Abstract
Two metabolites from the ascomycete fungus Septofusidium berolinense were recently identified as having antineoplastic activity [Ekiz, et al. (2015) J. Antibiot. (Tokyo)]. However, the basis for this activity is not known. One of the compounds [3,6-dihydroxy-2-propylbenzaldehyde (GE-1)] is a hydroquinone and the other [2-hydroxymethyl-3-propylcyclohexa-2,5-diene-1,4-dione (GE-2)] is a quinone. Because some hydroquinones and quinones act as topoisomerase II poisons, the effects of GE-1 and GE-2 on DNA cleavage mediated by human topoisomerase IIα were assessed. GE-2 enhanced DNA cleavage ~4–fold and induced scission with a site specificity similar to that of the anticancer drug etoposide. Similar to other quinone-based topoisomerase II poisons, GE-2 displayed several hallmark characteristics of covalent topoisomerase II poisons, including: 1) the inability to poison a topoisomerase IIα construct that lacks the N-terminal domain; 2) the inhibition of DNA cleavage when the compound was incubated with the enzyme prior to the addition of plasmid, and 3) the loss of poisoning activity in the presence of a reducing agent. In contrast to GE-2, GE-1 did not enhance DNA cleavage mediated by topoisomerase IIα except at very high concentrations. However, the activity and potency of the metabolite were dramatically enhanced under oxidizing conditions. Results suggest that topoisomerase IIα may play a role in mediating the cytotoxic effects of these fungal metabolites.
INTRODUCTION
Topoisomerase II regulates DNA under- and overwinding and resolves knots and tangles in the double helix.1-5 It also is an important target for several widely prescribed anticancer drugs.6-11 Although structurally diverse, all clinically relevant topoisomerase II-targeted drugs act by stabilizing covalent enzyme-cleaved DNA complexes (cleavage complexes) that are requisite intermediates in the catalytic cycle of the type II enzyme. These drugs are called topoisomerase II poisons because they convert this essential enzyme to a lethal enzyme that fragments the genome.6-12
A number of topoisomerase II-targeted drugs are derived from natural sources. For example, etoposide and teniposide are semi-synthetic derivatives of podophyllotoxin, which is found in the Mayapple plant.13,14 In addition, doxorubicin and daunorubicin, two related anthracyclines, are produced by the bacterium Streptomyces peucetius.8,15
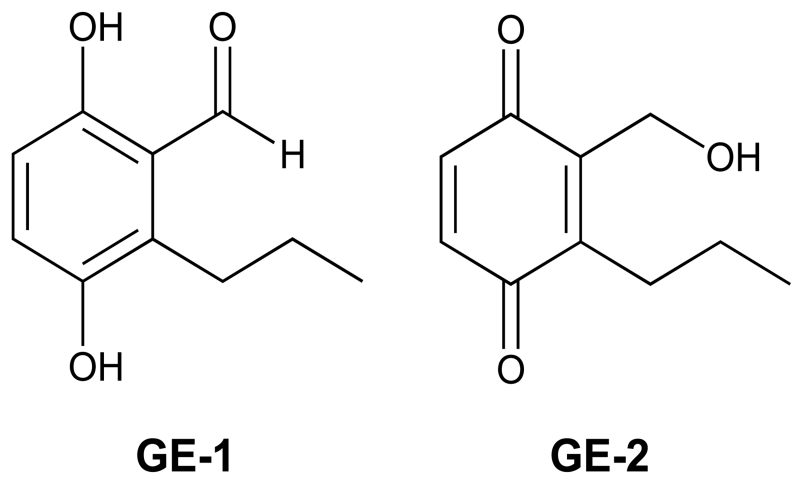
Fungi are a rich source of bioactive metabolites and many of them display antineoplastic activity in cancer models.16-19 Recently, two compounds that are cytotoxic to a variety of human cancer cell lines (IC50 values ranged from ~30-250 μM) were isolated from the filamentous soil fungus, Septofusidium berolinense.20 Based on mass spectroscopy and 13C and 1H NMR analysis, one of these metabolites was identified as 3,6-dihydroxy-2-propylbenzaldehyde (GE-1),20 which is a hydroquinone, and the other was identified as 2-hydroxymethyl-3-propylcyclohexa-2,5-diene-1,4-dione (GE-2),20,21 which is a quinone (Figure 1). At the present time, nothing is known about the targets that mediate the cytotoxicity of GE-1 and GE-2.
Figure 1.
Structures of two secondary metabolites isolated from the fungus Septofusidium berolinense. 3,6-dihydroxy-2-propylbenzaldehyde (GE-1) is a hydroquinone and 2-hydroxymethyl-3-propylcyclohexa-2,5-diene-1,4-dione (GE-2) is a quinone.
Several naturally occurring polyphenols act as topoisomerase II poisons.11,22 These include quinones (benzoquinone, curcumin oxidation products, and thymoquinone),23-26 hydroquinone,24,27 and catechols (quercetin, epigallocatechin gallate, hydroxytyrosol, and oleuropein).28-31 Some of these compounds work by a unique mechanism and are believed to enhance topoisomerase II-mediated DNA cleavage by forming covalent adducts with the enzyme. Consequently, they are referred to as covalent topoisomerase II poisons.7,11
Because GE-1 and GE-2 have structural elements consistent with some established topoisomerase II poisons, the effects of these fungal metabolites on the DNA cleavage activity of human topoisomerase IIα were assessed. Results strongly suggest that GE-2 is a covalent topoisomerase II poison. Although GE-1 does not enhance enzyme-mediated DNA scission under normal assay conditions, the presence of an oxidant converts the metabolite into an efficacious topoisomerase II poison. These findings suggest that topoisomerase IIα may be a cytotoxic target for GE-2 and potentially GE-1 in human cells.
EXPERIMENTAL PROCEDURES
Enzymes and Materials
Recombinant human topoisomerase IIα was expressed in Saccharomyces cerevisiae and purified as described previously.32-34 The catalytic core of human topoisomerase IIα (residues 431-1193) was a gift from J. Deweese and was expressed and purified as described previously.35 Enzymes were stored at −80 °C as a 1.5 mg/mL stock in 50 mM Tris-HCl, pH 7.9, 0.1 mM EDTA, 750 mM KCl, 5% glycerol. The residual concentration of dithiothreitol (DTT) was <2 μM in final reaction mixtures.
Negatively supercoiled pBR322 DNA was prepared from Escherichia coli using a Plasmid Mega Kit (Qiagen) as described by the manufacturer. Analytical grade etoposide was purchased from Sigma-Aldrich. Two quinone-type compounds, 3,6-dihydroxy-2-propylbenzaldehyde (GE-1) and 2-hydroxymethyl-3-propylcyclohexa-2,5-diene-1,4-dione (GE-2), were isolated from the ascomycete fungus S. berolinense as part of a search for new bioactive secondary metabolites.20 Analytical data for GE-1 and GE-2 are reported in Ekiz et al.20 Compounds were prepared as 50 mM stock solutions in 100% DMSO and stored at 4 °C.
DNA Cleavage
DNA cleavage reactions were performed as described by Fortune and Osheroff.36 Reaction mixtures contained 110 nM human topoisomerase IIα or 430 nM topoisomerase IIα catalytic core and 10 nM negatively supercoiled pBR322 DNA in a total of 20 μL of cleavage buffer [10 mM Tris-HCl (pH 7.9), 5 mM MgCl2, 100 mM KCl, 0.1 mM EDTA, and 2.5% (v/v) glycerol]. DNA cleavage reaction mixtures were incubated at 37 °C for 6 min, and enzyme-DNA cleavage complexes were trapped by the addition of 2 μL of 5% SDS followed by 2 μL of 250 mM EDTA (pH 8.0). Proteinase K (2 μL of a 0.8 mg/mL solution) was added, and samples were incubated at 45 °C for 30 min to digest the type II enzyme. Reaction samples were mixed with 2 μL of agarose loading dye [60% sucrose in 10 mM Tris-HCl pH 7.9, 0.5% bromophenol blue, and 0.5% xylene cyanol FF], heated at 45 °C for 2 min, and subjected to electrophoresis using 1% agarose gels in 40 mM Tris-acetate (pH 8.3) and 2 mM EDTA containing 0.5 μg/mL ethidium bromide. DNA bands were visualized by UV light and quantified using an Alpha Innotech digital imaging system. Double-stranded DNA cleavage was monitored by the conversion of negatively supercoiled plasmid to linear molecules. In some cases, EcoRI-digested plasmid was used as a control for double-strand cleavage (100%) and etoposide was used as positive control as an interfacial topoisomerase IIα poison.
DNA cleavage reactions were carried out in the presence of 0–1000 μM GE-1 or GE-2 or 100 μM etoposide. Unless stated otherwise, compounds were added last to reaction mixtures. In reactions that determined whether DNA cleavage by human topoisomerase IIα was reversible, 2 μL of 250 mM EDTA was added to samples prior to treatment with SDS. To determine whether cleaved DNA was protein-linked, proteinase K treatment was omitted. To examine the effects of a reducing agent (DTT) or oxidizing agent [(K3Fe(CN)6] on the actions of 250 μM GE-1 or GE-2 against topoisomerase IIα, 250 μM DTT or 50 μM K3Fe(CN)6 (final concentration) was incubated with the compounds for 10 min before their addition to DNA cleavage reaction mixtures.
To assess the effects of GE-2 on human topoisomerase IIα prior to the addition of DNA, the enzyme (110 nM final enzyme concentration) was incubated in the presence of 250 μM (final concentration) compound at 37 °C for 0–3 min in 15 μL of DNA cleavage buffer. DNA cleavage was initiated by the addition of 10 nM negatively supercoiled pBR322 DNA (final concentration) to reaction mixtures (20 μL final volume), and samples were incubated at 37 °C for 6 min. Reactions were stopped, and samples were processed and analyzed as above.
DNA Cleavage Site Utilization
DNA cleavage sites were mapped using the procedure of Hawtin et al.37 pBR322 DNA was linearized by treatment with HindIII, and terminal 5’-phosphates were removed and replaced with [32P]phosphate by treatment with calf intestinal alkaline phosphatase followed by T4 polynucleotide kinase and [γ-32P]ATP. The labeled DNA was digested using EcoRI, and the 4330 bp singly-end-[32P] labeled fragment was purified from the short EcoRI-HindIII fragment by passage through a CHROMA SPIN+TE-100 column (Clontech).
Reaction mixtures contained 4 nM [32P]-labeled 4330 bp DNA substrate and 44 nM human topoisomerase IIα in 50 μL of DNA cleavage buffer. Assays were carried out in the absence of compound, or in the presence of 10 μM etoposide or 0-1000 μM GE-1 or GE-2. Reactions were initiated by the addition of the topoisomerase IIα and were incubated at 37 °C for 1 min. DNA cleavage intermediates were trapped by addition of 5 μL of 5% SDS, followed by 3.75 μL of 250 mM EDTA (pH 8.0). Proteinase K (5 μL of a 0.8 mg/mL solution) was added and incubated at 45 °C for 30 min to digest the topoisomerase IIα. DNA products were precipitated in 100% ethanol and 3 M NaOAc, washed in 70% ethanol, dried, and resuspended in 6 μL of 40% formamide, 10 mM NaOH, 0.02% xylene cyanol FF, and 0.02% bromophenol blue. Samples were subjected to electrophoresis in a 6% denaturing polyacrylamide sequencing gel in 100 mM Tris-borate (pH 8.3), and 2 mM EDTA. The gel was dried and exposed to an imaging screen (Bio-Rad). [32P]-labeled DNA cleavage products were analyzed on a Pharos Molecular Imager FX (Bio-Rad).
Persistence of Cleavage Complexes
The persistence of topoisomerase IIα-DNA cleavage complexes was determined using the procedure of Gentry et al.38 Initial reactions contained 50 nM DNA and 550 nM topoisomerase IIα in a total of 20 μL of DNA cleavage buffer. Reactions were carried out in the absence or presence of 250 μM GE-2. Mixtures were incubated at 37 °C for 6 min and then diluted 20–fold with 37 °C DNA cleavage buffer. Aliquots (20 μL) were removed at times ranging from 0–24 h, and DNA cleavage was stopped by the addition of 2 μL of 5% SDS followed by 2 μL of 250 mM EDTA (pH 8.0). Samples were processed as described above for plasmid cleavage assays. The persistence of cleavage complexes was monitored by the maintenance of the linear reaction product over time.
RESULTS AND DISCUSSION
Effects of GE-1 and GE-2 on DNA Cleavage Mediated by Topoisomerase IIα
Humans encode two isoforms of topoisomerase II, α and β.1-5 Topoisomerase IIα is the isoform that is highly expressed in rapidly proliferating cells and is responsible for unlinking daughter chromosomes. The enzyme is also an important target for anticancer drugs.1-5,7 Therefore, topoisomerase IIα was used to analyze the effects of GE-1 and GE-2 on enzyme-mediated DNA cleavage.
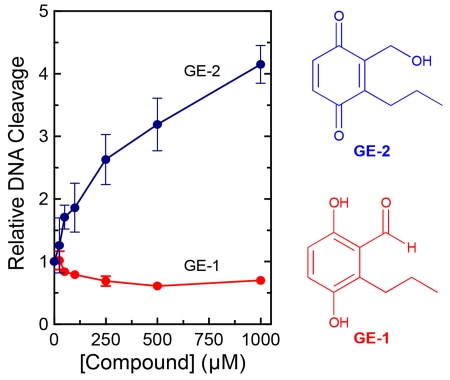
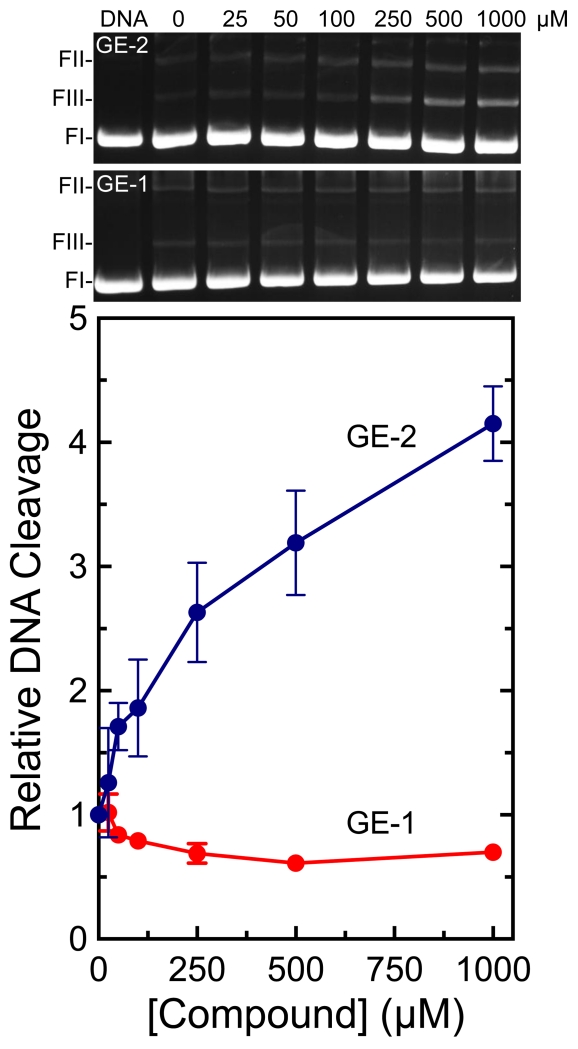
GE-2, which is a quinone, enhanced DNA cleavage mediated by topoisomerase IIα ~4–fold (Figure 2). In contrast, GE-1, which is a hydroquinone, displayed no ability to increase enzyme-mediated DNA cleavage at concentrations as high as 1 mM. Moreover, over this concentration range, GE-1 inhibited DNA scission by ~40%. Previous studies found that 1,4-hydroquinone could act as a topoisomerase II poison, but required concentrations that were 5-10–fold higher than that of the corresponding 1,4-benzoquinone.23,27 Therefore, the effects of 5 mM GE-1 on topoisomerase IIα-mediated DNA cleavage were assessed. At this concentration, GE-1 increased levels of DNA scission ~2.8–fold (data not shown). This activity of GE-1 seen at high concentrations may reflect low levels of redox cycling that converts the metabolite from a hydroquinone to a quinone under assay conditions.
Figure 2.
The effects of GE-1 and GE-2 on DNA cleavage mediated by human topoisomerase IIα. Double-stranded DNA cleavage levels in the presence of 0-1000 μM GE-1 (red) or GE-2 (blue) were calculated relative to a reaction that contained enzyme but no compound. A DNA control also is shown (DNA). Error bars represent standard deviations for at least three independent experiments. Ethidium bromide-stained agarose gels depicting DNA cleavage are shown (top). The mobility of negatively supercoiled DNA (form I; FI), nicked circular plasmid (form II; FII), and linear molecules (form III; FIII) are indicated. Gels are representative of five independent experiments.
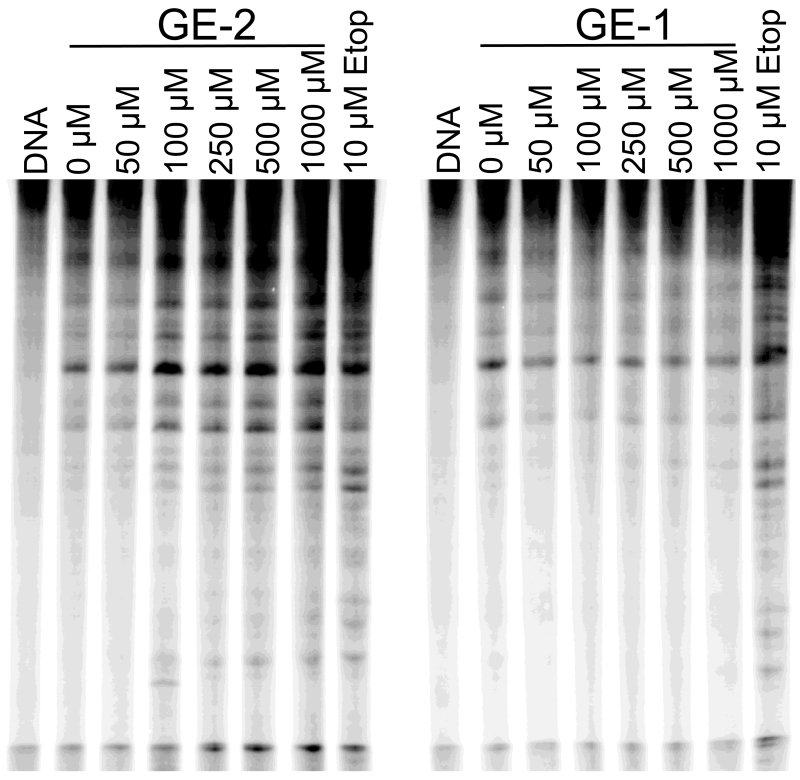
The effects of GE-1 and GE-2 (0-1 mM) on DNA cleavage site utilization by topoisomerase IIα are shown in Figure 3. GE-2 increased scission at several sites and produced a banding pattern similar to that of etoposide. Consistent with the results seen with plasmid DNA, GE-1 decreased enzyme-mediated scission at all observable sites.
Figure 3.
Effects of GE-1 and GE-2 on DNA cleavage site utilization by human topoisomerase IIα. An autoradiogram of a 6% polyacrylamide gel is shown. A singly 32P-end-labeled linear 4332 bp fragment of pBR322 was used as the cleavage substrate. DNA cleavage reactions were carried out in the presence of 0-1000 μM GE-1 or GE-2, or 10 μM etoposide (as a positive control, Etop). A DNA control also is shown (DNA). Results are representative of three independent experiments.
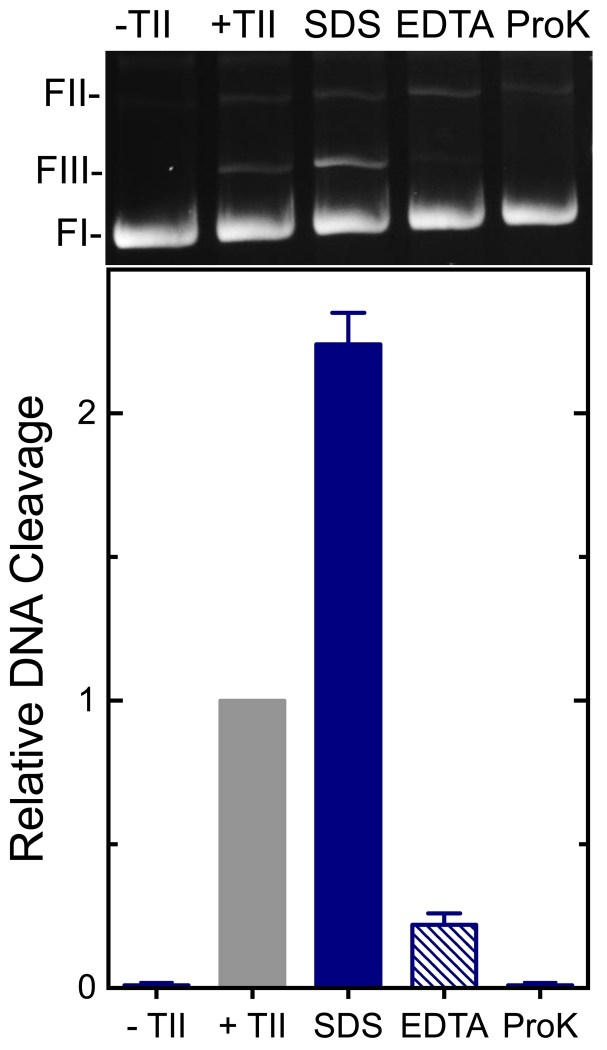
In order to ensure that DNA cleavage induced by GE-2 was mediated by topoisomerase IIα, three control reactions were performed in the presence of 250 μM metabolite (Figure 4). First, no DNA scission was observed when GE-2 was incubated with DNA in the absence of the type II enzyme (even at 1 mM, data not shown). Second, DNA cleavage was reversed when the active site Mg2+ ions were chelated with EDTA prior to trapping cleavage complexes with SDS. This reversibility is not consistent with an enzyme-independent reaction. Third, cleaved plasmid products were covalently linked to topoisomerase IIα. When proteinase K treatment was omitted, the free linear DNA band disappeared and was replaced by a lower mobility smear that extended to the origin.
Figure 4.
DNA cleavage induced by GE-2 is reversible and protein-linked. Assay mixtures contained DNA and 250 μM GE-2 in the absence of enzyme (-TII), DNA in the presence of topoisomerase IIα in the absence of GE-2 (+TII), or reaction mixtures that contained DNA, enzyme, and 250 μM GE-2 that were stopped with SDS (SDS). To determine whether the reaction was reversible, EDTA was added prior to SDS (EDTA). To determine whether the cleaved DNA was protein-linked, proteinase K treatment was omitted (ProK). The mobility of negatively supercoiled DNA (form I; FI), nicked circular plasmid (form II; FII), and linear molecules (form III; FIII) are indicated. Error bars represent the standard deviation of three independent experiments. A gel that is representative of three independent experiments is shown (top).
Finally, consistent with previous results with the quinone-based topoisomerase II poison thymoquinone,26 GE-2 induced stable cleavage complexes with the α isoform (data not shown). The t1/2 of DNA cleavage mediated in the presence of GE-2 was ≥ 24 h (levels of cleavage complex remaining = 58 ± 18%).
GE-2 Is a Covalent Topoisomerase II Poison
There are two types of topoisomerase II poisons, interfacial and covalent.7,9,11,39 Interfacial poisons act non-covalently at the cleavage-ligation active site of topoisomerase II, interact with both the protein and the DNA, and stabilize cleavage complexes by intercalating into the cut scissile bond.7,9,11,39 Covalent topoisomerase II poisons are reactive compounds that increase levels of enzyme-mediated DNA cleavage by adducting cysteine (and potentially other) residues that are outside of the active site.7,11 Although the mechanistic details of how this adduction alters DNA cleavage are not well understood, it is believed that covalent poisons act (at least in part) by closing a “gate” formed by the N-terminus of the type II enzyme.40,41 This gate normally closes during the DNA strand passage reaction, pushing a double helix through the DNA segment that is cleaved by topoisomerase II.1,6 Concomitant with the closure of the N-terminal protein gate, higher levels of enzyme-mediated DNA scission are induced.1,42
There are several hallmark characteristics of covalent topoisomerase II poisons that are not displayed by interfacial poisons.7,11 Because of their reliance on the topoisomerase II N-terminal domain, covalent poisons do not enhance DNA cleavage mediated by the catalytic core of topoisomerase II.43 Furthermore, although covalent poisons increase levels of DNA scission when added to enzyme-DNA complexes, they inhibit scission when incubated with the enzyme prior to the addition of the nucleic acid substrate.23,40,44 This may reflect the fact that closing the N-terminal protein gate prevents plasmid binding.40,41 Lastly, because covalent poisons require redox cycling as part of their adduction mechanism, incubation with reducing agents abolishes their reactivity.23,40
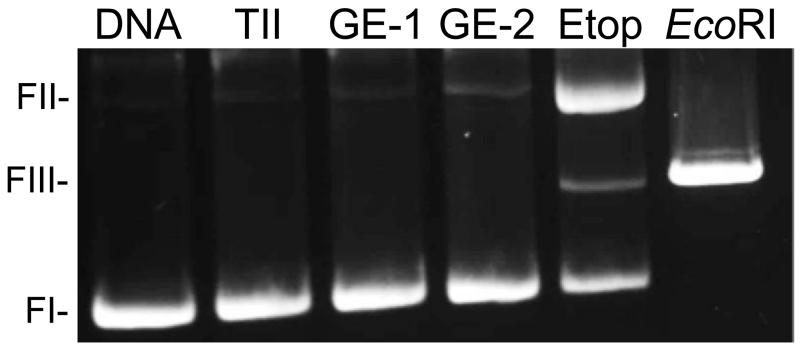
First, to assess the requirement for the N-terminal domain, the effects of 250 μM GE-1 and GE-2 on DNA cleavage mediated by the catalytic core of topoisomerase IIα were determined (Figure 5). Whereas etoposide enhanced DNA cleavage, no cleavage increase was observed in the presence of GE-1 or GE-2. In addition, neither fungal metabolite was able to diminish the effects of etoposide on DNA cleavage mediated by the catalytic core, suggesting that they were unable to compete with the interfacial poison at the active site of topoisomerase IIα (data not shown).
Figure 5.
GE-1 and GE-2 require the N-terminal domain in order to enhance DNA cleavage mediated by topoisomerase IIα. The effects of GE-1 and GE-2 on DNA cleavage mediated by the catalytic core of human topoisomerase IIα (which lacks both the C-terminal and N-terminal domains) are shown. DNA cleavage reactions were carried out using 250 μM metabolite or 100 μM etoposide. A DNA control (DNA) and a cleavage reaction carried out in the absence of compounds (TII) also are shown. An EcoRI-digested plasmid control (EcoRI) is shown to depict the position of linear DNA and represents 100% double-stranded DNA cleavage. The gel is representative of three independent experiments.
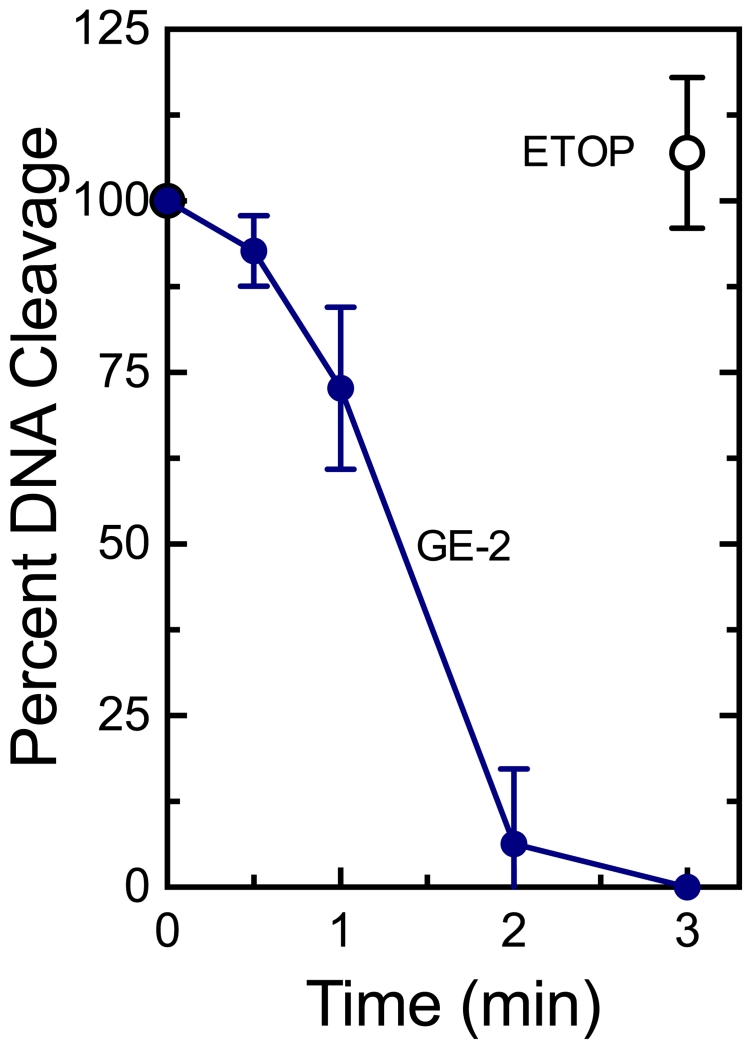
Second, 250 μM GE-2 was incubated with topoisomerase IIα prior to the addition of DNA (Figure 6). Consistent with the actions of covalent poisons, the metabolite rapidly inactivated enzyme-mediated DNA cleavage (t1/2 ≈ 1.5 min). In contrast, prior incubation with the interfacial poison etoposide (200 μM) had no effect on DNA cleavage at 3 min (Figure 6).
Figure 6.
GE-2 inactivates human topoisomerase IIα when incubated with the enzyme prior to the addition of DNA. Cleavage reactions were initiated after topoisomerase IIα was incubated with 250 μM GE-2 for 0-3 min (blue). Double-stranded DNA cleavage levels were calculated relative to scission induced when GE-2 and the enzyme were not incubated prior to initiation of the DNA cleavage assay. Results for a control reaction in which topoisomerase IIα was incubated with 200 μM etoposide for 3 min (Etop, open black) are shown. Error bars represent standard deviations for at least three independent experiments.
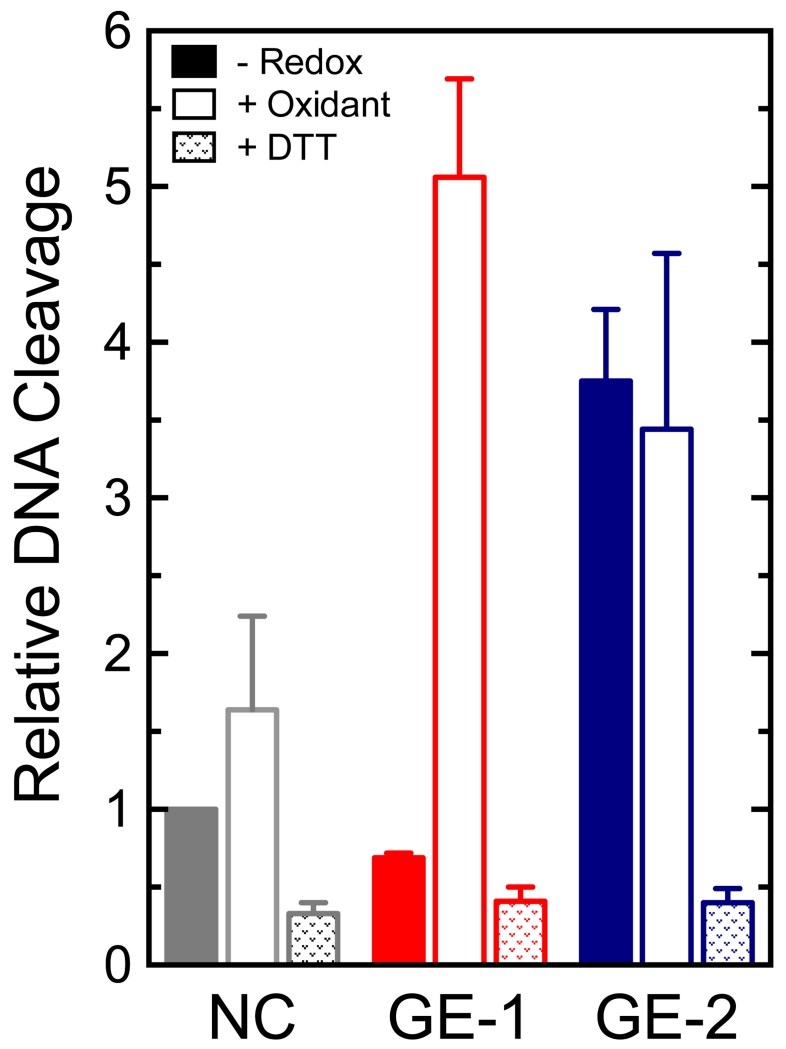
Third, the addition of DTT to GE-2 completely blocked its ability to enhance DNA cleavage mediated by topoisomerase IIα (Figure 7). In contrast, incubation of etoposide with DTT or other reducing agents has no significant effect on its activity against the human enzyme.30
Figure 7.
Effects of reducing and oxidizing reagents on the activities of GE-1 and GE-2 in topoisomerase IIα-mediated DNA cleavage reactions. DNA cleavage reactions were carried out in the absence of a reducing/oxidizing reagent (-Redox; solid bars), or in the presence of 50 μM K3Fe(CN)6 (+ oxidant; open bars) or 250 μM reducing agent (+DTT; stippled bars). Results are shown in the absence of compound [(NC); gray], or in the presence of 250 μM GE-1 (red) or GE-2 (blue). In reactions that contained the reducing or oxidizing reagent, metabolites were incubated with these reagents for 10 min prior to the start of the 6-min cleavage reaction. DNA cleavage levels were calculated relative to that of a control reaction that contained no metabolites or reducing/oxidizing reagents. Error bars represent standard deviations for at least three independent experiments.
Finally, the addition of oxidants to some catechols greatly enhances their potency as topoisomerase II poisons.31 Presumably, oxidation converts the catechols to more reactive quinone-based species45 that can more readily adduct the type II enzyme. Oxidation had no effect on the activity of GE-2, which already was a quinone (Figure 7). In marked contrast, the presence of an oxidant [K3Fe(CN)6] dramatically increased the activity of GE-1. Whereas GE-1 displayed no ability to enhance topoisomerase IIα-mediated cleavage at 1 mM (see Figure 2), oxidized GE-1 enhanced DNA scission ~5–fold at 250 μM (Figure 7).
SUMMARY.
Fungal metabolites are a rich source of compounds with biological activity.16-19 Recently, two compounds with antineoplastic activity were isolated from the filamentous soil fungus, S. berolinense. GE-2, a quinone-based compound, enhanced DNA cleavage mediated by topoisomerase IIα in a concentration range at which it was reported to be cytotoxic to human cancer cell lines.20 Furthermore, the compound displayed all of the hallmarks of a covalent topoisomerase II poison. GE-1, a hydroquinone-based compound, displayed little activity toward the human enzyme except at high concentrations. However, its activity and potency improved considerably under oxidizing conditions. Results of the present study suggest that topoisomerase IIα may play a role in mediating at least some of the antineoplastic effects of these fungal metabolites.
ACKNOWLEDGEMENTS
We are grateful to Rachel E. Ashley, Lorena Infante Lara, Elizabeth G. Gibson, and Jo Ann Byl for critical reading of the manuscript.
FUNDING
This research was supported by grant GM033944 (N.O.) from the National Institutes of Health, grants from TUBITAK (109S361) and EBILTEM (2012/BỊL/028) (E.B.), and grant 115Z349 (Z.T.) from the Turkish Scientific and Technical Research Assembly. K.R.V. was a trainee under NIH grants R25 GM062459 and T32 GM008320 and was supported in part by a Research Supplement to Grant GM033944 to Promote Diversity in Health-Related Research.
ABBREVIATIONS
- GE-1
3,6-dihydroxy-2-propylbenzaldehyde
- GE-2
2-hydroxymethyl-3-propylcyclohexa-2,5-diene-1,4-dione.
Footnotes
The authors declare no competing financial interests.
REFERENCES
- (1).Deweese JE, Osheroff MA, Osheroff N. DNA topology and topoisomerases: Teaching a “knotty” subject. Biochem. Mol. Biol. Educ. 2008;37:2–10. doi: 10.1002/bmb.20244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Nitiss JL. DNA topoisomerase II and its growing repertoire of biological functions. Nat. Rev. Cancer. 2009;9:327–337. doi: 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Liu Z, Deibler RW, Chan HS, Zechiedrich L. The why and how of DNA unlinking. Nucleic Acids Res. 2009;37:661–671. doi: 10.1093/nar/gkp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Vos SM, Tretter EM, Schmidt BH, Berger JM. All tangled up: How cells direct, manage and exploit topoisomerase function. Nat. Rev. Mol. Cell. Biol. 2011;12:827–841. doi: 10.1038/nrm3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Chen SH, Chan N-L, Hsieh T-S. New mechanistic and functional insights into DNA topoisomerases. Ann. Rev. Biochem. 2013;82:139–170. doi: 10.1146/annurev-biochem-061809-100002. [DOI] [PubMed] [Google Scholar]
- (6).Fortune JM, Osheroff N. Topoisomerase II as a target for anticancer drugs: When enzymes stop being nice. Prog. Nucleic Acid Res. Mol. Biol. 2000;64:221–253. doi: 10.1016/s0079-6603(00)64006-0. [DOI] [PubMed] [Google Scholar]
- (7).Deweese JE, Osheroff N. The DNA cleavage reaction of topoisomerase II: Wolf in sheep’s clothing. Nucleic Acids Res. 2009;37:738–748. doi: 10.1093/nar/gkn937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Pogorelcnik B, Perdih A, Solmajer T. Recent developments of DNA poisons - human DNA topoisomerase IIα inhibitors - as anticancer agents. Curr. Pharm. Des. 2013;19:2474–2488. doi: 10.2174/1381612811319130016. [DOI] [PubMed] [Google Scholar]
- (11).Ketron AC, Osheroff N. Phytochemicals as anticancer and chemopreventive topoisomerase II poisons. Phytochem. Rev. 2014;13:19–35. doi: 10.1007/s11101-013-9291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Pendleton M, Lindsey RH, Jr., Felix CA, Grimwade D, Osheroff N. Topoisomerase II and leukemia. Ann. N. Y. Acad. Sci. 2014;1310:98–110. doi: 10.1111/nyas.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hande KR. Etoposide: Four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer. 1998;34:1514–1521. doi: 10.1016/s0959-8049(98)00228-7. [DOI] [PubMed] [Google Scholar]
- (14).Baldwin EL, Osheroff N. Etoposide, topoisomerase II and cancer. Curr. Med. Chem. Anticancer Agents. 2005;5:363–372. doi: 10.2174/1568011054222364. [DOI] [PubMed] [Google Scholar]
- (15).Bodley A, Liu LF, Israel M, Seshadri R, Koseki Y, Giuliani FC, Kirschenbaum S, Silber R, Potmesil M. DNA topoisomerase II-mediated interaction of doxorubicin and daunorubicin congeners with DNA. Cancer Res. 1989;49:5969–5978. [PubMed] [Google Scholar]
- (16).Greve H, Mohamed I, Pontius A, Kehraus S, Gross H, König G. Fungal metabolites: Structural diversity as incentive for anticancer drug development. Phytochem. Rev. 2010;9:537–545. [Google Scholar]
- (17).Kharwar RN, Mishra A, Gond SK, Stierle A, Stierle D. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011;28:1208–1228. doi: 10.1039/c1np00008j. [DOI] [PubMed] [Google Scholar]
- (18).Cragg GM, Newman DJ. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Evidente A, Kornienko A, Cimmino A, Andolfi A, Lefranc F, Mathieu V, Kiss R. Fungal metabolites with anticancer activity. Nat. Prod. Rep. 2014;31:617–627. doi: 10.1039/c3np70078j. [DOI] [PubMed] [Google Scholar]
- (20).Ekiz G, Hames EE, Nalbantsoy A, Bedir E. Two rare quinone-type metabolites from the fungus Septofusidium berolinense and their biological activities. J. Antibiot. (Tokyo) 2015 doi: 10.1038/ja.2015.84. [DOI] [PubMed] [Google Scholar]
- (21).Ymele-Leki P, Cao S, Sharp J, Lambert KG, McAdam AJ, Husson RN, Tamayo G, Clardy J, Watnick PI. A high-throughput screen identifies a new natural product with broad-spectrum antibacterial activity. PLoS One. 2012;7:e31307. doi: 10.1371/journal.pone.0031307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Wang H, Mao Y, Chen AY, Zhou N, LaVoie EJ, Liu LF. Stimulation of topoisomerase II-mediated DNA damage via a mechanism involving protein thiolation. Biochemistry. 2001;40:3316–3323. doi: 10.1021/bi002786j. [DOI] [PubMed] [Google Scholar]
- (23).Lindsey RH, Jr., Bromberg KD, Felix CA, Osheroff N. 1,4-Benzoquinone is a topoisomerase II poison. Biochemistry. 2004;43:7563–7574. doi: 10.1021/bi049756r. [DOI] [PubMed] [Google Scholar]
- (24).Lindsey RH, Bender RP, Osheroff N. Stimulation of topoisomerase II-mediated DNA cleavage by benzene metabolites. Chem. Biol. Interact. 2005;153-154:197–205. doi: 10.1016/j.cbi.2005.03.035. [DOI] [PubMed] [Google Scholar]
- (25).Ketron AC, Gordon ON, Schneider C, Osheroff N. Oxidative metabolites of curcumin poison human type II topoisomerases. Biochemistry. 2013;52:221–227. doi: 10.1021/bi3014455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Ashley RE, Osheroff N. Natural products as topoisomerase II poisons: Effects of thymoquinone on DNA cleavage mediated by human topoisomerase IIα. Chem. Res. Toxicol. 2014;27:787–793. doi: 10.1021/tx400453v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Lindsey RH, Jr., Bender RP, Osheroff N. Effects of benzene metabolites on DNA cleavage mediated by human topoisomerase IIα: 1,4-Hydroquinone is a topoisomerase II poison. Chem. Res. Toxicol. 2005;18:761–770. doi: 10.1021/tx049659z. [DOI] [PubMed] [Google Scholar]
- (28).Austin CA, Patel S, Ono K, Nakane H, Fisher LM. Site-specific DNA cleavage by mammalian DNA topoisomerase II induced by novel flavone and catechin derivatives. Biochem J. 1992;282(Pt 3):883–889. doi: 10.1042/bj2820883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Bandele OJ, Clawson SJ, Osheroff N. Dietary polyphenols as topoisomerase II poisons: B ring and C ring substituents determine the mechanism of enzyme-mediated DNA cleavage enhancement. Chem. Res. Toxicol. 2008;21:1253–1260. doi: 10.1021/tx8000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Bandele OJ, Osheroff N. Epigallocatechin gallate, a major constituent of green tea, poisons human type II topoisomerases. Chem. Res. Toxicol. 2008;21:936–943. doi: 10.1021/tx700434v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Vann KR, Sedgeman CA, Gopas J, Golan-Goldhirsh A, Osheroff N. Effects of olive metabolites on DNA cleavage mediated by human type II topoisomerases. Biochemistry. 2015;54:4531–4541. doi: 10.1021/acs.biochem.5b00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Worland ST, Wang JC. Inducible overexpression, purification, and active site mapping of DNA topoisomerase II from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1989;264:4412–4416. [PubMed] [Google Scholar]
- (33).Wasserman RA, Austin CA, Fisher LM, Wang JC. Use of yeast in the study of anticancer drugs targeting DNA topoisomerases: Expression of a functional recombinant human DNA topoisomerase IIα in yeast. Cancer Res. 1993;53:3591–3596. [PubMed] [Google Scholar]
- (34).Kingma PS, Greider CA, Osheroff N. Spontaneous DNA lesions poison human topoisomerase IIα and stimulate cleavage proximal to leukemic 11q23 chromosomal breakpoints. Biochemistry. 1997;36:5934–5939. doi: 10.1021/bi970507v. [DOI] [PubMed] [Google Scholar]
- (35).Wendorff TJ, Schmidt BH, Heslop P, Austin CA, Berger JM. The structure of DNA-bound human topoisomerase IIα: Conformational mechanisms for coordinating inter-subunit interactions with DNA cleavage. J. Mol. Biol. 2012;424:109–124. doi: 10.1016/j.jmb.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Fortune JM, Osheroff N. Merbarone inhibits the catalytic activity of human topoisomerase IIα by blocking DNA cleavage. J. Biol. Chem. 1998;273:17643–17650. doi: 10.1074/jbc.273.28.17643. [DOI] [PubMed] [Google Scholar]
- (37).Hawtin RE, Stockett DE, Byl JA, McDowell RS, Nguyen T, Arkin MR, Conroy A, Yang W, Osheroff N, Fox JA. Voreloxin is an anticancer quinolone derivative that intercalates DNA and poisons topoisomerase II. PLoS One. 2010;5:e10186. doi: 10.1371/journal.pone.0010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Gentry AC, Pitts SL, Jablonsky MJ, Bailly C, Graves DE, Osheroff N. Interactions between the etoposide derivative F14512 and human type II topoisomerases: Implications for the C4 spermine moiety in promoting enzyme-mediated DNA cleavage. Biochemistry. 2011;50:3240–3249. doi: 10.1021/bi200094z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Pommier Y, Marchand C. Interfacial inhibitors: Targeting macromolecular complexes. Nat. Rev. Drug. Discov. 2012;11:25–36. doi: 10.1038/nrd3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Bender RP, Ham AJ, Osheroff N. Quinone-induced enhancement of DNA cleavage by human topoisomerase IIα: Adduction of cysteine residues 392 and 405. Biochemistry. 2007;46:2856–2864. doi: 10.1021/bi062017l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Mondrala S, Eastmond DA. Topoisomerase II inhibition by the bioactivated benzene metabolite hydroquinone involves multiple mechanisms. Chem. Biol. Interact. 2010;184:259–268. doi: 10.1016/j.cbi.2009.12.023. [DOI] [PubMed] [Google Scholar]
- (42).Robinson MJ, Osheroff N. Effects of antineoplastic drugs on the post-strand-passage DNA cleavage/religation equilibrium of topoisomerase II. Biochemistry. 1991;30:1807–1813. doi: 10.1021/bi00221a012. [DOI] [PubMed] [Google Scholar]
- (43).Lindsey RH, Pendleton M, Ashley RE, Mercer SL, Deweese JE, Osheroff N. Catalytic core of human topoisomerase IIα: Insights into enzyme-DNA interactions and drug mechanism. Biochemistry. 2014;53:6595–6602. doi: 10.1021/bi5010816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Wang JC. DNA topoisomerases. Annu. Rev. Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- (45).Vogna D, Pezzella A, Panzella L, Napolitano A, d’Ischia M. Oxidative chemistry of hydroxytyrosol: Isolation and characterisation of novel methanooxocinobenzodioxinone derivatives. Tetrahedron Lett. 2003;44:8289–8292. [Google Scholar]