Abstract
Background
Suspected food allergies are the cause of more than 200,000 visits to the emergency department annually. Racial differences in the prevalence of food allergy have also been reported, but the evidence is less conclusive. Researchers continue to struggle with the identification of food allergy for epidemiologic studies.
Objective
To explore racial differences in IgE-mediated food allergy (IgE-FA) in a birth cohort.
Methods
We used a panel of board-certified allergists to systematically identify IgE-FA to egg, milk, or peanut in a multiethnic birth cohort in which patient medical history, patient symptoms, and clinical data were available through 36 months of age.
Results
Of the 590 infants analyzed, 52.9% were male and 65.8% African American. Sensitization (serum specific IgE >0.35 IU/mL) to the food allergens was significantly higher for African American children compared with non–African American children as has been previously reported. No statistically significant racial/ethnic differences in IgE-FA were observed; however, a higher proportion of African American children were designated as having peanut allergy, and the percentage of African American children with an IgE level greater than 95% predictive decision points for peanut was 1.7% vs 0.5% for non–African American children. With the use of logistic regression, race/ethnicity was not significantly associated with IgE-FA (adjusted odds ratio, 1.12; 95% confidence interval, 0.58–2.17; P = .75) but was associated with sensitization to more than 1 of the food allergens (adjusted odds ratio, 1.80; 95% confidence interval, 1.22–2.65; P = .003).
Conclusion
We did not observe an elevated risk of IgE-FA for African American children, although established differences in sensitization were observed. Racial/ethnic differences in sensitization must be taken into consideration when investigating disparities in asthma and allergy.
Introduction
Suspected food allergies are the cause of more than 200,000 visits to the emergency department annually.1 Food allergies directly affect the quality of life of affected children and their families. There is compelling evidence that food allergy is increasing in the United States and other countries. Racial differences in the prevalence of food allergy have also been reported, but the evidence is less conclusive. Sicherer et al2 found a higher prevalence of self-reported seafood allergy among African Americans responding to a telephone survey using random digit dialing; however, other studies have reported no significant differences3 or report differences only in sensitization.4,5 Variations in the definition of food allergy for these studies may be the reason for the inconsistencies.
Researchers continue to struggle with the identification of food allergy for epidemiologic studies. Investigators typically use surveys or clinical evidence of sensitization. A limitation of using surveys to identify food allergy is that respondents may confuse food intolerance with an allergic reaction to food allergens.6 Measuring IgE is also less than ideal in that a clinical diagnosis of food allergy is based on symptoms that occur on the ingestion of food, which cannot be accurately predicted by sensitization.7 Although sensitization is a major risk factor for IgE-mediated food allergy (IgE-FA), a substantial number of sensitized persons will not respond to an oral food challenge, which is still considered the gold standard for the clinical diagnosis of IgE-FA.7,8 However, double-blind, placebo-controlled oral food challenges (DBPCFCs) are seldom feasible for large studies, and there is a substantial tendency for false-negative results because the test may not reproduce what occurred when the patient actually experienced the adverse reaction(s).7 We did not find any studies that suggested a racial difference in food allergy based on evidence using oral food challenges, yet evidence of a racial difference in the prevalence of food allergy could help researchers explain persistent racial differences in asthma and allergy prevalence, morbidity, and mortality. As an alternative to self-report of food allergy, the use of sensitization to food allergens alone as a surrogate measure, or the conduct of DBPCFCs, we established a physician panel of allergists to systematically identify cases of food allergy in a multiethnic birth cohort in which a bundled portfolio of patient medical history, clinical data, and self-reported symptoms was available.9 The objective of the present analysis is to explore whether African Americans have more IgE-FA compared to other racial/ethnic groups in the cohort, using the results of the physician panel to identify cohort members with IgE-FA.
Methods
Study Cohort
All aspects of this research were approved by institutional review boards responsible for the conduct of research in human subjects at Henry Ford Health System (HFHS) and Georgia Regents University. Eligibility and recruitment for the Wayne County Health, Environment, Allergy, and Asthma Longitudinal Study (WHEALS) birth cohort are described in a previous publication.10 The WHEALS cohort was established to identify environmental factors related to the development of allergy and asthma in infancy and childhood. Recruited participants were pregnant women aged 21 to 45 years who were seen for prenatal care in 1 of 5 HFHS obstetric clinics from September 1, 2003, through November 26, 2007. The women resided in the city or western suburbs of Detroit as defined by zip code, spoke English well enough to provide written informed consent, and had plans to stay in the Detroit area for 2 years after delivery. Collection of blood and interview data for the cohort has been described previously and is summarized below.9,10
Blood Collection
Placental cord blood specimens were collected after delivery and analyzed for total serum IgE level. In addition, research field staff collected venous blood samples from infants during home visits at 1, 6, and 12 months of age for total serum IgE measurements and during a 24-month clinic visit to measure total and allergen specific serum IgE. Specific serum IgE measurements were determined for 11 allergens, including the food allergens milk, egg, and peanut. Serum IgE measurements were conducted using the Pharmacia UniCAP system (Phadia; Thermo Fisher Scientific Inc, Waltham, Massachusetts). Food sensitization was defined as a food specific IgE level of 0.35 IU/mL or higher.
Skin Prick Tests
During a 24-month clinic visit, infants underwent skin prick tests (SPTs) for food allergens to milk, egg, and peanut. SPTs were performed using a Duotip-test device (Lincoln Diagnostics Inc, Decatur, Illinois). A positive SPT result to a food allergen was defined as having a wheal diameter 3 mm or greater than the saline control test. The allergy testing was performed under the guidance of a board-certified allergist (C.N., R.M., E.Z.). SPT results were discussed with the patient's guardian at the time of the testing. At the parent/guardian's request, a letter with the testing results was sent to the child's pediatrician. A standardized assessment sheet was completed by the allergist at the 2-year visit and sent to the study's principal investigator, who reviewed the results to see whether there was a positive allergic reaction and/or history that required further follow-up as noted by the evaluating allergist.
Parent Interviews
Field staff interviewed parents when the infant was 1, 6, 12, and 24 months of age. These interviews addressed infants' episodes of illness, atopic dermatitis or eczema, allergy and allergic reactions, and rashes and also collected information related to family medical history. Parents were also asked about any prescriptions or medical visits for the infant. A follow-up parent interview was administered when the child was 3 to 6 years of age (mean age, 4.3 years), including specific items on infant food avoidance, gastrointestinal symptoms, and onset and duration of reactions to food. Data collected during these interviews were supplemented by a review of the infant's medical record. For infants born outside the HFHS, medical records were requested and reviewed. Medical record reviews included assessments of all clinical encounters, with an emphasis on any diagnoses of food allergy, possible allergic disease diagnoses for related conditions (eg, eczema), any reports of foods causing symptoms or health problems, and the infant age at time of diagnosis or parental report of symptoms.
Determination of Food Allergy
A physician panel of 2 board-certified generalist allergists (C.N., R.M.) was established to classify cohort participants as to the presence or absence of IgE-FA to milk, egg, and peanut.9 The physician panel was presented with data collected from the parent interview, which included information on infant gastrointestinal symptoms suggestive of food intolerance or food allergy; other potential allergic reactions, such as rash, respiratory difficulty, wheezing, or colic; any report of food avoidance; and clinical assessments (serum total and specific IgE and SPT results). For efficiency, infant data were forwarded to the panel only if 1 or more of the following criteria were met for milk, egg, or peanut allergens: (1) 1 or more specific IgE levels of 0.35 IU/mL or higher, (2) a positive SPT result, or (3) parental report of infant symptoms potentially related to food allergy plus at least one specific IgE level of 0.10 IU/mL or higher (eTable 1 and eTable 2). This level of IgE was not used to define sensitization but was used to ensure that those infants with even a low probability of IgE-FA would be reviewed by our physician panel.
eTable 1.
Geometric mean total IgE by race for subgroups of study sample by racea
| Status | Sample size | Geometric total IgE, mean (SE) |
|||
|---|---|---|---|---|---|
| All | African American | Non–African American | P valueb | ||
| Final determination is IgE-FA | 52 | 101.8 (4.5) | 98.4 (4.4) | 110.7 (5.3) | .99 |
| Final determination is no IgE-FA | |||||
| No potential for food allergy | 310 | 11.3 (3.2) | 12.1 (3.2) | 10.3 (3.2) | .12 |
| Met criteria for panel review | 280 | 52.9 (3.3) | 51.8 (3.3) | 55.8 (3.3) | .61 |
| Panel consensus is no IgE-FA | 228 | 46.7 (3.0) | 45.7 (3.0) | 48.9 (2.8) | .42 |
Abbreviation: IgE-FA, IgE-mediated food allergy.
These subgroups correspond to Figure 1.
P value for African American vs non–African American comparison.
eTable 2.
Guidelines Provided to Physician Panel for the Determination of IgE-Mediated Food Allergy in a Birth Cohort
| Allergen | Highly probable | Likely | Unlikely |
|---|---|---|---|
| Milk | Specific IgE ≥15 IU/mLa AND a SPT ≥ negative control OR a convincing medical history AND SPT >3 mm OR specific IgE >0.1 IU/mL | Convincing medical history AND specific IgE ≥0.1 IU/mL | No, equivocal, or weak medical history AND specific IgE <2 OR SPT ≤3 mm |
| Egg | Specific IgE ≥7 IU/mLa AND a SPT ≥ negative control OR a convincing medical history AND SPT >3 mm OR specific IgE >0.1 IU/mL | Equivocal or weak medical history AND specific IgE ≥2 IU/mL <95% PD points OR SPT >4 mm | No, equivocal, or weak medical history AND specific IgE <2 OR SPT ≤3 mm |
| Peanut | Specific IgE ≥14 IU/mLa AND a SPT ≥ negative control AND a convincing history AND SPT >3 mm OR specific IgE >0.1 IU/mL | Equivocal or weak history AND specific IgE ≥2 IU/mL <95% PD points OR SPT >4 mm | No, equivocal, or weak history AND specific IgE <2 OR SPT ≤3 mm |
Abbreviations: PD, predictive decision; SPT, skin prick test.
Adapted from Adverse Reactions to Food Committee of American Academy of Allergy, Asthma & Immunology. Work Group Report: Oral Food Challenge Testing. J Allergy Clin Immunol. 2009; 123(6 suppl):S365–S383.
Infant data were presented to the panel in a standardized, web-based format. To enhance standardization in classifying infants as to the presence of IgE-FA, physicians were asked to combine professional experience with investigator-developed protocols based on the Guidelines for the Diagnosis and Management of Food Allergy in the United States.11,12 Infants were classified as highly probable, likely, and unlikely to have had IgE-FA. A third allergist (R.E.) independently reviewed and ruled on discordant decisions.
Statistical Analysis
The study population for these analyses includes cohort children with 6- or 12-month interview data, data from the clinic visit at 2 to 3 years, and specific IgE measures at 2 to 3 years. For this analysis, atopic phenotypes included food allergy as determined by physician panel (highly probable or likel”); allergen specific IgE to egg, milk, or peanut; positive SPT results to egg, milk, or peanut; and the 95% predicted decision (PD) points for IgE established by Sampson et al.13 These are derived from “specific IgE antibody levels recommended for identifying patients with a >95% probability of reacting to a food challenge” (pg 895).13 The χ2 tests were used to make subgroup comparisons of participant characteristics for binary and categorical variables, and t tests were used for comparison of continuous variables.
Logistic regression was used to calculate odds ratios (ORs) and corresponding 95% confidence intervals (CIs) to describe the association between race and food allergy and between race and sensitization, adjusting for potential confounders. Independent variables included in the model were reported before delivery and included yearly household income less than $40,000, maternal educational level of high school or less, and unmarried status. In addition, from the 1- and 6-month survey, we collected mother's report of infant race, infant sex, breastfeeding for less than 6 months, infant exposure to environmental tobacco smoke during mother's pregnancy, and current smoking in child's household. Independent variables were retained in the final model if their inclusion changed the estimate of effect between race and food allergy or race and sensitization by more than 15%.14
Results
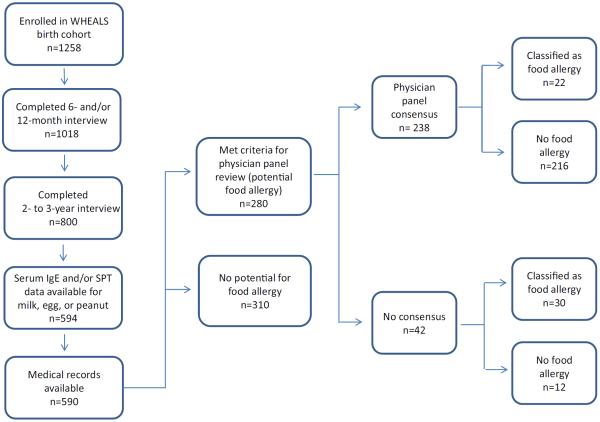
Of the 1258 infants enrolled in the study, 590 (46.9%) were included in the analysis (Fig 1). Demographic characteristics of included participants are provided in Table 1. The mean (SD) maternal age at infant birth was 29.8 (5.2) years, and 52.9% of the infants included in the analysis were male. A total of 65.8% of the study sample self-reported non-Hispanic black/African American race. The remaining 34.2% were categorized as non–black/African American and included 138 white infants (68.3%), 30 Hispanic/Latino infants (14.9%), and 34 infants of other races (16.8%), which included 1 American Indian/Alaska Native infant, 17 Asian infants, 12 Middle Eastern infants, and 4 who reported more than one race. Compared with the 668 not included in the analysis, those included in the analysis were significantly more likely to be male (P = .03) and have parents who were married (P = .02) with higher levels of education and household income (both P < .001).
Figure 1.
Study population. SPT indicates skin prick test; WHEALS, Wayne County Health, Environment, Allergy, and Asthma Longitudinal Study.
Table 1.
Characteristics of the 1,258 study participantsa
| Characteristic | Included in analysis (n = 590) | Not included in analysis (n = 668) | P value |
|---|---|---|---|
| Maternal age at infant birth, mean (SD), y | 29.8 (5.2) | 29.4 (5.3) | .18 |
| Race/ethnicity | .11 | ||
| Non-Hispanic black/African American | 388 (65.8) | 434 (65.0) | |
| All othersb | 202 (34.2) | 234 (35.0) | |
| Infant sexc | .03 | ||
| Male | 312 (52.9) | 312 (46.8) | |
| Female | 278 (47.1) | 355 (53.2) | |
| Medicaid | .72 | ||
| Yes | 187 (31.7) | 170 (32.7) | |
| No | 403 (68.3) | 350 (67.3) | |
| Marital status | .02 | ||
| Married | 382 (64.7) | 391 (58.5) | |
| Unmarried | 208 (35.3) | 277 (41.5) | |
| Maternal education level greater than bachelor's of science | <.001 | ||
| Yes | 196 (33.2) | 155 (23.2) | |
| No | 394 (66.8) | 513 (76.8) | |
| Annual household income <40,000 | <.001 | ||
| Yes | 190 (32.2) | 287 (43.0) | |
| No | 322 (54.6) | 308 (46.1) | |
| Refused/don't know | 78 (13.2) | 73 (10.9) | |
| Parental history of asthma or allergy | .89 | ||
| Yes | 246 (45.4) | 279 (45.8) | |
| No | 296 (54.6) | 330 (54.2) | |
| Breastfed for >6 months | .008 | ||
| Yes | 156 (26.8) | 97 (19.9) | |
| No | 427 (7.32) | 391 (80.1) | |
| Maternal smoking during pregnancy | .06 | ||
| Yes | 59 (10.0) | 90 (13.5) | |
| No | 531 (90.0) | 578 (86.5) | |
| Current smoker in householdd | .48 | ||
| Yes | 120 (20.3) | 38 (18.1) | |
| No | 470 (79.7) | 172 (81.9) | |
| Complementary food <4 months | .83 | ||
| Yes | 233 (39.5) | 176 (38.9) | |
| No | 357 (60.5) | 277 (61.1) |
Data are presented as number (percentage) unless otherwise indicated.
Includes 138 white infants (68.3%), 30 Hispanic/Latino infants (14.9%), and 34 infants of other races (16.8%).
One infant with missing data.
Current at 2- to 3-year interview.
Table 2 gives the estimated prevalence of IgE-FA, by race, as determined by the allergist panel (C.N., R.M.). The panel reached consensus for 238 (85.0%) of 280 infants, whereas 42 decisions (15.0%) were finalized with input from a third allergist (R.E.). Of the 41 children found to have IgE-FA (Table 2), 12 (29.3%) had at least one medical encounter documented in the medical record with an International Classification of Diseases, Ninth Revision (ICD-9), code for food allergy according to retrospective medical record review.
Table 2.
Estimated prevalence of food allergy and sensitization to egg, milk, or peanut and IgE PD points15 for each food allergen, restricted to infants with measures of specific IgE
| Milk (n = 566)a |
Egg (n = 567) |
Peanut (n = 560) |
Any(n = 558)b |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| African American, no. (%) | Non–African American, no. (%) | P value | African American, no. (%) | Non–African American, no. (%) | P value | African American, no. (%) | Non–African American, no. (%) | P value | African American, no. (%) | Non–African American, no. (%) | P value | |
| IgE-FAc | 6 (1.6) | 3 (1.5) | .99 | 20 (5.4) | 11 (5.6) | .95 | 17 (4.7) | 7 (3.6) | .54 | 27 (7.4) | 14 (7.2) | .91 |
| IgE ≥0.35 IU/mL | 128 (34.8) | 51 (25.8) | .03 | 101 (27.4) | 38 (19.2) | .03 | 50 (13.7) | 15 (7.7) | .03 | 157 (43.2) | 63 (32.3) | .01 |
| IgE ≥95% PD points | 3 (0.8) | 0 (0) | .56 | 8 (2.2) | 5 (2.5) | .78 | 6 (1.7) | 1 (0.5) | .43 | 13 (3.6) | 6 (3.1) | .75 |
Abbreviations: IgE-FA, IgE-mediated food allergy; PD, predictive decision.
Number with specific IgE measures for this food allergen, including 24, 23, and 30 infants with missing specific IgE data for milk, egg, and peanut, respectively.
Denominator is persons with specific IgE measures for all 3 food allergens and excludes 32 infants missing at least one food specific IgE.
As determined by physician panel and combines highly probable and likely.
Prevalence for milk and egg IgE-FA was similar by race (Table 2). A higher proportion of African American infants were designated as having peanut allergy, but this finding was not significantly different by race. An IgE level of 0.35 IU/mL or greater to each of 3 food allergens was significantly higher for African American children compared with non–African American children. There was no difference in the percentage of children meeting IgE 95% PD points for milk and egg by race. Although not statistically significant (P = .43), the percentage of African American children with IgE levels greater than 95% PD points for peanut, however, was 3 times higher than that of non–African American children (1.7% vs 0.5% for African Americans and non–African Americans).
Table 3 gives the results of logistic regression analyses to determine the independent association between ethnicity and the outcomes of IgE-FA (by physician panel review) and by sensitization, adjusting for potential confounders. For the model using IgE-FA as the dependent variable, no variables changed the estimate between race/ethnicity and IgE-FA by more than 15%. A final model included only race/ethnicity with an adjusted OR of 1.12 (95% CI, 0.58–2.17; P = .75). For an IgE level of 0.35 IU/mL or higher, the adjusted OR was 1.80 (95% CI, 1.22–2.65; P = .003) for a model containing race/ethnicity and marital status.
Table 3.
Results of logistic regression for the association of race to food allergy and to sensitization to milk, egg, or peanut among children in the WHEALS birth cohorta
| Variable | Black/African American |
|
|---|---|---|
| aOR (95% CI) | P value | |
| IgE-mediated food allergyb | 1.12c (0.58–2.17) | .75 |
| IgE ≥0.35 IU/mL | 1.80d (1.22–2.65) | .003 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; WHEALS, Wayne County Health Environment Allergy and Asthma Longitudinal Study.
Variables available for logistic regression analysis were those listed in Table 1. Variables were retained in the final model if their inclusion changed the estimate of effect between race and the dependent variable (food allergy as determined by physician panel or food sensitization defined as specific IgE level ≥0.35 IU/mL) by 15% or more.13
As determined by physician panel.
No variables changed the aOR by 15% or more for food allergy as determined by physician panel.
Adjusted for mother's marital status.
Discussion
Obtaining accurate food allergy prevalence estimates for epidemiologic studies is challenging. The gold standard of DBPCFC is seldom feasible in a large-scale study. Potential study limitations can result from the use of questionnaires. For example, recall bias is a potential limitation to this study. We believe this type of bias has been minimized due to the collection of clinical and objective data periodically at key time points that span the child's lifetime (birth through 3–6 years of age). In addition, physician panel members took into consideration clinical data and report of symptoms. A major limitation of questionnaires is the difficulty respondents may have in being able to distinguish a variety of adverse food reactions from an IgE-mediated response to ingested foods. The National Health Interview Survey asks about “food or digestive allergy,” but without report of a physician diagnosis, the problem of misclassification remains. In light of these problems, we used a physician panel to obtain an estimate of IgE-FA for research purposes. The question of an increased risk of food allergy for persons of African ancestry has been difficult to assess because of problems with the outcome selected and the way the outcome is defined. It is clear that food allergy and sensitization are not the same. Because sensitization is a very important and quantifiable component of food allergy, investigators often use this outcome as an indicator of the risk of food allergy. Most studies finding racial differences in sensitization have inferred that there may be racial differences in food allergy as well. Using multivariate logistic regression, we did not observe a statistically significant elevated risk of IgE-FA for African Americans, although the previously established elevated risk of sensitization was observed.
There is no consensus in the literature regarding racial differences in food allergy. Although early reports3,16 cite a higher rate of shellfish allergy among African American respondents compared with European Americans, the overall prevalence of any food allergy was similar by race. Previous studies5,17 used the National Health and Nutrition Examination Survey to explore patterns of food allergy and reported an increased risk for “non-Hispanic blacks,” males, and children. In this study, likely food allergy and probable food allergy were determined using previously published cut points for serum specific IgE. Using surveys from the National Center for Health Statistics, Branum et al4 reported that increases in food allergy from 1997 through 2007 were evident across 3 racial/ethnic groups (non-Hispanic white, non-Hispanic black, and Hispanic), but non-Hispanic white children had the highest reported prevalence of food allergy using the National Health Interview Survey. Sicherer et al15 examined medical records of children seen in an urban hospital–based pediatric clinic and reported that black children were significantly more likely than other racial/ethnic groups (consisting primarily of Hispanic and multiracial children) to be allergic to peanut and shellfish and to be allergic to more than one food. Authors noted that confirmation of food allergy was evident for less than half of the children. Gupta et al18 found that non-Hispanic black and Asian children were more likely to have parental report of food allergy than non-Hispanic white children but were less likely to have it confirmed. Hannaway et al,19 however, reported a lower prevalence of food allergy among nonwhite students compared with white students using prescriptions for injectable epinephrine on file in school records. Systematic reviews on the topic also differ, with some indicating there is a racial difference20 and others reporting no evidence.21 Problems cited in these reviews included use of different definitions for food allergy, including sensitization, self-report of a physician diagnosis, and the results of a food challenge.
Joseph et al22 previously published on racial differences in physiologic parameters related to asthma. They noted that the association between spirometric factors, such as response to methacholine, and IgE appeared to differ by race. In that analysis conducted among youth of a middleclass suburb, the authors described how total IgE increased with increasing methacholine reactivity for European American children, as might be expected, but they did not observe a similar trend for African American youth. They concluded that the association of IgE to asthma indicators differed by race. In the present analysis, we have come to a similar conclusion. IgE is influenced by factors related to ancestry, and therefore, its association with certain outcomes (asthma and allergy) must be described in that context.23 The known tendency for African Americans to have higher IgE levels must come into play when investigating racial disparities in biological parameters for asthma and allergy.23 When using cut points beyond the traditional 0.35 IU/mL or higher, vestiges of difference still persist but are difficult to interpret without larger sample sizes. For example, in Table 2, IgE levels for peanut are 77.9% and 240% higher using cut points of 0.35 IU/mL or higher and 95% PD points, respectively, for African Americans vs non–African Americans. This finding is similar to what was reported for the Boston cohort, in which African Americans were found to have a higher prevalence of sensitization to peanut. Kumar et al,24 however, focused only on sensitization, although for peanut, the investigators dichotomized serum IgE levels using greater than 5 kUA/L as a cutoff, which is associated with a higher likelihood of clinical reactivity.
Although food allergy is increasing, the condition remains difficult to study, and questions remain regarding racial differences in prevalence. More research is needed that can provide guidance in the appropriate interpretation of serum specific IgE and corresponding PD points, given African ancestry. Because only 3% to 6% of infants will develop food allergy in childhood, larger ethnically diverse cohorts are also needed to fully explore food allergy etiology and risk.
Acknowledgments
We acknowledge the families that have participated in WHEALS. We appreciate your trust. We also thank the research staff who labored diligently at data collection. We appreciate your hard work.
Funding Sources: The studies reported in this publication were supported by the grants R01AI50681 and 1R21A1080066-01A1 from the National Institutes of Health.
Footnotes
Disclosures: Authors have nothing to disclose.
Supplementary Data Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.anai.2015.12.019.
References
- [1].Clark S, Espinola J, Rudders SA, Banerji A, Camargo CA., Jr Frequency of US emergency department visits for food-related acute allergic reactions. J Allergy Clin Immunol. 2011;127:682–683. doi: 10.1016/j.jaci.2010.10.040. [DOI] [PubMed] [Google Scholar]
- [2].Sicherer SH, Scott H, Muñoz-Furlong M, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112:1203–1207. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- [3].Vierk KA, Koehler KM, Fein SB, Street DA. Prevalence of self-reported food allergy in American adults and use of food labels. J Allergy Clin Immunol. 2007;119:1504–1510. doi: 10.1016/j.jaci.2007.03.011. [DOI] [PubMed] [Google Scholar]
- [4].Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124:1549–1555. doi: 10.1542/peds.2009-1210. [DOI] [PubMed] [Google Scholar]
- [5].Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2010;126:798–806. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sampson HA. Adverse Reactions to Foods. In: Adkinson NF Jr, Yuninger JW, Busse WW, Bochner BS, Holgate ST, Simons FER, editors. Middleton's Allergy Principles & Practice. 6th ed Mosby; Philadelphia: 2007. pp. 1619–1644. [Google Scholar]
- [7].Asero R, Ballmer-Weber BK, Beyer K, et al. IgE-mediated food allergy diagnosis: Current status and new perspectives. Mol Nutr Food Res. 2007;51:135–147. doi: 10.1002/mnfr.200600132. [DOI] [PubMed] [Google Scholar]
- [8].Bock SA. Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Pediatrics. 1987;79:683–688. [PubMed] [Google Scholar]
- [9].Ezell JM, Ownby DR, Zoratti EM, et al. Using a physician panel to estimate food allergy prevalence in a longitudinal birth cohort. Ann Epidemiol. 2014;24:551–553. doi: 10.1016/j.annepidem.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Johnson C, Havstad S, Wegienka G, Zoratti E, Ownby D. The impact of Caesarian Section on the relationship between allergen exposure and allergen-specific IgE at age 2 years. J Allergy Clin Immunol. 2013;131(suppl 2):AB129. [Google Scholar]
- [11].Ben-Shoshan M, Harrington DW, Soller L, et al. A population-based study on peanut, tree nut, fish, shellfish, and sesame allergy prevalence in Canada. J Allergy Clin Immunol. 2010;125:1327–1335. doi: 10.1016/j.jaci.2010.03.015. [DOI] [PubMed] [Google Scholar]
- [12].Boyce JA, Assa'ad A, Burks AW, et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol. 2010;126:1105–1118. doi: 10.1016/j.jaci.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- [14].Rothman K, Greenland S. Modern Epidemiology. 2nd ed Lippincott-Raven Publishers; 1998. [Google Scholar]
- [15].Sicherer SH, Burks AW, Sampson HA. Clinical features of acute allergic reactions to peanut and tree nuts in children. Pediatrics. 1998;102(suppl 2):364–370. doi: 10.1542/peds.102.1.e6. [DOI] [PubMed] [Google Scholar]
- [16].Liu AH, Sicherer SH, Wood RA, et al. In the United States, black male children have an increased risk of food allergy: Results from NHANES 2005–2006. J Allergy Clin Immunol. 2009;123(suppl 2):S267. [Google Scholar]
- [17].Taylor-Black S, Wang J. The prevalence and characteristics of food allergy in urban minority children. Ann Allergy Asthma Immunol. 2012;109:431–437. doi: 10.1016/j.anai.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–e17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- [19].Hannaway PJ, Connelly ME, Cobbett RM, Dobrow PJ. Differences in race, ethnicity, and socioeconomic status in schoolchildren dispensed injectable epinephrine in 3 Massachusetts school districts. Ann Allergy Asthma Immunol. 2005;95:143–148. doi: 10.1016/S1081-1206(10)61203-3. [DOI] [PubMed] [Google Scholar]
- [20].Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- [21].Greenhawt M, Weiss C, Conte ML, Doucet M, Engler A, Camargo CA., Jr Racial and ethnic disparity in food allergy in the United States: a systematic review. J Allergy Clin Immunol Pract. 2013;1:378–386. doi: 10.1016/j.jaip.2013.04.009. [DOI] [PubMed] [Google Scholar]
- [22].Joseph CL, Ownby DR, Peterson EL, Johnson CC. Racial differences in physiologic parameters related to asthma among middle-class children. Chest. 2000;117:1336–1344. doi: 10.1378/chest.117.5.1336. [DOI] [PubMed] [Google Scholar]
- [23].Ownby D. Clinical significance of Immunoglobulin E. In Allergy Principals and Practice. In: Middleton EJ, Reed CE, Ellis EF, Adkinson N, Yunginger J, Busse W, editors. In Allergy Principal and Practice. 2 ed Mosby, Inc; St. Louis: 1998. [Google Scholar]
- [24].Kumar R, Tsai HJ, Hong X, et al. Race, ancestry, and development of food-allergen sensitization in early childhood. Pediatrics. 2011;128:e821–e829. doi: 10.1542/peds.2011-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]