Abstract
Background: Coronary artery disease (CAD) is associated with cognitive decrements and risk of later dementia, but it is not known if shared genetic factors underlie this association. We tested whether polygenic risk for CAD was associated with cognitive ability in community-dwelling cohorts of middle-aged and older adults.
Methods: Individuals from Generation Scotland: Scottish Family Health Study (GS:SFHS, N = 9865) and from the Lothian Birth Cohorts of 1921 (LBC1921, N = 517) and 1936 (LBC1936, N = 1005) provided cognitive data and genome-wide genotype data. Polygenic risk profile scores for CAD were calculated for all of the cohorts using the largest available genome-wide association studies (GWAS) data set, the CARDIoGRAM consortium (22 233 cases and 64 762 controls). Polygenic risk profile scores for CAD were then tested for their association with cognitive abilities in the presence and absence of manifest cardiovascular disease.
Results: A meta-analysis of all three cohorts showed a negative association between CAD polygenic risk and fluid cognitive ability (β = −0.022, P = 0.016), verbal intelligence (β = −0.024, P = 0.011) and memory (β = −0.021, P = 0.028).
Conclusions: Increased polygenic risk for CAD is associated with lower cognitive ability in older adults. Common genetic variants may underlie some of the association between age-related cognitive decrements and the risk for CAD.
Keywords: Coronary artery disease, polygenic traits, cognition, ageing, dementia, genetics
Key Messages
Coronary artery disease is associated with lower cognitive ability.
This study examines the association between polygenic risk for coronary artery disease and cognitive ability in a large sample ( N = 11 387).
The findings imply a shared genetic architecture between coronary artery disease and lower cognitive ability.
Identifying associated pathways may provide novel drug targets for these common conditions.
Introduction
Age-related cognitive decline is an important aspect of health in older people. Accelerated cognitive ageing is associated with greater mortality and morbidity, less independence, lower quality of life and increased dementia risk. 1 Since dementia is a substantial and growing burden on ageing populations, 2 it is important to understand the mechanisms underpinning cognitive ability and ageing.
Some cognitive abilities decline on average as people grow older, including aspects of memory, speed of thinking and abstract reasoning. 1 Age-related cognitive decline shows substantial variation in the population 3,4 and this has spurred attempts to discover the factors influencing cognitive abilities in later life. Genetic and environmental risk factors both make a substantial contribution to people’s differences in their level of cognitive ability. Twin studies suggest that the heritability of cognitive ability is above 50% in adulthood, including in older age. 5–7 Genome-wide association studies (GWAS) suggest that around one-third of the variation in cognitive ability is accounted for by common genetic variants. 8,9
A proportion of the variation in cognitive ability levels is probably due to disease states. Coronary artery disease (CAD) is one type of cardiovascular disease (CVD) robustly associated with reduced cognitive ability. 10–15 It tends to be assumed that the direction of the causation between CAD and cognitive ability is that more CAD leads to lower cognitive ability. There is considerable evidence indicating the reverse direction also, whereby lower cognitive ability in childhood represents a risk factor for CAD and is associated with higher morbidity and mortality for CAD. 16,17 Whereas there is a substantial environmental contribution to CAD, family and twin studies suggest that CAD is also substantially heritable, with approximately 40% to 50% of its susceptibility being accounted for by genetic factors. 18 Based on a heritability of 40%, recent GWAS studies have shown that 45 confirmed CAD susceptibility loci explained 6% of the additive genetic variance for CAD, which increased to 10.6% after the addition of 104 nominally associated variants. 19
In summary, CAD and cognition are correlated phenotypically, and population variation in each is caused by genetic and environmental factors. Luciano et al.20 showed that some associations between risk factors for CVD and cognitive ability are explained by genetic factors. Therefore, it is possible that the association between CAD and cognitive ability may in part be due to shared genetic variation; that is, some genetic risk factors might confer risk of both CAD and lower cognitive ability level. In order to test whether there is a genetic correlation between CAD and cognitive ability, cohorts consisting of cognitively healthy individuals with both genome-wide genotyping and cognitive data are required.
Because of their phenotypic correlation and the heritable and polygenic nature of both CAD and cognitive ability, we hypothesized that these traits have shared genetic causes. We tested the hypothesis using a polygenic risk profiling approach, 21 using the Lothian Birth Cohorts (LBCs) and the Generation Scotland: Scottish Family Health Study Cohort (GS:SFHS) in the presence and absence of manifest CVD.
Methods
Cohorts and cognitive measures
GS:SFHS
GS:SFHS is a recently available population-based cohort of over 21 000 people. 22,23 Genome-wide single nucleotide polymorphism (SNP) data were ascertained for 9865 individuals, 5790 female and 4075 male, with a mean (standard deviation; SD) age of 52.2 (13.6) years. Details of GS:SFHS are provided in the Supplementary material (available as Supplementary data at IJE online) and the derived cognitive measures are shown in Table 1 . In short, the cohort included measures of verbal intelligence ( N = 9697), memory ( N = 9748), verbal fluency ( N = 9753) and processing speed ( N = 9732). A derived measure of fluid cognitive ability ( N = 9630) was obtained through a principal component analysis (PCA) of the measures for memory, verbal fluency and processing speed, and extracting the first unrotated principal component.
Table 1.
Cognitive abilities derived from the cognitive test battery of GS:SFHS, LBC1921 and LBC1936
| GS:SFHS | LBC1921 | LBC1936 | |
|---|---|---|---|
| Fluid cognitive ability |
|
||
| Verbal intelligence | Mill Hill Vocabulary Test | National Adult Reading Test | National Adult Reading Test |
| Memory | Logical Memory † | Total Logical Memory ‡ | |
| Processing speed | Digit Symbol-Coding * |
|
|
Test references are shown in the supplemental methods .
† Wechsler Memory Scale-III.
*Wechsler Adult Intelligence Scale-III.
‡ Wechsler Memory Scale-Revised.
LBC1921 and LBC1936
The LBCs of 1921 and 1936 are longitudinal studies providing lifelong cognitive data between the ages of 11 and 79, and genome-wide SNP data for a total of 1522 older individuals (LBC1921 = 517 individuals, 302 female and 215 male, LBC1936 = 1005 individuals, 496 female and 509 male). 24,25 The Lothian Birth Cohorts were originally identified through contacting individuals living in the Edinburgh area of Scotland (Lothian) who were born in 1921 or 1936 and might therefore have taken part in the Scottish Mental Surveys (SMS) at age about 11 years in 1932 or 1947, respectively. In these surveys, almost every child born in those years and attending school in Scotland completed the Moray House Test No. 12 (MHT) assessment of general intelligence. All participants of both LBCs completed an additional cognitive test battery around the ages of 79 and 83 in LBC1921 and age 70 in LBC1936, including a measure of verbal intelligence (total N = 1515). Derived measures of memory (total N = 1477), processing speed (total N = 1253) and fluid cognitive ability (total N = 1491) were obtained through a PCA and extracting the first unrotated principal component. Details of the LBCs and derived cognitive measures are shown in the Supplementary material (available as Supplementary data at IJE online) and in Table 1 .
Genotyping
DNA extracted from venous blood from the participants was genotyped at the Wellcome Trust Clinical Research Facility using the Illumina HumanOmniExpressExome-8 v1.0 DNA Analysis Beadchip and Infinium chemistry (GS:SFHS), 26,27 and the Illumina 610-Quadv1 whole-genome SNP array (LBC’s). The sample collection, quality control and genotyping process is described elsewhere in more detail. 8,23 Four (five for GS:SFHS) MDS components were calculated and extracted using the SNP data set to control for population stratification. Scree plots of the first 10 MDS components for population stratification in GS:SFHS and the first four in both LBCs are shown in Supplementary Figure 1a–c (available as Supplementary data at IJE online).
Creating CAD polygenic risk scores
The method to create polygenic risk scores (PGRS) has been described previously. 21 In summary, this method calculates SNP effect sizes from published genetic association data and calculates the genome-wide weighted sum of the alleles that an individual carries. This sum, the polygenic risk score, then serves as an index of the genetic load for a specific disease. Summary statistics from the CARDIoGRAM consortium (22 233 cases and 64 762 controls) were used to create the CAD PGRS. Details of the methods used by CARDIoGRAM can be found elsewhere. 28 The training sample data results for each SNP were thresholded at cut-offs of 0.01, 0.05, 0.1, 0.5 and 1. After linkage disequilibrium pruning (based on r 2 > 0.25 within a 200-SNP sliding window), the five resulting SNP sets were used to construct a risk profile for the subjects in GS:SFHS, LBC1921 and LBC1936, using the cumulative sum of each SNP allele dose multiplied by the log of the odds ratio derived from CARDIoGRAM. Raw genotype data were used to create the risk scores for both LBCs and GS:SFHS. The number of SNPs included in each threshold in the three cohorts can be found in Supplementary Table 1 (available as Supplementary data at IJE online). Throughout the paper, only the data using the cut-off of 0.5 will be reported as this threshold has shown to be the most predictive threshold in a large independent sample (UK Biobank, n = 112 151, Supplementary Table 2 , available as Supplementary data at IJE online).
Statistical analysis
All statistical analyses were conducted in the R statistical software package. 29 Point-biserial correlation coefficients were calculated between self-reported CVD and the cognitive phenotypes. At all five SNP thresholds, linear regression models were created between CAD PGRS and self-reported CVD, as proof of principle, and the cognitive phenotypes, adjusting for the four MDS components for population stratification, age and sex in both LBC1921 and LBC1936.
The GS:SFHS models were also adjusted for family structure by fitting a univariate linear mixed model which estimates the genetic and environmental variance, using the ASReml program. 30 The inverse of a relationship matrix was created by using pedigree kinship information to fit the family structure as a random effect. The cognitive scores were used as the dependent variable and age, sex, the five MDS components and the PGRS were used as fixed effects. Wald’s conditional F-test was used to calculate P -values for the fixed effects. Additional analyses were performed adjusting the fluid cognitive ability models for verbal intelligence and the verbal intelligence models for fluid cognitive ability.
An analysis by age group (<40 years, 40–60 years, >60 years) in GS:SFHS was performed to test if CAD PGRS had different associations with cognition in different age groups. An analysis excluding participants with self-reported CVD was performed to test if any associations with cognitive ability were being driven by participants with CVD.
A meta-analysis, using the meta package, of the results across studies was then conducted in order to synthesize the findings for maximum statistical power and to check for heterogeneity. A fixed-effects model was used in which the standardized regression coefficients were weighted by the inverse of their squared standard error and pooled to provide a summary estimate across both cohorts, based on the method of the DerSimonian-Laird estimator. 31 We tested for the presence and magnitude of between-study heterogeneity using Cochran’s Q and the I 2 statistic, respectively. 32
To test whether different CAD SNPs are contributing to different cognitive traits, a linear regression was conducted between 25 (of 45) CAD SNPs that were available in the largest data set (GS:SFHS), adjusting for age, sex and five MDS components for population stratification.
Results
A positive CVD history was observed in 30.2 % of LBC1921 ( n = 156), 24.6 % of LBC1936 ( n = 247) and 4.7 % of GS:SFHS ( n = 471). All cognitive traits were approximately normally distributed, with outliers > ± 3.5 SDs from the mean excluded. There were many (9 of 15) small point-biserial correlations between self-reported history of CVD and lower scores for the cognitive phenotypes in the larger GS:SFHS and LBC1936 cohorts, but not in LBC1921, though the latter had similar effect sizes ( Table 2 ). Phenotypic correlations between the different cognitive ability traits in each cohort can be found in Supplementary Table 3a–c (available as Supplementary data at IJE online). Correlations between the polygenic risk scores in each cohort can be found in Supplementary Table 4a–c (available as Supplementary data at IJE online).
Table 2.
Phenotypic correlations (point-biserial) for self-reported cardiovascular disease and each of the cognitive traits for the three cohorts
|
Self-reported cardiovascular disease
|
|||
|---|---|---|---|
| GS:SFHS | LBC1921 | LBC1936 | |
| VI | 0.01 | −0.08 | −0.07 * |
| Memory | −0.07** | −0.07 | −0.10** |
| PS | −0.16** | −0.14 * | −0.14** |
| VF | −0.02 * | −0.08 | −0.05 |
| G fluid | −0.12** | −0.05 | −0.13** |
VI, verbal intelligence; PS, processing speed; VF, Verbal Fluency test; G fluid , fluid cognitive ability.
* P < 0.05; ** P < 0.005.
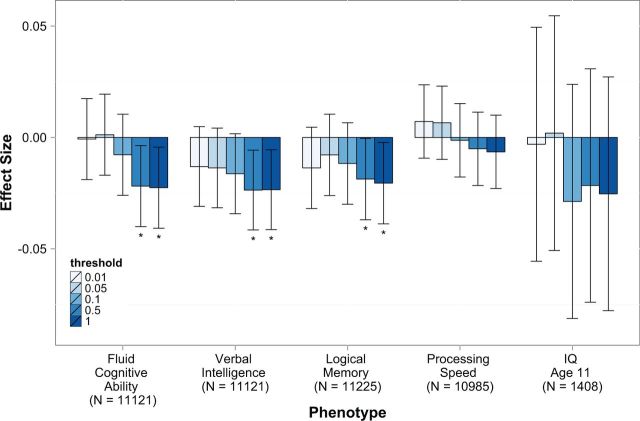
A fixed-effects meta-analysis of the cognitive phenotypes in all three cohorts showed that CAD polygenic risk was positively associated with self-reported CVD at all SNP inclusion thresholds ( Table 3 , Figure 1 ). CAD polygenic risk was associated with fluid cognitive ability (β = −0.0213, P = 0.0195), with verbal intelligence (β = −0.0238, P = 0.0107) and with memory (β = −0.0186, P = 0.0461). No heterogeneity was reported except for CVD history at a threshold of P < 0.01 (I 2 = 72.7%, P = 0.03) ( Supplementary Table 5 , available as Supplementary data at IJE online).
Table 3.
Effect size and significance of meta-analysis correlations between CAD polygenic risk scores and cognitive abilities or self-reported history of cardiovascular disease
| CAD genetic risk scores: SNPs with P -values < | Fluid cognitive ability | Verbal intelligence | Memory | Processing speed | IQ age 11 | CVD history | |
|---|---|---|---|---|---|---|---|
| n = 11121 | n = 11121 | n = 11225 | n = 10985 | n = 1408 | n = 7930 | ||
| 1 | β | −0.0220 | −0.0237 | −0.0205 | −0.0063 | −0.0253 | 0.1806 |
| z | −2.41 | −2.53 | −2.19 | −0.74 | −0.081 | 4.15 | |
| P -value | 0.0161 | 0.0113 | 0.0284 | 0.4567 | 0.3444 | 3.33 × 10 − 5 | |
| 0.5 | β | −0.0213 | −0.0238 | −0.0186 | −0.0049 | −0.0216 | 0.1737 |
| z | −2.34 | −2.55 | −1.99 | −0.59 | −0.807 | 4.00 | |
| P -value | 0.0195 | 0.0107 | 0.0461 | 0.559 | 0.4197 | 6.40 × 10 − 5 | |
| 0.1 | β | −0.0072 | −0.0160 | −0.0114 | −0.0007 | −0.0287 | 0.1818 |
| z | −0.79 | −1.71 | −1.22 | −0.09 | −1.07 | 4.20 | |
| P -value | 0.429 | 0.0865 | 0.2207 | 0.9313 | 0.2843 | 2.70 × 10 − 5 | |
| 0.05 | β | 0.0014 | −0.0135 | −0.0077 | 0.0070 | 0.0019 | 0.1341 |
| z | 0.16 | −1.45 | −0.82 | 0.83 | 0.07 | 3.12 | |
| P -value | 0.8759 | 0.1477 | 0.411 | 0.406 | 0.9429 | 0.0018 | |
| 0.01 | β | −0.0003 | −0.0128 | −0.0135 | 0.0078 | −0.003 | 0.1006 |
| z | −0.03 | −1.38 | −1.44 | 0.92 | −0.1137 | 2.33 | |
| P -value | 0.9742 | 0.1716 | 0.1489 | 0.3571 | 0.9095 | 0.0194 | |
Effects with P < 0.05 are shown in bold.
CAD, coronary artery disease; CVD history, history of cardiovascular disease; β, standardized regression coefficient.
Figure 1.
Meta-analysis of associations between coronary artery disease polygenic risk and cognitive traits at five SNP thresholds. Plot shows estimates with 95% confidence intervals; results with P -values below 0.05 are indicated by an asterisk.
Individual analysis of the three cohorts showed that CVD status was associated with CAD PGRS in GS:SFHS (β = 0.202, P = 0.0013) and LBC1921 (β = 0.231, P = 0.0225). No association was found between CVD status and CAD PGRS in LBC1936 and LBC1921 ( Supplementary Tables 6 and Supplementary Data , available as Supplementary data at IJE online).
In GS:SFHS, CAD PGRS showed an association with the Mill Hill Vocabulary (MHV) test (β = −0.03, P = 0.004), with Memory (β = −0.02, P = 0.035) and with fluid cognitive ability (β = −0.02, P = 0.020). No associations were found for the other cognitive phenotypes. The direction of effect for all associations of the cognitive phenotypes with CAD PGRS was negative and occurred in the proposed direction of the hypothesis: greater CAD PGRS is associated with lower cognitive test scores. A subset without individuals with self-reported CVD showed comparable results ( Supplementary Table 6 ). No associations were found for the age group below 40 years ( n = 1831), or the group between 40 and 60 years ( n = 5204). In the age group above 60 years ( n = 2825) CAD PGRS showed an association with fluid cognitive ability (β = −0.037, P = 0.044), as well as verbal fluency (VF) (β = −0.063, P = 0.001) ( SupplementaryTable 7 , available as Supplementary data at IJE online).
Additional analyses adjusting the verbal intelligence models for fluid cognitive ability showed that verbal intelligence remains associated with CAD polygenic risk (β = −0.009, P = 0.29). Fluid cognitive ability is not associated with CAD polygenic risk after adjusting for verbal intelligence (β = −0.02, P = 0.03). No associations were found between CAD PGRS and the cognitive phenotypes in LBC1921 and LBC1936 ( Supplementary Table 8 , available as Supplementary data at IJE online).
In GS:SFHS, no associations were found between 25 SNPs, which passed a threshold for association for CAD, and the cognitive traits ( Supplementary Table 9 , available as Supplementary data at IJE online).
Discussion
Based on SNP associations from a large GWAS study of CAD, PGRS were created for CAD in the three independent cohorts measuring cognition in middle to old age. CAD PGRS was associated with CVD history at all SNP thresholds in a meta-analysis of all three cohorts. This study found widespread phenotypic associations between CVD history and the cognitive phenotypes in GS:SFHS and LBC1936; people with CVD tended to have lower cognitive ability. In the smaller LBC1921, most associations showed similar-sized correlation coefficients. This study found a negative association between common genetic variants for CAD and fluid cognitive ability in GS:SFHS and in a meta-analysis of all three cohorts. Processing speed and memory were the only traits showing an association in LBC1921, the smallest of the cohorts. No associations were found in LBC1936, though the effects were in the expected direction and comparable to the effects in GS:SFHS. CAD PGRS were also negatively correlated with measures of vocabulary and logical memory in GS:SFHS.
Cognitive ability tends to be lower in individuals suffering from CAD, 10,33 which is supported by our results showing a negative correlation between CVD history and the cognitive phenotypes, and more recent studies have demonstrated that a decline in cognitive abilities may be seen in individuals at high risk for CAD. 34 The results of this DNA-based study suggest that the phenotypic association between CAD and cognitive ability has some shared genetic aetiologies. This is supported by the family-based genetic analysis results of Luciano et al.20 on GS:SFHS.
The GS:SFHS analysis by age group showed negative associations in the age group above 60 years between CAD PGRS and MHV, VF and fluid cognitive ability. This suggests that the decline in cognitive abilities happens when individuals reach the age where they are more at risk for CAD. This is supported by results from the American Heart Association, who showed that the prevalence of CAD doubles in older individuals. 35 When fitting an interaction term for age and CAD polygenic risk, we only found an interaction for verbal fluency.
Although the direction of causation tends to be assumed to be from more CAD to less cognitive capability, there is evidence here and elsewhere to lead us to consider the reverse association too. It is now widely replicated that lower general cognitive ability from childhood and young adulthood predicts more morbidity and mortality from CVD in later life. 17,36–39 It is notable that in the present meta-analysis, verbal intelligence was associated with CAD PGRS. This type of vocabulary test tends to indicate peak previous cognitive ability rather than being an indicator of cognitive ageing. Therefore, it is possible that this result represents some genetic confounding such that some of the same genes result in lower vocabulary scores and more CAD. However, we did not find an association for IQ at age 11 with CAD PGRS. It is also conceivable that the CAD PGRS is in part picking up some of the genetic contribution to lifetime-stable cognitive differences and that such cognitive differences are associated with creating lifestyles and environments that are more or less conducive to CAD, as we discuss elsewhere. 16,40
Fluid cognitive ability and verbal intelligence are moderately correlated with each other (r = 0.34). The additional analyses adjusting both verbal intelligence and fluid cognitive ability for each other shows greater attenuation for fluid cognitive ability after controlling for verbal intelligence than the reverse. This could be because verbal intelligence is psychometrically a more reliable measure. Also, these results could indicate that the association with CAD is more due to the stable trait of cognitive ability, typically well assessed by vocabulary measures, rather than to any cognitive decline that might be captured by the more age- and illness-sensitive fluid cognitive ability.
Among the study’s strengths were the large sample size of GS:SFHS, the detailed cognitive testing of different domains in the cohorts, the geographical homogeneity of the three cohorts, the access to the results of a large meta-analysis of GWAS studies of CAD, and the rare ability to examine the association of CAD with cognitive ability data in childhood and older age in both LBCs.
The present study has some limitations. The use of self-reported CVD may have led to misclassification of CVD conditions, causing a likely bias toward the null hypothesis. 33 This study is unable to test for a direction of causation between CAD and cognitive ability. Raw P -values are presented for the PGRS associations. We acknowledge that multiple tests were performed, but it is difficult to determine the appropriate test to correct for multiple testing because both the traits as the polygenic risk scores are highly correlated ( Supplementary Tables 3a–c and Supplementary Data , available as Supplementary data at IJE online).
This study failed to replicate the associations found in GS:SFHS between CAD PGRS and cognition in both LBCs. The sample size of LBC1921 is substantially smaller than the sample sizes of GS:SFHS and LBC1936; therefore LBC1921 probably does not have enough power to detect differences. Together with the higher mean age and the greater comorbidity, this might explain the differences in results. An explanation for the absence of associations in LBC1936 might be the lack of an association between self-reported CVD and CAD PGRS. Nevertheless, the meta-analysis of the associations in all three cohorts supports the overall findings and conclusion.
The present study suggests that CAD genetic risk is negatively associated with cognitive ability in healthy population- based cohorts. These findings were made in general population cohorts and were independent of CAD pathology. These findings suggest that CAD and cognitive ability share some common genetic aetiology. Further annotation of the shared genetic architecture and its associated biological pathways may provide novel drug targets for both disorders.
Funding
This work was supported by Wellcome Trust Strategic Award [104036/Z/14/Z]. Generation Scotland has received core funding from the Chief Scientist Office of the Scottish Government Health Directorates [CZD/16/6] and the Scottish Funding Council [HR03006]. The research was supported by a programme grant from Age UK (Disconnected Mind) and by grants from the Biotechnology and Biological Sciences Research Council (BBSRC). The work was undertaken by the University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross-council Lifelong Health and Wellbeing Initiative [MR/K026992/1]. Funding from the Medical Research Council (MRC) and BBSRC is gratefully acknowledged.
Supplementary Material
Acknowledgements
We are grateful to all the families who took part, to the general practitioners and the Scottish School of Primary Care for their help in recruiting them, and to the whole Generation Scotland team, including Professor Blair Smith, Dr Lynne Hocking, Dr Sandosh Padmanabhan, Professor David Porteous, interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, healthcare assistants and nurses. For the Lothian Birth Cohorts (LBC1921 and LBC1936), we thank Paul Redmond for database management assistance; Alan Gow, Martha Whiteman, Alison Pattie, Michelle Taylor, Janie Corley, Caroline Brett and Caroline Cameron for data collection and data entry; nurses and staff at the Wellcome Trust Clinical Research Facility, where blood extraction and genotyping was performed; staff at the Lothian Health Board, and the staff at the SCRE Centre, University of Glasgow. This research has been conducted using the UK Biobank Resource (application 7898).
Conflict of interest: All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1. Salthouse T . Consequences of age-related cognitive declines . Ann Rev Psychol 2012. ; 63 : 201 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prince M, Knapp M, Guerchet M, et al. . Overview – Dementia UK . London: : Dementia UK; , 2014. . [Google Scholar]
- 3. Deary IJ, Corley J, Gow AJ, et al. . Age-associated cognitive decline . Br Med Bull 2009. ; 92 : 135 – 52 . [DOI] [PubMed] [Google Scholar]
- 4. Plassman BL, Williams JW, Burke JR, Holsinger T, Benjamin S . Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life . Ann Intern Med 2010. ; 153 : 182 – 93 . [DOI] [PubMed] [Google Scholar]
- 5. McClearn GE, Johansson B, Berg S, et al. . Substantial genetic influence on cognitive abilities in twins 80 or more years old . Science 1997. ; 276 : 1560 – 63 . [DOI] [PubMed] [Google Scholar]
- 6. Deary IJ, Spinath FM, Bates TC . Genetics of intelligence . Eur J Hum Genet 2006. ; 14 : 690 – 700 . [DOI] [PubMed] [Google Scholar]
- 7. Pedersen NL, Plomin R, Nesselroade JR, McClearn GE . A quantitative genetic analysis of cognitive abilities during the second half of the life span . Psychol Sci 1992. ; 3 : 346 – 53 . [Google Scholar]
- 8. Davies G, Tenesa A, Payton A, et al. . Genome-wide association studies establish that human intelligence is highly heritable and polygenic . Mol Psychiatry 2011. ; 16 : 996 – 1005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies G, Armstrong N, Bis J, et al. . Genetic contributions to variation in general cognitive function: a meta-analysis of genome-wide association studies in the CHARGE consortium . Mol Psychiatry 2015. ; 20:183 – 92 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abete P, Della-Morte D, Gargiulo G, et al. . Cognitive impairment and cardiovascular diseases in the elderly. A heart-brain continuum hypothesis . Ageing Res Rev 2014. ; 18c : 41 – 52 . [DOI] [PubMed] [Google Scholar]
- 11. Muller M, Grobbee D, Aleman A, Bots M, Van der Schouw Y . Cardiovascular disease and cognitive performance in middle-aged and elderly men . Atherosclerosis 2007. ; 190 : 143 – 49 . [DOI] [PubMed] [Google Scholar]
- 12. Haring B, Leng X, Robinson J, et al. . Cardiovascular disease and cognitive decline in postmenopausal women: results from the Women’s Health Initiative Memory Study . J Am Heart Assoc 2013. ; 2 : e000369 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Verhaegen P, Borchelt M, Smith J . Relation between cardiovascular and metabolic disease and cognition in very old age: cross-sectional and longitudinal findings from the Berlin aging study . Health Psychol 2003. ; 22 : 559 . [DOI] [PubMed] [Google Scholar]
- 14. Singh-Manoux A, Sabia S, Lajnef M, et al. . History of coronary heart disease and cognitive performance in midlife: the Whitehall II study . Eur Heart J 2008. ; 29 : 2100 – 07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rafnsson SB, Deary IJ, Smith FB, Whiteman MC, Fowkes FG . Cardiovascular diseases and decline in cognitive function in an elderly community population: the Edinburgh Artery Study . Psychosom Med 2007. ; 69 : 425 – 34 . [DOI] [PubMed] [Google Scholar]
- 16. Deary IJ, Weiss A, Batty GD . Intelligence and Personality as Predictors of Illness and Death. How Researchers in Differential Psychology and Chronic Disease Epidemiology Are Collaborating to Understand and Address Health Inequalities . Psychol Sci Public Interest 2010. ; 11 : 53 – 79 . [DOI] [PubMed] [Google Scholar]
- 17. Hart C, Taylor M, Davey Smith G, et al. . Childhood IQ and cardiovascular disease in adulthood: prospective observational study linking the Scottish Mental Survey 1932 and the Midspan studies . Soc Sci Med 2004. ; 59 : 2131 – 38 . [DOI] [PubMed] [Google Scholar]
- 18. Peden JF, Farrall M . Thirty-five common variants for coronary artery disease: the fruits of much collaborative labour . Hum Mol Genet 2011. ; 20 : R198 – 205 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deloukas P, Kanoni S, Willenborg C, et al. . Large-scale association analysis identifies new risk loci for coronary artery disease . Nat Genet 2013. ; 45 : 25 – 33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luciano M, Batty GD, McGilchrist M, et al. . Shared genetic aetiology between cognitive ability and cardiovascular disease risk factors: Generation Scotland’s Scottish family health study . Intelligence 2010. ; 38 : 304 – 13 . [Google Scholar]
- 21. Purcell SM, Wray NR, Stone JL, et al. . Common polygenic variation contributes to risk of schizophrenia and bipolar disorder . Nature 2009. ; 460 : 748 – 52 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith BH, Campbell A, Linksted P, et al. . Cohort profile: Generation Scotland: Scottish Family Health Study (GS: SFHS). The study, its participants and their potential for genetic research on health and illness . Int J Epidemiol 2013. ; 42:689 – 70 . [DOI] [PubMed] [Google Scholar]
- 23. Smith BH, Campbell H, Blackwood D, et al. . Generation Scotland: the Scottish Family Health Study; a new resource for researching genes and heritability . BMC Med Genet 2006. ; 7 : 74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deary IJ, Gow AJ, Pattie A, Starr JM . Cohort profile: The Lothian Birth Cohorts of 1921 and 1936 . Int J Epidemiol 2012. ; 41 : 1576 – 84 . [DOI] [PubMed] [Google Scholar]
- 25. Deary IJ, Gow AJ, Taylor MD, et al. . The Lothian Birth Cohort 1936: a study to examine influences on cognitive ageing from age 11 to age 70 and beyond . BMC Geriatr 2007. ; 7 : 28 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gunderson KL . Whole-genome genotyping on bead arrays . In: Dufva M. (ed). DNA Microarrays for Biomedical Research . New York NY: : Humana; , 2009. . [Google Scholar]
- 27. Kerr SM, Campbell A, Murphy L, et al. . Pedigree and genotyping quality analyses of over 10 000 DNA samples from the Generation Scotland: Scottish Family Health Study . BMC Med Genet 2013. ; 14 : 38 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schunkert H, König IR, Kathiresan S, et al. . Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease . Nat Genet 2011. ; 43 : 333 – 38 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. R Development Core Team . R: A Language and Environment for Statistical Computing . Vienna: : R Foundation for Statistical Computing; , 2012. . [Google Scholar]
- 30. Gilmour AR, Gogel B, Cullis B, Thompson R . Asreml User Guide Release 3.0 . Hemel Hempstead, UK: : VSN International; , 2009. . [Google Scholar]
- 31. DerSimonian R, Laird N . Meta-analysis in clinical trials . Control Clin Trials 1986. ; 7 : 177 – 88 . [DOI] [PubMed] [Google Scholar]
- 32. Higgins J, Thompson SG, Deeks JJ, Altman DG . Measuring inconsistency in meta-analyses . BMJ 2003. ; 327 : 557 – 60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zheng L, Mack WJ, Chui HC, et al. . Coronary artery disease is associated with cognitive decline independent of changes on magnetic resonance imaging in cognitively normal elderly adults . J Am Geriatr Soc 2012. ; 60 : 499 – 504 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Joosten H, van Eersel ME, Gansevoort RT, Bilo HJ, Slaets JP, Izaks GJ . Cardiovascular risk profile and cognitive function in young, middle-aged, and elderly subjects . Stroke 2013. ; 44 : 1543 – 49 . [DOI] [PubMed] [Google Scholar]
- 35. Go AS, Mozaffarian D, Roger VL, et al. . Heart Disease and Stroke Statistics—2014 Update: A Report From the American Heart Association . Circulation 2014. ; 129 : 399 – 410 . [DOI] [PubMed] [Google Scholar]
- 36. Hagger-Johnson G, Mõttus R, Craig LC, Starr JM, Deary IJ . Pathways from childhood intelligence and socioeconomic status to late-life cardiovascular disease risk . Health Psychol 2012. ; 31 : 403 . [DOI] [PubMed] [Google Scholar]
- 37. Hagger-Johnson GE, Shickle DA, Deary IJ, Roberts BA . Direct and indirect pathways connecting cognitive ability with cardiovascular disease risk: Socioeconomic status and multiple health behaviors . Psychosom Med 2010. ; 72 : 777 – 85 . [DOI] [PubMed] [Google Scholar]
- 38. Batty GD, Shipley MJ, Dundas R, et al. . Does IQ explain socio-economic differentials in total and cardiovascular disease mortality? Comparison with the explanatory power of traditional cardiovascular disease risk factors in the Vietnam Experience Study . Eur Heart J 2009. ; 30:1903 – 09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Batty GD, Shipley MJ, Mortensen LH, Gale CR, Deary IJ . IQ in late adolescence/early adulthood, risk factors in middle-age and later coronary heart disease mortality in men: the Vietnam Experience Study . Eur J Cardiovasc Prev Rehabil 2008. ; 15 : 359 – 61 . [DOI] [PubMed] [Google Scholar]
- 40. Luciano M, Mõttus R, Harris S, et al. . Predicting cognitive ability in ageing cohorts using Type 2 diabetes genetic risk . Diabet Med 2014. ; 31 : 714 – 20 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.