic Pathways in Plants, vol 42. Proceedings of the Phytochemical Society of Europe. Netherlands: Springer; 1999. pp. 37–51. [Google Scholar]
- Leegood RC. Roles of the bundle sheath cells in leaves of C3 plants. J Exp Bot. 2008;59(7):1663–1673. doi: 10.1093/jxb/erm335. [DOI] [PubMed] [Google Scholar]
- Lindahl M, Tabak S, Cseke L, Pichersky E, Andersson B, Adam Z. Identification, characterization, and molecular cloning of a homologue of the bacterial FtsH protease in chloroplasts of higher plants. J Biol Chem. 1996;271(46):29329–29334. doi: 10.1074/jbc.271.46.29329. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- Majeran W, van Wijk KJ. Cell-type-specific differentiation of chloroplasts in C4 plants. Trends Plant Sci. 2009;14(2):100–109. doi: 10.1016/j.tplants.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Malone S, Chen ZH, Bahrami AR, Walker RP, Gray JE, Leegood RC. Phosphoenolpyruvate carboxykinase in Arabidopsis: changes in gene expression, protein and activity during vegetative and reproductive development. Plant Cell Physiol. 2007;48(3):441–450. doi: 10.1093/pcp/pcm014. [DOI] [PubMed] [Google Scholar]
- Manetas Y. Probing corticular photosynthesis through in vivo chlorophyll fluorescence measurements: evidence that high internal CO2 levels suppress electron flow and increase the risk of photoinhibition. Physiol Plantarum. 2004;120(3):509–517. doi: 10.1111/j.0031-9317.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Marshall DM, Muhaidat R, Brown NJ, Liu Z, Stanley S, Griffiths H, Sage RF, Hibberd JM. Cleome, a genus closely related to Arabidopsis, contains species spanning a developmental progression from C3 to C4 photosynthesis. Plant J. 2007;51(5):886–896. doi: 10.1111/j.1365-313X.2007.03188.x. [DOI] [PubMed] [Google Scholar]
- Meierhoff K, Westhoff P. Differential biogenesis of photosystem II in mesophyll and bundle-sheath cells of monocotyledonous NADP-malic enzyme-type C4 plants: the non-stoichiometric abundance of the subunits of photosystem II in the bundle-sheath chloroplasts and the translational activity of the plastome-encoded genes. Planta. 1993;191(1):23–33. doi: 10.1007/BF00240892. [DOI] [Google Scholar]
- Moons A, Valcke R, Van Montagu M. Low-oxygen stress and water deficit induce cytosolic pyruvate orthophosphate dikinase (PPDK) expression in roots of rice, a C3 plant. Plant J. 1998;15(1):89–98. doi: 10.1046/j.1365-313X.1998.00185.x. [DOI] [PubMed] [Google Scholar]
- Oukarroum A, Strasser RJ, Schansker G. Heat stress and the photosynthetic electron transport chain of the lichen Parmelina tiliacea (Hoffm.) Ach. in the dry and the wet state: differences and similarities with the heat stress response of higher plants. Photosynth Res. 2012;111(3):303–314. doi: 10.1007/s11120-012-9728-7. [DOI] [PubMed] [Google Scholar]
- Raven JA. Long-term functioning of enucleate sieve elements: possible mechanisms of damage avoidance and damage repair. Plant Cell Environ. 1991;14(2):139–138. doi: 10.1111/j.1365-3040.1991.tb01330.x. [DOI] [Google Scholar]
- Sage RF, Christin PA, Edwards EJ. The C4 plant lineages of planet Earth. J Exp Bot. 2011;62(9):3155–3169. doi: 10.1093/jxb/err048. [DOI] [PubMed] [Google Scholar]
- Schansker G, Toth SZ, Strasser RJ. Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim Biophys Acta. 2005;1706(3):250–261. doi: 10.1016/j.bbabio.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Strasser R, Tsimilli-Michael M, Dangre D, Rai M. Biophysical phenomics reveals functional building blocks of plants systems biology: a case study for the evaluation of the impact of mycorrhization with Piriformospora indica. In: Varma A, Oelmüller R, editors. Advanced Techniques in Soil Microbiology. Berlin Heidelberg: Springer; 2007. pp. 319–341. [Google Scholar]
- Strasser R, Tsimilli-Michael M, Srivastava A. Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou G, Govindjee, editors. Chlorophyll a Fluorescence, vol 19. Advances in Photosynthesis and Respiration. Netherlands: Springer; 2004. pp. 321–362. [Google Scholar]
- Strasser RJ, Srivastava A. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria*. Photochem Photobiol. 1995;61(1):32–42. doi: 10.1111/j.1751-1097.1995.tb09240.x. [DOI] [Google Scholar]
- Strasser RJ, Tsimilli-Michael M, Qiang S, Goltsev V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim Biophys Acta. 2010;1797(6–7):1313–1326. doi: 10.1016/j.bbabio.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Takabayashi A, Ishikawa N, Obayashi T, Ishida S, Obokata J, Endo T, Sato F. Three novel subunits of Arabidopsis chloroplastic NAD(P)H dehydrogenase identified by bioinformatic and reverse genetic approaches. Plant J. 2009;57(2):207–219. doi: 10.1111/j.1365-313X.2008.03680.x. [DOI] [PubMed] [Google Scholar]
- Tietz S, Wild A. Investigations on the phosphoenolpyruvate carboxylase activity of spruce needles relative to the occurrence of novel forest decline. J Plant Physiol. 1991;137(3):327–331. doi: 10.1016/S0176-1617(11)80140-9. [DOI] [Google Scholar]
- Tsimilli-Michael M, Strasser RJ. Mycorrhiza: Genetics and Molecular Biology, Eco-function, Biotechnology, Eco-physiology, and Structure and Systematics. 2008. In vivo assessment of stress impact on plant’s vitality: applications in detecting and evaluating the beneficial role of mycorrhization on host plants; pp. 679–703. [Google Scholar]
- Voll LM, Zell MB, Engelsdorf T, Saur A, Wheeler MG, Drincovich MF, Weber AP, Maurino VG. Loss of cytosolic NADP-malic enzyme 2 in Arabidopsis thaliana is associated with enhanced susceptibility to Colletotrichum higginsianum. New Phytol. 2012;195(1):189–202. doi: 10.1111/j.1469-8137.2012.04129.x. [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Franceschi VR, Pyankov VI, Edwards GE. Anatomy, chloroplast structure and compartmentation of enzymes relative to photosynthetic mechanisms in leaves and cotyledons of species in the tribe Salsoleae (Chenopodiaceae) J Exp Bot. 1999;50(341):1779–1717. doi: 10.1093/jxb/50.341.1779. [DOI] [Google Scholar]
- Walker RP, Chen Z-H, Técsi LI, Famiani F, Lea PJ, Leegood RC. Phosphoenolpyruvate carboxykinase plays a role in interactions of carbon and nitrogen metabolism during grape seed development. Planta. 1999;210(1):9–18. doi: 10.1007/s004250050648. [DOI] [PubMed] [Google Scholar]
- Wittmann C, Pfanz H, Loreto F, Centritto M, Pietrini F, Alessio G. Stem CO2 release under illumination: corticular photosynthesis, photorespiration or inhibition of mitochondrial respiration? Plant Cell Environ. 2006;29(6):1149–1158. doi: 10.1111/j.1365-3040.2006.01495.x. [DOI] [PubMed] [Google Scholar]
- Wojciech M, Zybailov B, Ytterberg AJ, Dunsmore J, Sun Q, Wijk KJ. Consequences of C4 differentiation for chloroplast membrane proteomes in maize mesophyll and bundle sheath cells. Mol Cell Proteomics. 2008;7:30. doi: 10.1074/mcp.M800016-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiotis C, Manetas Y. Sinks for photosynthetic electron flow in green petioles and pedicels of Zantedeschia aethiopica: evidence for innately high photorespiration and cyclic electron flow rates. Planta. 2010;232(2):523–531. doi: 10.1007/s00425-010-1193-y. [DOI] [PubMed] [Google Scholar]
- Yiotis C, Petropoulou Y, Manetas Y. Evidence for light-independent and steeply decreasing PSII efficiency along twig depth in four tree species. Photosynthetica. 2009;47(2):231. doi: 10.1007/s11099-009-0036-1. [DOI] [Google Scholar]
- Yoshimura Y, Kubota F, Ueno O. Structural and biochemical bases of photorespiration in C4 plants: quantification of organelles and glycine decarboxylase. Planta. 2004;220(2):307–317. doi: 10.1007/s00425-004-1335-1. [DOI] [PubMed] [Google Scholar]
- Yu GH, Li W, Yuan ZY, Cui HY, Lv CG, Gao ZP, Han B, Gong YZ, Chen GX. The effects of enhanced UV-B radiation on photosynthetic and biochemical activities in super-high-yield hybrid rice Liangyoupeijiu at the reproductive stage. Photosynthetica. 2012;51(1):33–44. doi: 10.1007/s11099-012-0081-z. [DOI] [Google Scholar]
- Yusuf MA, Kumar D, Rajwanshi R, Strasser RJ, Tsimilli-Michael M, Govindjee SNB. Overexpression of gamma-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: physiological and chlorophyll a fluorescence measurements. Biochim Biophys Acta. 2010;1797(8):1428–1438. doi: 10.1016/j.bbabio.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Zhang CJ, Chen L, Shi DW, Chen GX, Lu CG, Wang P, Wang J, Chu HJ, Zhou QC, Zuo M, Sun L. Characteristics of ribulose-1,5-bisphosphate carboxylase and C4 pathway key enzymes in flag leaves of a super-high-yield hybrid rice and its parents during the reproductive stage. S Afr J Bot. 2007;73(1):22–28. doi: 10.1016/j.sajb.2006.05.002. [DOI] [Google Scholar]


