Abstract
We conducted a randomized controlled trial (RCT) of an early palliative care intervention (ENABLE: Educate, Nurture, Advise, Before Life Ends) for persons with advanced cancer and their family caregivers. Not all patient participants had a caregiver coparticipant; hence, we explored whether there were relationships between patient survival, having an enrolled caregiver, and caregiver outcomes prior to death. One hundred and twenty‐three patient‐caregiver dyads and 84 patients without a caregiver coparticipant participated in the ENABLE early versus delayed (12 weeks later) RCT. We collected caregiver quality‐of‐life (QOL), depression, and burden (objective, stress, and demand) measures every 6 weeks for 24 weeks and every 3 months thereafter until the patient's death or study completion. We conducted survival analyses using log‐rank and Cox proportional hazards models. Patients with a caregiver coparticipant had significantly shorter survival (Wald = 4.31, HR = 1.52, CI: 1.02–2.25, P = 0.04). After including caregiver status, marital status (married/unmarried), their interaction, and relevant covariates, caregiver status (Wald = 6.25, HR = 2.62, CI: 1.23–5.59, P = 0.01), being married (Wald = 8.79, HR = 2.92, CI: 1.44–5.91, P = 0.003), and their interaction (Wald = 5.18, HR = 0.35, CI: 0.14–0.87, P = 0.02) were significant predictors of lower patient survival. Lower survival in patients with a caregiver was significantly related to higher caregiver demand burden (Wald = 4.87, CI: 1.01–1.20, P = 0.03) but not caregiver QOL, depression, and objective and stress burden. Advanced cancer patients with caregivers enrolled in a clinical trial had lower survival than patients without caregivers; however, this mortality risk was mostly attributable to higher survival by unmarried patients without caregivers. Higher caregiver demand burden was also associated with decreased patient survival.
Keywords: Advanced cancer, family caregivers, patient survival
Introduction
We conducted a fast track randomized controlled trial (RCT) with 207 newly diagnosed advanced cancer patients and 123 family caregivers to test the ENABLE III (Educate, Nurture, Advise, Before Life Ends) nurse‐led, telephone‐based palliative care intervention. This RCT demonstrated that concurrent palliative care initiated soon after a diagnosis of advanced cancer had beneficial effects on patient survival and caregiver depression and burden compared with initiating this intervention 12 weeks later 1, 2. Because we enrolled patient participants with and without family caregivers, we had a “natural” experiment 3 allowing us to examine whether the presence of a caregiver and a caregiver's wellbeing and burden might also be related to patient survival.
Caregivers perform a plethora of health‐related tasks that appear vital for advanced cancer patients' survival. They spend an average of 8 h/day 4 tracking and treating symptoms; administering medications and breathing treatments; coordinating medical appointments; participating in advance care planning and other healthcare decision‐making; providing emotional and spiritual support; preparing meals; managing finances; and performing domestic home duties 5, 6, 7, 8. Over time, however, caregivers may become burdened by performing these tasks.
Part of this burden relates to caregivers feeling untrained and unprepared 6, 8, 9, while other parts relate to witnessing someone close to them struggle with serious illness. Thus, caregivers become prone to depression 10, anxiety 11, and poor physical health 12, 13. As the Institute of Medicine 14 and others 15, 16 have reported, this poor caregiver health can attenuate a caregiver's ability to provide care to cancer patients. This line of reasoning served as the impetus for us to include a parallel intervention specifically for family caregivers in the ENABLE III trial. Nurse coaches provided one‐on‐one support and education to caregivers about caregiving tasks, and about how to cope with their care recipients' struggle with serious illness. We believed this would improve caregivers' wellbeing and performance thus benefiting cancer patients' wellbeing and survival. Indeed, the main results of our trial demonstrated that caregivers had lower depressed mood and stress burden 2.
However, two unanswered questions remained: (1) What relationship exists between having a family caregiver and a patient's survival? (2) What relationships exist between a caregiver's outcomes (quality‐of‐life [QOL], depression, and burden) and a patient's survival? To address these questions, we conducted a secondary analysis of our clinical trial data. We hypothesized that having a caregiver would be associated with increased patient survival, and that higher caregiver QOL and lower caregiver depression and burden would be associated with increased patient survival.
Methods
This was a secondary analysis of the ENABLE III 1, 2 “fast track” RCT 17. Individuals with newly diagnosed, recurrent, or progressive metastatic cancer and their caregivers (if they had one and were willing to enroll) were randomly assigned to receive the intervention as soon as possible after diagnosis (early group) or 12 weeks later (delayed group). The study protocol, data and safety monitoring plan were approved by the Norris Cotton Cancer Center/Dartmouth College and the Veterans Administration (VA) Medical Center, White River Junction, Vermont institutional review boards. The trial was registered in clinicaltrials.gov (Identifier NCT01245621).
Sample and setting
Patient and caregiver participants in the ENABLE III trial were recruited between October 11, 2010 and March 5, 2013 from the Norris Cotton Cancer Center at Dartmouth‐Hitchcock Medical Center (DHMC), affiliated DHMC outreach clinics, and the White River Junction, Vermont VA Medical Center. Patient inclusion criteria were: (1) age >18 years; (2) within 30–60 days of being informed by a treating oncologist of a new diagnosis, recurrence, or progression of an advanced‐stage cancer; (3) oncologist‐estimated prognosis of 6–24 months; (4) English speaking; and (5) able to complete baseline questionnaires. Patients were excluded if: they scored <4 on the Callahan Cognitive Screen; 18 had an active Axis I psychiatric condition (e.g., schizophrenia, bipolar disorder) or substance use disorder; or had uncorrectable hearing disorder or unreliable telephone service. Patient participants were asked to nominate a family caregiver, defined as “a person who knows you well and is involved in your medical care” to participate in a parallel intervention. Patients were not excluded if they did not have a participating caregiver. There were no other caregiver eligibility criteria. After completing baseline measures, patients and their caregiver coparticipants, if they had one, were randomly assigned to receive early or delayed intervention.
The ENABLE III early palliative care intervention
The ENABLE III intervention was initially developed in 1998 as a Robert Wood Johnson demonstration project to integrate early palliative care with oncology care and has now been refined and evaluated in two large multisite RCTs 1, 2, 19, 20, 21. Details of the intervention are described elsewhere 1, 2, 20 and on the National Cancer Institute's Research‐tested Intervention Programs website (rtips.cancer.gov/rtips/index.do). Briefly, the ENABLE III intervention consisted of: (1) an outpatient palliative care assessment following National Consensus Guidelines 22 (caregivers invited to attend); (2) a series of individualized phone sessions delivered weekly by nurse coaches following a guidebook called Charting Your Course (patients: 6 sessions; caregivers: 3 sessions); and (3) monthly follow‐up to reinforce previous content as needed or to address new issues. Patients and caregivers received their one‐on‐one sessions with a separate nurse coach. The first three patient and caregiver sessions addressed decision‐making and problem‐solving strategies (based upon the principles of Problem‐solving Treatment and the COPE program) 23, 24, 25, 26, communication skills, advance care planning, and symptom management. The patient portion of ENABLE III included three more sessions that incorporated the Outlook life review intervention developed by Steinhauser and colleagues 27, 28.
Data collection and measures
Caregiver outcome measures were collected by telephone by a study coordinator blinded to group assignment at baseline and every 6 weeks for 24 weeks and then every 12 weeks until the patient's death or study completion. Caregiver QOL was measured using the 35‐item Caregiver QOL‐Cancer Scale (CQOL‐C) (score range 0–140: higher scores = worse QOL) 29. Domains of the CQOL‐C include physical, emotional, and spiritual wellbeing related to caregiving and relationship quality with the care recipient. Internal consistency has been reported as 0.91 and a test–retest reliability as 0.95. Caregiver depressed mood was measured using the 20‐item Center for Epidemiological Studies Depression (CES‐D) scale (score range 0–60; higher scores = higher depressed mood; >16 = clinically significant depression) 30, 31. Caregiver burden was measured using the 14‐item Montgomery Borgatta Caregiver Burden Scale (MBCB) that includes objective, demand, and stress burden subscales (subscales α = 0.88, 0.74 and 0.84, respectively) 32, 33. Objective burden is defined as interference with the caregiver's private, social and recreational time, and normal daily routine (e.g., restrictions on vacations and trips, amount of time for friends, amount of personal privacy). Demand burden is defined as the degree of strain on caregivers due to feeling that their care recipients are overly demanding (e.g., attempts by care recipient to manipulate caregiver, unreasonable care recipient demands and requests). Stress burden is defined as the emotional strain felt by caregivers due to caregiving tasks (e.g., life tension, anxiety, depression about the care recipient relationship).
Statistical methods
t‐Tests and Pearson's chi‐square were used to examine demographic and baseline patient outcome differences between patients with and patients without a caregiver. Variables with significant differences between groups were included as covariates in survival analyses (i.e., variables significantly associated with having a family caregiver). Cox proportional hazards regression analyses 34 were used to model: (1) the association between caregiver coparticipant presence/absence and survival (with and without adjustment for baseline covariates) and (2) the association between caregivers' QOL, depressed mood, and burden (objective, demand, and stress burden) measured both at baseline and at the last occasion before patients' death and patient survival. Patients with missing covariate data were excluded as needed for each Cox model.
Results
Table 1 lists caregiver demographic characteristics. The mean age was 59.4 years. Most caregivers were female (78.0%, n = 96); White race (92.7%, n = 114); Protestant (33.3%, n = 41); married or living with a partner (91.9%, n = 113); employed full or part time (49.6%, n = 61); and were the patient's spouse/partner (75.6%, n = 93). The diagnoses of the care recipients were lung (43.1%, n = 53), gastrointestinal (25.2%, n = 31), genitourinary (8.1%, n = 10), breast (8.1%, n = 10), hematologic (5.7%, n = 7), and other solid tumor cancers (10.6%, n = 13).
Table 1.
Caregiver Demographic Characteristics (N = 123)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Mean | 59.4 | |
| SD | 11.7 | |
| Sex | ||
| Female | 96 | 78.0 |
| Male | 26 | 21.1 |
| Missing | 1 | .8 |
| Race | ||
| White people | 114 | 92.7 |
| Other | 5 | 4.1 |
| Missing/no response | 4 | 3.3 |
| Marital Status | ||
| Married or living with partner | 113 | 91.9 |
| Never Married | 4 | 3.3 |
| Divorced or separated | 3 | 2.4 |
| Widowed | 2 | 1.6 |
| Missing/no response | 1 | .8 |
| Education | ||
| High school or GED; some college or technical school | 70 | 56.9 |
| ≥ College graduate | 51 | 41.5 |
| <High school graduate | 1 | .8 |
| Missing/no response | 1 | .8 |
| Employment status | ||
| Full or part time | 61 | 49.6 |
| Retired | 35 | 28.5 |
| Not employed | 25 | 20.3 |
| Missing/no response | 1 | .8 |
| Religious affiliation | ||
| Protestant | 41 | 33.3 |
| Catholic | 36 | 29.3 |
| Jewish | 2 | 1.6 |
| None | 23 | 18.7 |
| Other | 15 | 12.2 |
| Missing/no response | 6 | 4.9 |
| Relationship to Patient | ||
| Spouse/partner | 93 | 75.6 |
| Sibling | 7 | 5.7 |
| Son or daughter | 14 | 11.4 |
| Parent | 7 | 5.7 |
| Other | 1 | .8 |
| Missing/no response | 1 | .8 |
| Primary disease site of patient | ||
| Lung | 53 | 43.1 |
| GI | 31 | 25.2 |
| GU | 10 | 8.1 |
| Breast | 10 | 8.1 |
| Hematologic | 7 | 5.7 |
| Other solid tumor | 13 | 10.6 |
Table 2 lists patient characteristics and compares groups of patients with (n = 123) and without caregivers (n = 84). Compared to patients without caregivers, patients with caregivers were older (62.4 vs. 65.7, P = 0.02); male (38.1% vs. 62.6%, P < 0.01); married or living with a partner (53.6% vs. 73.2%, P < 0.01); and were more likely to have a living will or durable power of attorney (34.5% vs. 49.6%, P = 0.05). There were no differences in patients' other demographics, Charlson scores, Karnofsky Performance Status (KPS), symptom impact, depressed mood (CES‐D), QOL (FACIT‐Pal), clinical trial enrollment, presence of a do‐not‐resuscitate (DNR) order, or intervention group.
Table 2.
Patient characteristics
| Characteristic | All patients (N = 207) | Patients with a caregiver (N = 123) | Patients without a caregiver (N = 84) | P a | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age, years | |||||||
| Mean | 64.3 | 62.4 | 62.4 | 0.02 | |||
| SD | 9.9 | 9.9 | 9.9 | ||||
| Male gender | 109 | 52.7 | 77 | 62.6 | 32 | 38.1 | <0.01 |
| Marital Status | |||||||
| Married | 135 | 65.2 | 90 | 73.2 | 45 | 53.6 | <0.01 |
| Not married | 72 | 34.8 | 33 | 26.8 | 39 | 46.4 | |
| Education | |||||||
| <High school graduate | 11 | 5.3 | 6 | 4.9 | 5 | 6.0 | 0.92 |
| High school graduate | 111 | 53.6 | 67 | 54.5 | 44 | 52.4 | |
| College graduate | 85 | 41.1 | 50 | 40.7 | 35 | 41.7 | |
| Race | |||||||
| White people | 200 | 96.6 | 118 | 96.7 | 82 | 97.6 | 0.31 |
| Black people | 1 | .5 | 0 | 0 | 1 | 1.2 | |
| Other | 5 | 2.4 | 4 | 3.3 | 1 | 1.2 | |
| Missing | 1 | .5 | 1 | .01 | 0 | 0 | |
| Religion | |||||||
| Catholic | 65 | 31.4 | 34 | 27.6 | 31 | 36.9 | 0.41 |
| Protestant | 63 | 30.4 | 42 | 34.1 | 21 | 25.0 | |
| Jewish | 1 | .5 | 1 | .8 | 0 | 0 | |
| None | 44 | 21.3 | 25 | 20.3 | 19 | 22.6 | |
| Other | 28 | 13.5 | 16 | 13.0 | 12 | 14.3 | |
| Missing | 6 | 2.9 | 5 | 4.1 | 1 | 1.2 | |
| Employment Status | |||||||
| Employed | 49 | 23.7 | 27 | 22.0 | 22 | 26.2 | 0.56 |
| Retired | 99 | 47.8 | 61 | 49.6 | 38 | 45.2 | |
| Not Employed | 58 | 28.0 | 35 | 28.5 | 23 | 27.4 | |
| Student | 1 | .5 | 0 | 0 | 1 | 1.2 | |
| Medical insurance | |||||||
| Medicare | 104 | 50.2 | 64 | 52.0 | 40 | 48.2 | 0.47 |
| Private/Commercial | 71 | 34.3 | 42 | 34.1 | 29 | 34.9 | |
| Military | 19 | 9.2 | 12 | 9.8 | 7 | 8.4 | |
| Medicaid | 7 | 3.4 | 4 | 3.3 | 3 | 3.6 | |
| Uninsured | 5 | 2.4 | 1 | .8 | 4 | 4.8 | |
| Missing | 1 | .5 | 0 | 0 | 1 | 1.2 | |
| Ever smoked | 145 | 70.1 | 87 | 70.7 | 58 | 69.0 | 0.80 |
| Diagnosis | |||||||
| Lung | 88 | 42.5 | 53 | 43.1 | 35 | 41.7 | 0.68 |
| Gastrointestinal tract | 50 | 24.2 | 31 | 25.2 | 19 | 22.6 | |
| Breast | 23 | 11.1 | 10 | 8.1 | 13 | 15.5 | |
| Other solid tumor | 20 | 9.7 | 12 | 10.6 | 8 | 9.5 | |
| Genitourinary tract | 16 | 7.7 | 10 | 8.1 | 6 | 7.1 | |
| Hematologic malignancies | 10 | 4.8 | 7 | 5.7 | 3 | 3.6 | |
| Charlson score | 6.3 | 1.7 (SD) | 6.2 | 1.6 (SD) | 6.3 | 2.0 (SD) | 0.65 |
| Karnofsky Performance Status | 81.0 | 10.3 (SD) | 81.1 | 11.0 (SD) | 80.8 | 9.3 (SD) | 0.84 |
| FACIT‐Pal (Baseline) | 126.2 | 21.3 | 126.6 | 19.7 (SD) | 125.6 | 23.4 (SD) | 0.75 |
| CES‐D (Baseline) | 14.2 | 10.1 | 13.3 | 8.8 (SD) | 15.5 | 11.6 (SD) | 0.14 |
| QUAL‐E, Symptom Impact Subscale (Baseline) | 11.7 | 3.7 (SD) | 11.6 | 3.7 (SD) | 11.8 | 3.6 (SD) | 0.61 |
| In a clinical trial at enrollment | 27 | 13.0 | 16 | 13.0 | 11 | 13.1 | 0.71 |
| Advance directive in chart at enrollment | |||||||
| Living will or durable power of attorney | 89 | 43.0 | 60 | 49.6 | 29 | 34.5 | 0.05 |
| DNR order | 20 | 9.7 | 13 | 11.7 | 7 | 8.5 | 0.63 |
| Early intervention group | 104 | 50.2 | 61 | 51.2 | 42 | 49.4 | 0.95 |
Fisher's exact or Pearson's chi‐square test for categorical variables and t‐test for continuous variables. DNR, do‐not‐resuscitate.
Caregiver status and patient survival
In a Cox regression model that included the caregiver status predictor and no covariates, having a caregiver coparticipant was associated with reduced patient survival (n = 207, Wald(1) = 4.31, HR = 1.52, CI: 1.02–2.25, P = 0.04) (see Fig. 1). In a model that included covariates correlating significantly with caregiver status (patient age, patient gender, marital status [married/unmarried], and advance directive status [see Table 2]), caregiver status was not associated with reduced survival (n = 205, Wald(1) = 1.33, HR = 1.28, CI: 0.84–1.96, P = 0.25); however, marital status was significantly associated with reduced survival (n = 205, Wald(1) = 4.33, HR = 1.62, CI: 1.03–2.56, P = 0.04). Because caregiver status was significantly associated with marital status, we conducted a Cox regression analysis that included caregiver status, marital status, and their interaction as simultaneous predictors while covarying patient age, gender, and advance directive status. In this model, caregiver status (n = 205, Wald(1) = 6.25, HR = 2.62, CI: 1.23–5.59, P = 0.01), marital status (n = 205, Wald(1) = 8.79, HR = 2.92, CI: 1.44–5.91, P = 0.003), and their interaction (n = 205, Wald(1) = 5.18, HR = 0.35, CI: 0.14–0.87, P = 0.02) were significant predictors of survival. The interaction took the form such that caregiver status was a significant predictor of survival among unmarried but not among married patients. Hence, when Cox analyses were conducted separately, caregiver status was a significant predictor of reduced survival among unmarried patients (Wald = 5.19, HR = 2.44, CI: 1.13–5.25, P = 0.02), but not among married patients (Wald = 0.37, HR = 0.86, CI: 0.52–1.42, P = 0.55). In order to illustrate this effect, a fourfold categorical variable was created (caregiver present/married, caregiver present/unmarried, caregiver absent/married, caregiver absent/unmarried). When this variable was entered in a Cox regression model covarying patient age, gender, and advance directive status, it was a significant predictor of patient survival (Wald = 8.79, P = 0.02). Kaplan–Meier survival curves were generated for these four groups (see Fig. 2). Unmarried patients without a caregiver (indicated by the solid blue line) experienced better survival than the other three groups.
Figure 1.
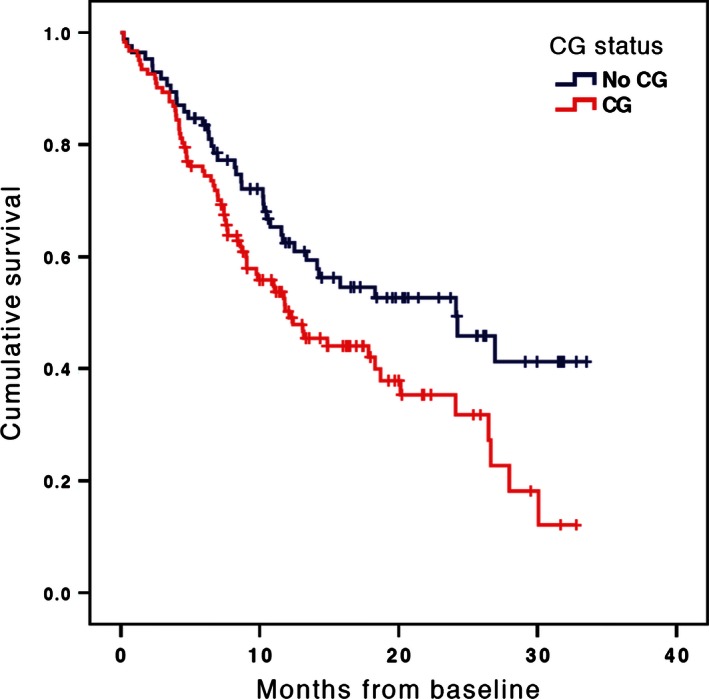
Patient survival curves by caregiver coparticipant presence/absence. Cox proportional hazards model with no covariates.
Figure 2.
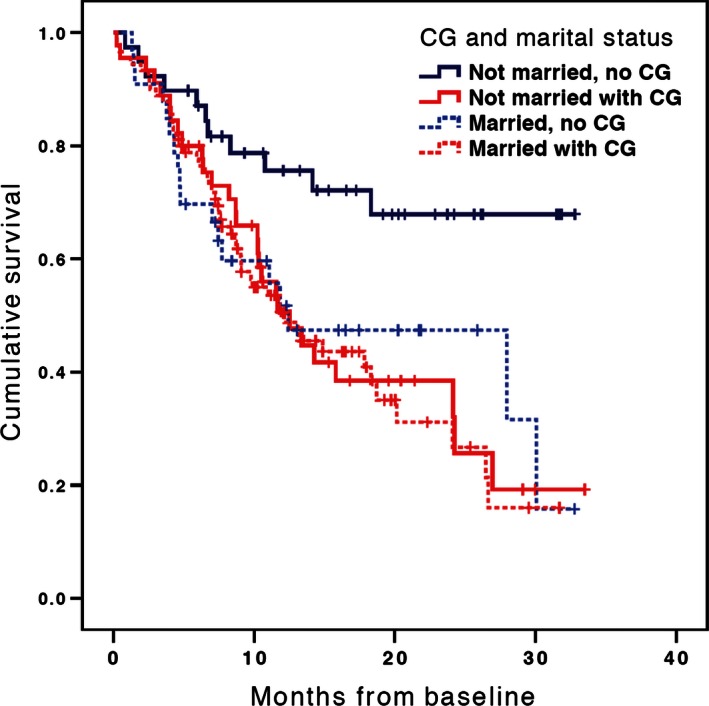
Adjusted survival curves by caregiver and marital status. Cox proportional hazards model adjusted for patient age, gender, and presence of an advance directive and/or durable power of attorney.
Caregiver QOL, depressed mood, and burden and patient survival
At baseline, caregiver QOL, depressed mood, and burden were not predictive of patient survival. At the last measurement period before death, only caregiver demand burden (n = 77, Wald(1) = 4.87, CI: 1.01–1.20, P = 0.03) (Fig. 3) was significantly related to decreased survival. Caregiver QOL (n = 93, Wald(1) = 1.14, CI: 0.99–1.02, P = 0.29), depressed mood (n = 93, Wald(1) = 1.22, CI: 0.99–1.04, P = 0.27), objective (n = 81, Wald(1) = 1.68, CI: 0.97–1.15, P = 0.20) and stress burden (n = 91, Wald(1) = 1.77, CI: 0.97–1.16, P = 0.18) were not significant.
Figure 3.
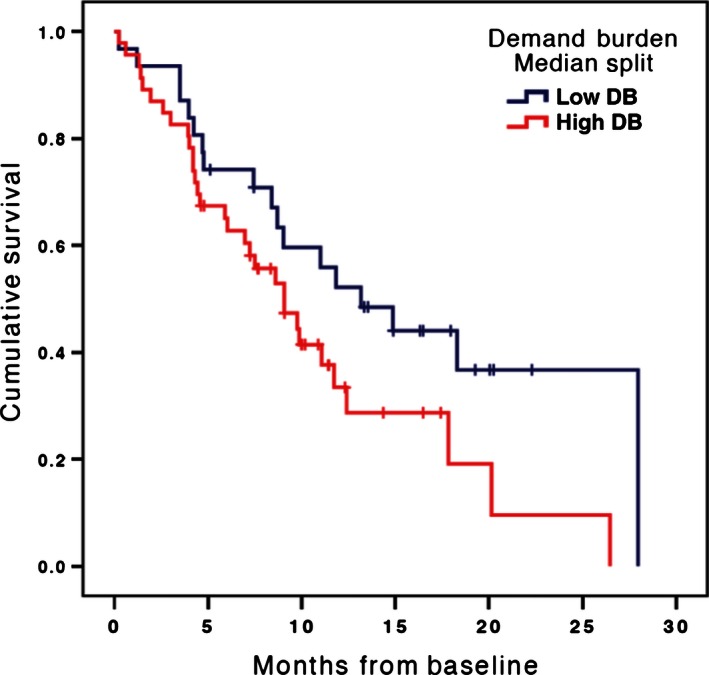
Adjusted survival curves by high and low caregiver burden using median split. Cox proportional hazards model adjusted for intervention group.
Post Hoc analysis
In our RCT, we collected two measures of a patients' social support that could be related to having a caregiver: the Multidimensional Scale of Perceived Social Support 35 and the Social Well‐being subscale of the FACIT‐Pal 36. Although both measures were related to caregiver status such that patients with caregivers had higher ratings of social support, neither measure was associated with patient survival (both with and without covariates). When these social support measures were included as covariates along with gender, age, and advance directive status, the results for caregiver and marital status remained essentially unchanged as shown in these results: caregiver status (n = 203, Wald(1) = 6.90, HR = 2.89, CI: 1.31–6.40, P = 0.009), marital status (n = 203, Wald(1) = 9.10, HR = 3.11, CI: 1.49–6.49, P = 0.003), and their interaction (n = 203, Wald(1) = 5.62, HR = 0.33, CI: 0.13–0.83, P = 0.02).
Comment
We conducted a secondary analysis of the ENABLE III RCT data to examine whether advanced cancer patients' higher survival would be associated with having a family caregiver, higher caregiver quality‐of‐life (QOL), and lower caregiver depression. Contrary to our hypothesis, patients with a caregiver coparticipant had lower survival compared to those without. This finding was mostly attributable to the higher survival of unmarried patients without caregiver coparticipants. Of interest, higher caregiver demand burden was associated with lower survival, while caregiver QOL, depression, objective burden, and stress burden were not. This is the first study to show a significant relationship between the survival of advanced cancer patients and family caregivers' presence and burden.
It is unclear why patients with a caregiver had shorter survival compared with those patients in the trial who did not. We offer two possible explanations. First, patients who had a high‐disease burden might have had more daily health needs that required the presence and assistance of a family caregiver. This high‐disease burden might itself be associated with shorter survival; hence having a caregiver might represent sicker patients with a poorer prognosis. However, the available data we collected of potential markers of disease severity did not support this explanation. We found no detectable differences between those with and without a caregiver in baseline cancer diagnoses, Charlson scores, KPS scores, symptom impact, depressed mood, QOL, or clinical trial enrollment (see Table 2). It could be that there are other markers of disease severity that would better predict patient survival and needing a family caregiver. Thus, we recommend that this be explored in future studies.
A second potential explanation for our results could relate to patients' self‐perceived burden on their family caregivers 37, 38, 39, 40. When debilitating illnesses, such as cancer, constrain a patients' ability to care for themselves thereby necessitating assistance from family members, it is possible that these patients begin to see themselves as an undue burden on others. There is some evidence to suggest that this reluctance to burden others may result in depression 40 and impact one's health behaviors and preferences for treatment. For example, a study by Lee and colleagues 38 of 326 patient‐caregiver dyads with distant stage cancers found that both higher patient self‐perceived burden and higher caregiver burden scores were associated with lower preferences for life‐sustaining treatments. While evidence is lacking in our study to directly support the mechanism of a patient's reluctance to burden others, it is notable that compared to patients without caregivers, a higher proportion of patients with caregivers had a living will or durable power of attorney (see Table 2).
When conducting these analyses, we found it puzzling that just over half of the patient samples with no caregiver coparticipants were married (54%). This appears to challenge the adequacy of caregiver coparticipation as a proxy variable for caregiver status since it is reasonable to assume that these married patients may have been receiving some kind of assistance from their spouse. To clarify this puzzle, we included marital status in the Cox regression analysis as a predictor in the 4‐group Kaplan–Meier curves (Fig. 2). This analysis revealed two important insights. First, being married was highly associated with having a caregiver coparticipant (Table 2). Second, the addition of marital status along with caregiver status as predictor variables in the Cox regression showed that the significant association with survival was maintained in the same negative direction, such that having a spouse was also associated with lower survival. This is consistent with the 4‐group Kaplan–Meier curves demonstrating that those patients who were unmarried with no caregiver coparticipant had the better survival in comparison to everyone else who was either married and/or had a caregiver coparticipant. Our interpretation of this is that those 54% of married patients with no caregiver coparticipant did in reality have a spouse who may have been providing support, however, this continued to be associated with decreased survival.
We propose several explanations for these findings. First, while numerous studies report a protective effect of marriage on cancer mortality 41, 42, 43, 44, 45, 46, 47, other studies find marriage associated with lower survival 48 (which is consistent with our findings). Alternatively, the relationship may vary depending on the history of a person's marital status: for example, in a meta‐analysis of the association of social networks with cancer mortality 49, survival was lowest for individuals who were never married compared to individuals who were divorced, separated or widowed. In our analyses, marital status was operationalized as married/unmarried and hence it is likely that our unmarried patient group reflects a sample that is heterogeneous with regard to marital status history, making it difficult to interpret how marital status drives the findings.
Second, despite controlling for gender in our analyses, unmeasured factors associated with gender may explain our strong study results. Lending support for this hypothesis, a meta‐analysis of 1,365 nonsmall cell lung cancer patients by Siddiqui and colleagues 50 found that unmarried females had higher overall survival than both married and unmarried males. This is especially compelling given that in our study, patients without a caregiver were largely unmarried and female.
Finally, our measure of marital status does not necessarily indicate the quality of patients' relationships and social support networks. A substantial body of literature has shown that the quality of relationships and social support have a significant impact on patients' health and survival 51, 52. In post hoc analyses, social support did correlate with having a caregiver coparticipant. However, neither the MSPSS nor the FACIT‐Pal social well‐being subscale was related to patient survival. Furthermore, inclusion of these social support measures did not change the results of the final model. This suggests that while the constructs of “social support” and “family caregiver” have related features, there are dimensions of having a family caregiver not related to social support that are associated with patient survival. Future research should examine how different types of social support, including the unique type of social support delivered by family caregivers, influence patients' health and longevity.
For patients with a family caregiver, lower survival was associated with higher caregiver demand burden. That is, the patients of caregivers who perceived care recipients and their care to be overly demanding had a higher risk of death. Mirroring the first explanation above, patients with more severe and progressive life‐limiting illness may have a greater need for assistance from caregivers that would impact a caregiver's normal daily routine and sense of a patient's overly demanding situation; however, we found no differences in these patients' markers of disease severity at baseline.
While provocative, these findings are subject to several limitations. First, this was not a prospective, planned analysis. Second, as is common across studies of the seriously ill, we experienced significant caregiver attrition (32%) that could have resulted in a selection bias. It is reasonable to conjecture that those caregivers experiencing higher burden discontinued the study; however, as reported in the trial's primary paper 2, we found no significant associations between attrition and caregiver demographics and outcomes. Third, this study included few caregivers and patients of a minority racial group, whose burden has been shown to differ from Whites 53, thus limiting generalizability. Fourth, it is not entirely known what proportion of patients who did not elect to have a caregiver participate did in fact have close family and friends who assisted them in some way with their care. In other words, patients with or without caregiver co‐participants may not equate with actual caregivers available to patients. Future studies will benefit from having more detailed information about family support regardless of whether patients elect a caregiver to co‐participate.
To conclude, we believe the results of this analysis are surprising relative to current literature and raise more questions than are answered. However, these findings are important as they serve to challenge our assumptions about the impact of family caregiving on patient outcomes. We echo what the Institute of Medicine 6, 54 and others 16, 55 have recently emphasized, namely that there is a critical need to prioritize and place special emphasis on research and interventions aimed at better understanding and supporting family caregiving for the critically ill and dying. The scope of this need is vast, as most of the over half million individuals with advanced cancer who are in their last year of life 56 have a family member or close friend who assists them on a daily basis. Not only are these family caregivers encumbered with delivering nearly all of a patient's daily care, they are burdened with witnessing someone close to them struggle with life‐limiting illness. Hence, it is imperative that palliative and oncology clinicians' work together to ensure that these caregivers are supported in their role. Understanding how to best provide this support will be greatly benefited by continuing to improve our understanding of the complex dynamic between seriously ill patients and their family caregivers.
Conflict of Interest
The authors have no conflict of interests to disclose.
Acknowledgments
The parent study was supported by National Institute for Nursing Research (R01NR011871‐01), Cancer and Leukemia Group B Foundation Clinical Scholar Award, Foundation for Informed Medical Decision‐Making, the Norris Cotton Cancer Center pilot funding, and the Dartmouth‐Hitchcock, Section of Palliative Medicine. Dr. J. Nicholas Dionne‐Odom is a postdoctoral fellow supported by the University of Alabama at Birmingham Cancer Prevention and Control Training Program (5R25CA047888), by a National Palliative Care Research Center Career Development Award, and by the MSM/TU/UAB Comprehensive Cancer Center Partnership (U54CA118948). Kathleen D. Lyons, ScD is supported by a Mentored Research Scholar Grant in Applied and Clinical Research (MRSG 12‐113‐01 – CPPB) from the American Cancer Society. Dr. Bakitas is a recipient of a National Palliative Care Research Center Junior Career Development Award.
Cancer Medicine 2016; 5(5): 853–862
References
- 1. Bakitas, M. A. , Tosteson T. D., Li Z., Lyons K. D., Hull J. G., Li Z., et al. 2015. Early Versus Delayed Initiation of Concurrent Palliative Oncology Care: Patient Outcomes in the ENABLE III Randomized Controlled Trial. J. Clin. Oncol. 33:1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dionne‐Odom, J. N. , Azuero A., Lyons K. D., Hull J.G., T. Tosteson, Li Z., et al. 2015. Benefits of Early Versus Delayed Palliative Care to Informal Family Caregivers of Patients With Advanced Cancer: outcomes From the ENABLE III Randomized Controlled Trial. J. Clin. Oncol. 33:1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dunning, T. 2012. Natural experiments in the Social Sciences: a design‐based approach. Cambridge University Press, New York. [Google Scholar]
- 4. Yabroff, K. R. , and Kim Y.. 2009. Time costs associated with informal caregiving for cancer survivors. Cancer 115(18 Suppl):4362–4373. [DOI] [PubMed] [Google Scholar]
- 5. Stenberg, U. , Ruland C. M., and Miaskowski C.. 2010. Review of the literature on the effects of caring for a patient with cancer. Psychooncology 19:1013–1025. [DOI] [PubMed] [Google Scholar]
- 6. Institute of Medicine . Retooling for an aging America: building the health care workforce. National Academies Press, Washington, DC2008. [PubMed] [Google Scholar]
- 7. AARP with the United Hospital Fund . 2012. Home alone: family caregivers providing complex chronic care. AARP Public Policy Institute, Washington DC. [Google Scholar]
- 8. van Ryn, M. , Sanders S., Kahn K., et al. 2011. Objective burden, resources, and other stressors among informal cancer caregivers: a hidden quality issue? Psychooncology 20:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adelman, R. D. , Tmanova L. L., Delgado D., Dion S., and Lachs M. S.. 2014. Caregiver burden: a clinical review. JAMA 311:1052–1060. [DOI] [PubMed] [Google Scholar]
- 10. Hudson, P. L. , Thomas K., Trauer T., Remedios C., and Clarke D.. 2011. Psychological and social profile of family caregivers on commencement of palliative care. J. Pain Symptom Manage. 41:522–534. [DOI] [PubMed] [Google Scholar]
- 11. Hodges, L. J. , Humphris G. M., and Macfarlane G.. 2005. A meta‐analytic investigation of the relationship between the psychological distress of cancer patients and their carers. Soc. Sci. Med. 60:1–12. [DOI] [PubMed] [Google Scholar]
- 12. Palos, G. R. , Mendoza T. R., Liao K. P., et al. 2011. Caregiver symptom burden: the risk of caring for an underserved patient with advanced cancer. Cancer 117:1070–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perkins, M. , Howard V. J., Wadley V. G., et al. 2013. Caregiving strain and all‐cause mortality: evidence from the REGARDS study. J. Gerontol. B Psychol. Sci. Soc. Sci. 68:504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Institute of Medicine . Delivering high quality cancer care: charting a new course for a system in crisis. National Academies Press, Washington DC2013. [PubMed] [Google Scholar]
- 15. Park, S. M. , Kim Y. J., Kim S., et al. 2010. Impact of caregivers' unmet needs for supportive care on quality of terminal cancer care delivered and caregiver's workforce performance. Support. Care Cancer 18:699–706. [DOI] [PubMed] [Google Scholar]
- 16. Evercare and National Alliance for Caregiving . Caregivers in decline: a close‐up look at the health risks of caring for a loved one. National Alliance for Caregiving, Bethesda, MD2006. [Google Scholar]
- 17. Farquhar, M. , Higginson I. J., and Booth S.. 2009. Fast‐track trials in palliative care: an alternative randomized controlled trial design. J. Palliat. Med. 12:213. [DOI] [PubMed] [Google Scholar]
- 18. Callahan, C. M. , Unverzagt F. W., Hui S. L., Perkins A. J., and Hendrie H. C.. 2002. Six‐item screener to identify cognitive impairment among potential subjects for clinical research. Med. Care 40:771–781. [DOI] [PubMed] [Google Scholar]
- 19. Bakitas, M. , Lyons K., Hegel M., et al. 2009. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II Randomized Controlled Trial. JAMA 302:741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bakitas, M. , Lyons K., Hegel M., et al. 2009. Project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: baseline findings, methodological challenges, and solutions. Palliat. Support Care. 7:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bakitas, M. , Stevens M., Ahles T., et al. 2004. Project ENABLE: a palliative care demonstration project for advanced cancer patients in three settings. J. Palliat. Med. 7:363–372. [DOI] [PubMed] [Google Scholar]
- 22. National Consensus Project . Clinical Practice Guidelines for Quality Palliative Care. National Consensus Project for Quality Palliative Care, Pittsburgh, PA2013. [Google Scholar]
- 23. Hegel, M. T. , Dietrich A. J., Seville J. L., and Jordan C. B.. 2004. Training residents in problem‐solving treatment of depression: a pilot feasibility and impact study. Fam. Med. 36:204–208. [PubMed] [Google Scholar]
- 24. Unützer, J. , Katon W., Callahan C. M., et al. 2002. Collaborative care management of late‐life depression in the primary care setting: a randomized controlled trial. JAMA 288:2836–2845. [DOI] [PubMed] [Google Scholar]
- 25. McMillan, S. C. , Small B. J., Weitzner M., et al. 2005. Impact of coping skills intervention with family caregivers of hospice patients with cancer. Cancer 106:214–222. [DOI] [PubMed] [Google Scholar]
- 26. McMillan, S. C. , and Small B. J.. 2007. Using the COPE intervention for family caregivers to improve symptoms of hospice homecare patients: a clinical trial. Oncol. Nurs. Forum 34:313–321. [DOI] [PubMed] [Google Scholar]
- 27. Steinhauser, K. E. , Alexander S. C., Byock I. R., George L. K., Olsen M. K., and Tulsky J. A.. 2008. Do preparation and life completion discussions improve functioning and quality of life in seriously ill patients? Pilot randomized control trial. J. Palliat. Med. 11:1234–1240. [DOI] [PubMed] [Google Scholar]
- 28. Steinhauser, K. E. , Alexander S. C., Byock I. R., George L. K., and Tulsky J. A.. 2009. Seriously ill patients' discussions of preparation and life completion: an intervention to assist with transition at the end of life. Palliat. Support Care. 7:393–404. [DOI] [PubMed] [Google Scholar]
- 29. Weitzner, M. , Jacobsen P. B., Wagner H., Friedland J., and Cox C.. 1999. The caregiver quality of life index‐cancer (CQOL‐C) scale: development and validation of an instrument to measure quality of life of the family of caregiver of patients with cancer. Qual. Life Res. 8:55–63. [DOI] [PubMed] [Google Scholar]
- 30. Radloff, L. 1977. The CES‐D scale: a self‐report depression scale for research in the general population. Appl. Psychol. Meas. 1:385–401. [Google Scholar]
- 31. Okun, A. , Stein R. E., Bauman L. J., and Silver E. J.. 1996. Content validity of the Psychiatric Symptom Index, CES‐depression Scale, and State‐Trait Anxiety Inventory from the perspective of DSM‐IV. Psychol. Rep. 79(3 Pt 1):1059–1069. [DOI] [PubMed] [Google Scholar]
- 32. Montgomery, R. , Gonyea J., and Hooyman N.. 1985. Caregiving and the Experience of Subjective and Objective Burden. Fam. Relat. 34:19–26. [Google Scholar]
- 33. Montgomery, R. , Borgatta E., and Borgatta M.. 2000. Societal and family change in the burden of care Pp. 27–54 in Liu B. and Kendig H., eds. Who should care of the elderly? An east‐west value divide. World Scientific, River Edge, NJ. [Google Scholar]
- 34. Kleinbaum, D. , and Klein M.. 2012. Survival Analysis, 3rd ed Springer, New York. [Google Scholar]
- 35. Zimet, G. D. , Powell S. S., Farley G. K., Werkman S., and Berkoff K. A.. 1990. Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. J. Pers. Assess. 55:610–617. [DOI] [PubMed] [Google Scholar]
- 36. Lyons, K. D. , Bakitas M., Hegel M. T., Hanscom B., Hull J., and Ahles T. A.. 2009. Reliability and validity of the Functional Assessment of Chronic Illness Therapy‐Palliative care (FACIT‐Pal) scale. J. Pain Symptom Manage. 37:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McPherson, C. J. , Wilson K. G., and Murray M. A.. 2007. Feeling like a burden to others: a systematic review focusing on the end of life. Palliat. Med. 21:115–128. [DOI] [PubMed] [Google Scholar]
- 38. Lee, J. E. , Shin D. W., Cho J., et al. 2015. Caregiver burden, patients' self‐perceived burden, and preference for palliative care among cancer patients and caregivers. Psychooncology 24:1545–1551. [DOI] [PubMed] [Google Scholar]
- 39. Akazawa, T. , Akechi T., Morita T., et al. 2010. Self‐perceived burden in terminally ill cancer patients: a categorization of care strategies based on bereaved family members' perspectives. J. Pain Symptom Manage. 40:224–234. [DOI] [PubMed] [Google Scholar]
- 40. Chochinov, H. M. , Kristjanson L. J., Hack T. F., Hassard T., McClement S., and Harlos M.. 2007. Burden to others and the terminally ill. J. Pain Symptom Manage. 34:463–471. [DOI] [PubMed] [Google Scholar]
- 41. Inverso, G. , Mahal B. A., Aizer A. A., Donoff R. B., Chau N. G., and Haddad R. I.. 2015. Marital status and head and neck cancer outcomes. Cancer 121:1273–1278. [DOI] [PubMed] [Google Scholar]
- 42. Aizer, A. A. , Chen M. H., McCarthy E. P., et al. 2013. Marital status and survival in patients with cancer. J. Clin. Oncol. 31:3869–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mahdi, H. , Kumar S., Munkarah A. R., Abdalamir M., Doherty M., and Swensen R.. 2013. Prognostic impact of marital status on survival of women with epithelial ovarian cancer. Psychooncology 22:83–88. [DOI] [PubMed] [Google Scholar]
- 44. Abern, M. R. , Dude A. M., and Coogan C. L.. 2012. Marital status independently predicts testis cancer survival–an analysis of the SEER database. Urol. Oncol. 30:487–493. [DOI] [PubMed] [Google Scholar]
- 45. Wang, L. , Wilson S. E., Stewart D. B., and Hollenbeak C. S.. 2011. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: does marriage affect cancer survival by gender and stage? Cancer Epidemiol. 35:417–422. [DOI] [PubMed] [Google Scholar]
- 46. Pan, I. W. , Ferguson S. D., and Lam S.. 2015. Patient and treatment factors associated with survival among adult glioblastoma patients: a USA population‐based study from 2000‐2010. J. Clin. Neurosci. 22:1575–1581. [DOI] [PubMed] [Google Scholar]
- 47. Baine, M. , Sahak F., Lin C., Chakraborty S., Lyden E., and Batra S. K.. 2011. Marital status and survival in pancreatic cancer patients: a SEER based analysis. PLoS ONE 6:e21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brusselaers, N. , Mattsson F., Johar A., et al. 2014. Marital status and survival after oesophageal cancer surgery: a population‐based nationwide cohort study in Sweden. BMJ Open. 4:e005418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pinquart, M. , and Duberstein P. R.. 2010. Associations of social networks with cancer mortality: a meta‐analysis. Crit. Rev. Oncol. Hematol. 75:122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Siddiqui, F. , Bae K., Langer C. J., et al. 2010. The influence of gender, race, and marital status on survival in lung cancer patients: analysis of Radiation Therapy Oncology Group trials. J. Thorac. Oncol. 5:631–639. [DOI] [PubMed] [Google Scholar]
- 51. Kroenke, C. H. , Kubzansky L. D., Schernhammer E. S., Holmes M. D., and Kawachi I.. 2006. Social networks, social support, and survival after breast cancer diagnosis. J. Clin. Oncol. 24:1105–1111. [DOI] [PubMed] [Google Scholar]
- 52. Yang, H. C. , and Schuler T. A.. 2009. Marital quality and survivorship: slowed recovery for breast cancer patients in distressed relationships. Cancer 115:217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Siefert, M. L. , Williams A. L., Dowd M. F., Chappel‐Aiken L., and McCorkle R.. 2008. The caregiving experience in a racially diverse sample of cancer family caregivers. Cancer Nurs. 31:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Institute of Medicine . Dying in America: improving quality and honoring individual preferences near the end of life. National Academies Press, Washington, DC2014. [PubMed] [Google Scholar]
- 55. Centers for Disease Control and Prevention . Assuring Health Caregivers. Kimberly‐Clark Corporation, Neenah, WI2008. [Google Scholar]
- 56. Howlader, N. , Noone A., Krapcho M., et al. 2013. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute, Bethesda, MD. [Google Scholar]


