Abstract
Smooth muscle cells (SMCs) in the rat carotid artery leave the quiescent state and proliferate after balloon catheter injury. The precise signals responsible for this SMC mitogenesis need to be elucidated. Although platelet-derived growth factor (PDGF), a potent SMC mitogen, is released from activated platelets, damaged endothelium, and macrophages, it cannot be solely responsible for this proliferation. In search of other SMC growth factors, we have examined several proteins of the coagulation cascade. At nanomolar concentrations, factors X, Xa, and protein S promote cultured rat aortic SMC mitosis. In contrast, factor IX is only weakly mitogenic, whereas factor VII and protein C fail to stimulate SMC division. Protein S, the most mitogenic of these coagulation cascade factors, stimulates DNA synthesis in cultured SMCs with a time course similar to that of PDGF-AA and without the delay observed for transforming growth factor beta. Antistasin and tick anticoagulant peptide, two specific factor Xa inhibitors, inhibit SMC mitogenesis due to Xa and protein S. Coagulation factors that possess mitogenic activity may contribute to intimal SMC proliferation after vascular injury as a result of angioplasty or vascular compromise during atherogenesis.
Full text
PDF

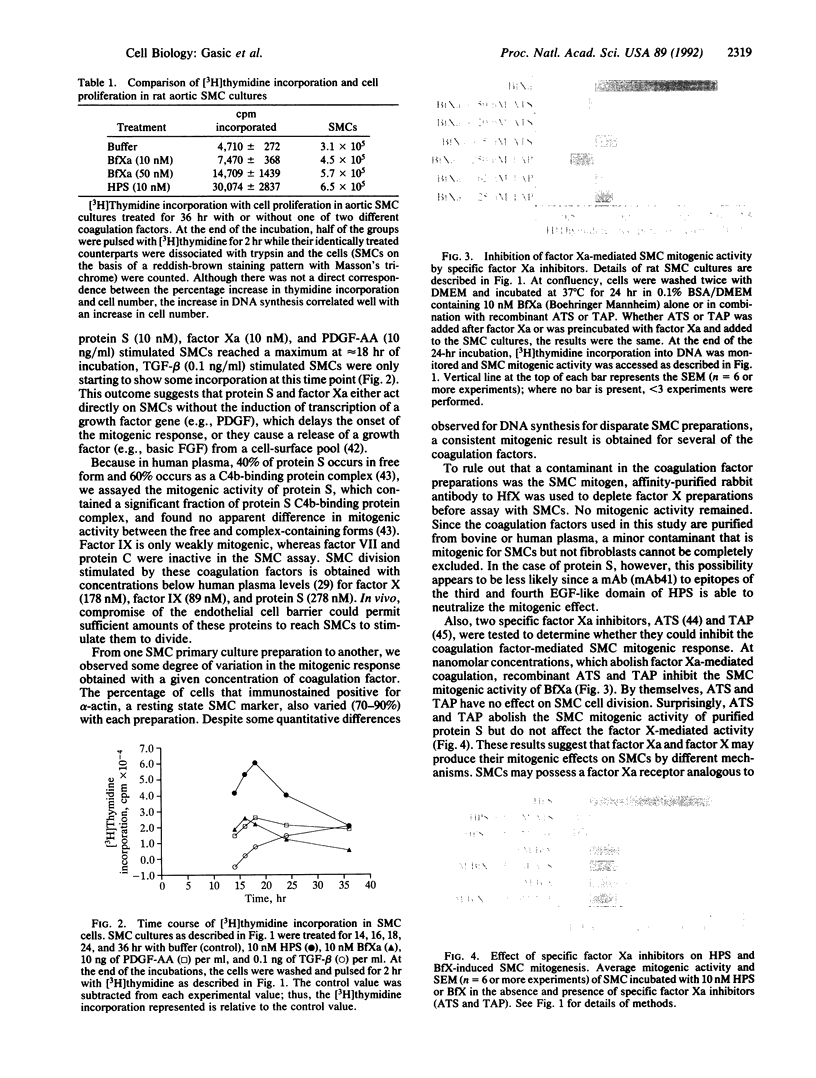

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong M. L., Heistad D. D. Animal models of atherosclerosis. Atherosclerosis. 1990 Nov;85(1):15–23. doi: 10.1016/0021-9150(90)90178-l. [DOI] [PubMed] [Google Scholar]
- Battegay E. J., Raines E. W., Seifert R. A., Bowen-Pope D. F., Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990 Nov 2;63(3):515–524. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Gown A. M. Atheroma: the artery wall and the environment. Int Rev Exp Pathol. 1980;21:55–118. [PubMed] [Google Scholar]
- Bowen-Pope D. F., Hart C. E., Seifert R. A. Sera and conditioned media contain different isoforms of platelet-derived growth factor (PDGF) which bind to different classes of PDGF receptor. J Biol Chem. 1989 Feb 15;264(5):2502–2508. [PubMed] [Google Scholar]
- Brunner G., Gabrilove J., Rifkin D. B., Wilson E. L. Phospholipase C release of basic fibroblast growth factor from human bone marrow cultures as a biologically active complex with a phosphatidylinositol-anchored heparan sulfate proteoglycan. J Cell Biol. 1991 Sep;114(6):1275–1283. doi: 10.1083/jcb.114.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. H., Campbell G. R. Biology of the vessel wall and atherosclerosis. Clin Exp Hypertens A. 1989;11(5-6):901–913. doi: 10.3109/10641968909035381. [DOI] [PubMed] [Google Scholar]
- Dahlbäck B., Hildebrand B., Malm J. Characterization of functionally important domains in human vitamin K-dependent protein S using monoclonal antibodies. J Biol Chem. 1990 May 15;265(14):8127–8135. [PubMed] [Google Scholar]
- Dahlbäck B., Lundwall A., Stenflo J. Primary structure of bovine vitamin K-dependent protein S. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4199–4203. doi: 10.1073/pnas.83.12.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B. Purification of human C4b-binding protein and formation of its complex with vitamin K-dependent protein S. Biochem J. 1983 Mar 1;209(3):847–856. doi: 10.1042/bj2090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F. Reconstructing the evolution of vertebrate blood coagulation from a consideration of the amino acid sequences of clotting proteins. Cold Spring Harb Symp Quant Biol. 1987;52:869–874. doi: 10.1101/sqb.1987.052.01.095. [DOI] [PubMed] [Google Scholar]
- Esmon C. T. The regulation of natural anticoagulant pathways. Science. 1987 Mar 13;235(4794):1348–1352. doi: 10.1126/science.3029867. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R. Studies of hypercholesterolemia in the nonhuman primate. II. Fatty streak conversion to fibrous plaque. Arteriosclerosis. 1984 Jul-Aug;4(4):341–356. doi: 10.1161/01.atv.4.4.341. [DOI] [PubMed] [Google Scholar]
- Fanelli C., Aronoff R. Restenosis following coronary angioplasty. Am Heart J. 1990 Feb;119(2 Pt 1):357–368. doi: 10.1016/s0002-8703(05)80028-6. [DOI] [PubMed] [Google Scholar]
- Ferns G. A., Reidy M. A., Ross R. Balloon catheter de-endothelialization of the nude rat carotid. Response to injury in the absence of functional T lymphocytes. Am J Pathol. 1991 Apr;138(4):1045–1057. [PMC free article] [PubMed] [Google Scholar]
- Friedman M. H. A biologically plausible model of thickening of arterial intima under shear. Arteriosclerosis. 1989 Jul-Aug;9(4):511–522. doi: 10.1161/01.atv.9.4.511. [DOI] [PubMed] [Google Scholar]
- Friedman R. J., Stemerman M. B., Wenz B., Moore S., Gauldie J., Gent M., Tiell M. L., Spaet H. The effect of thrombocytopenia on experimental arteriosclerotic lesion formation in rabbits. Smooth muscle cell proliferation and re-endothelialization. J Clin Invest. 1977 Nov;60(5):1191–1201. doi: 10.1172/JCI108872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie B., Furie B. C. The molecular basis of blood coagulation. Cell. 1988 May 20;53(4):505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Gajdusek C., Carbon S., Ross R., Nawroth P., Stern D. Activation of coagulation releases endothelial cell mitogens. J Cell Biol. 1986 Aug;103(2):419–428. doi: 10.1083/jcb.103.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Golden M. A., Au Y. P., Kirkman T. R., Wilcox J. N., Raines E. W., Ross R., Clowes A. W. Platelet-derived growth factor activity and mRNA expression in healing vascular grafts in baboons. Association in vivo of platelet-derived growth factor mRNA and protein with cellular proliferation. J Clin Invest. 1991 Feb;87(2):406–414. doi: 10.1172/JCI115011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton J. R., Karnovsky M. J. Smooth muscle cell proliferation in the occluded rat carotid artery: lack of requirement for luminal platelets. Am J Pathol. 1979 Mar;94(3):585–602. [PMC free article] [PubMed] [Google Scholar]
- Hanke H., Strohschneider T., Oberhoff M., Betz E., Karsch K. R. Time course of smooth muscle cell proliferation in the intima and media of arteries following experimental angioplasty. Circ Res. 1990 Sep;67(3):651–659. doi: 10.1161/01.res.67.3.651. [DOI] [PubMed] [Google Scholar]
- Huang C. L., Ives H. E. Growth inhibition by protein kinase C late in mitogenesis. 1987 Oct 29-Nov 4Nature. 329(6142):849–850. doi: 10.1038/329849a0. [DOI] [PubMed] [Google Scholar]
- Ip J. H., Fuster V., Israel D., Badimon L., Badimon J., Chesebro J. H. The role of platelets, thrombin and hyperplasia in restenosis after coronary angioplasty. J Am Coll Cardiol. 1991 May;17(6 Suppl B):77B–88B. doi: 10.1016/0735-1097(91)90942-3. [DOI] [PubMed] [Google Scholar]
- Liu M. W., Roubin G. S., King S. B., 3rd Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989 Jun;79(6):1374–1387. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Bowen-Pope D. F., Hart C. E., Wilcox J. N., Schwartz S. M. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990 Nov;111(5 Pt 1):2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda J., Ross R. Atherogenesis during low level hypercholesterolemia in the nonhuman primate. I. Fatty streak formation. Arteriosclerosis. 1990 Mar-Apr;10(2):164–177. doi: 10.1161/01.atv.10.2.164. [DOI] [PubMed] [Google Scholar]
- Nutt E., Gasic T., Rodkey J., Gasic G. J., Jacobs J. W., Friedman P. A., Simpson E. The amino acid sequence of antistasin. A potent inhibitor of factor Xa reveals a repeated internal structure. J Biol Chem. 1988 Jul 25;263(21):10162–10167. [PubMed] [Google Scholar]
- Paigen B., Holmes P. A., Novak E. K., Swank R. T. Analysis of atherosclerosis susceptibility in mice with genetic defects in platelet function. Arteriosclerosis. 1990 Jul-Aug;10(4):648–652. doi: 10.1161/01.atv.10.4.648. [DOI] [PubMed] [Google Scholar]
- Reddick R. L., Read M. S., Brinkhous K. M., Bellinger D., Nichols T., Griggs T. R. Coronary atherosclerosis in the pig. Induced plaque injury and platelet response. Arteriosclerosis. 1990 Jul-Aug;10(4):541–550. doi: 10.1161/01.atv.10.4.541. [DOI] [PubMed] [Google Scholar]
- Reilly C. F. Rat vascular smooth muscle cells immortalized with SV40 large T antigen possess defined smooth muscle cell characteristics including growth inhibition by heparin. J Cell Physiol. 1990 Feb;142(2):342–351. doi: 10.1002/jcp.1041420217. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Ross R. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of WHHL and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1990 Sep-Oct;10(5):680–687. doi: 10.1161/01.atv.10.5.680. [DOI] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W. Cellular interactions, growth factors, and smooth muscle proliferation in atherogenesis. Ann N Y Acad Sci. 1990;598:102–112. doi: 10.1111/j.1749-6632.1990.tb42282.x. [DOI] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Foy L., Bowen-Pope D. F., Ross R. Derivation and properties of platelet-derived growth factor-independent rat smooth muscle cells. Am J Pathol. 1990 Jun;136(6):1417–1428. [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M., Heimark R. L., Majesky M. W. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990 Oct;70(4):1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Reidy M. A. Common mechanisms of proliferation of smooth muscle in atherosclerosis and hypertension. Hum Pathol. 1987 Mar;18(3):240–247. doi: 10.1016/s0046-8177(87)80006-0. [DOI] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Friedman J., Abraham J. A., Fiddes J. C., Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987 Jan 15;325(6101):257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Singh T. M., Kadowaki M. H., Glagov S., Zarins C. K. Role of fibrinopeptide B in early atherosclerotic lesion formation. Am J Surg. 1990 Aug;160(2):156–159. doi: 10.1016/s0002-9610(05)80297-1. [DOI] [PubMed] [Google Scholar]
- Sueishi K., Yasunaga C., Castellanos E., Kumamoto M., Tanaka K. Sustained arterial injury and progression of atherosclerosis. Ann N Y Acad Sci. 1990;598:223–231. doi: 10.1111/j.1749-6632.1990.tb42294.x. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Fridman R., Sullivan R., Sasse J., Klagsbrun M. Aortic endothelial cells synthesize basic fibroblast growth factor which remains cell associated and platelet-derived growth factor-like protein which is secreted. J Cell Physiol. 1987 Jun;131(3):402–408. doi: 10.1002/jcp.1041310312. [DOI] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Waller B. F., Pinkerton C. A., Orr C. M., Slack J. D., VanTassel J. W., Peters T. Restenosis 1 to 24 months after clinically successful coronary balloon angioplasty: a necropsy study of 20 patients. J Am Coll Cardiol. 1991 May;17(6 Suppl B):58B–70B. doi: 10.1016/0735-1097(91)90940-b. [DOI] [PubMed] [Google Scholar]
- Waxman L., Smith D. E., Arcuri K. E., Vlasuk G. P. Tick anticoagulant peptide (TAP) is a novel inhibitor of blood coagulation factor Xa. Science. 1990 May 4;248(4955):593–596. doi: 10.1126/science.2333510. [DOI] [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Williams L. T., Schwartz S. M., Gordon D. Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J Clin Invest. 1988 Sep;82(3):1134–1143. doi: 10.1172/JCI113671. [DOI] [PMC free article] [PubMed] [Google Scholar]






