Abstract
Aims
The primary aim of this study is to provide data to inform the design of a randomized controlled clinical trial (RCT) of a palliative care (PC) intervention in heart failure (HF). We will identify an appropriate study population with a high prevalence of PC needs defined using quantifiable measures. We will also identify which components a specific and targeted PC intervention in HF should include and attempt to define the most relevant trial outcomes.
Methods
An unselected, prospective, near‐consecutive, cohort of patients admitted to hospital with acute decompensated HF will be enrolled over a 2‐year period. All potential participants will be screened using B‐type natriuretic peptide and echocardiography, and all those enrolled will be extensively characterized in terms of their HF status, comorbidity, and PC needs. Quantitative assessment of PC needs will include evaluation of general and disease‐specific quality of life, mood, symptom burden, caregiver burden, and end of life care. Inpatient assessments will be performed and after discharge outpatient assessments will be carried out every 4 months for up to 2.5 years. Participants will be followed up for a minimum of 1 year for hospital admissions, and place and cause of death. Methods for identifying patients with HF with PC needs will be evaluated, and estimates of healthcare utilisation performed.
Conclusion
By assessing the prevalence of these needs, describing how these needs change over time, and evaluating how best PC needs can be identified, we will provide the foundation for designing an RCT of a PC intervention in HF.
Keywords: Heart failure, Palliative care
Background
Heart failure (HF) is common, affecting 1–2% of the general population with the prevalence rising to over 10% in those aged over 80 years.1, 2 HF leads to a reduced life expectancy, with 5 and 10 year survival rates of 50 and 10% ,respectively, reported in epidemiological studies.3, 4 These poor survival rates have led to comparisons with cancer.5 Therefore, it would seem intuitive that some patients with HF may benefit from specialist palliative care (PC), given the poor survival and high symptom and care‐giver burden associated with this condition. The World Health Organization (WHO) defines PC as an approach that improves the quality of life (QOL) of patients and their families facing the problem associated with life‐threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial, and spiritual.6 Contemporary HF guidelines have begun to recommend that PC be considered in patients with advanced HF refractory to optimal, or maximally tolerated, contemporary, disease‐modifying drug and device therapies, and where the resultant symptom burden is high and prognosis poor.7, 8, 9, 10 These recommendations are, however, based on relatively limited evidence and no substantial and systematic study of the actual and specific PC needs of patients with HF (and their caregivers) has been undertaken. It is possible that despite a reduced life‐expectancy, many patients with HF do not require specific or specialist PC—for example those who remain only slightly functionally limited by symptoms before a sudden death (and sudden death is relatively more common in patients with mild symptoms).11 However, a proportion of patients with HF almost certainly do require PC but, as yet, we do not know how large or small this group is.
Although many small studies and recent systematic reviews of these have suggested likely unmet PC needs of patients with HF,12, 13 the true prevalence of these needs is difficult to quantify for a number of reasons. As well as being small, most of the studies included highly selected patients or described cohorts with HF and a variety of other conditions. Secondly, the patients with HF were often not clearly described, and because natriuretic peptides and echocardiography were rarely used, the diagnosis of HF in some is uncertain. Furthermore, any differences in PC needs between HF with preserved ejection fraction (HF‐PEF), which accounts for half of all cases in some cohorts,14, 15 and HF with reduced ejection fraction (HF‐REF) have not been described. Most studies to date have been cross‐sectional and have not, therefore, described how PC needs change over time. Finally, many of the existing studies have used qualitative techniques, meaning their findings cannot be quantified or translated to other populations. A systematic study describing the prevalence of PC needs (and the specifics of these), using quantifiable measures, in an unselected, real‐life cohort of patients with HF, is required. As HF is a condition known to fluctuate, a single assessment, cross‐sectional study design is unlikely to identify those with a sustained need for a palliative‐intervention, and serial measurement is essential.
Whether PC is a worthwhile intervention in HF is unknown because no substantial randomized controlled clinical trial (RCT) has been performed. An RCT of PC in terminal conditions is, however, possible. One RCT of an early PC intervention in patients with lung cancer has been performed, with a suggestion that early PC can improve QOL and reduce healthcare utilisation.16 However, a more recent RCT of early PC in patients with advanced cancer did not show a significant improvement in the primary outcome of QOL in the early PC arm.17 While some of these results are encouraging, the symptoms and needs of patients with HF who are approaching the end of life may be quite different from patients with cancer, and the design of a potential RCT of PC in HF must consider this. Measures for assessing PC needs in patients with HF require further evaluation before being applied in a large RCT in HF. Another notable trial was conducted in patients deemed terminally ill as a result of a number of different illness and were housebound (33% of study participants had HF, 47% had cancer, and 21% had chronic obstructive airways disease). This trial compared usual care to usual care plus PC at home, delivered by a multi‐disciplinary team.18 Participants in the PC arm experienced significantly greater patient satisfaction, reduced use of medical services and healthcare costs, and were more likely to die at home than in a hospital. Although this study was well designed and informative, the cohort was mixed and the characteristics of the patients with HF were not clearly described. In particular, there was no differentiation between HF‐PEF and HF‐REF and no description of HF treatment. More recently, a single centre, RCT of PC in HF, randomized 72 outpatients with HF‐REF NYHA class III/IV to receive either early PC and HF care together or standard care.19 Assessments of QOL and symptoms were made at 1, 3 and 6 months and patients were followed up for HF hospitalisations for 6 months. The PC arm experienced greater improvements in QOL and had fewer HF hospitalisations. These results are encouraging, however, the numbers of patients were small and patients with HF‐PEF were not represented. A large RCT of PC in HF is needed to build upon these preliminary findings. Before such a trial can be designed, suitable outcome measures and tools for identifying patients with PC needs must be assessed in a real‐life population of patients with HF.
Study aims
Ultimately, the goal of this study is to provide the foundation for an RCT of a PC intervention in HF. This will include characterisation of an appropriate study population, identifying the components of a specific and targeted PC intervention, and defining potential trial outcomes. To achieve this goal, we will address the following specific points:
Describe and quantify the supportive and PC needs of a cohort of patients hospitalized with HF (and the needs of their caregivers). Specifically, describe and quantify general and disease‐specific QOL, symptom burden, caregiver burden, mood, performance status, and preferences for end of life (EOL) care of these patients.
Evaluate whether estimated poor prognosis or recognized assessment tools used in other populations (e.g. patients with cancer) can identify patients with hospitalized HF who have PC needs.
Describe the healthcare utilisation of patients hospitalized with HF, and describe any differences between those with and without PC needs.
Using the findings from items 1–3, design an RCT of a PC intervention for HF. In particular, we will identify an appropriate population with a high PC need (and describe how to identify this need), define the components of an HF‐specific and targeted PC intervention, and suggest potential outcome measures for such a trial.
Rationale and study design
Describing the supportive and palliative care needs of patients with heart failure
Although there have been descriptions of unmet supportive and PC needs of patients with HF, these needs have not been described using reproducible and quantifiable measures in a ‘real world’ HF population. Any such description should take into account the WHO definition of PC,6 and therefore not only make an assessment of EOL care needs, but also the QOL of patients and their caregivers, mood and symptom burden.
Quality of life
It is unclear which QOL tool is best in patients with HF, although a combination of a HF specific and a generic questionnaire may be optimal.20 One of the most commonly used HF specific questionnaires is the Kansas City Cardiomyopathy Questionnaire (KCCQ).21 This has been widely used in a number of HF studies and has been well validated.22, 23, 24, 25 A variety of generic QOL assessment tools are available and one of the most widely used is the SF‐36,26 which has been validated in a variety of populations including HF.27, 28 This has been shortened to a 12 question format, while retaining validity,29 in the form of the Short Form 12 (SF‐12).29, 30
Heart failure not only affects patients' QOL, but also that of their caregivers.31 Caregiver QOL can be readily assessed using a generic tool, such as SF‐12, but assessing ‘caregiver burden’ within a family as a result of HF can also be assessed using the Zarit Burden Interview.32 This is the most widely used and validated caregiver assessment tool.33 This tool also includes an assessment of financial strain placed on the caregiver. This is an important question, as patients within the last 6 months of life are potentially entitled to financial support in some countries.
Symptoms
Assessing on‐going symptoms should also form part of an assessment of potential PC needs in HF patients nearing end‐of‐life. HF trials tend to focus on the symptoms of dyspnoea, fatigue, and oedema. However, it has recently been shown that patients with HF can develop a multitude of other symptoms including pain, anxiety, low mood, constipation, anorexia, nausea, insomnia, and persistent cough.34, 35 There are recognized tools to help make an objective measurement of symptom burden but these have not been extensively evaluated in HF (although they have been in other diseases). The Edmonton Symptom Assessment System (ESAS)36 has been validated in cancer37, 38 and has previously been used in a study of patients with HF.39 Scrutiny of the content of the ESAS and this prior study suggest that it is able to quantify many of the symptoms experienced in HF.
Mood assessment
The WHO definition of PC states that PC should identify and treat psychosocial and physical problems. Therefore, any assessment of PC needs should detail how QOL, symptom burden, and end‐of‐life care potentially affect a patient's mood. Indeed, in one RCT of PC use in lung cancer, mood assessment was used as an outcome measure. Depression, which is common in HF,40 can affect QOL41 and is associated with higher morbidity and mortality.42, 43, 44 A validated screening questionnaire for depression is the Hospital Anxiety and Depression Scale (HADS).45 This has been used in a variety of populations,46 and is validated in HF.47
End of life care
Most patients with HF die from cardiovascular (CV) causes, the majority of which are from either worsening HF or sudden cardiac death.48 There is some evidence that patients with more severe symptoms of HF are more likely to die from worsening HF, whereas less symptomatic patients are more likely to suffer sudden cardiac death.11, 49 Not every patient with HF who dies will have unmet palliative needs, e.g. those who die from sudden unexpected cardiac death with little functional limitation. However, patients with progressive HF may benefit from palliative intervention, and how these individuals are best identified is currently unknown.
The majority of HF patients currently die in hospital. Place of death was recorded in the Assessment of Treatment with Lisinopril and Survival (ATLAS) trial with more than 50% of patients dying in hospital. Among those who died out of hospital, the mode of death was most likely to be sudden.50 These findings were similar to those of the Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) where 58% of patients died in hospital, 29% died at home, and 7% in an extended care facility.51 Although these clinical trials are informative, they included selected populations and are not representative of ‘real world’ patients with HF. A recent analysis of data from death certificates from England and Wales reported that over 60% of patients dying from HF died in hospital and <20% died at home.52 However, HF is under‐reported on death certificates,53, 54 and this finding may not reflect the true experience of patients with HF. Preferred place of death when recovery seemed unlikely was described in a study of 80 patients hospitalized with HF which reported that 50% wished to be cared for at home, 40% wished to remain in hospital, and 10% were unsure.55 Data comparing preferred place of death to actual place of death are lacking in an unselected cohort of patients with HF.
Patient preference for EOL care has been identified as a priority for research into PC need in HF.10, 56 Previous studies suggest that patients often change their mind about preferred place of death, and there is also poor agreement with their caregiver on this issue.57 Patients also change their mind about resuscitation status.58, 59 A recent study showed that HF patients were willing to discuss EOL issues and that patients were willing to trade QOL for length of life,60 which is contrary to previous studies.61, 62 Although these studies are informative, they are based on selected cohorts of patients.
Any EOL assessment should not only assess preference for and actual place of death, but also the patient and caregiver experience of dying, wherever that occurs. The Views of Informal Carers for the Evaluation of Services (VOICES) postal questionnaire has been designed to evaluate relative's experience of EOL care of the patients in the last few months of life.63 This questionnaire has been validated, and a recent review by the United Kingdom's Department of Health has identified this as an appropriate measurement tool and the tool of choice in a survey of EOL care.64
Identification of patients with palliative care needs
Before an RCT of PC use in HF can be planned, there is a need to further explore how patients with PC needs can be identified and which require the additional services of a specialist PC service. HF Guidelines suggest using the following factors to identify patients with HF and PC needs: frequent admission to hospital with decompensated HF; weight loss and cachexia; the need for frequent or on‐going intravenous therapy; chronic poor QOL with New York Heart Association (NYHA) class IV symptoms; and a clinical judgement that the patient is close to the EOL.7, 10 However, predicting prognosis is notoriously difficult and is recognized as a barrier to PC referral in HF.65 A number of prognostic models have been described from various HF cohorts.66 Unfortunately, most models were developed in chronic ambulatory populations (as opposed to acutely hospitalized patients) and many were based on patients not receiving contemporary pharmacotherapy, or did not include important prognostic factors such as renal function, B‐type natriuretic peptide (BNP)67 (or NT pro BNP) or troponin.68, 69 Use of prognostic models has been suggested as a way of identifying HF patients who are approaching EOL.70 However, while these models may predict death, there is no evidence to suggest that prognostic models correlate well with PC needs.71 This question requires further exploration.72
One approach to try and specifically identify and assess the PC needs of HF patients is to use tools currently in development for cancer patients, acknowledging that these require assessment in patients with HF. The Needs Assessment Tool (progressive disease – cancer) (NAT‐PD‐C)73 has been designed specifically to assess PC needs in cancer patients. It was designed based upon a literature review of needs of patients and their caregivers. This assessment is made on a single page and completed by the patient's healthcare professional. The NAT‐PD‐C has been validated in cancer patients74, 75 and has been adapted for use in HF with the creation of the Needs Assessment Tool Progressive Disease Heart Failure (NAT‐PD‐HF), with reliability testing and construct validation.76 However, this tool has yet to be evaluated in a substantial cohort of patients with HF, and its value in identifying PC needs in patients with HF is as yet unconfirmed. Therefore, we will carry out these evaluations of the NAT‐PD‐HF.
Performance status has been used by PC clinicians in both clinical practice and research as an indication for the likely need for PC services.77, 78, 79 The Karnofksy Performance Scale (KPS)80 is regarded by many as the gold standard tool for use in cancer patients.77, 78 This instrument has been simplified and validated in the form of the Australia‐Modified Karnofksy Performance Scale (AKPS).81 The AKPS has been developed for use in cancer, and review of it suggests that it should also provide a suitable assessment of performance status in patients with HF.
An overview of the study design and the outcome measures used are illustrated in Figure 1, Panel A.
Figure 1.
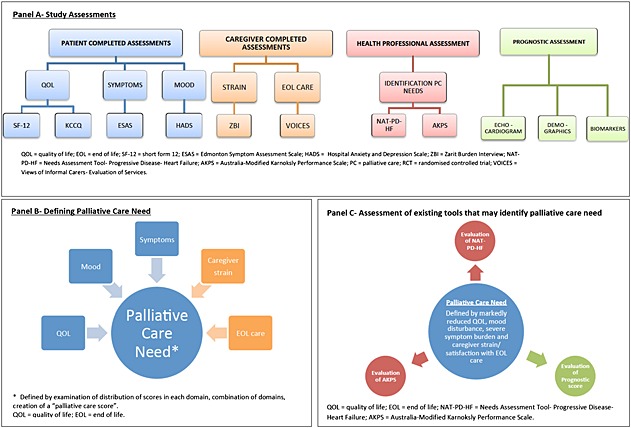
Study design.
Study protocol
This will be a 2‐year prospective observational study of near‐consecutive patients admitted to hospital with HF. An outline is described in Figure 2. Patients will be extensively characterized during their inpatient stay by collecting echocardiographic (Table 1), demographic, and physiological data, as well as a detailed past medical history. Patient symptom burden, mood, and QOL will be assessed during the index admission and repeatedly during follow up. The burden on caregivers will also be assessed. Patient preference for place of death (and actual place of death, if death occurs), as well as resuscitation preference, will be recorded. Health care utilisation will be evaluated.
Figure 2.
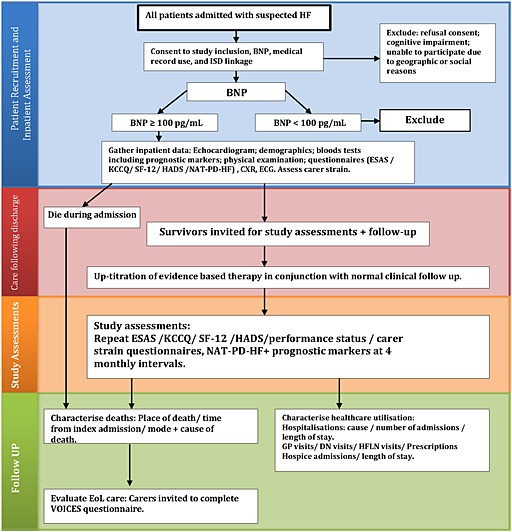
Study protocol.
Table 1.
Echocardiographic protocol and data
| Protocol | Measurement | ||
|---|---|---|---|
| Window | Doppler | 2D/M‐mode | |
| Parasternal | |||
| Long axis | MV & AV colour flow | IVSd, LVEDD, LVPWd | LV end diastolic dimension (cm/m2) |
| IVSs, LVESD, LVPWs, LVOT, LA | LV end systolic dimension (cm/m2) | ||
| RV inflow | TV CW + colour flow | ||
| Short axis | |||
| Base | AV, TV & PV colour flow | ||
| MV | MV colour flow | ||
| Papillary muscle | 2D endocardial & epicardial area | LV mass index (g/m2) | |
| apex | |||
| Apical | |||
| 4 chamber | MV annulus TDI + LV inflow PW | LV volume diastole + systole, LAA | LV EF (%) |
| MV colour flow, TV colour flow | LV diastolic volume (ml/m2) | ||
| LV systolic volume (ml/m2) | |||
| LV stroke volume (ml) | |||
| Cardiac output (L/min) | |||
| LV diastolic parameters (E, E/e’, IVRT, E/A) | |||
| Left atrial volume (ml/m2) | |||
| Valve assessment of structure and function | |||
| 2 chamber | MV colour flow | LV volume diastole + systole, LAA | LV EF (%) |
| Left atrial volume (ml/m2) | |||
| LV diastolic volume (ml/m2) | |||
| LV systolic volume (ml/m2) | |||
| 5 chamber | AV CW + PW + IVRT, AV colour flow | ||
| Long axis | MV colour flow | ||
| RV | TAPSE, RAA | TAPSE | |
| Right atrial area (mm2) | |||
| Subcostal | |||
| 4 chamber | |||
| IVC & hepatic veins | IVC diameter | RVSP | |
AV, aortic valve; CW, continuous wave; E, early diastolic filling; e’, early lengthening velocity; IVC, inferior vena cava; IVRT, isovolumic relaxation time; IVSd, intraventricular septal diastole; IVSs, intraventricular septum systole; LVEDd, left ventricular end‐diastolic dimension; LVESD, left ventricular end‐systolic dimension; LVPWd, left ventricular posterior wall diastole; LVEDs, left ventricle posterior wall systole; EF, ejection fraction; LAA, left atrial area; LV, left ventricle; MV, mitral valve; PV, pulmonary valve; PW, pulsed wave; RV, right ventricle; RVSP, right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion.
Patient recruitment
Near consecutive patients admitted to the Western Infirmary in Glasgow with suspected HF will be screened for inclusion in the study. HF will be defined according to the European Society of Cardiology (ESC) guidelines.10 The Western Infirmary acts as a community hospital for the North and West of the city, serving a population of about 250 000. All patients admitted with a primary diagnosis of clinically suspected HF will be approached and asked for permission to access their medical records and to link their record through National Health Service Scotland Information Services Division (ISD), allowing identification and cause of hospital readmission and death (including place of death). Plasma BNP will be measured to aid the diagnosis of HF (and will provide prognostic information). A finger prick (12 μL) sample of blood will be analysed for BNP using a validated, point of care, capillary blood sample analysis (Alere HeartCheck System). Those with a BNP <100 pg/mL will be excluded.10 In addition to elevated BNP, patients must meet the ESC echocardiographic criteria for the diagnosis of HF.10, 82 Patients with a confirmed diagnosis of HF will be invited to participate in the study, and a further sample of blood and urine will be taken and stored for later batched analysis of biomarkers, which have prognostic importance in HF. In a previous study collecting data on near‐consecutive HF admissions, we recruited almost 350 patients at the Western Infirmary over a 2‐year period.83 and we anticipate similar recruitment in this study. Based on our earlier study, we anticipate that approximately 30% of patients will die in the first year of follow‐up and approximately 15% per year thereafter.
Inclusion and exclusion criteria are detailed in Table 2.
Table 2.
Inclusion/ exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Admitted to hospital with a primary diagnosis of acute decompensated HF | • Refusal to participate |
| • Age ≥18 years | • Unable to provide informed consent/complete study assessments |
| • Fulfilling the ESC diagnostic criteria for the diagnosis of HF | ○ Confusion/dementia |
| • HF‐REF, HF‐PEF and valvular HF will be included | ○ Learning difficulties |
| ○ Unable to read or write English language | |
| ○ Moribund | |
| • Readmission | |
| • Geographical reasons, not from catchment area | |
| • Isolated cor pulmonale | |
| • Acute coronary syndrome complicated by pulmonary oedema |
ESC, European Society of Cardiology; HF, heart failure; HF‐REF, heart failure with reduced ejection fraction; HF‐PEF, heart failure with preserved ejection fraction.
Patient assessment
Detailed clinical data and data used in validated models of mortality prediction in HF will be gathered during the index hospitalisation.66 A full echocardiographic examination will be carried out according to the European Association of Echocardiography guidelines,84 and assessment of known prognostic variables will be recorded (Table 1). Left ventricular ejection fraction will be measured using Simpson's biplane method.85 Before discharge from hospital, patients will complete the KCCQ and SF‐12 questionnaires to assess QOL and the ESAS questionnaire to assess their current symptom burden. Mood assessment will be made with the HADS questionnaire. Patients' caregivers will be invited to complete the Zarit Burden Interview to assess caregiver burden. Performance status will be evaluated using the AKPS. The NAT‐PD‐HF will be used to assess the palliative needs of the patient. One of the investigators will also ask about preferred place of death and resuscitation preference, in a sensitive way, in patients thought to be near to death and in those who exhibit significant deterioration during follow‐up. Patients will be asked to consider their preference for place of care, specifically, patients will be asked ‘If your health was to deteriorate in the future, such that you required other people to care for you, where would you prefer that care to take place?’ Patients will then be given the following options to choose from, after explaining this is a hypothetical discussion: in their own home; a nursing or care home; hospital; hospice; or undecided. Patients will then be asked to consider their preferred place of care for EOL treatment, specifically, patients will be asked ‘If you were to think about the last few days of hours of life, would you have a strong opinion or preference for where that care took place?’ Patients will then be given the following options to choose from, after explaining this is a hypothetical discussion: in their own home; a nursing or care home; hospital; hospice; or undecided. Finally, patients will be asked, after an explanation of what resuscitation is, to consider their preference for resuscitation. Specifically, patients will be asked ‘Do you have a strong opinion or preference to be resuscitated or not to be resuscitated in the event of a cardiac arrest’. Patient will be asked to pick an option from ‘for active resuscitation, not for resuscitation, or undecided’. The researcher will only ask these questions where it is felt to be appropriate, in patients approaching the EOL, and only after making explicitly clear that the questions relate to research and are not for clinical purposes. While we recognize the limitations in predicting likely death in HF, our Ethics Committee does not think it appropriate that we approach all patients about this question.
Study assessments
Following discharge, patients will be reviewed at an outpatient clinic by a cardiologist, a Heart Failure Liaison Nurse (HFLN), or both, where evidence‐based therapy will be optimized in accordance with ESC guidelines.10 Patients will be invited to attend for study assessments at 4‐monthly intervals following discharge for a maximum follow‐up period of 2.5 years. At these visits, KCCQ, SF‐12, HADS, and ESAS questionnaires will be completed, to detail any potential change in QOL, mood, and symptom burden over time. EOL preferences will also be re‐evaluated (as appropriate), including preferred place of care/death and resuscitation preference. At these study assessments, prognostic markers will be updated and the NAT‐PD‐HF will be reassessed. To ensure a complete follow‐up as possible, we will contact those unable to attend study visits by telephone and offer home visits where possible.
Follow‐up
All patients who consent will be ‘flagged’ using ISD linkage, ensuring complete follow‐up (all will be followed up for a minimum of 12 months). PC could potentially alter Healthcare utilisation.16 Therefore, the number of hospital admissions, length of stay, cause of admission, general practitioner (GP) visits, district nurse (DN) visits, hospice admissions, HFLN visits, and prescription costs will be recorded. These data including date, cause and location of death will be available from ISD and GP records.
Relatives of deceased patients will be asked to complete the VOICES EOL postal questionnaire. Relatives will be written to and given the opportunity to opt out prior to the questionnaire being posted.
Data handling and statistical analysis
All data will be managed and analysed by the Robertson Centre for Biostatistics (University of Glasgow) and the Data and Biostatistics Centre of the UK Clinical Research Collaboration Glasgow Clinical Trials Unit (CTU). Baseline and follow‐up data (including patient and carer QOL) will be entered into a case report form and then into the study database by experienced data entry staff. All data will be stored, managed, and analysed according to CTU standard operating procedures that comply with appropriate legal and regulatory requirements. We will define patients in need of PC as those reporting a substantial reduction in QOL, marked mood disturbance, or severe symptoms (as measured by NYHA class, KCCQ, and ESAS), especially if there is associated caregiver strain (Figure 1, Panel B). We will test whether the NAT‐PD‐HF and AKPS tools (and a prognostic score derived from standard clinical assessments) identify these patients. Much of the analysis will be descriptive, using different scores and combinations of scores from the various patient‐reported outcomes to define a need for PC (Figure 1, Panel C). We will also assess temporal variations in QOL, patient symptom burden, and PC needs for the cohort as a whole and for different patient sub‐groups. The relationship between the need for PC as identified by the NAT‐PD‐HF and other clinical and QOL instruments will be analysed using logistic regression models. The relationship between baseline characteristics, QOL data, and mortality will be assessed using logistic regression and time to event analyses. The additional value of repeated QOL assessments will be evaluated using time varying covariate Cox models.
Ethical considerations
This study will be conducted according to the principles outlined in the Declaration of Helsinki.86 The study protocol has been approved by the West of Scotland Research Ethics Committee. All participants will be given over 24 h to read the patient information letter and consider if they wish to participate before provide written consent. Patient burden and load have been considered, and patient reported outcome measures have been chosen to limit the burden placed on participants. Burden of follow‐up study visits has been reduced by offering participants home visits or providing door‐to‐door transport. Our local Ethics Committee has asked that we only ask preferred place of death in patients thought to be near to death and in those who exhibit significant deterioration during follow‐up. While we recognize the limitations in predicting likely death in HF, our Ethics Committee does not think it appropriate that we approach all patients about this question.
Discussion
Performing research in people with serious illness is difficult, with poor recruitment, and high rates of participant dropout common,87 perhaps explaining why few studies of PC in HF have been longitudinal, quantitative, or performed in large cohorts. Barnes et al. described their experience of recruiting a large cohort of elderly patients with HF into a longitudinal study,88 and reported such problems. They experienced difficulties with recruitment and retention of elderly patients, with only 30% of patients approached agreeing to participate. One of the main challenges they had was the reliance on a gate‐keeper to recruit participants, in their case the GP (but in other similar studies often hospital specialists).89 They found some GPs to be over‐restrictive in recruitment, excluding potential participants for reasons outside the protocol‐specified exclusion criteria, particularly elderly patients living in care facilities. Other GPs did not utilize the exclusion criteria, and therefore ineligible patients were also approached. These factors can introduce selection bias, reducing the generalisability of results. One of the key strengths of our study is the reduction in selection bias by approaching a consecutive, and thus recruiting a relatively unselected, cohort of hospitalized patients. We will reduce the aforementioned issues by not relying on a gate‐keeper physician to recruit participants, as a member of the study team will screen all admissions for potential participants.
Another potential issue highlighted by Barnes et al. was the identification of patients with HF. They searched patient databases for the diagnostic code ‘heart failure’ and combining this with a search for patients on a regular loop diuretic. Although this screening method is efficient and has the advantage of high sensitivity, specificity is likely low. The diagnosis of HF is difficult, especially HF‐PEF,90 and usually requires the combination of clinical signs and symptoms, natriuretic peptides, and echocardiography.10 This highlights a further strength of our study as all potential participants will be screened using natriuretic peptides and echocardiography, making the cohort one of the best described contemporary HF populations in the field of palliative care.
Participation burden has also been identified as a potential issue affecting retention of participants in any longitudinal study of patients with HF,88 with some outcome measures, such as filling in questionnaires or attending study assessments, becoming more challenging for participants to complete as their condition progresses and their general health deteriorates. This has been considered, and measures and study design chosen to reduce participant burden as much as possible. This is one of the main aims of this study, to pilot the use of many of the outcome measures over time in a frail population, to help inform the design of a large RCT of early PC intervention in patients with HF.
The main weakness of our study is the exclusion criteria. We will exclude patients with cognitive impairment such that they are unable to provide informed consent or complete study assessments, or those unable to complete study assessments because of language or communication difficulties. Although these criteria are necessary, we will potentially exclude not only a common group of patients,91 but those with some of the greatest needs.92 Targeting inpatients could also result in some patients being too unwell to participate; however, we feel the strength of recruiting an unselected hospital cohort outweighs this potential weakness.
Conclusion
This study will be one of the largest assessing PC needs in a largely unselected cohort of patients admitted to hospital with HF, and will be one of the best‐described contemporary HF cohorts. We will assess the prevalence of PC needs and describe how these needs change over time, and assess whether those with PC needs can be identified. In doing, so we will provide the foundation for designing a large RCT of an early PC intervention in HF.
Conflict of interest
None declared.
Funding
This project is supported by a project grant from the British Heart Foundation; Grant number PG/13/17/30050.
Campbell, R. T. , Jackson, C. E. , Wright, A. , Gardner, R. S. , Ford, I. , Davidson, P. M. , Denvir, M. A. , Hogg, K. J. , Johnson, M. J. , Petrie, M. C. , and McMurray, J. J. (2015), Palliative care needs in patients hospitalized with heart failure (PCHF) study: rationale and design. ESC Heart Failure, 2: 25–36. doi: 10.1002/ehf2.12027.
References
- 1. Davies M, Hobbs F, Davis R, Kenkre J, Roalfe AK, Hare R, Wosornu D, Lancashire RJ. Prevalence of left‐ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet 2001; 358: 439–444. [DOI] [PubMed] [Google Scholar]
- 2. McDonagh TA, Morrison CE, Lawrence A, Ford I, Tunstall‐Pedoe H, McMurray JJ, Dargie HJ. Symptomatic and asymptomatic left‐ventricular systolic dysfunction in an urban population. Lancet 1997; 350: 829–833. [DOI] [PubMed] [Google Scholar]
- 3. MacIntyre K, Capewell S, Stewart S, Chalmers JW, Boyd J, Finlayson A, Redpath A, Pell JP, McMurray JJ. Evidence of improving prognosis in heart failure: trends in case fatality in 66 547 patients hospitalized between 1986 and 1995. Circulation 2000; 102: 1126–1131. [DOI] [PubMed] [Google Scholar]
- 4. Mosterd A, Cost B, Hoes AW, de Bruijne MC, Deckers JW, Hofman A, Grobbee DE. The prognosis of heart failure in the general population: The Rotterdam Study. Eur Heart J 2001; 22: 1318–1327. [DOI] [PubMed] [Google Scholar]
- 5. Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More 'malignant' than cancer? Five‐year survival following a first admission for heart failure. Eur J Heart Fail 2001; 3: 315–322. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organisation. World Health Organisation: definition of palliative care. 2012. http://www.who.int/cancer/palliative/definition/en/ (accessed November 2014)
- 7. Jaarsma T, Beattie JM, Ryder M, Rutten FH, McDonagh T, Mohacsi P, Murray SA, Grodzicki T, Bergh I, Metra M, Ekman I, Angermann C, Leventhal M, Pitsis A, Anker SD, Gavazzi A, Ponikowski P, Dickstein K, Delacretaz E, Blue L, Strasser F, McMurray J. Palliative care in heart failure: a position statement from the palliative care workshop of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2009; 11: 433–443. [DOI] [PubMed] [Google Scholar]
- 8. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr. , Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B, American College of Cardiology, American Heart Association Task Force on Practice Guidelines, American College of Chest Physicians, International Society for Heart and Lung Transplantation, Heart Rhythm Society. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 2005; 112: e154–235. [DOI] [PubMed] [Google Scholar]
- 9. Goodlin SJ, Hauptman PJ, Arnold R, Grady K, Hershberger RE, Kutner J, Masoudi F, Spertus J, Dracup K, Cleary JF, Medak R, Crispell K, Pina I, Stuart B, Whitney C, Rector T, Teno J, Renlund DG. Consensus statement: palliative and supportive care in advanced heart failure. J Card Fail 2004; 10: 200–209. [DOI] [PubMed] [Google Scholar]
- 10. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 11. MERIT‐HF Study Group . Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). Lancet 1999; 353: 2001–2007. [PubMed] [Google Scholar]
- 12. Barclay S, Momen N, Case‐Upton S, Kuhn I, Smith E. End‐of‐life care conversations with heart failure patients: a systematic literature review and narrative synthesis. The British Journal of General Practice : The Journal of the Royal College of General Practitioners 2011; 61: e49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Low J, Pattenden J, Candy B, Beattie JM, Jones L. Palliative care in advanced heart failure: an international review of the perspectives of recipients and health professionals on care provision. J Card Fail 2011; 17: 231–252. [DOI] [PubMed] [Google Scholar]
- 14. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 15. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med 2006; 355: 260–269. [DOI] [PubMed] [Google Scholar]
- 16. Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med 2010; 363: 733–742. [DOI] [PubMed] [Google Scholar]
- 17. Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, Moore M, Rydall A, Rodin G, Tannock I, Donner A, Lo C. Early palliative care for patients with advanced cancer: a cluster‐randomised controlled trial. Lancet 2014; 383: 1721–1730. [DOI] [PubMed] [Google Scholar]
- 18. Brumley R, Enguidanos S, Jamison P, Seitz R, Morgenstern N, Saito S, McIlwane J, Hillary K, Gonzalez J. Increased satisfaction with care and lower costs: results of a randomized trial of in‐home palliative care. J Am Geriatr Soc 2007; 55: 993–1000. [DOI] [PubMed] [Google Scholar]
- 19. Brannstrom M, Boman K. Effects of person‐centred and integrated chronic heart failure and palliative home care. PREFER: a randomized controlled study. Eur J Heart Fail 2014; 16: 1142–1151. [DOI] [PubMed] [Google Scholar]
- 20. Berry C, McMurray J. A review of quality‐of‐life evaluations in patients with congestive heart failure. Pharmacoeconomics 1999; 16: 247–271. [DOI] [PubMed] [Google Scholar]
- 21. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000; 35: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 22. Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation 2004; 110: 546–551. [DOI] [PubMed] [Google Scholar]
- 23. Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS, Cardiovascular outcomes research consortium monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005; 150: 707–715. [DOI] [PubMed] [Google Scholar]
- 24. Heidenreich PA, Spertus JA, Jones PG, Weintraub WS, Rumsfeld JS, Rathore SS, Peterson ED, Masoudi FA, Krumholz HM, Havranek EP, Conard MW, Williams RE. Cardiovascular outcomes research consortium. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol 2006; 47: 752–756. [DOI] [PubMed] [Google Scholar]
- 25. Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P, Spertus JA. Identifying heart failure patients at high risk for near‐term cardiovascular events with serial health status assessments. Circulation 2007; 115: 1975–1981. [DOI] [PubMed] [Google Scholar]
- 26. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36) I. Conceptual framework and item selection. Med Care 1992; 30: 473–483. [PubMed] [Google Scholar]
- 27. Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ 1993; 306: 1437–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ware JE Jr, Bayliss MS, Rogers WH, Kosinski M, Tarlov AR. Differences in 4‐year health outcomes for elderly and poor, chronically ill patients treated in HMO and fee‐for‐service systems. Results from the Medical Outcomes Study. JAMA 1996; 276: 1039–1047. [PubMed] [Google Scholar]
- 29. Ware J Jr, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–233. [DOI] [PubMed] [Google Scholar]
- 30. Jenkinson C, Layte R, Jenkinson D, Lawrence K, Petersen S, Paice C, Stradling J. A shorter form health survey: can the SF‐12 replicate results from the SF‐36 in longitudinal studies? J Public Health Med 1997; 19: 179–186. [DOI] [PubMed] [Google Scholar]
- 31. Iqbal J, Francis L, Reid J, Murray S, Denvir M. Quality of life in patients with chronic heart failure and their carers: a 3‐year follow‐up study assessing hospitalization and mortality. Eur J Heart Fail 2010; 12: 1002–1008. [DOI] [PubMed] [Google Scholar]
- 32. Zarit SH, Reever KE, Bach‐Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980; 20: 649–655. [DOI] [PubMed] [Google Scholar]
- 33. Whalen KJ, Bucholz SW. The Reliability, Validity and Feasibility of Tools Used to Screen for Caregiver Burden: a Systematic Review. JBI Lib Syst Rev 2009; 7: 1372–1429. [DOI] [PubMed] [Google Scholar]
- 34. Nordgren L, Sorensen S. Symptoms experienced in the last six months of life in patients with end‐stage heart failure. European Journal of Cardiovascular Nursing : Journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology 2003; 2: 213–217. [DOI] [PubMed] [Google Scholar]
- 35. Janssen DJ, Spruit MA, Wouters EF, Schols JM. Daily symptom burden in end‐stage chronic organ failure: a systematic review. Palliat Med 2008; 22: 938–948. [DOI] [PubMed] [Google Scholar]
- 36. Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991; 7: 6–9. [PubMed] [Google Scholar]
- 37. Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer 2000; 88: 2164–2171. [DOI] [PubMed] [Google Scholar]
- 38. Moro C, Brunelli C, Miccinesi G, Fallai M, Morino P, Piazza M, Labianca R, Ripamonti C. Edmonton symptom assessment scale: Italian validation in two palliative care settings. Supportive Care in Cancer : Official Journal of the Multinational Association of Supportive Care in Cancer 2006; 14: 30–37. [DOI] [PubMed] [Google Scholar]
- 39. Opasich C, Gualco A, De Feo S, Barbieri M, Cioffi G, Giardini A, Majani G. Physical and emotional symptom burden of patients with end‐stage heart failure: what to measure, how and why. J Cardiovasc Med 2008; 9: 1104–1108. [DOI] [PubMed] [Google Scholar]
- 40. Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta‐analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006; 48: 1527–1537. [DOI] [PubMed] [Google Scholar]
- 41. Dekker RL, Lennie TA, Albert NM, Rayens MK, Chung ML, Wu JR, Song EK, Moser DK. Depressive symptom trajectory predicts 1‐year health‐related quality of life in patients with heart failure. J Card Fail 2011; 17: 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jiang W, Alexander J, Christopher E, Kuchibhatla M, Gaulden LH, Cuffe MS, Blazing MA, Davenport C, Califf RM, Krishnan RR, O'Connor CM. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001; 161: 1849–1856. [DOI] [PubMed] [Google Scholar]
- 43. Vaccarino V, Kasl SV, Abramson J, Krumholz HM. Depressive symptoms and risk of functional decline and death in patients with heart failure. J Am Coll Cardiol 2001;38:199‐205. [DOI] [PubMed] [Google Scholar]
- 44. Albert NM, Fonarow GC, Abraham WT, Gheorghiade M, Greenberg BH, Nunez E, O'Connor CM, Stough WG, Yancy CW, Young JB. Depression and clinical outcomes in heart failure: an OPTIMIZE‐HF analysis. Am J Med 2009; 122: 366–373. [DOI] [PubMed] [Google Scholar]
- 45. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 46. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002; 52: 69–77. [DOI] [PubMed] [Google Scholar]
- 47. Haworth JE, Moniz‐Cook E, Clark AL, Wang M, Cleland JG. An evaluation of two self‐report screening measures for mood in an out‐patient chronic heart failure population. Int J Geriatr Psychiatry 2007; 22: 1147–1153. [DOI] [PubMed] [Google Scholar]
- 48. Carson P, Anand I, O'Connor C, Jaski B, Steinberg J, Lwin A, Lindenfeld J, Ghali J, Barnet JH, Feldman AM, Bristow MR. Mode of death in advanced heart failure: the Comparison of Medical, Pacing, and Defibrillation Therapies in Heart Failure (COMPANION) trial. J Am Coll Cardiol 2005; 46: 2329–2334. [DOI] [PubMed] [Google Scholar]
- 49. O'Connor CM, Miller AB, Blair JE, Konstam MA, Wedge P, Bahit MC, Carson P, Haass M, Hauptman PJ, Metra M, Oren RM, Patten R, Pina I, Roth S, Sackner‐Bernstein JD, Traver B, Cook T, Gheorghiade M, Efficacy of Vasopressin Antagonism in heart Failure Outcome Study with Tolvaptan investigators. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction: results from Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) program. Am Heart J 2010; 159: 841–849 e841. [DOI] [PubMed] [Google Scholar]
- 50. Poole‐Wilson PA, Uretsky BF, Thygesen K, Cleland JG, Massie BM, Ryden L, Atlas Study Group . Mode of death in heart failure: findings from the ATLAS trial. Heart 2003; 89: 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Olshansky B, Wood F, Hellkamp AS, Poole JE, Anderson J, Johnson GW, Boineau R, Domanski MJ, Mark DB, Lee KL, Bardy GH, SCD‐HeFT Investigators. Where patients with mild to moderate heart failure die: results from the Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT). Am Heart J 2007; 153: 1089–1094. [DOI] [PubMed] [Google Scholar]
- 52. Laribi S, Aouba A, Nikolaou M, Lassus J, Cohen‐Solal A, Plaisance P, Pavillon G, Jois P, Fonarow GC, Jougla E, Mebazaa A, GREAT network Trends in death attributed to heart failure over the past two decades in Europe. Eur J Heart Fail 2012; 14: 234–239. [DOI] [PubMed] [Google Scholar]
- 53. Murdoch DR, Love MP, Robb SD, McDonagh TA, Davie AP, Ford I, Capewell S, Morrison CE, McMurray JJ. Importance of heart failure as a cause of death. Changing contribution to overall mortality and coronary heart disease mortality in Scotland 1979‐1992. Eur Heart J 1998; 19: 1829–1835. [DOI] [PubMed] [Google Scholar]
- 54. Engelfriet PM, Hoogenveen RT, Boshuizen HC, van Baal PH. To die with or from heart failure: a difference that counts: is heart failure underrepresented in national mortality statistics? Eur J Heart Fail 2011; 13: 377–383. [DOI] [PubMed] [Google Scholar]
- 55. Formiga F, Chivite D, Ortega C, Casas S, Ramon JM, Pujol R. End‐of‐life preferences in elderly patients admitted for heart failure. QJM 2004; 97: 803–808. [DOI] [PubMed] [Google Scholar]
- 56. Metra M, Ponikowski P, Dickstein K, McMurray JJ, Gavazzi A, Bergh CH, Fraser AG, Jaarsma T, Pitsis A, Mohacsi P, Bohm M, Anker S, Dargie H, Brutsaert D, Komajda M, Heart Failure Association of the European Society of Cardiology Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2007; 9: 684–694. [DOI] [PubMed] [Google Scholar]
- 57. Agar M, Currow DC, Shelby‐James TM, Plummer J, Sanderson C, Abernethy AP. Preference for place of care and place of death in palliative care: are these different questions? Palliat Med 2008; 22: 787–795. [DOI] [PubMed] [Google Scholar]
- 58. Krumholz HM, Phillips RS, Hamel MB, Teno JM, Bellamy P, Broste SK, Califf RM, Vidaillet H, Davis RB, Muhlbaier LH, Connors AF Jr, Lynn J, Goldman L. Resuscitation preferences among patients with severe congestive heart failure: results from the SUPPORT project. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Circulation 1998; 98: 648–655. [DOI] [PubMed] [Google Scholar]
- 59. Janssen DJ, Spruit MA, Schols JM, Cox B, Nawrot TS, Curtis JR, Wouters EF. Predicting changes in preferences for life‐sustaining treatment among patients with advanced chronic organ failure. Chest 2012; 141: 1251–1259. [DOI] [PubMed] [Google Scholar]
- 60. Brunner‐La Rocca HP, Rickenbacher P, Muzzarelli S, Schindler R, Maeder MT, Jeker U, Kiowski W, Leventhal ME, Pfister O, Osswald S, Pfisterer ME, Rickli H, Investigators T‐C. End‐of‐life preferences of elderly patients with chronic heart failure. Eur Heart J 2012; 33: 752–759. [DOI] [PubMed] [Google Scholar]
- 61. Rector TS, Tschumperlin LK, Kubo SH, Bank AJ, Francis GS, McDonald KM, Keeler CA, Silver MA. Use of the Living with Heart failure Questionnaire to ascertain patients' perspectives on improvement in quality of life versus risk of drug‐induced death. J Card Fail 1995; 1: 201–206. [DOI] [PubMed] [Google Scholar]
- 62. Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. The Journal of Heart and Lung Transplantation : The Official Publication of the International Society for Heart Transplantation 2001; 20: 1016–1024. [DOI] [PubMed] [Google Scholar]
- 63. Hunt KJ, Shlomo N, Addington‐Hall J. End‐of‐life care and achieving preferences for place of death in England: results of a population‐based survey using the VOICES‐SF questionnaire. Palliat Med 2014; 28: 412–421. [DOI] [PubMed] [Google Scholar]
- 64. Department of Health : Office for National Statistics. National Bereavement Survey (VOICES). 2011 ; http://www.ons.gov.uk/ons/rel/subnational‐health1/national‐bereavement‐survey‐‐voices‐/2012/stb‐‐‐national‐bereavement‐survey‐2012.html. (November 2014)
- 65. Hanratty B, Hibbert D, Mair F, May C, Ward C, Capewell S, Litva A, Corcoran G. Doctors' perceptions of palliative care for heart failure: focus group study. BMJ 2002; 325: 581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Alba AC, Agoritsas T, Jankowski M, Courvoisier D, Walter SD, Guyatt GH, Ross HJ. Risk prediction models for mortality in ambulatory patients with heart failure: a systematic review. Circ Heart Fail 2013; 6: 881–889. [DOI] [PubMed] [Google Scholar]
- 67. Di Angelantonio E, Chowdhury R, Sarwar N, Ray KK, Gobin R, Saleheen D, Thompson A, Gudnason V, Sattar N, Danesh J. B‐type natriuretic peptides and cardiovascular risk: systematic review and meta‐analysis of 40 prospective studies. Circulation 2009; 120: 2177–2187. [DOI] [PubMed] [Google Scholar]
- 68. Hudson MP, O'Connor CM, Gattis WA, Tasissa G, Hasselblad V, Holleman CM, Gaulden LH, Sedor F, Ohman EM. Implications of elevated cardiac troponin T in ambulatory patients with heart failure: a prospective analysis. Am Heart J 2004; 147: 546–552. [DOI] [PubMed] [Google Scholar]
- 69. de Antonio M, Lupon J, Galan A, Vila J, Urrutia A, Bayes‐Genis A. Combined use of high‐sensitivity cardiac troponin T and N‐terminal pro‐B type natriuretic peptide improves measurements of performance over established mortality risk factors in chronic heart failure. Am Heart J 2012; 163: 821–828. [DOI] [PubMed] [Google Scholar]
- 70. Adler ED, Goldfinger JZ, Kalman J, Park ME, Meier DE. Palliative care in the treatment of advanced heart failure. Circulation 2009; 120: 2597–2606. [DOI] [PubMed] [Google Scholar]
- 71. Haga K, Murray S, Reid J, Ness A, O'Donnell M, Yellowlees D, Denvir MA. Identifying community based chronic heart failure patients in the last year of life: a comparison of the Gold Standards Framework Prognostic Indicator Guide and the Seattle Heart Failure Model. Heart 2012; 98: 579–583. [DOI] [PubMed] [Google Scholar]
- 72. Hogg KJ, Jenkins SM. Prognostication or identification of palliative needs in advanced heart failure: where should the focus lie? Heart 2012; 98: 523–524. [DOI] [PubMed] [Google Scholar]
- 73. Waller A, Girgis A, Currow D, Lecathelinais C, Palliative Care Research Program team. Development of the palliative care needs assessment tool (PC‐NAT) for use by multi‐disciplinary health professionals. Palliat Med 2008; 22: 956–964. [DOI] [PubMed] [Google Scholar]
- 74. Waller A, Girgis A, Lecathelinais C, Scott W, Foot L, Sibbritt D, Currow D, Palliative Care Research Program t. Validity, reliability and clinical feasibility of a Needs Assessment Tool for people with progressive cancer. Psychooncology 2010; 19: 726–733. [DOI] [PubMed] [Google Scholar]
- 75. Waller A, Girgis A, Johnson C, Lecathelinais C, Sibbritt D, Forstner D, Liauw W, Currow DC. Improving outcomes for people with progressive cancer: interrupted time series trial of a needs assessment intervention. J Pain Symptom Manage 2012; 43: 569–581. [DOI] [PubMed] [Google Scholar]
- 76. Waller A, Girgis A, Davidson PM, Newton PJ, Lecathelinais C, Macdonald PS, Hayward CS, Currow DC. Facilitating needs‐based support and palliative care for people with chronic heart failure: preliminary evidence for the acceptability, inter‐rater reliability, and validity of a needs assessment tool. J Pain Symptom Manage 2013; 45: 912–925. [DOI] [PubMed] [Google Scholar]
- 77. Nikoletti S, Porock D, Kristjanson LJ, Medigovich K, Pedler P, Smith M. Performance status assessment in home hospice patients using a modified form of the Karnofsky Performance Status Scale. J Palliat Med 2000; 3: 301–311. [DOI] [PubMed] [Google Scholar]
- 78. Kaasa T, Wessel J. The Edmonton Functional Assessment Tool: further development and validation for use in palliative care. J Palliat Care 2001; 17: 5–11. [PubMed] [Google Scholar]
- 79. Abernethy AP, Currow DC, Hunt R, Williams H, Roder‐Allen G, Rowett D, Shelby‐James T, Esterman A, May F, Phillips PA. A pragmatic 2 x 2 x 2 factorial cluster randomized controlled trial of educational outreach visiting and case conferencing in palliative care‐methodology of the Palliative Care Trial [ISRCTN 81117481]. Contemp Clin Trials 2006; 27: 83–100. [DOI] [PubMed] [Google Scholar]
- 80. Karnofsky D, Abelmann W, Craver L, Burchenal J. The use of nitrogen mustard in hte palliative treatment of cancer. Cancer 1948; 1: 634–656. [Google Scholar]
- 81. Abernethy AP, Shelby‐James T, Fazekas BS, Woods D, Currow DC. The Australia‐modified Karnofsky Performance Status (AKPS) scale: a revised scale for contemporary palliative care clinical practice [ISRCTN81117481]. BMC Palliative Care 2005; 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite‐Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007; 28: 2539–2550. [DOI] [PubMed] [Google Scholar]
- 83. Jackson CE, Myles RC, Tsorlalis IK, Dalzell JR, Spooner RJ, Rodgers JR, Bezlyak V, Greenlaw N, Ford I, Cobbe SM, Petrie MC, McMurray JJ. Profile of microvolt T‐wave alternans testing in 1003 patients hospitalized with heart failure. Eur J Heart Fail 2012; 14: 377–386. [DOI] [PubMed] [Google Scholar]
- 84. Evangelista A, Flachskampf F, Lancellotti P, Badano L, Aguilar R, Monaghan M, Zamorano J, Nihoyannopoulos P. European Association of Echocardiography recommendations for standardization of performance, digital storage and reporting of echocardiographic studies. European Journal of Echocardiography: the Journal of the Working Group on Echocardiography of the European Society of Cardiology 2008; 9: 438–448. [DOI] [PubMed] [Google Scholar]
- 85. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography : Official Publication of the American Society of Echocardiography 2005; 18: 1440–1463. [DOI] [PubMed] [Google Scholar]
- 86. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 87. Hudson P, Aranda S, McMurray N. Randomized controlled trials in palliative care: overcoming the obstacles. Int J Palliat Nurs 2001; 7: 427–434. [DOI] [PubMed] [Google Scholar]
- 88. Barnes S, Gott M, Payne S, Parker C, Seamark D, Gariballa S, Small N. Recruiting older people into a large, community‐based study of heart failure. Chronic Illn 2005; 1: 321–329. [DOI] [PubMed] [Google Scholar]
- 89. Janssen DJ, Wouters EF, Schols JM, Spruit MA. Self‐perceived symptoms and care needs of patients with severe to very severe chronic obstructive pulmonary disease, congestive heart failure or chronic renal failure and its consequences for their closest relatives: the research protocol. BMC Palliative Care 2008; 7: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Campbell RT, McMurray JJ. Comorbidities and differential diagnosis in heart failure with preserved ejection fraction. Heart Fail Clin 2014; 10: 481–501. [DOI] [PubMed] [Google Scholar]
- 91. Gure TR, Blaum CS, Giordani B, Koelling TM, Galecki A, Pressler SJ, Hummel SL, Langa KM. Prevalence of cognitive impairment in older adults with heart failure. J Am Geriatr Soc 2012; 60: 1724–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Formiga F, Olmedo C, Lopez‐Soto A, Navarro M, Culla A, Pujol R. Dying in hospital of terminal heart failure or severe dementia: the circumstances associated with death and the opinions of caregivers. Palliat Med 2007; 21: 35–40. [DOI] [PubMed] [Google Scholar]


