Abstract
Context
Polycystic ovary syndrome (PCOS) is a chronic condition with metabolic manifestations spanning the reproductive years.
Objective
We sought to examine glucose metabolism, irrespective of the presence of obesity in a cohort of adolescent girls with PCOS.
Design
One hundred adolescents were assessed for PCOS in a multi-specialty adolescent PCOS program. PCOS was diagnosed by Androgen Excess Society criteria. Oral glucose tolerance testing (OGTT), homeostatic model assessment of insulin resistance, and androgen and lipid profiles were performed for those meeting criteria.
Results
Sixty-six adolescents (mean age 15.8 ± 0.2 yrs, range 13.0–18.6) had confirmed PCOS, and were eligible for inclusion in our analysis. Abnormal glucose metabolism was present in 12 of 66 (18.2%) subjects: 2 (3.0%) impaired fasting glucose, 10 (15.2%) impaired glucose tolerance (IGT), and 1 (1.5%) type 2 diabetes. IGT was the most common abnormality, occurring with equal frequency in obese (OB, mean body mass index (BMI) 36.9 ± 0.8 kg/m2) and non-obese (NOB, mean BMI 24.5 ± 0.6 kg/m2) adolescents (p = 0.3). In a subgroup analysis, NOB adolescents with IGT (NOB-IGT) had similar mean 2-h insulin, high density lipoprotein, C-reactive protein, and testosterone levels to the OB cohort despite marked differences in BMI (p < 0.001) and % body fat (p = 0.002). However, the NOB-IGT group had a lower mean fasting insulin level than the OB cohort (p = 0.04).
Conclusion
Abnormal glucose metabolism is highly prevalent in adolescents with PCOS. In particular, IGT occurs across the spectrum of BMI. A screening OGTT should be considered for adolescents diagnosed with PCOS, independently of their BMI.
Keywords: adolescence, OGTT, PCOS
Polycystic ovary syndrome (PCOS) is a chronic condition clinically characterized by irregular menses and hyperandrogenism. It affects approximately 7% of reproductive age women (1, 2). While PCOS is highly prevalent, diagnosis and treatment are often delayed until infertility prompts evaluation in adulthood. A history of PCOS is associated with an increased risk for future type 2 diabetes (3, 4). Although most complications of PCOS occur in adulthood, the clinical manifestations of PCOS may begin at the time of gonadarche, when the ovaries begin to synthesize sex steroids and support follicular development. Remarkably, PCOS remains under-evaluated during adolescence because the overlapping symptoms of physiologic puberty are deemed to obscure the clinical picture.
Few studies have sought to examine the diagnosis of PCOS and presence of metabolic risk in adolescents with symptoms of irregular menses. In particular, impaired glucose tolerance (IGT), the most prevalent (31–35%) glucose abnormality in women with PCOS (4, 5), has not been examined systematically in adolescents. Most studies involving adolescents have been either limited in size or lacked comprehensive metabolic evaluation. One study reported that 9 of 27 (33%) PCOS adolescents had IGT (6). However, this analysis predominantly included very obese (OB) subjects [mean body mass index (BMI), 38.4 kg/m2 ± 8.8]. In another report characterizing 70 adolescents with PCOS, glucose tolerance testing was only performed on those subjects with additional diabetes risk factors, such as obesity or a family history of type 2 diabetes mellitus (T2DM). Of the 33 adolescents tested, IGT was present in two (6%) subjects (7). However, in adult women with PCOS, IGT occurs independently of excess body weight (8). In addition, metabolic risk and future T2DM is insufficiently captured by fasting markers of impaired glucose metabolism in adults with PCOS (4–6, 9).
We herein report the largest number of adolescents with PCOS examined for impaired glucose metabolism, irrespective of traditional risk factors for T2DM. Over a 2-yr period we evaluated and performed metabolic testing for 100 adolescents referred to our monthly PCOS program to document the clinical presentation and early metabolic abnormalities in girls with irregular menstrual patterns, hirsutism, and abnormal ovarian morphology.
Subjects and methods
Subjects
We studied 100 girls, aged 11.7–19.2 yrs, who were consecutively referred to a single multi-specialty adolescent program for evaluation of PCOS from 2008 to 2010. The main indication for referral was irregular menses beyond the first 1.5 postmenarchal years or primary amenorrhea as defined by no menstruation 3 yrs post-thelarche (10). A few subjects were referred for clinical hyperandrogenism despite regular appearing menses. As a result, our cohort included many lean and non-obese (NOB) adolescents. All referred adolescents were 11 yrs or older, but were only considered for inclusion in this study if they were over 13 yrs of age and had Tanner stage 5 pubertal development.
PCOS diagnostic criteria
We applied the 2006 criteria for PCOS diagnosis from the Androgen Excess Society (AES), as guidelines for diagnosis specific to adolescents are not yet established. Diagnosis of PCOS in adolescents is more difficult than in adults due to changes occurring in physiological puberty (11). However, hirsuitism and hyperandrogenemia are uncommon in the general adolescent population (12). The AES criteria require the presence of hyperandrogenemia or hyperandrogenism in addition to irregular menses or ultrasound findings of morphologic changes consistent with polycystic ovaries (PCO) (8). Biochemical screening of a morning 17-hydroxyprogesterone, total testosterone, dehydroepiandrosterone/sulfate (DHEA/S), prolactin, and thyrotropin stimulating hormone (TSH) were used to exclude conditions that may mimic symptoms of PCOS, such as non-classical adrenal 21-hydyroxylase deficiency, androgen-secreting tumors, hyperprolactinemia, and thyroid dysfunction. If a morning 17-hydroxyprogesterone value was ≥200 ng/dL, then an ACTH stimulation test was performed. No adolescents within this cohort were receiving valproic acid, an anti-seizure medication known to affect androgen metabolism (13).
In this study, we defined biochemical hyperandrogenemia as a serum total testosterone above the normal reference range for tanner stage 5 on a commercially available high performance liquid chromatrography/mass spectrometry (HPLC/MS) assay, reported as a total testosterone ≥39 ng/dL. PCO, as assessed by transabdominal ultrasound (TAUS), was defined as at least one ovary with a volume of greater than 10 cm3 (14) in the absence of a dominant follicle (9, 15). Ovarian volume was calculated using the simplified formula for a prolate ellipsoid (16). A single radiologist reported each TAUS, and was blinded to subject medical history. TAUS was used since transvaginal ultrasound is invasive for adolescents. The TAUS technique is not sufficiently sensitive for imaging the size or number of follicles, particularly in obese subjects. Furthermore, the appearance of multicystic ovaries, which is a physiologic occurrence in puberty, may be mistaken for PCO (11). Hence, the alternative criterion for PCO (12 or more follicles measuring 2–9 mm in diameter) was not applied to our cohort (17).
Metabolic exclusion criteria
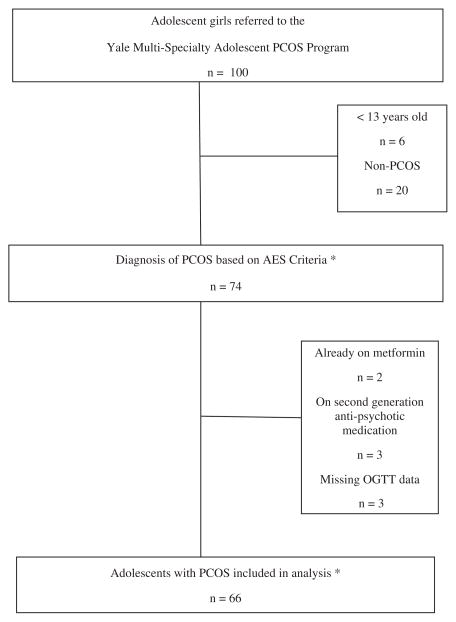
Since the primary outcome measure was impaired glucose metabolism, any patient receiving metformin or a second generation anti-psychotic medication at the time of first visit was excluded from the analysis, due to the effect of these medications on insulin sensitivity (18). Patients were also excluded from analysis if they declined an oral glucose tolerance test (OGTT), or if any OGTT data were missing (for inclusion and exclusion of analysis, see Fig. 1). Our final sample included 66 patients who met the PCOS criteria.
Fig. 1.
Flow chart of the prospective cohort study.
Protocol
On the first visit to the Multi-Specialty Adolescent PCOS Program, every patient received the same work-up, including a complete history and physical exam by the same Pediatric Endocrinologist and Gynecologist. The presence of acanthosis nigricans was recorded, and hirsutism was assessed using the modified Ferriman and Gallwey score (19). Ethnicity data were self-reported. The body weight of each subject was measured to the nearest 0.1 kg with a digital scale which was integrated in a bioimpedance analyzer (Tanita TBF-310, Arlington Heights, IL, USA). Percent body fat was also assessed by the bioimpedance analyzer. TAUS using a curved 5–2 mHz transducer was performed by a single radiologist, blinded to patient phenotype and medical history. A fasting metabolic and hormonal work up was done between 08:00 and 10:00 h, including a 2-h 75 g OGTT with measurement of serum glucose and insulin levels at time 0 and 2 h. Assays for total testosterone and androgen profiling, sex hormone binding globulin (SHBG), and lipid profile were performed. The clinical cohort study was approved by the Yale School of Medicine institutional review board. Each subject signed an age appropriate assent/consent form and a parent of each subject gave written informed consent.
Assays
Plasma glucose levels were determined by the glucose oxidation method, and insulin levels were determined by chemiluminescence radioimmunoassay (RIA) within the Yale Clinical Laboratories. All hormone labs were analyzed by the commercial lab Esoterix, Inc. (Austin, TX, USA). Total testosterone, androstenedione, and 17α-hydroxyprogesterone were measured by HPLC/MS, and serum free testosterone was determined by equilibrium dialysis. Sex hormone binding globulin, dehydroepiandrosterone (DHEA), and DHEAS were determined by RIA. Follicular stimulating hormone (FSH) and luteinizing hormone (LH) were determined by electrochemiluminescent assay.
Data analysis
The baseline clinical characteristics of adolescents meeting PCOS diagnosis were reported as prevalence or mean values within the cohort of 66 girls. Within the adolescent population, obesity was defined by a BMI in the 95th percentile or higher for age, as designated by the Centers for Disease Control (CDC). BMI percentiles and BMI Z-scores were calculated using the method and standard curves from the CDC (www.cdc.gov/growthcharts/). As a BMI Z-score of 2.5 or greater is another definition for obesity, it was compared to the analysis with BMI percentiles. Glucose abnormalities were assessed according to 2011 American Diabetes Association (ADA) criteria (20). Impaired fasting glucose (IFG) was defined as a fasting glucose of 100–125 mg/dL, and IGT was defined as a 2-h postload glucose of at least 140 mg/dL and <200 mg/dL. T2DM was defined as either a 2-h postload glucose more than 200 mg/dL, or a fasting glucose of over 125 mg/dL on more than two occasions (20). The homeostasis model assessment for insulin resistance (HOMA-IR) was calculated as fasting insulin concentration (μU/mL) multiplied by fasting glucose concentration (mg/dL) divided by 405, assuming that normal young subjects have an insulin resistance of 1, as previously described (21). Log transformation were done for variables that showed skewed distribution and could not assume normality, including insulin levels, HOMA-IR, C-reactive protein (CRP), and testosterone values. For log transformed variables, the geometric means with 95% confidence intervals (CI) were reported.
Adolescents with PCOS were divided into NOB (n = 23) and OB (n = 43) cohorts, with analysis of their glucose tolerance status and metabolic risk factors. Metabolic parameters for the NOB and OB cohorts were compared using the t-test, and comparison of IGT frequency between NOB and OB cohorts was done using Fisher’s exact test. Each cohort was further subdivided by whether adolescents were found to have IGT or normal glucose tolerance (NGT). One adolescent with type 2 diabetes was excluded from this subgroup analysis. Metabolic characteristics were reported for four subgroups: NOB-NGT (n = 18), NOB-IGT (n = 5), OB-NGT (n = 37), and OB-IGT (n = 5), and baseline comparisons of the metabolic characteristics among four subgroups were done using the Kruskal–Wallis one way analysis of variance. The Bonferroni method was used in a multi-comparison adjustment for selected pair-wise comparisons between subgroups. Box plots of the log transformed fasting and 2-h postchallenge insulin levels were plotted for each subgroup. Metabolic parameters for the NOB-IGT (n = 5) and OB (n = 42) (both NGT and IGT) groups were compared using the Wilcoxon rank sum test. All statistical analyses were performed using SAS 9.2 (Cary, NC, USA). We also examined whether a family history of T2DM within a first degree relative was associated with obesity and status of glucose tolerance using Fisher’s exact test.
Results
Clinical characteristics of adolescents with PCOS
The clinical and biochemical characteristics of the 66 patients meeting inclusion standards and PCOS diagnostic criteria by the AES are summarized in Table 1. Irregular menses occurred in 92% of patients, hence 61 of the 66 patients also met NIH criteria for diagnosis. Acne, hirsutism, and PCO were also found in the majority of patients. Two-thirds of the patients presented with acanthosis nigricans. Thirty-five percent of the cohort was NOB according to BMI percentiles (mean BMI 24.5 ± 0.6 kg/m2). The OB subjects had a mean BMI of 36.9 ± 0.8 kg/m2.
Table 1.
Baseline clinical characteristics of PCOS adolescents meeting inclusion standards
| Number of subjects | 66 |
| Age, yrs; mean ± SEM (range) | 15.8 ± 0.2 (13.0–18.6) |
| Gynecological age, yrs, mean ± SEM (range)* | 3.9 ± 0.2 (1.5–9.0) |
| BMI, kg/m2; mean ± SEM (range) | 32.6 ± 0.9 (19.2–47.0) |
| BMI percentile >95th (%) | 43 (65) |
| Ethnicity, no. (%): | |
| Caucasian, NH | 37 (56) |
| Hispanic | 16 (24) |
| African-American | 5 (8) |
| Asian | 1 (1) |
| Mixed race | 4 (6) |
| Not listed | 3 (5) |
| First degree relative with T2DM, no. (%)† | |
| Yes | 16 (24) |
| No | 41 (62) |
| Unknown (adopted) | 4 (6) |
| Presentation, no. (%) | |
| Irregular menses | 61 (92) |
| Hirsuitism | 53 (84) |
| Acne | 48 (81) |
| Acanthosis nigricans† | 39 (67) |
| PCO morphology on US | 44 (68) |
| Laboratory results (mean ± SEM)‡ | |
| Total testosterone, ng/dL | 53 ± 3 |
| Androstendione, ng/dL | 194 ± 10 |
| DHEAS, μg/dL | 149 ± 10 |
| LH/FSH ratio | 2.0 ± 0.2 |
| 17-Hydroxyprogesterone, ng/dL | 75 ± 6 |
BMI, body mass index; DHEAS, dehydroepiandrosterone; FSH, follicular stimulating hormone; LH, luteinizing hormone; PCOS, polycystic ovary syndrome; T2DM, type 2 diabetes mellitus.
Gynecological age calculated for n = 60, 6 subjects had primary amenorrhea.
Numbers may not add up to total because of missing data in 5 subjects.
Normal range of assay for total testosterone 20–38 ng/dL, androstenedione 50–224 ng/dL, DHEAS 44–248 μg/dL, and 17-hydroxyprogesterone 20–265 ng/dL.
Prevalence of glucose intolerance
Overall, our PCOS cohort had an 18.2% prevalence of abnormal glucose metabolism. IGT was the most common glucose abnormality identified in our PCOS subjects, for which the prevalence was 15.2%. Two patients (3%) were found to have IFG; one of whom had NGT while the other had IGT. One adolescent had T2DM (1.5%) by the 2-h glucose criterion, although her fasting glucose was in the normal range.
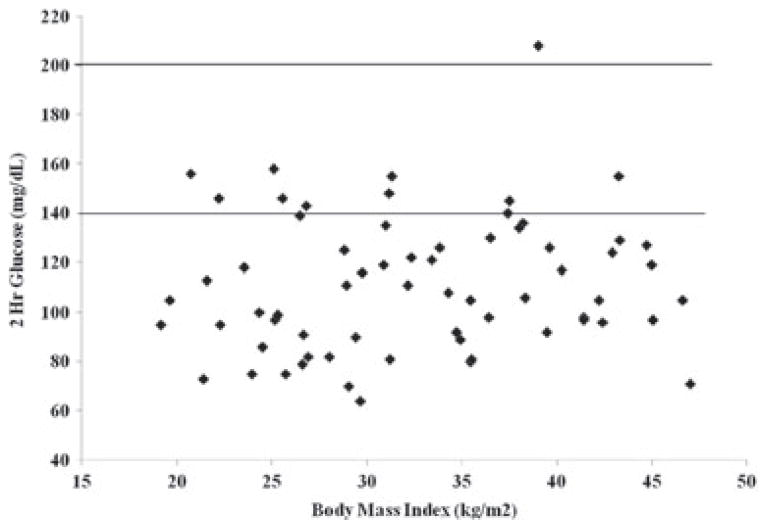
Glucose intolerance across the BMI spectrum
IGT occurred across the spectrum of BMI, as represented in Fig. 2. This distribution remained consistent when displayed with BMI percentiles or BMI Z-scores because there were many lean and normal weight subjects. There were an equal number of NOB and OB girls found to have IGT. In a sub-group analysis based on BMI percentiles, the prevalence of IGT in our NOB cohort was 21.7 vs. 11.6% in the OB cohort (p = 0.3).
Fig. 2.
Impaired glucose tolerance, as defined by a 2-h stimulated serum glucose >140 mg/dL, occurred across the spectrum of body mass index in adolescents with polycystic ovary syndrome. One adolescent met criteria for type 2 diabetes, based on a 2-h serum glucose >200 mg/dL.
Comparison between NOB and OB subjects
Adolescents with PCOS were divided into OB (n = 43) and NOB (n = 23) cohorts, with analysis of their glucose tolerance status and metabolic risk factors. The OB cohort had a mean BMI of 36.9 ± 0.8 kg/m2 and NOB cohort had a mean BMI of 24.5 ± 0.6 kg/m2. OB adolescents were younger (mean age of 15.6 ± 0.2 yrs) than the NOB cohort (age of 16.3 ± 0.3 yrs, p = 0.04). The mean fasting glucose levels of the OB and NOB cohorts were similar (87 ± 1 vs. 84 ± 2 mg/dL, p = 0.1), but the mean fasting insulin levels were significantly higher in the OB cohort at 16 μU/mL vs. 8.0 μU/mL in the NOB cohort (95% CI 12, 19; 6, 10; p < 0.001). Although there was overlap in HOMA-IR scores between the two cohorts, the mean HOMA-IR was over twofold higher in the OB than NOB cohort (3.3 vs. 1.6, 95% CI 2.6, 4.1; 1.3, 2.1; p < 0.001). For the postglucose challenge, the mean 2-h glucose levels were similar between cohorts (114 ± 4 vs. 106 ± 6 mg/dL, p = 0.3), but the mean 2-h insulin level of the OB cohort was significantly higher that of the NOB cohort (105 vs. 61 μU/mL, 95% CI 81, 136; 45, 82; p = 0.01). The OB cohort had lower mean high density lipoprotein (HDL) and higher CRP levels relative to the NOB cohort (HDL: 44 ± 2 vs. 54 ± 2 mg/dL, p = 0.002; CRP: 2.7 vs. 0.5 mg/dL, 95% CI 1.9, 3.8; 0.3, 1.1; p < 0.0001). The OB adolescents had similar testosterone levels relative to the adolescents in the NOB cohort (49 vs. 46 ng/dL, 95% CI 1.42, 56; 38, 55; p = 0.7).
Information regarding a family history of T2DM within a first degree relative was available for 57 of 66 patients. Among 16 patients with a positive family history, 75% (12/16) were obese. Among 41 patients without a family history of T2DM, 61% (25/41) were obese. In our cohort, obesity was not associated with T2DM in a first degree relative (p = 0.4).
Comparison between NGT and IGT subjects
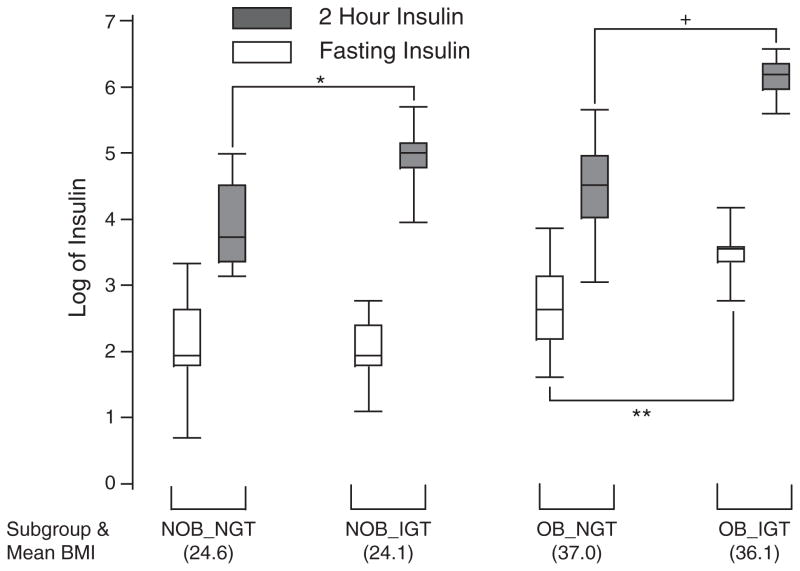
To compare how adolescents with IGT differ from adolescents with NGT within each NOB and OB cohort, we separated the NOB-IGT and OB-IGT subjects from their respective cohorts. Baseline characteristics and metabolic indices for each group are shown in Table 2. Compared with their NGT counterparts, the NOB-IGT and OB-IGT adolescents had similar mean BMI and percent body fat. Despite having similar fasting insulin levels (7 vs. 8 μU/mL, 95% CI 4, 13; 6, 11; p = 1), the NOB-IGT group had mean 2-h insulin levels nearly threefold higher than those of the NOB-NGT group (140 vs. 48 μU/mL, 95% CI 78, 240; 37, 64; p = 0.007). The Box plots of log transformed fasting and 2-h insulin levels for each group are shown in Fig. 3. The NOB-IGT group trended toward lower mean HDL and higher CRP levels than the NOB-NGT group, although this finding was not statistically significant (Table 2). The testosterone levels in the NOB-NGT, NOB-IGT, and OB-NGT groups were very similar (Table 2). The OB-IGT group had the highest mean testosterone level (74, 95% CI, 44, 123), which was not significantly different from the other three groups.
Table 2.
Clinical and biochemical characteristics of subjects by obesity and glucose tolerance status. Data is expressed as either number, mean ± SEM or geometric mean (95% CI) for log transformed data
| NOB NGT |
NOB IGT |
OB NGT |
OB IGT |
|
|---|---|---|---|---|
| Subjects, no. | 18 | 5 | 37 | 5* |
| Age, yr (mean ± SEM) | 16.4 ± 0.3 | 15.9 ± 0.5 | 15.7 ± 0.2 | 14.7 ± 0.7 |
| BMI, kg/m2 (mean ± SEM) | 24.6 ± 0.7 | 24.1 ±1.1 | 37.0 ± 0.9 | 36.1 ± 2.3+ |
| Body fat, % (mean ± SEM) | 32.6 ± 1.7 | 31.6 ± 3.2 | 45.7 ± 0.6 | 45.0 ± 1.5+ |
| Acanthosis Nigracans, no. (%)† | 4 (29) | 3 (75) | 27 (77) | 4 (100) |
| First degree relative with T2DM, no. (%) | 2 (13) | 2 (50) | 9 (29) | 3 (60) |
| Fasting glucose, mg/dL (mean ± SEM) | 83 ± 2 | 87 ± 6 | 87 ± 1 | 84 ± 3 |
| Fasting insulin, μU/mL (geometric mean, 95% CI) | 8 (6, 11) | 7 (4, 13) | 14 (11, 18) | 33 (21, 51)**,+ |
| HOMA (geometric mean, 95% CI) | 1.7 (1.2, 2.2) | 1.6 (0.8, 3.1) | 3.0 (2.4, 3.8) | 6.8 (4.4, 10.4)+ |
| 2-h insulin, μU/mL (geometric mean, 95% CI) | 48 (37, 64) | 137 (78, 240)* | 86 (69, 109) | 463 (334, 641)**,+ |
| HDL, mg/dL (mean ± SEM) | 56 ± 3 | 46 ± 6 | 45 ± 2 | 38 ± 5** |
| CRP, mg/dL (mean ± SEM) | 0.89 ± 0.4 | 2.67 ± 1.87 | 3.54 ± 0.64 | 6.03 ± 1.42 |
| Testosterone, ng/dL (geometric mean, 95% CI) | 46 (37, 59) | 42 (33, 55) | 45 (40, 52) | 74 (44, 123) |
BMI, body mass index; CRP, C-reactive protein; CI, confidence intervals; HDL, high density lipoprotein; HOMA, homeostatic model assessment; IGT, impaired glucose tolerance; NGT, normal glucose tolerance; NOB, non-obese; OB, obese; T2DM, Type 2 Diabetes Mellitus.
Subject with T2DM was not included in sub-group analysis.
Data missing for 9 subjects (2 from NOB-NGT, 1 from NOB-IGT, 6 from OB-NGT).
p-value < 0.001 for comparison of NOB-IGT vs. NOB-NGT.
p-value < 0.05 for comparison of OB-IGT vs. OB-NGT.
p-value ± 0.01 for comparison of OB-IGT vs. NOB-IGT.
Fig. 3.
Box plot of log transformed fasting and 2-h stimulated insulin levels for each sub-group characterized in Table 2. IGT, impaired glucose tolerance; NGT, normal glucose tolerance; NOB, non-obese; OB, obese. *NOB-NGT vs. NOB-IGT, p = 0.007; **OB-NGT vs. OB-IGT, p < 0.04; +OB-NGT vs. OB-IGT, p < 0.01.
Among 16 patients with a positive family history of T2DM in a first degree relative, 69% (11/16) had normal glucose tolerance. Among 41 patients without a family history of T2DM, 88% (36/41) had normal glucose tolerance. In our cohort, glucose tolerance status was not associated with T2DM in a first degree relative (p = 0.1), although our cohort was not powered to address fully this potential association.
Comparison between NOB-IGT and OB subjects
In order to evaluate the relative severity of metabolic abnormalities in the NOB PCOS adolescents, we compared the NOB-IGT group to the overall OB cohort. The NOB-IGT group had similar 2-h insulin levels to the OB cohort (137 vs. 106 μU/mL, 95% CI 78, 240; 81, 138; p = 0.4), despite significant differences in BMI (p < 0.001) and percent body fat (p = 0.002). The NOB-IGT group had a lower mean fasting insulin level than the OB cohort (7 vs. 16 μU/mL, 95% CI 4, 13; 13, 20; p = 0.04). However, for HDL, CRP and testosterone, the NOB-IGT group was not statistically different than the OB cohort (HDL: 46 ± 6 vs. 44 ± 2 mg/dL, p = 0.7; CRP: 1.5 vs. 2.7 mg/dL, 95% CI 0.3, 6.7; 1.9, 3.8; p = 0.4; testosterone: 42 vs. 48 ng/dL, 95% CI 33, 55; 42, 56; p = 0.7).
Discussion
In our adolescent cohort of PCOS subjects, one of seven girls has IGT, suggesting that young age does not confer protection from the metabolic disturbances commonly seen in adults with PCOS. IGT is by far the most common glucose abnormality in our cohort, and presents equally in NOB and OB adolescents. Our discovery that IGT occurs across the spectrum of BMI is a novel finding in adolescence and consistent with prior studies in adult women with PCOS (4).
IGT is indicative of increased peripheral insulin resistance, and present in our NOB adolescents who have otherwise unremarkable fasting glucose and insulin levels. Their IGT is associated with significant 2-h hyperinsulinemia, representing compensatory pancreatic secretion in response to increased peripheral insulin resistance. Importantly, the degree of 2-h hyperinsulinemia in NOB IGT individuals is similar to the overall OB cohort, despite the significantly higher mean BMI (37 vs. 24 kg/m2) and higher fasting insulin (16 vs. 7 μU/mL of the OB cohort. Our data suggest that exaggerated postprandial hyperinsulinemia (as represented by an elevated 2-h insulin level during the OGTT) may be one of the earliest biochemical manifestations of PCOS-mediated metabolic dysfunction, occurring regardless of weight. This finding is supported by Sir-Petermann et al. in their comparison of prepubertal and pubertal daughters of women with PCOS relative to normal controls. They showed that 2-h insulin levels were higher during all Tanner stages in the daughters of women with PCOS compared with the daughters of normal controls (22). By Tanner stage 4 and 5, three of the 35 PCOS daughters had IGT vs. none in the control daughter cohort (n = 30). In contrast, mean fasting levels of insulin were not statistically different between the groups in all but one of the development stages.
The mechanism of insulin resistance in the NOB PCOS phenotype is understudied, but one hypothesis is that insulin resistance results from hyperandrogenemia (23, 24). Circulating androgen levels were found to have a strong correlation with insulin secretion during an OGTT in a study of twenty NOB women with PCOS and paired control women (25). In a proof of concept study, regularly menstruating, NOB women were treated with methyltestosterone. They developed an increase in peripheral insulin resistance as demonstrated by hyperglycemic and euglycemic hyperinsulinemic clamps performed before and after treatment (26). However, treatment with flutamide, an androgen receptor blocker, for 18 months did not alter mean glucose or insulin levels during an OGTT, in NOB adolescents with PCOS, despite a marked decrease in androgen levels (27). Our data was not sufficiently powered to examine the relationship between testosterone levels and hyperinsulinemia. Hence, further studies are needed to delineate the role of androgens in promoting insulin resistance.
Irregular menses may be the first physiologic sign that the ovary is maladaptive in its response to physiologic or pathophysiologic rises in insulin levels. The metabolic implications of oligo/amenorrhea in adolescence are underestimated by clinicians when irregular menses are thought merely to represent immaturity of the hypothalamic – pituitary – gonadal axis. This may be especially true of NOB adolescents, because their weight does not indicate a concern for metabolic abnormalities. Current guidelines of the ADA recommend a screening OGTT in adolescents only if they are OB and have two additional traditional diabetes risk factors (20). While the ADA includes PCOS as one of the traditional risk factors for diabetes, the recommended OGTT is conditioned upon the presence of obesity. One of the 66 adolescents had T2DM on OGTT despite a normal fasting blood sugar. Since she was also very OB (BMI 39 kg/m2) the current guidelines for an OGTT would have picked up her diagnosis of diabetes. However, we found that many NOB PCOS adolescents demonstrated impaired stimulated glucose and insulin levels despite normal range fasting values. Even though these patients did not have T2DM, they may benefit from closer monitoring or metformin therapy. Our findings are consistent with multiple studies of women and adolescents with PCOS, in whom fasting markers of impaired glucose metabolism were insufficient to demonstrate metabolic risk (4–6, 8).
In our cohort, obesity contributed to higher fasting insulin levels and HOMA-IR, which is consistent with prior literature. In general, we also found that obesity was associated with lower HDL and higher CRP levels in our adolescent population. However, the NOB PCOS adolescents with IGT had HDL and CRP levels similar to the OB adolescents.
To our knowledge, this is the largest cohort of adolescent girls with PCOS examined for abnormalities in glucose metabolism using an OGTT. A defining characteristic of our cohort is the significant number of NOB patients attending our program since menstrual irregularity was the main reason for referral, not obesity. We carefully included only girls over the age of 13 yrs with Tanner stage 5 development in order to identify the early metabolic abnormalities associated with the PCOS phenotype, separate from changes during puberty. We did not include the presence of acne to fulfill a clinical definition of hyperandrogenism since acne may be representative of peri-puberty. Our results regarding impaired glucose metabolism may not be generalizable to adolescents for whom different definitions of PCOS are applied.
Our PCOS program represents a single referral center within the New England area of the United States, and our population is predominantly Caucasian. We had insufficient diversity to determine any differences in PCOS presentation or impaired glucose metabolism attributable to ethnicity or race. Since we serve a primary clinical role in the care of adolescents with PCOS, only a single OGTT, with its understood coefficient of variability, was performed for each subject. Of note, a prior study on OGTT reproducibility in children found that even if IGT status was discordant on serial OGTTs, a single finding of IGT was associated with metabolic characteristics that imply higher risk for T2DM (28). Finally, our program did not recruit control adolescents without PCOS for metabolic analysis, so we rely on literature references for this comparison.
In summary, we found a high prevalence of abnormal glucose metabolism in adolescent girls diagnosed with PCOS, and IGT occurred with equal frequency in NOB and OB adolescents. An evaluation of glucose metabolism is warranted even in NOB adolescents, when irregular menses occur in the presence of hyperandrogenism/emia and/or PCO morphology on ultrasound. Since fasting glucose levels and HOMA did not indicate risk for IGT in our population, we support an OGTT for adolescents diagnosed with PCOS, regardless of their BMI. Even though menstrual irregularity is often deemed physiologic in adolescent girls, it may be the first indicator of underlying insulin resistance in the setting of PCOS.
Acknowledgments
We thank the clinical and administrative staff of the Yale Multi-disciplinary PCOS Program (Yale MAPP), and we are especially grateful to all of the adolescents who participated in this study. We thank Dr. Lynwood Hammers (Hammers Healthcare Imaging, New Haven, CT) for expert analysis of all ultrasound imaging. We also thank Dr. Kasia Lipska, who contributed to the critique of this manuscript. Grant support provided by National Institutes of Health (K23 DK 074439).
References
- 1.Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 3.Solomon CG, Hu FB, Dunaif A, et al. Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. JAMA. 2001;286:2421–2426. doi: 10.1001/jama.286.19.2421. [DOI] [PubMed] [Google Scholar]
- 4.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 5.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141–146. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 6.Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:1017–1023. doi: 10.1210/jcem.87.3.8305. [DOI] [PubMed] [Google Scholar]
- 7.Bekx MT, Connor EC, Allen DB. Characteristics of adolescents presenting to a multidisciplinary clinic for polycystic ovarian syndrome. J Pediatr Adolesc Gynecol. 2010;23:7–10. doi: 10.1016/j.jpag.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Azziz R, Carmina E, Dewailly D, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 9.Fulghesu AM, Angioni S, Portoghese E, et al. Failure of the homeostatic model assessment calculation score for detecting metabolic deterioration in young patients with polycystic ovary syndrome. Fertil Steril. 2006;86:398–404. doi: 10.1016/j.fertnstert.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 10.ACOG Committee Opinion No. 349. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. ACOG Committee on Adolescent Health Care. Obstet Gynecol. 2006;108:1323–8. doi: 10.1097/00006250-200611000-00059. [DOI] [PubMed] [Google Scholar]
- 11.Carmina E, Oberfield SE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol. 2010;203:201.e1–201.e5. doi: 10.1016/j.ajog.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Hickey M, Doherty DA, Atkinson H, et al. Clinical, ultrasound and biochemical features of polycystic ovary syndrome in adolescents: implications for diagnosis. Hum Reprod. 2011;26:1469–1477. doi: 10.1093/humrep/der102. [DOI] [PubMed] [Google Scholar]
- 13.Joffe H, Cohen LS, Suppes T, et al. Longitudinal follow-up of reproductive and metabolic features of valproate-associated polycystic ovarian syndrome features: a preliminary report. Biol Psychiatry. 2006;60:1378–1381. doi: 10.1016/j.biopsych.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Mortensen M, Rosenfield RL, Littlejohn E. Functional significance of polycystic-size ovaries in healthy adolescents. J Clin Endocrinol Metab. 2006;91:3786–3790. doi: 10.1210/jc.2006-0835. [DOI] [PubMed] [Google Scholar]
- 15.ACOG Practice Bulletin. Polycystic ovary syndrome. Obstet Gynecol. 2009;114:936–949. doi: 10.1097/AOG.0b013e3181bd12cb. [DOI] [PubMed] [Google Scholar]
- 16.Swanson M, Sauerbrei EE, Cooperberg PL. Medical implications of ultrasonically detected polycystic ovaries. J Clin Ultrasound. 1981;9:219–222. doi: 10.1002/jcu.1870090504. [DOI] [PubMed] [Google Scholar]
- 17.Jonard S, Robert Y, Cortet-Rudelli C, Pigny P, Decanter C, Dewailly D. Ultrasound examination of polycystic ovaries: is it worth counting the follicles? Hum Reprod. 2003;18:598–603. doi: 10.1093/humrep/deg115. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. . Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- 19.Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815–830. doi: 10.1016/0002-9378(81)90746-8. [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2011;34:S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Sir-Petermann T, Codner E, Perez V, et al. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:1923–1930. doi: 10.1210/jc.2008-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasquali R, Stener-Victorin E, Yildiz BO, et al. PCOS Forum: research in polycystic ovary syndrome today and tomorrow. Clin Endocrinol. 2011;74:424–433. doi: 10.1111/j.1365-2265.2010.03956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruni V, Metella D, Nannini S, Balzi D, Nuvolone D. Polycystic ovary syndrome in adolescence. Ann NY Acad Sci. 2010;1205:175–184. doi: 10.1111/j.1749-6632.2010.05648.x. [DOI] [PubMed] [Google Scholar]
- 25.Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab. 1983;57:356–359. doi: 10.1210/jcem-57-2-356. [DOI] [PubMed] [Google Scholar]
- 26.Diamond MP, Grainger D, Diamond MC, Sherwin RS, Defronzo RA. Effects of methyltestosterone on insulin secretion and sensitivity in women. J Clin Endocrinol Metab. 1998;83:4420–4425. doi: 10.1210/jcem.83.12.5333. [DOI] [PubMed] [Google Scholar]
- 27.Ibáñez L, Potau N, Marcos MV, de Zegher F. Treatment of hirsutism, hyperandrogenism, oligomenorrhea, dyslipidemia, and hyperinsulinism in nonobese, adolescent girls: effect of flutamide. J Clin Endocrinol Metab. 2000;85:3251–3255. doi: 10.1210/jcem.85.9.6814. [DOI] [PubMed] [Google Scholar]
- 28.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab. 2008;93:4231–4237. doi: 10.1210/jc.2008-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]