Abstract
Background
Regular intake of vitamin C/ascorbate reduces blood pressure (BP) in hypertensives. High-dose intravenous vitamin C (IVC) achieves higher plasma levels; however, there is a paucity of research on acute BP effects. Our study is the first to investigate the effect of high-dose IVC, with or without concomitant i.v. nutrients, on BP during i.v. treatment.
Methods
A cohort of adult patients scheduled to receive IVC treatment for infection, cancer or fatigue, as prescribed by their treating doctor, participated at a Melbourne clinic, Australia. Ambulatory BP was assessed every 10 min over 90 min during i.v. treatment. Patients received 15–100 g of IVC alone or in addition to i.v. vitamin B, glutathione, magnesium or zinc. BP change over time adjusted for baseline BP, IVC dosage, i.v. treatment and BMI was analysed.
Results
A total of 77 mostly normotensive patients participated, with a third receiving IVC alone (42±20 g), and two-thirds also received other i.v. nutrients. IVC alone (>30 g) reduced the mean BP up to 8–9 mmHg in prehypertensive patients. In contrast, concomitant intravenous vitamin B12 (IVB12) significantly increased the mean BP by 11–13 mmHg. Comparison of BP change during IVC versus IVC+IVB12 indicated a highly significant difference [systolic blood pressure: mean difference (SD)=16.6 (17.8) mmHg, P<0.001; diastolic blood pressure: mean difference (SD)=12.5 (16.7) mmHg, P=0.003].
Conclusion
Our study suggests an acute BP-reducing effect of high-dose IVC, particularly with dosages above 30 g, and in patients with prehypertension and normal BMI. Furthermore, our study indicated a marked and clinically relevant hypertensive effect of IVB12, suggesting routine BP monitoring during i.v. therapy in clinical practice.
Keywords: ascorbate, blood pressure, cobalamin, intravenous, parenteral, vitamin B12, vitamin C
Introduction
Epidemiological research has linked higher vitamin C status with lower blood pressure (BP), reducing BP by 2–4 mmHg with every 50 μmol/l increase in vitamin C plasma levels 1. In addition, a meta-analysis of 29 randomized-controlled clinical trials including more than 1400 participants showed that oral supplementation of 500 mg of vitamin C (ascorbic acid) per day for 8 weeks resulted in an average reduction of 3.8±1.5 mmHg systolic (P<0.01) and 1.5±1.4 mmHg diastolic (P=0.04), and 4.9 mmHg systolic in hypertensive patients (P<0.01) compared with placebo 2.
In contrast, no significant acute BP effect was observed after 2 h of a 2 g dose of oral vitamin C supplementation 3. However, a protective BP-lowering effect was observed after 3 g daily of an oral sustained-release vitamin C supplementation for 2 weeks in a group of 60 healthy normotensive healthy adults exposed to a stressor (Trier Social Stress Test) 4. While the placebo group experienced a 35 mmHg systolic blood pressure (SBP) spike within 1 min of the stress test, the vitamin C group experienced a lower BP spike of 27 mmHg (mean difference±SD=8.3±2.2 mmHg; P<0.001) 4. In addition, BP returned to prestress test levels within 40 min in the vitamin C group, whereas the placebo group’s BP remained slightly elevated (5 mmHg, P<0.001), indicating a slower recovery time, which has been associated with greater cardiovascular risk 4,5.
There is a paucity of research on the effect of intravenously administered vitamin C on BP. One study explored the acute effect within 30 min of a bolus of 1 or 2 g (6 or 12 mmol) of intravenous vitamin C (IVC) and observed an immediate but short-term BP reduction of 10–15 mmHg systolic at 10–20 min with return to baseline after IVC administration in patients with mild hypertension 6.
Intestinal absorption of orally administered vitamin C is tightly regulated by a dose-dependent active transport process to a threshold plasma level (50–100 μmol/l), whereby absorption of doses of greater than 1 g/day is less than 50%, with excretion of the remainder in the urine 7,8. In contrast, intravenous administration of high doses of ascorbate (1–200 g) can lead to plasma concentrations of up to 20 mmol/l, up to 100-fold greater than by oral administration within 1–2 h of administration 9,10.
IVC therapy is used to boost immune function and as part of integrative cancer treatment 11,12; however, much higher dosages between 10 and 100 g per day (150–710 mg/kg/day) are used than generally tolerable with oral vitamin C intake. High-dose IVC therapy boosts the immune system by promoting antiviral, antibacterial and anticancer activities, helps recovery from injury and surgery, alleviates disease-related fatigue and improves quality of life 11–15.
IVC has a high safety profile. In a survey of 199 practitioners using IVC (mean dose 28 g, median 50 g, range 1–200 g per treatment) mainly for infection or cancer, it was found that 1% (101 out of 9328 patients) reported side effects, including minor fatigue (n=59), change in mental status (n=21) and vein irritation/phlebitis (n=6) 16. Two deaths related to IVC therapy were because of known risk factors, renal impairment and glucose-6-phosphate-dehydrogenase deficiency. Patients with these risk factors should be excluded from IVC therapy.
Our study is the first to systematically assess the acute effect on BP of high-dose IVC alone and in conjunction with other standard i.v. nutrients including vitamin B12 and other B vitamins, glutathione, zinc and magnesium in a cohort of patients treated for a variety of conditions in an integrative medical practice.
Methods
Participants and study design
A cohort of adult patients, scheduled to receive IVC treatment for infection, cancer or fatigue, as prescribed by their treating doctor at the National Institute of Integrative Medicine (NIIM) clinic, Melbourne, Australia, were recruited between March 2014 and May 2015. Patients with renal impairment, defined by abnormal levels of estimated glomerular filtration, albumin, creatinine or blood urea nitrogen levels, or patients with glucose-6-phosphate-dehydrogenase deficiency were not eligible because of contraindications to IVC therapy. Patients with arrhythmia, or those not able to provide informed consent, were also excluded.
Participation was not restricted by the reason for IVC therapy or underlying illness, or the number of previous IVC treatments received. Ninety percent of patients scheduled to receive IVC treatment consented to participate in the BP study.
Consenting participants’ sociodemographic data, reason for IVC treatment, concomitant therapies (hyperthermia, chemotherapy, any medications), treatment schedule, including dose of vitamin C, number of IVC treatments and time period, and concomitant intravenous agents (type, dosage) were obtained.
Blood pressure monitoring
Baseline BP was recorded before start of the intravenous drip using an oscillometric ambulatory BP monitor (Mobil-O-Graph; IEM GmbH, Stolberg, Germany) 17 with appropriate-sized brachial cuffs on the opposite arm with the intravenous drip with the patient seated and the arm at the heart level. The Mobil-O-graph device was programmed to record BP at 10 min intervals throughout the duration of the intravenous treatment (up to 1.5 h). Participants were advised to keep the arm still through the cuff inflation.
Intravenous treatments
Patients received ambulant treatment in the i.v. clinic at NIIM. Pharmaceutical-grade sodium ascorbate solution (Biological Therapies, Melbourne, Victoria, Australia) was diluted in H2O as necessary to provide the target dose at an infusion rate of 500 ml/h (8.5 ml/min) or 20 drops/ml (170 drops/min). IVC dosage was less than or equal to 1.3 g/kg bodyweight up to a maximum of 60 g per at least 60 kg patient. Up to 30 g of sodium ascorbate is usually diluted in 500 ml H2O and administered within 1 h, whereas 60 g of sodium ascorbate is diluted in 750 ml H2O and administered within 1.5–2 h. Patients’ IVC tolerance levels are gradually built up, usually starting with 15 g (75 mmol) of IVC in the first session, up to 30 g (150 mmol) of IVC in the second session and up to 60 g (300 mmol) of IVC in the third and consecutive sessions. A number of participants received additional i.v. treatments alongside the ascorbate solution. A variety of combinations of additional i.v. treatments administered included an average of 1 g (3 mmol) of glutathione solution, 2.47 mg (20 mmol) of magnesium sulphate in 5 ml and 5.1 mg of zinc chloride in 2 ml or 400 mg (1.4 mmol) of α-linoleic acid, as well as B vitamins (Table 1). B vitamins were administered as an intravenous vitamin B (IVB) dose (B1 – 10 mg, B2 – 5 mg, B3 – 100 mg, B5 – 20 mg and B6 – 50 mg), as vitamin B forte =B1 – 250 mg, B2 – 5 mg, B3 – 50 mg, B5 – 50 mg, B6 – 100 mg, B12 – 1 mg (0.7 mmol cyanocobalamin) or as methylcobalamin injection vitamin B12 (10 mg, 7 mmol, in 2 ml).
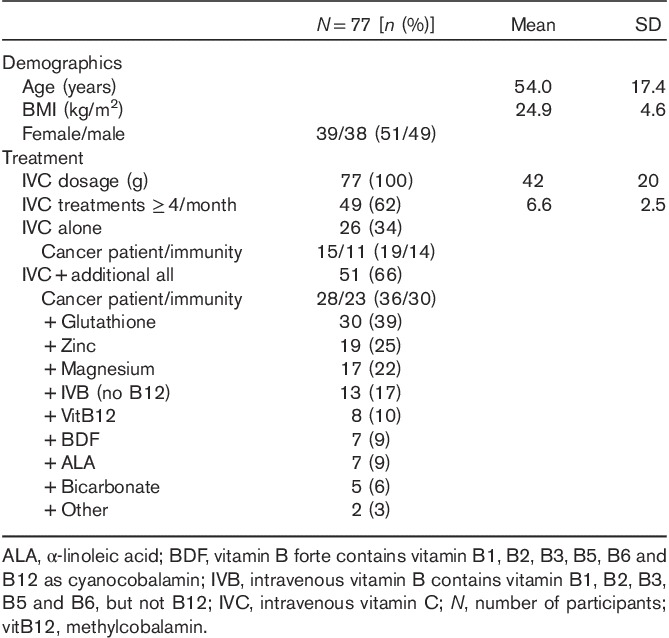
Table 1.
Baseline characteristics
Intravenous therapy administration was performed by experienced registered NIIM clinical nurses according to a doctor’s prescription. Administration of sodium ascorbate injection followed the protocol provided by Biological Therapies at the Injectable Nutrients Workshop supported by the Australasian College of Medical Nutrition. Care of patients consenting to take part in the study followed standard clinical practice.
Statistical analyses
Baseline characteristics were descriptively analysed. Primary outcome measures were SBP and diastolic blood pressure (DBP) at all time points compared with baseline by analysis of covariance, adjusting for baseline BP, IVC dosage, i.v. treatment or BMI. Extreme outlier BP values, because of movement during cuff inflation, were removed. We also carried out subgroup analyses by IVC dosage, baseline BP, BMI and concomitant intravenous agents, and explored BP changes by health condition (cancer vs. infection/fatigue). All analyses were carried out using IBM SPSS, version 22 (SPSS Inc., Chicago, Illinois, USA).
A sample size calculation indicated that 50 participants (n=25 per group) were sufficient to detect a significant and clinically relevant difference of 8 mmHg (SD=10) in BP between groups with 80% power and 95% confidence.
The study received ethics approval from the NHMRC registered HREC committee at NIIM (Trial number 252).
Results
Participants
A total of 77 adult patients participated in the study. Baseline characteristics are shown in Table 1. Participants’ mean age was 54±17 years, with a mean average BP of 124/78 mmHg at baseline, with three participants on BP medication and only one participant reporting major cardiovascular issues in the past. No participants were smokers and the sample had an average mean BMI of 25 kg/m2. A third (n=26, 34%) received IVC alone, whereas two-thirds (n=51, 66%) received additional treatments plus IVC. The average IVC dosage was 42±20 g (range 15–100 g), with treatment lasting an average of 80 min. About half (56%) of the participants received treatment for cancer, whereas 44% underwent IVC treatment for general immunity (colds/flus), Lyme disease and chronic fatigue. About two-thirds of the participants (n=49, 62%) had received IVC treatment more than once per week, with an average of 6.6±2.5 times per month (Table 1).
Blood pressure
The mean changes in SBP and DBP compared with baseline were analysed every 10 min throughout the i.v. treatment, with the main changes observed at three time points (20–30/50–60/70–75 min; Tables 2 and 3).
Table 2.
Systolic blood pressure mean changes within groups and mean differences between groups at three time points compared with baseline
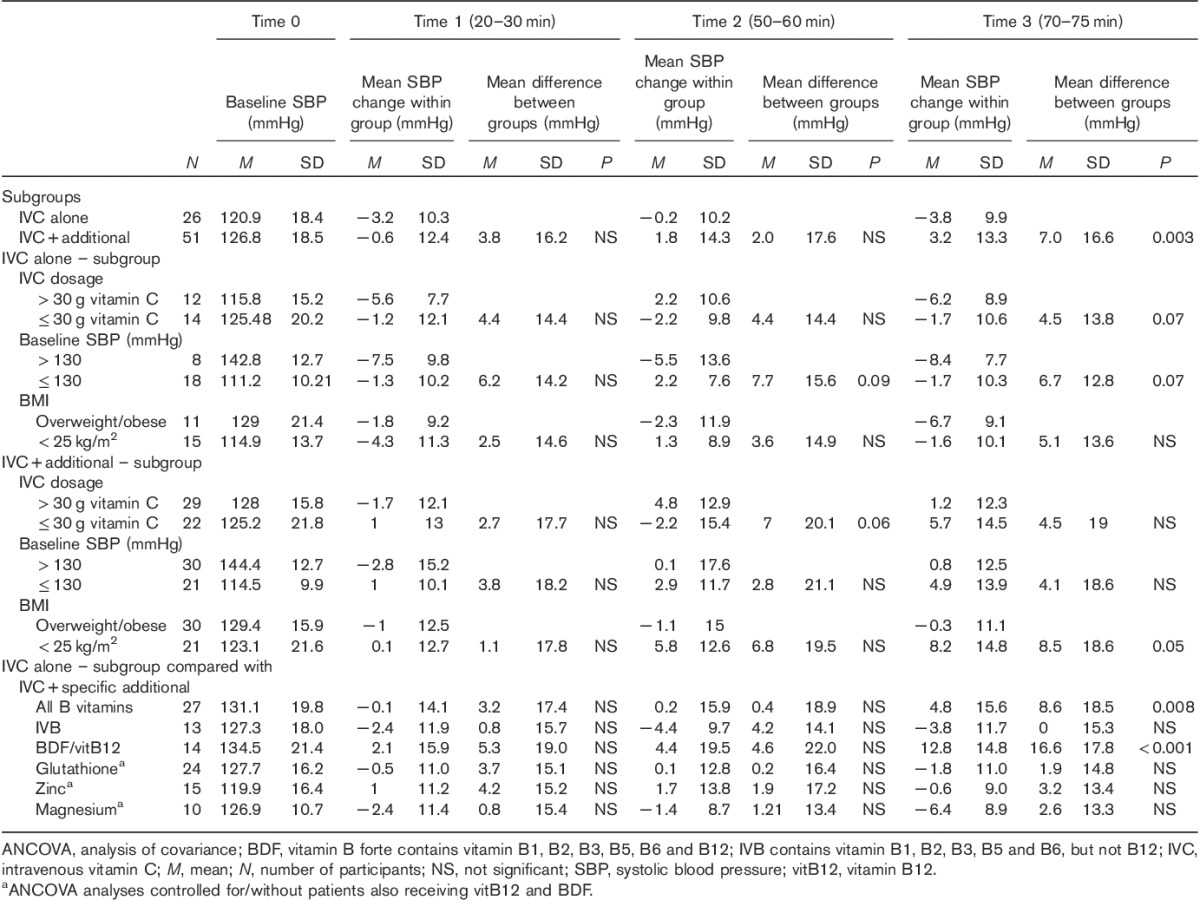
Table 3.
Diastolic blood pressure mean changes within groups and mean differences between groups at three time points compared with baseline
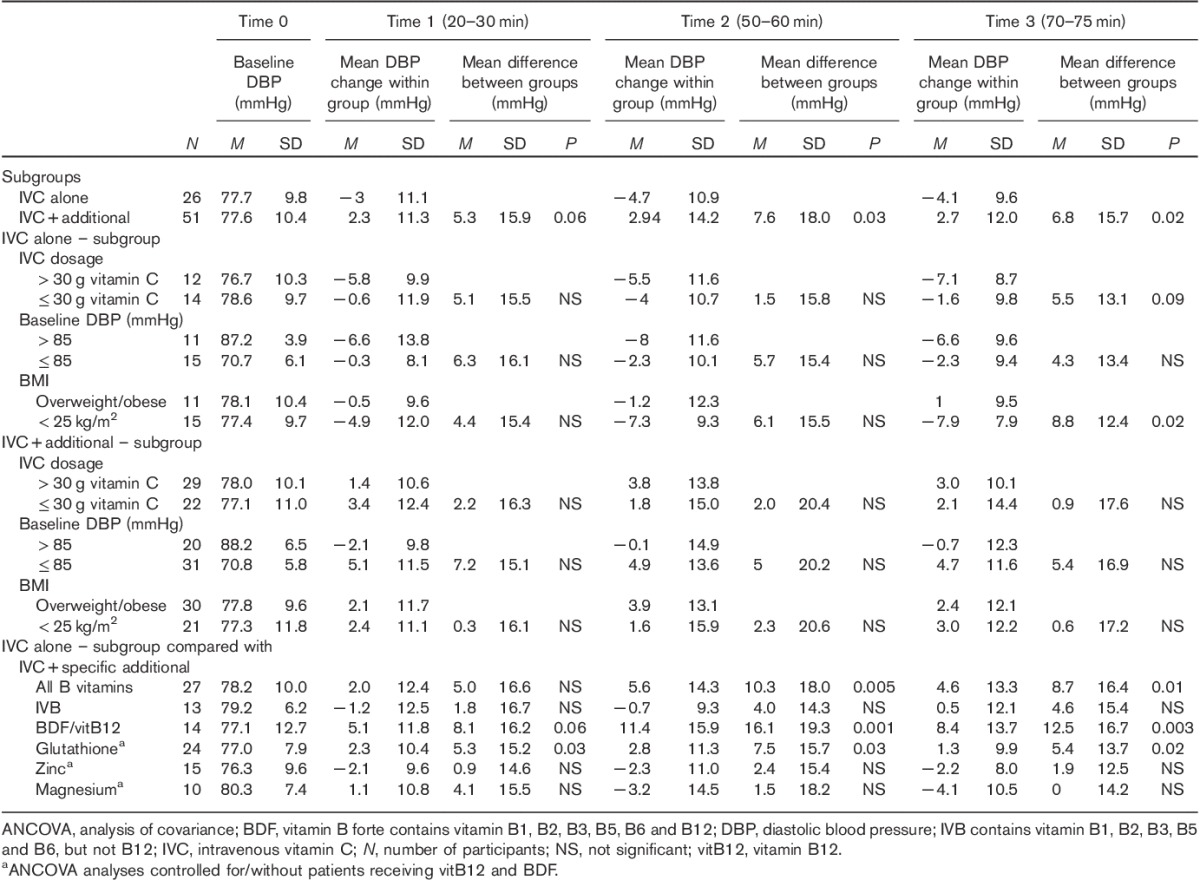
In the majority of patients, SBP decreased during the first 20–30 min of i.v. treatment and increased at 50–60 min. However, BP response at 70–75 min was significantly dependent on whether concomitant treatments were administered in addition to IVC. While the mean BP decreased in the group receiving IVC alone at the end of i.v. treatment, BP increased when additional treatments were administered (mean difference SBP±SDtime3=7.0±16.6 mmHg; P=0.003; mean difference DBP±SDtime3=6.8±15.7 mmHg; P=0.02) (Figs 1a and 2a and Tables 2 and 3).
Fig. 1.
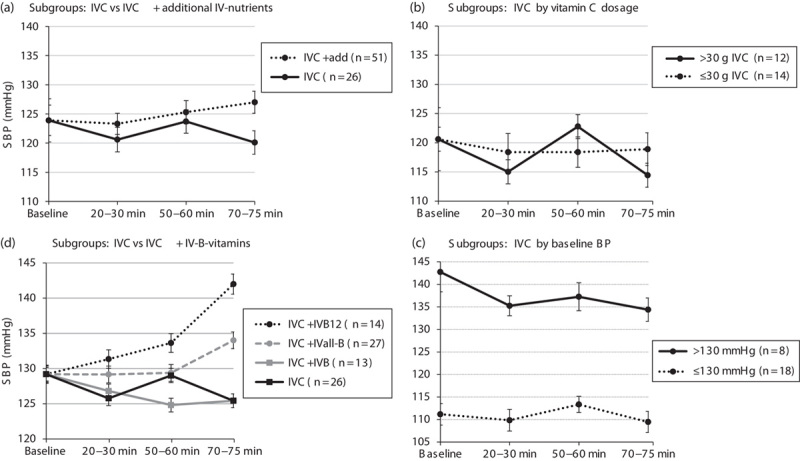
Systolic blood pressure (SBP) change over time during i.v. treatment. (a) Subgroups: IVC alone versus IVC+additional i.v. nutrients; (b) IVC alone by vitamin C dosage; (c) IVC alone by baseline BP; and (d) IVC alone versus IVC+IVB vitamins. (a, b, d) Adjusted for baseline BP. Statistically significant difference between subgroups compared with IVC: *P<0.01, **P<0.001. add, additional i.v. nutrients; BP, blood pressure; IVB, intravenous vitamin B contains vitamin B1, B2, B3, B5, B6 but not B12; IVB12, intravenous vitamin B12 as methylcobalamin (7 mmol) or in vitamin B forte (BDF) as cyanocobalamin (0.7 mmol); IVall-B, intravenous-all-inclusive vitamin B12; IVC, intravenous vitamin C.
Fig. 2.
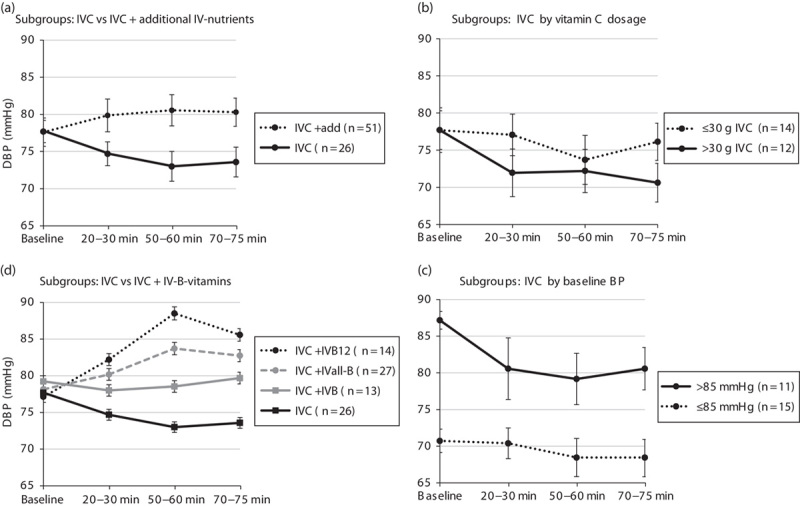
Diastolic blood pressure (DBP) change over time during i.v. treatment. (a–d) For details and abbreviations, see Fig. 1.
Therefore, main analyses were separated into two groups with patients receiving IVC alone (n=26) or IVC plus additional treatments (n=51). Data were further analysed by IVC dosage, baseline BP, and BMI.
IVC treatment alone
The mean BP in all patients receiving IVC alone (n=26) changed only slightly over the course of the treatment. However, analysis by IVC dosage showed a marked difference BP pattern/change over time.
While BP tended to decrease more over time in patients receiving higher concentrations of IVC (>30 g), the mean BP did not alter appreciably in patients receiving less than 30 g of IVC (mean difference SBP±SDtime3=4.5±13.8 mmHg; P=0.07; mean difference DBP±SDtime3=5.5±13.1 mmHg; P=0.09) (Figs 1b and 2b and Tables 2 and 3).
Baseline BP also influenced response to IVC treatment. In prehypertensive patients (SBP/DBP>130/85 mmHg), SBP decreased by an average of 8–9 mmHg and DBP by 6–7 mmHg over the course of the IVC treatment, whereas BP remained steady in normotensive patients (SBP/DBP≤130/85 mmHg) (mean difference SBP±SDtime3=6.7±12.8 mmHg; P=0.07; mean difference DBP±SDtime3=5.5±13.1 mmHg; P=NS) (Figs 1c and 2c and Tables 2 and 3).
BMI significantly influenced DBP response to IVC treatment, whereby DBP decreased by almost 8 mmHg in the healthy-weight group (BMI≤25 kg/m2), but did not alter appreciably in the overweight/obese group (mean difference DBP±SDtime3=8.8±12.4 mmHg; P=0.02) (Table 3).
Subgroup analyses by health condition (cancer vs. infection or fatigue) did not show any appreciable difference in BP change between the groups (data not shown).
IVC and additional i.v. treatments
In the subgroup of patients receiving IVC and additional treatments, IVC dosage (mean difference SBP±SDtime2=7.0±20.1 mmHg; P=0.06) and BMI (mean difference SBP±SDtime3=8.5±18.6 mmHg; P=0.05) slightly influenced SBP response at selected time points (Table 2).
Further analyses indicated that administration of B vitamins, in particular vitamin B12 independent of dosage, was responsible for a significantly different BP response over time compared with IVC alone.
While patients’ BP decreased with IVC alone, their BP increased markedly with the addition of vitamin B12 (vitamin B forte or vitamin B12) (mean difference SBP±SDtime3=16.6±17.8 mmHg; P<0.001; mean difference DBP±SDtime3=12.5±16.7 mmHg; P=0.003) (Figs 1d and 2d and Tables 2 and 3).
In contrast, administration of glutathione (SBP), magnesium, zinc and B vitamins other than vitamin B12 did not alter the BP response pattern appreciably compared with IVC alone (Table 2).
Discussion
Our study is the first to report on acute BP effects of high-dose IVC over a 1.5 h period with or without concomitant nutrient treatment. We found IVC to significantly reduce BP of up to 6–7 mmHg systolic and diastolic if administered at dosages above 30 g and up to 8–9 mmHg in patients with prehypertension (SBP/DBP≥130/85 mmHg) at baseline. In contrast, administration of intravenous vitamin B12 (IVB12) solutions significantly increased BP response by up to 13 mmHg systolic and 11.5 mmHg diastolic during and at the end of i.v. treatment.
The results obtained with IVC alone were consistent with the literature, whereby a bolus of 1–2 g of IVC reduced BP by up to 10–15 mmHg in the first 10–20 min and BP returned back to baseline at 30 min 6. In our study, for the first time, we continued the assessment of BP every 10 min until 90 min and observed a prolonged BP-reducing effect of IVC. Although the reduction in BP within the first 10–20 min may be primarily attributed to a direct vasodilatory physiological effect, described as venodilation 18, BP reduction observed after 70–90 min is likely attributable to pharmacokinetically plausible vitamin C absorption and vasodilation because of nitric oxide release 9,19,20. Pharmacokinetic studies of IVC administration observed peak plasma levels within the first 90 min, with plasma levels reaching 13350 μmol/l for 50 g of IVC 9.
In addition, the greater mean BP-reductive effect of vitamin C in hypertensives compared with normotensive patients is consistent with the literature 2. Essential hypertension, associated with endothelial dysfunction because of an impaired nitric oxide/l-arginine pathway and impaired vasodilation can be restored by vitamin C 21.
Furthermore, a significantly greater reduction in DBP of 8–9 mmHg observed in patients within the healthy weight range (BMI≤25 kg/m2) compared with the overweight/obese group is congruent with the literature, whereby obesity-related insulin resistance and higher levels of epinephrine are associated with impaired endothelial function, reduced NO production and thus increased BP 22.
Moreover, our study is the first to describe the marked increase in BP response when IVB12 is administered. The mean BP increased significantly up to 12–16 mmHg systolic and diastolic independent of the dosage of vitamin B12 (0.7–7 mmol). This marked increase in BP – although likely attenuated by the coadministration of IVC in our patient cohort – could potentially lead to problems in already hypertensive patients.
The production of norepinephrine, which can stimulate angiotensin-II production, which in turn influences BP, has been suggested as a possible mechanism for the increase in BP with IVB12 23. Animal studies have found higher serum levels of norepinephrine (noradrenaline) in the adrenal medulla of rats receiving methylcobalamin (methyl-vitamin B12) 24. In addition, excess norephinephrine levels stimulate the sympathetic nervous system, leading to increased cortisol production, which has also been linked to increases in BP 25.
The lack of a significant BP reduction with the administration of i.v. glutathione observed in our study may have been because of a lower dosage of 3 mmol compared with 6 and 12 mmol in the study by Ceriello et al. 6 and may have been confounded by the concomitant administration of other nutrients.
Our study had some limitations related to its observational nature and being the first study of its kind, therefore including variability in health conditions and disease status of participants, IVC dosage regime, baseline BP, etc. Although we controlled for some of the variables in the analysis and explored heterogeneity by subgroup analysis, future studies could focus on more congruent groups.
Furthermore, it would be of interest to explore the acute effects of single i.v. nutrients on BP, in particular, the influence of IVB12. The results of our study imply that BP should be monitored routinely during the administration of IVB12, in particular, in hypertensive patients, for safety reasons.
Second, measurement of selected biomarkers that influence BP levels such as cortisol, insulin and norepinephrine could shed more light on the mechanisms of BP change observed with IVB12.
Third, a controlled study comparing IVC administration compared with a saline solution as placebo could tease out any i.v. nutrient-specific effects on BP, albeit no changes in BP were observed within 10–20–30 min of saline administration in the study by Ceriello et al. 6.
Finally, as our study has been the first to measure the effect of IVC and other nutrients during treatment on BP for a prolonged period of 90 min, further extension of BP measurements and vitamin C plasma levels would broaden insights into maximal BP changes in correlation to vitamin C peak plasma levels.
Conclusion
Our study is the first to examine the acute effects of high-dose IVC treatment on BP across 90 min of treatment and the first to incorporate additional i.v. nutrients in conjunction with IVC. Our results suggest IVC to reduce BP by 6–7 mmHg if dosages of more than 30 g are administered and up to 8–9 mmHg in patients with prehypertension (SBP/DBP≥130/85 mmHg).
Furthermore, our study is the first to describe the marked BP-increasing effect by 12–16 mmHg of IVB12, suggesting routine BP monitoring during i.v. therapy in clinical practice.
Acknowledgements
The authors acknowledge all participants and the NIIM nurses and staff who supported this study. All authors are employees of NIIM; NIIM is a not-for-profit DGR-endorsed charitable organization.
Author contributions: K.R. and A.S. conceptualized the study. Data were collected by N.T. N.T. and K.R. carried out data analysis and interpretation. K.R. and N.T. prepared the manuscript. All authors approved the final version.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ness AR, Khaw K-T, Bingham S, Day NE. Vitamin C status and blood pressure. J Hypertens 1996; 14:503–508. [PubMed] [Google Scholar]
- 2.Juraschek SP, Guallar E, Appel LJ, Miller ER., 3rd Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2012; 95:1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffy SJ, Gokce N, Holbrook M, Huang A, Frei B, Keaney JF, Jr., Vita JA. Treatment of hypertension with ascorbic acid. Lancet 1999; 354:2048–2049. [DOI] [PubMed] [Google Scholar]
- 4.Brody S, Preut R, Schommer K, Schürmeyer TH. A randomized controlled trial of high dose ascorbic acid for reduction of blood pressure, cortisol, and subjective responses to psychological stress. Psychopharmacology (Berl) 2002; 159:319–324. [DOI] [PubMed] [Google Scholar]
- 5.Schuler JL, O’Brien WH. Cardiovascular recovery from stress and hypertension risk factors: a meta-analytic review. Psychophysiology 1997; 34:649–659. [DOI] [PubMed] [Google Scholar]
- 6.Ceriello A, Giugliano D, Quatraro A, Lefebvre PJ. Anti-oxidants show an anti-hypertensive effect in diabetic and hypertensive subjects. Clin Sci (Lond) 1991; 81:739–742. [DOI] [PubMed] [Google Scholar]
- 7.Graumlich JF, Ludden TM, Conry-Cantilena C, Cantilena LR, Jr, Wang Y, Levine M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm Res 1997; 14:1133–1139. [DOI] [PubMed] [Google Scholar]
- 8.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA 1996; 93:3704–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 2004; 140:533–537. [DOI] [PubMed] [Google Scholar]
- 10.Verrax J, Calderon PB. The controversial place of vitamin C in cancer treatment. Biochem Pharmacol 2008; 76:1644–1652. [DOI] [PubMed] [Google Scholar]
- 11.Fritz H, Flower G, Weeks L, Cooley K, Callachan M, McGowan J, et al. Intravenous vitamin C and cancer: a systematic review. Integr Cancer Ther 2014; 13:280–300. [DOI] [PubMed] [Google Scholar]
- 12.Lee WJ. The prospects of vitamin C in cancer therapy. Immune Netw 2009; 9:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oudemans-van Straaten HM, Spoelstra-de Man AM, de Waard MC. Vitamin C revisited. Crit Care 2014; 18:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikirova N, Hunninghake R. Effect of high dose vitamin C on Epstein–Barr viral infection. Med Sci Monit 2014; 20:725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carr AC, Vissers MC, Cook JS. The effect of intravenous vitamin C on cancer- and chemotherapy-related fatigue and quality of life. Front Oncol 2014; 4:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One 2010; 5:e11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Qattan KK, Khan I, Alnaqeeb MA, Ali M. Mechanism of garlic (Allium sativum) induced reduction of hypertension in 2 K-1C rats: a possible mediation of Na/H exchanger isoform-1. Prostaglandins Leukot Essent Fatty Acids 2003; 69:217–222. [DOI] [PubMed] [Google Scholar]
- 18.Grossmann M, Dobrev D, Himmel HM, Ravens U, Kirch W. Ascorbic acid-induced modulation of venous tone in humans. Hypertension 2001; 37:949–954. [DOI] [PubMed] [Google Scholar]
- 19.Vanhoutte PM. Endothelium and control of vascular function. State of the art lecture. Hypertension 1989; 13 (Pt 2):658–667. [DOI] [PubMed] [Google Scholar]
- 20.Rees DD, Palmer RM, Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci USA 1989; 86:3375–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation 1998; 97:2222–2229. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 1996; 97:2601–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep 2007; 9:228–235. [DOI] [PubMed] [Google Scholar]
- 24.Masoud EAM, Bakar AMA, Ossama AM, Dhiab NAAH. Methylcobalamin has an effect on hypothalamic–hypophyseal–adrenal axis. Mol Pharmacol 2010; 1:2. [Google Scholar]
- 25.Kelly J, Mangos G, Williamson P, Whitworth J. Cortisol and hypertension. Clin Exp Pharmacol Physiol 1998; 25 (S1):S51–S56. [DOI] [PubMed] [Google Scholar]


