Abstract
Riluzole is the only FDA approved drug for the treatment of amyotrophic lateral sclerosis (ALS). However, the drug affords moderate protection to ALS patients, extending life for a few months by a mechanism that remains controversial. In the presence of riluzole, astrocytes increase the production of factors protective to motor neurons. The stimulation of trophic factor production by motor neuron associated cells may contribute to riluzole’s protective effect in ALS. Here, we investigated the effects of media conditioned by astrocytes and Schwann cells acutely or chronically incubated with riluzole on trophic factor-deprived motor neuron survival. While acute riluzole incubation induced CT-1 secretion by astrocytes and Schwann cells, chronic treatment stimulated a significant decrease in trophic factor production compared to untreated cultures. Accordingly, conditioned media from astrocytes and Schwann cells acutely treated with riluzole protected motor neurons from trophic factor deprivation-induced cell death. Motor neuron protection was prevented by incubation with CT-1 neutralizing antibodies. In contrast, conditioned media from astrocytes and Schwann cells chronically treated with riluzole was not protective. Acute and chronic treatment of mice with riluzole showed opposite effects on trophic factor production in spinal cord, sciatic nerve and brain. There was an increase in the production of CT-1 and GDNF in the spinal cord and CT-1 in the sciatic nerve during the first days of treatment with riluzole, but the levels dropped significantly after chronic treatment with the drug. Similar results were observed in brain for CT-1 and BDNF while there was no change in GDNF levels after riluzole treatment. Our results reveal that riluzole regulates long-lasting processes involving protein synthesis, which may be relevant for riluzole therapeutic effects. Changing the regimen of riluzole administration to favor the acute effect of the drug on trophic factor production by discontinuous long-term treatment may improve the outcome of ALS patient therapy.
Keywords: Riluzole, ALS, astrocytes, Schwann cells, motor neurons, cardiotrophin-1, BDNF, GDNF, amyotrophic lateral sclerosis
Introduction
Amyotrophic lateral sclerosis (ALS) is a devastating and incurable neurological disease, characterized by the degeneration of pyramidal neurons in the motor cortex and motor neurons in the brain stem and spinal cord. ALS patients live without medical intervention an average of one to five years following diagnosis. Riluzole is the only drug that shows a small but consistent protective effect both in patients and transgenic mice models of ALS (Bensimon et al., 1994; Gurney et al., 1996; Gurney et al., 1998; Lacomblez et al., 1996a; Lacomblez et al., 1996b; Miller et al., 2012; Orrell, 2010). Riluzole has been shown to block sodium channels, activate G-proteins and reduce glutamate toxicity (Bellingham, 2011; Cheah et al., 2010; Doble, 1997; Meininger et al., 2000). However, the primary mechanism by which riluzole exerts its protective effects in ALS remains unknown.
Astrocytes and other glial cells produce trophic factors that support motor neuron survival (Ang et al., 1993; Arce et al., 1998; Eagleson and Bennett, 1986; Eagleson et al., 1985; Schnaar and Schaffner, 1981). Riluzole enhances the astrocytic production of these factors in cell culture (Peluffo et al., 1997). The incubation of cortical astrocytes with riluzole stimulates the production of brain derived neurotrophic factor (BDNF), glia derived neurotrophic factor (GDNF) and nerve growth factor (NGF) (Mizuta et al., 2001). Schwann cells also produce trophic factors that promote axon regeneration following exercise and nerve injury (Wilhelm et al., 2012; Xu et al., 2013). Cardiotrophin-1 (CT-1) is a potent motor neuron trophic factor that prevents motor neuron death after axotomy and delays death in animal models of ALS produced by muscle (Bordet et al., 2001; Pennica et al., 1996).
Initial studies on the effect of riluzole lead us and others to postulate that riluzole may exert at least in part its protective effects on motor neurons indirectly, by promoting trophic factor production by astrocytes and other glial cells (Mizuta et al., 2001; Peluffo et al., 1997). Here we investigated the effects of short and long time incubation with riluzole on trophic factor production by Schwann cells and astrocytes. Both cell types play an important role in cellular maintenance of motor neurons. We investigated also concentrations of trophic factors produced in brain, spinal cord, and sciatic nerve of C57BL/6J mice at different time points after continuous or discontinuous riluzole administration and correlated the in vivo results to specific glial cell responses. Our findings provide new insights on the potential mechanism of action of riluzole as a therapeutic treatment for ALS and possibly spinal cord injury.
Materials and Methods
Cell culture
Astrocytes
Spinal cords from one to three days old Sprague Dawley rats were used to prepare astrocytes as described previously (Peluffo et al., 1997; Saneto and Vellis, 1987). Once confluent (approximately 5-8 days), cells were shaken at 300 RPM for 2 days then treated with 10 μM arabinose C. Following a couple days recovery, cells were split and seeded on a 60mm dish at a density of one million cells or half a million for a 35mm dish. Cells were maintained in culture for no more than 22 days.
Motor neurons
Rat embryo motor neurons were purified using 6% OPTI prep and further purified using immunoaffinity. Cells were cultured in neurobasal medium containing glutamine, glutamate, β-mercaptoethanol, and B27 supplement (Gibco-Invitrogen) as previously described (Estevez et al., 1998; Pettmann and Henderson, 1998; Raoul et al., 1999) in the presence or absence of brain-derived neurotrophic factor (BDNF, 1 ng/mL), glial-derived neurotrophic factor (GDNF, 0.1 ng/mL), and cardiotrophin 1 (CT-1, 10 ng/mL). Motor neuron survival was determined by counting by hand in four well plates (Nunc) or by calcein staining (Molecular Probes, Invitrogen) according to manufacturer’s instruction. Extracellular calcein was quenched with 100 μg/mL hemoglobin and the images were captured using the RUNNER (Trophos, Marseilles, France). Data was analyzed using Tina software (Trophos).
Schwann Cells
Primary Schwann cells were isolated from the sciatic nerves of newborn Sprague Dawley rats with modifications as previously described (Brockes et al., 1979; Thaxton et al., 2011). 250,000 cells (35 mm dish) or 500,000 cells (60 mm dish) were grown in D10M (10% FBS, 20 μg/ml pituitary extracts and 2μM forskolin in DMEM) on poly-L-lysine coated plates (200 μg/mL) until confluency. Media was changed three days before the experiment was started. Experiments were conducted on Schwann cells that were passaged no more than four times.
Conditioned Media
Astrocytes or Schwann cells were treated with 1 μM riluzole for indicated time period. For chronic treatments, media was changed with fresh riluzole every 2-3 days. Media was removed and cells were washed with DPBS prior to addition of conditioning media (L15 supplemented with sodium bicarbonate (22 mM), conalbumin (0.1 mg/ml), putrescine (0.1 mM), insulin (5 μg/ml), and sodium selenite (31 nM). Astrocytes or Schwann cells conditioned this media for 24 hours prior to collection. Conditioned media was further diluted in motor neuron media prior to plating motor neurons. Motor neurons were cultured in the presence of conditioned media for three days then counted.
PCR Analysis
RNA was extracted from Schwann cells plated on a 35mm dish using Trizol. 1 μg RNA was used to synthesize cDNA using Superscript III (Invitrogen) according to manufacturer’s instruction. 2 μl of cDNA product was used as template for qPCR analysis. CT-1, BDNF, and GDNF levels were measured using Taq Man probes and master mix according to manufacturer’s instruction.
Animal Studies
Male C57BL/6J mice were given riluzole treated water (100 μg/ml) for given time points. Water was replaced with freshly prepared riluzole every 2-3 days. Brain, spinal cord, and sciatic nerve were removed for subsequent analysis. Untreated mice were sacrificed for control.
ELISA
Brain, spinal cord, and sciatic nerve were homogenized in PBS containing PMSF, and protease inhibitor cocktail. Samples were diluted to a concentration of 175 μg/mL and analyzed using ELISA [CT-1 (R & D Systems), BDNF and GDNF (Abnova)]. ELISA was preformed according to manufacturer’s instructions.
Results
Riluzole treatment stimulates glial cells to produce CT-1
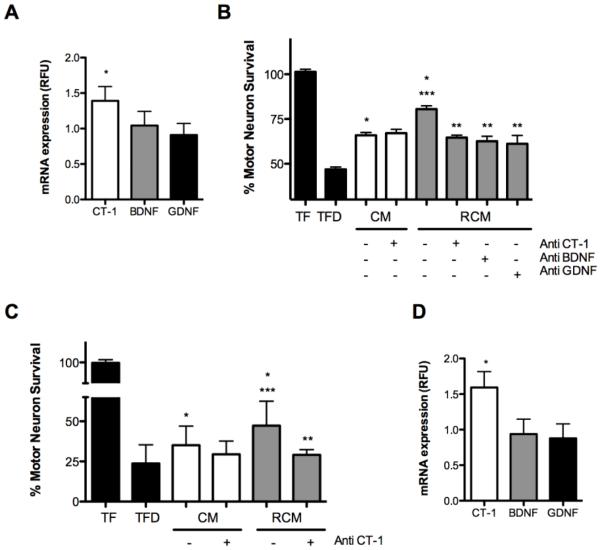
Riluzole stimulates trophic factor production by astrocytes (Mizuta et al., 2001; Peluffo et al., 1997). Incubation of motor neurons with media that was previously conditioned by astrocytes in the presence of 1-10 μM riluzole significantly increases motor neuron survival when compared to cells cultured in conditioned media from untreated astrocytes (Peluffo et al., 1997). Since the trophic factors BDNF, GDNF and CT-1 are critical to motor neuron survival, we investigated the effect of riluzole on production of these trophic factors by astrocytes using conditions previously described (Peluffo et al., 1997). There was a small, but statistically significant change in CT-1 mRNA levels with no change in mRNA levels of BDNF, and GDNF of riluzole treated astrocytes when compared to untreated controls (Fig. 1A). Since mRNA levels do not reflect protein levels, neutralizing antibodies to BDNF, GDNF and CT-1 were used to determine the effect of each trophic factor present in conditioned media on motor neuron survival. Motor neurons were cultured in the absence of trophic factors and survival was assessed after 24 h in culture. Incubation of motor neurons with media that was previously conditioned by astrocytes for 24 h partially protected motor neurons from trophic factor deprivation-induced cell death (Fig. 1B). This protection was significantly increased by conditioned media from astrocytes that were incubated in the presence of riluzole for 24 h prior to media conditioning (Fig. 1B). Antibodies against CT-1 had no effect on motor neuron survival in the presence of conditioned media from untreated astrocytes, but blocked the additional protection afforded by the media from riluzole-treated astrocytes (Fig. 1A), suggesting that CT-1 production is stimulated by riluzole. As expected, addition of antibodies against GDNF or BDNF also reversed the protection provided by riluzole treated astrocyte conditioned media (Fig. 1B) since these factors are basally secreted by astrocytes (Dougherty et al., 2000; Fulmer et al., 2014; Jean et al., 2008; Schaar et al., 1993). These results imply that in addition to stimulate the production of BDNF and GDNF by cortical astrocytes (Mizuta et al., 2001), riluzole also induces the synthesis and release of CT-1 by spinal astrocytes.
Figure 1. Riluzole induces cardiotrophin-1 production in astrocytes and Schwann cells.
Confluent astrocytes (A) were treated with riluzole for 24 h, RNA was extracted and the levels of CT-1, BDNF and GDNF were quantified, normalized against β-actin and expressed as relative levels respect to untreated cells. Conditioned media from astrocytes (B) and Schwann cells (C) was obtained by incubating the cells in the presence or absence of riluzole for 24 h, replacing the media and leaving the cells to condition the fresh media in the absence of riluzole for additional 24 h. Motor neurons were plated in the presence of the trophic factors BDNF, GDNF, and CT-1 (TF) or in the absence of trophic factors (trophic factor deprivation, TFD). When indicated, trophic factor-deprived motor neurons were plated in the presence of 1/10 dilution (A) or 1/25 dilution (C) of either untreated conditioned media (CM) or riluzole-conditioned media (RCM) and in the presence or absence of neutralizing antibody against CT-1 (Anti CT-1), BDNF (Anti BDNF) and GDNF (Anti GDNF). Motor neurons were cultured for 3 d then counted by hand or using Calcein AM. Confluent Schwann cells (D) were treated with riluzole for 24 h, RNA was extracted and the levels of CT-1, BDNF and GDNF were quantified as described in A. Values are the mean of at least 3 experiments. For B and C, *p<0.05 vs TFD, **p<0.05 vs RCM, *** p<0.05 vs CM. For A and D, *p<0.05 vs BDNF or GDNF. Statistical analysis was performed using one-way ANOVA followed by Bonferroni post-test.
Schwann cells provide trophic factor support for motor neurons under stress conditions (Wilhelm et al., 2012; Xu et al., 2013). Therefore we investigated if riluzole could also stimulate trophic factor production in these cells at the same concentrations of drug used for astrocytes. Initial experiments were conducted to determine the dilution of riluzole-treated and untreated Schwann cell conditioned media that would provide discernable differences in trophic factor support. Due to a higher level of trophic support in Schwann cell conditioned media, a 1:25 dilution was selected to perform subsequent experiments compare to the 1:10 dilution applied to conditioned media from astrocytes (Sup. Fig. 1A). Incubation of Schwann cells with riluzole for 24 h prior to conditioning the media significantly increased the protection provided to motor neurons in comparison to media from untreated cells (Fig. 1C). Neutralizing antibodies against CT-1 completely abolished the protection afforded by conditioned media from riluzole-treated Schwann cells (Fig. 1C). In the same conditions, neutralizing antibodies against BDNF and GDNF had no effect on motor neuron survival (Sup. Fig. 1B). In contrast, neutralizing antibodies against GDNF but not BDNF, reduced motor neuron survival in untreated conditioned media (Sup. Fig. 1B). In agreement with these observations, the levels of CT-1 messenger RNA were significantly increased after 24 h incubation with riluzole, while no changes were detected for GDNF and BDNF messenger RNAs (Fig. 1D). These results reveal that Schwann cells in culture produce CT-1 and that the production and release of CT-1 is enhanced by riluzole. Incubation of astrocytes and Schwann cells with riluzole had no effect on cellular morphology or number (Sup. Fig. 1 A and B). Together, these results show that riluzole treatment increases trophic factor deprived-motor neuron survival by inducing release of CT-1 by both astrocytes and Schwann cells.
Chronic riluzole treatment decreases trophic factor production by glial cells
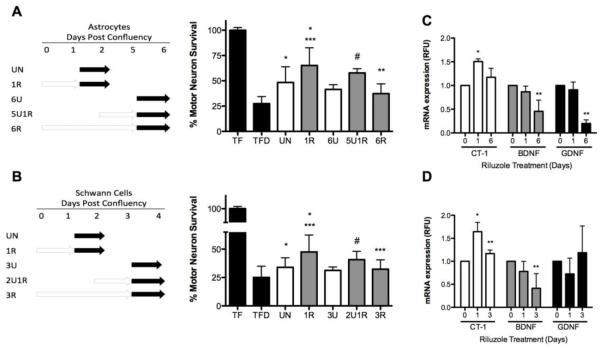
Since incubation of astrocytes and Schwann cells with riluzole for 24 h showed an increase in trophic factor production, we investigated whether longer incubation periods would results on higher and more stable production of trophic factors by glial cells. Surprisingly, incubation of astrocytes with riluzole for up to 6 days prior to media conditioning resulted in a significant reduction of the protection provided to motor neurons when compared with conditioned media from astrocyte cultures incubated with riluzole for 24 h (Fig. 2A). To determine if the loss of the astrocytes response to riluzole was the result of the chronic incubation with riluzole or astrocyte senescence, parallel astrocyte cultures were maintained in the same conditions, but in the absence of riluzole. These cultures were then treated with riluzole for 24 h prior to conditioning the media (5U1R, as described in Fig. 2A). Conditioned media from these cultures had a motor neuron trophic activity comparable to that of conditioned media from acute riluzole treated astrocyte (1R, Fig. 2A) and increases motor neuron survival above six days untreated astrocytes. These results reveal that conditioned media derived from astrocytes after chronic incubation with riluzole had reduced trophic support.
Figure 2. Chronic riluzole treatment reduces trophic factor production in astrocytes and Schwann cells.
Motor neurons were plated in the presence (TF) or absence of trophic factors (TFD), or with media conditioned by astrocytes (A) or Schwann cells (B) for the indicated periods of time (white arrows). Riluzole was then replaced with new media without riluzole and 24 h later conditioned media was collected (black arrows). Motor neurons were plated with conditioned media from astrocytes (A), cultured in the absence of riluzole for one (UN) or 6 days (6U) or presence of riluzole for 24 h (1R) or 6 days (6R), or cultured for 5 days prior to 24 h incubation with riluzole (5U1R). Motor neurons were cultured for 3 days in the conditioned media then counted by hand or using Calcein AM. (B) Motor neurons were plated in conditioned media from Schwann cells. (B) cultured in the absence of riluzole for one day (UN) or 3 days (3U) or presence of riluzole for 24 h (1R), 3 days (3R), and for 2 days and then treated with riluzole for 24 h (2U1R). Motor neurons were cultured for 3 days in described conditioned media then counted by hand or using Calcein AM. (C) Astrocytes were incubated without (0) or with riluzole for 24 h (1) and 6 days (6) before RNA was extracted. The relative changes in mRNA expression of CT-1 (white) BDNF (grey) and GDNF (black) were determined using β-actin to normalize. (D) Schwann cells were incubated without (0) or with riluzole for 24 h (grey) and 3 days (grey checkers) before determining the relative changes in mRNA expression of CT-1 (white) BDNF (grey) and GDNF (black) were determined as described in C. Values are the mean ± SD of at least 3 independent experiments. For A and B, statistical analysis was preformed using ANOVA followed by Bonferroni multiple comparison’s test, *p<0.05 vs TFD, **p<0.05 vs 1R, *** p<0.005 vs UN. # p<0.05 vs 6U. For C and D, *p<0.05 vs. 0 day riluzole, **p<0.05 vs. 1 day riluzole.
When the same experimental design was applied to Schwann cells, after 5 days in culture followed by 24 h incubation with riluzole the cells failed to respond to the drug (5U1R). However, conditioned media from acute treatment (1R, Sup. Fig. 1C) was indeed protective. Quantitative RT-PCR showed a significant reduction in CT-1 levels after 3 days of continuous incubation with riluzole while after 6 days in culture the cells became senescent. Therefore, conditioned media from Schwann cells incubated with riluzole for 3 days was considered a long-term treatment with the drug. In agreement, this conditioned media showed reduced motor neuron trophic factor support (Fig. 2B), while the control riluzole treatment, 2 days in culture followed by 24 h incubation with the drug (2U1R, Fig. 2B) maintained motor neuron survival to levels comparable to acute riluzole incubation (1R, Fig. 2B) and increased motor neuron survival above 3 day untreated cells. Long-term incubation of astrocytes and Schwann cell produced no change on the morphology or proliferation of the cells (Sup. Fig. 2 A and C). These observations revealed that the long-term effects of riluzole were not due to loss of cell responsiveness to the drug. In addition, the expression of CT-1 and BDNF messenger RNA in Schwann cells was decreased after long-term incubation with riluzole compared with one-day incubation, with the expression of BDNF mRNA lower than untreated control (Fig. 2D). In astrocytes, long-term incubation with riluzole decreased expression of CT-1 compared with cultures treated for one day with the drug. Interestingly, BDNF and GDNF, but not CT-1 mRNA expression was below untreated control in astrocytes after 6 days riluzole incubation (Fig. 2C). These results suggest that the effect of riluzole on the regulation of trophic factors mRNA expression is trophic factor and cell type specific. Together these findings suggest that chronic exposure to riluzole significantly reduces trophic factor production by glial cells, abolishing the protective effect observed on trophic factor deprived-motor neurons.
Acute and chronic riluzole treatment has opposite effects on trophic factor production in vivo
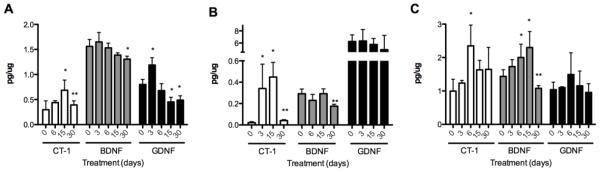
The effects of riluzole on trophic factor production were investigated in spinal cord, whole brain and sciatic nerve of C57BL/6J male mice after treatment with riluzole in the drinking water for various time periods. In the spinal cord, riluzole significantly increased CT-1 levels after 15 days of continuous treatment. However, the increase was only transitory since CT-1 production was reduced to basal levels after 30 days of treatment (Fig 3A). In contrast, riluzole did not stimulate BDNF production at any time, but induced a significant decrease of BDNF levels after 30 days of treatment (Fig. 3A). GDNF production was significantly increased after 3 days of riluzole treatment and returned to basal levels after 6 days of continuous administration of the drug. Surprisingly, GDNF production decreased below control levels after 15 and 30 days of continuous treatment (Fig. 3A). These observations cannot be attributed to differences in drug intake since there were no changes in the water consumption by the mice throughout the study (6.5-7.5 ml/animal/day, independently of the treatment). Thus, these findings are consistent with the repressive effects on BDNF, GDNF, and CT-1 production observed in cell culture after long-term incubation of astrocytes with riluzole.
Figure 3. Effects of riluzole on trophic factor levels in vivo.
Male C57BL/6J mice were treated with riluzole (100 μg/ml) in the drinking water for the number of days indicated in the figures. At the indicated times the mice were sacrificed, the tissues harvested and stored at −80°C. Spinal cord (A) sciatic nerve (B) and brain (whole cerebrum) (C) were homogenized and CT-1 (white), BDNF (grey) and GDNF (black) protein levels were quantified by ELISA. Values are the mean of at least 3 animals from at least 2 independent studies performed by duplicate. Statistical analysis was performed using 1-way ANOVA followed by Bonferroni post-test for each trophic factor, * p<0.001 vs control **p<0.05 vs 15 days.
In the sciatic nerve, treatment with riluzole increased CT-1 levels at 3 and 15 days. However, CT-1 levels were significantly decreased below control levels after 30 days of continuous treatment with the drug (Fig. 3B). Riluzole did not increase GDNF or BDNF concentration in the sciatic nerve but BDNF level was significantly decreased after 30 days of riluzole treatment. These findings are consistent with the repressive effects on BDNF and CT-1 production observed in Schwann cells following chronic treatment of riluzole in culture.
In the brain, CT-1 levels increased only at 6 days, returning to basal levels at 15 and 30 days of continuous riluzole administration. Riluzole did not stimulate significant changes in GDNF concentration at any time, but increased BDNF concentrations in the brain after 6 and 15 days treatment. After 30 days of continuous riluzole administration, the BDNF concentration was reduced below control levels (Fig. 3C). These results reveal that acute riluzole treatment activated the production of distinct combinations of CT-1, GDNF and BDNF in spinal cord, sciatic nerve and brain at different times. However, the chronic treatment with riluzole led to a significant reduction of trophic factor production, in some cases below the control levels.
Discontinuous riluzole treatment increases long-term CT-1 production in spinal cord
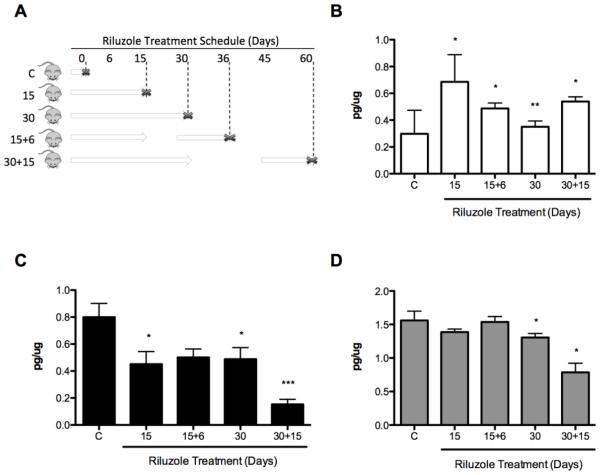
The opposite effects of the chronic and acute administration of riluzole in the animals lead us to investigate 1) whether the acute stimulatory effect could be maintained by discontinuous administration of the drug, and 2) whether the chronic inhibitory effect could be reversed. As shown in Fig. 3, trophic factor levels were elevated between 3 and 15 days then reduced by 15 or 30 days depending on the trophic factor and the tissue. Based on these results, animals were continuously administered riluzole for 15 and 30 days then riluzole was removed for 15 days. The treatment was then reinitiated for additional 6 or 15 days to avoid the chronic inhibitory effect (Fig. 4A). Trophic factor levels were studied in spinal cord from treated animals. After the second period of riluzole administration, CT-1 concentration was significantly increased as compared to the untreated control independently of whether the drug was given for 15 or 30 days during the initial period (Fig. 4B). In the case of GDNF, continuous treatment with riluzole for 15 or 30 days decreased the trophic factors levels. This decrease could not be reverted by discontinuing the treatment for 15 days and treating with the drug for additional 6 or 15 days (Fig. 4C). Moreover, treatment of animals for 30 days with 15 days resting period and 15 additional days with riluzole showed significantly more reduction in GDNF concentrations than animal continuously treated with riluzole for 30 days (Fig. 4C). The concentration of BDNF was not increase respect to the control in any condition (Fig. 4D). Similar to GDNF, BDNF levels in the spinal cord were significantly reduced after treatment of animals with riluzole for 30 days with a 15 days rest period than after 30 days of continuous administration (Fig. 4D). In combination, these observations suggest a complex regulation of the expression of trophic factors by riluzole.
Figure 4. Discontinuous riluzole treatment maintains higher levels of trophic factors.
(A) Riluzole treatment schedule. Animals were administered riluzole (grey arrows) in their drinking water (100 μg/ml) continuously for 15 or 30 days then riluzole was removed for another 15 days before receiving a second dose of riluzole for additional 6 or 15 days. At the indicated time points the mice were sacrificed (x), the tissues were harvested and stored at −80°C. Spinal cord was homogenized and CT-1 (B), GDNF (C) and BDNF (D) concentrations were quantified by ELISA. Values are the mean of at least 4 mice with the determinations performed by duplicate. Statistical analysis was performed using ANOVA followed by Bonferroni post-test. * p<0.001 vs control **p<0.05 vs 15 days, #p<0.05 vs 3 days, ## p<0.05 vs 30 days.
Discussion
Riluzole is the only FDA approved drug for the treatment of ALS. However, the primary mechanism by which riluzole offers protection remains unknown. In both spinal cord organotypic and enriched motor neuron cultures riluzole protects motor neurons from glutamate receptor hyperstimulation-induced death (Estevez et al., 1995; Rothstein and Kuncl, 1995). These original observations are supported by new evidence of the anti-excitotoxic effect of the drug in ALS patients (Bellingham, 2011; Cifra et al., 2013; Foerster et al., 2013; Vucic et al., 2013). However, these same reports conclude that the effects of riluzole on glutamate metabolism are not sufficient to explain its protective effects in ALS. After the failure of the gabapentin clinical trial, riluzole was postulated to act on pathways other than the glutamatergic transmission (Meininger et al., 2000). Riluzole stimulates trophic factor production by astrocytes (Mizuta et al., 2001; Peluffo et al., 1997). However, the effect of riluzole on trophic factor production in other cell types was not investigated. Motor neuron survival is supported by trophic factors released by Schwann cells during development (Henderson et al., 1994) and nerve regeneration (Xu et al., 2013) but whether Schwann cells supported motor neuron survival in adult normal conditions remained unknown. Here, we show for the first time that Schwann cells produce CT-1 in these conditions. Although Schwann cell production of BDNF and GDNF contribute also to motor neurons survival in culture, CT-1 was the only factor induced in Schwann cells following riluzole treatment. Results using conditioned media from riluzole-treated Schwann cells support CT-1 as the major trophic factor induced by riluzole, since neutralizing antibodies against CT-1 reduced motor neuron survival to levels comparable to those of untreated conditioned media. Similar findings were obtained with conditioned media from riluzole-treated astrocytes (Fig. 1). Previous reports shows that riluzole induces secretion of NGF, BDNF, and GDNF by cultured cortical astrocytes (Mizuta et al., 2001). In contrast with these observations, acute incubation of spinal cord astrocytes with riluzole led to a significant increase in secretion of CT-1 but not BDNF or GDNF (Fig. 3). However, in the brain of mice after acute treatment with riluzole, CT-1 and BDNF production was induced, without affecting GDNF levels. These discrepancies between the cell culture and in vivo tissue effects may be due to direct or indirect effects of riluzole on other cell types. The difference in trophic factor production in the examined tissue suggests that responses to riluzole depend on the cell specific population within the corresponding tissue. Surprisingly, long-term riluzole treatment of Schwann cells and astrocytes with riluzole resulted in a significant reduction of trophic activity (Fig. 2), suggesting a biphasic stimulatory-inhibitory effect of riluzole on the production of trophic factors.
The regulation of the concentration of different trophic factors by oral administration of riluzole to mice was tissue and time dependent (Fig 3). While some trophic factors were initially increased over basal concentrations, all trophic factors concentrations dropped to basal levels and in some cases below basal levels after longer treatment with riluzole. In agreement with the cell culture results, CT-1 was the only trophic factor consistently induced after acute treatment of mice with riluzole in spinal cord, sciatic nerve and brain (Fig. 3). BDNF and GDNF concentrations were initially increased in some tissues, but not others. However, chronic treatment reversed to basal or below control levels for all three trophic factors (Fig. 3). The half-life of riluzole in vivo is measured in hours. In the nervous system, riluzole is removed at the level of the blood-brain barrier by the P-glycoprotein (Dulin et al., 2013; Milane et al., 2010; Milane et al., 2007; Milane et al., 2009). In patients and models of ALS both drug efflux transporters, P-glycoprotein and breast cancer-resistant protein (BCRP) are increased. Gene deletion of P-glycoprotein or pharmacological inhibition of these transporters provides a small protective effect when riluzole is administer at disease onset in the G93A mouse model of ALS (Jablonski et al., 2014). This enhanced protection by riluzole is consistent with our observation of acute effects increasing trophic factor production. However, decreased concentrations of riluzole in the nervous system could explain the return of the trophic factor concentrations to basal control levels, but not below those levels, suggesting there is a secondary inhibitory effect mediated by riluzole itself or by a metabolic product of riluzole that remains to be identified.
Riluzole is undergoing clinical trials for the treatment of spinal cord injury and repair. In order to maximize its therapeutic effects riluzole must be administered within hours of injury (Nogradi et al., 2007). Thus, riluzole may be effective for treatment in spinal cord injury and repair in these conditions because trophic factor production by astrocytes is induced locally at the critical time to prevent neuronal death and to facilitate axonal regeneration and growth (Bergerot et al., 2004; Li et al., 1994). These findings suggest that induction of the specific trophic factors in the right cells could be critical to achieve the desired beneficial therapeutic effect in spinal trauma. Most animal studies were conducted using continuous administration of riluzole for 2-3 weeks (Bergerot et al., 2004; Dennys et al., 2014; Nogradi et al., 2007; Nogradi and Vrbova, 2001), which could result in a decreased of the potential beneficial effects. However, it is also possible that the long-term treatment with riluzole induces an initial increase in trophic support, which helps axon regenerate through the affected area. Conversely, the chronic effect of riluzole could also be beneficial in this context, by decreasing the local production of trophic factors, which allows axons to leave the affected area to interact with the right targets.
Trophic factors have shown to be protective in animal models of ALS. However, the translation to clinical trials resulted in failure. Most trophic factors investigated in clinical trials for ALS have been unsuccessful at extending patient survival (Group, 1996) These trials used invasive delivery methods to ensure the trophic factor of interest would cross the blood brain barrier (Dennys et al., 2014; Kastin et al., 2003). In summary, there are multiple factors that can explain trophic factors failure in clinical trails, including secondary effects due to systemic administration (Gould and Oppenheim, 2011). The success of riluzole may be due to the stimulation of the local production of trophic factor by motor neuron associated cells. However, independently of the mechanism of riluzole action, here we show that riluzole regulates protein expression and it has opposite effects on the production of trophic factors. Early stimulation of trophic factor production is followed by a decrease, in some cases below basal levels, which strongly suggest the second phase is not a consequence of loss of activity or habituation but inhibition of trophic factor production. Modifying the dosing regimen of riluzole may maximize the protective effects of the glial-derived trophic activity necessary to improve treatment of patients with ALS. We showed that discontinuous administration of riluzole maintained elevated CT-1 levels in the spinal cord in vivo (Fig. 4). However, these same protocols of discontinuous riluzole treatment did not prevent down regulation of BDNF or GDNF. In the case of GDNF, after 15 days treatment with riluzole with a 15 days rest period, the concentrations of the trophic factor were not affected by further administration of the drug. In contrast, when the initial period of riluzole administration was 30 days, the additional treatment with riluzole further decreased the concentrations of GDNF, suggesting that some of the chronic effects of riluzole could be irreversible or take a very long time to reverse.
Riluzole readily crosses the blood brain barrier and in addition to stimulate trophic factor production in glial cells, it has direct effects on motor neurons and maybe other yet unknown protein synthesis-dependent effects. Optimizing the administration protocols could avoid potential negative chronic effects such as the decrease of trophic factors levels described here. In addition, it is possible that the modest effects of riluzole in ALS are due to a combination of acute protective effects and chronic negative effects. However, this biphasic effect may be actually beneficial in other conditions such as spinal cord injury. The initial protective effect of riluzole could help neuronal survival and axon growth through the damaged area. The secondary inhibition of trophic factor production might allow axons to leave the damaged area and reach their targets, thus reversing the damage. Optimizing the administration conditions for riluzole would depend on the pathology to be treated and the development of good peripheral markers of riluzole action in the central nervous system to adjust dosage according to the response of the patient.
Supplementary Material
Highlights.
Riluzole stimulates CT-1 expression by glial cells
Short term riluzole treatment increases trophic factor levels in vitro and in vivo
Long-term riluzole treatment reduces trophic factors levels in vitro and in vivo
Reversion of the discontinuous riluzole administration different for the trophic factors
Acknowledgements
The authors would like to thank Mandi Gandelman for her technical assistance with quantitative PCR. The investigations were supported by NIH/NINDS grant NS36761 (AGE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ang LC, Bhaumick B, Juurlink BHJ. Neurite promoting activity of insulin-like growth factor I and nerve growth factor on spinal motoneurons is astrocyte dependent. Brain Res Dev Brain Res. 1993;74:83–88. doi: 10.1016/0165-3806(93)90086-p. [DOI] [PubMed] [Google Scholar]
- Arce V, Pollock RA, Philippe JM, Pennica D, Henderson CE, deLapeyriere O. Synergistic effects of schwann- and muscle-derived factors on motoneuron survival involve GDNF and cardiotrophin-1 (CT-1) J Neurosci. 1998;18:1440–1448. doi: 10.1523/JNEUROSCI.18-04-01440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS neuroscience & therapeutics. 2011;17:4–31. doi: 10.1111/j.1755-5949.2009.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. The New England journal of medicine. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- Bergerot A, Shortland PJ, Anand P, Hunt SP, Carlstedt T. Co-treatment with riluzole and GDNF is necessary for functional recovery after ventral root avulsion injury. Exp Neurol. 2004;187:359–366. doi: 10.1016/j.expneurol.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Bordet T, Lesbordes JC, Rouhani S, Castelnau-Ptakhine L, Schmalbruch H, Haase G, Kahn A. Protective effects of cardiotrophin-1 adenoviral gene transfer on neuromuscular degeneration in transgenic ALS mice. Hum Mol Genet. 2001;10:1925–1933. doi: 10.1093/hmg/10.18.1925. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Cheah BC, Vucic S, Krishnan AV, Kiernan MC. Riluzole, neuroprotection and amyotrophic lateral sclerosis. Current medicinal chemistry. 2010;17:1942–1199. doi: 10.2174/092986710791163939. [DOI] [PubMed] [Google Scholar]
- Cifra A, Mazzone GL, Nistri A. Riluzole: what it does to spinal and brainstem neurons and how it does it. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2013;19:137–144. doi: 10.1177/1073858412444932. [DOI] [PubMed] [Google Scholar]
- Dennys CN, Franco MC, Estevez AG. Trophic factor production by glial cells in the treatment of amyotrophic lateral sclerosis. J Biomed Eng. 2014;1:8. [Google Scholar]
- Doble A. Effects of riluzole on glutamatergic neurotransmission in the mammalian central nervous system, and other pharmacological effects. Reviews in Comtemporary Pharmacology. 1997;8:213–225. [Google Scholar]
- Dougherty KD, Dreyfus CF, Black IB. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol Dis. 2000;7:574–585. doi: 10.1006/nbdi.2000.0318. [DOI] [PubMed] [Google Scholar]
- Dulin JN, Moore ML, Grill RJ. The dual cyclooxygenase/5-lipoxygenase inhibitor licofelone attenuates p-glycoprotein-mediated drug resistance in the injured spinal cord. J Neurotrauma. 2013;30:211–226. doi: 10.1089/neu.2012.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleson KL, Bennett MR. Motoneurone survival requirements during development: the change from immature astrocyte dependence to myotube dependence. Developmental Brain Research. 1986;29:161–172. doi: 10.1016/0165-3806(86)90092-1. [DOI] [PubMed] [Google Scholar]
- Eagleson KL, Raju TR, Bennett MR. Motoneuron survival is induced by immature astrocytes from developing avian spinal cord. Dev Brain Res. 1985;17:95–104. doi: 10.1016/0165-3806(85)90135-x. [DOI] [PubMed] [Google Scholar]
- Estevez AG, Spear N, Manuel SM, Radi R, Henderson CE, Barbeito L, Beckman JS. Nitric oxide and superoxide contribute to motor neuron apoptosis induced by trophic factor deprivation. J Neurosci. 1998;18:923–931. doi: 10.1523/JNEUROSCI.18-03-00923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez AG, Stutzmann J-M, Barbeito L. Protective effect of riluzole on excitatory amino acid-mediated neurotoxicity in motoneuron-enriched cultures. European Journal of Pharmacology. 1995;280:47–53. doi: 10.1016/0014-2999(95)00186-o. [DOI] [PubMed] [Google Scholar]
- Foerster BR, Pomper MG, Callaghan BC, Petrou M, Edden RA, Mohamed MA, Welsh RC, Carlos RC, Barker PB, Feldman EL. An imbalance between excitatory and inhibitory neurotransmitters in amyotrophic lateral sclerosis revealed by use of 3-T proton magnetic resonance spectroscopy. JAMA neurology. 2013;70:1009–1016. doi: 10.1001/jamaneurol.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulmer CG, VonDran MW, Stillman AA, Huang Y, Hempstead BL, Dreyfus CF. Astrocyte-derived BDNF supports myelin protein synthesis after cuprizone-induced demyelination. J Neurosci. 2014;34:8186–8196. doi: 10.1523/JNEUROSCI.4267-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TW, Oppenheim RW. Motor neuron trophic factors: therapeutic use in ALS? Brain Res Rev. 2011;67:1–39. doi: 10.1016/j.brainresrev.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group ACTS. A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. ALS CNTF Treatment Study Group. Neurology. 1996;46:1244–1249. doi: 10.1212/wnl.46.5.1244. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Cutting FB, Zhai P, Doble A, Taylor CP, Andrus PK, Hall ED. Benefit of vitamin E, riluzole, and gabapentin in a transgenic model of familial amyotrophic lateral sclerosis. Ann Neurol. 1996;39:147–157. doi: 10.1002/ana.410390203. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Fleck TJ, Himes CS, Hall ED. Riluzole preserves motor function in a transgenic model of familial amyotrophic lateral sclerosis. Neurology. 1998;50:62–66. doi: 10.1212/wnl.50.1.62. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC, et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Jablonski MR, Markandaiah SS, Jacob D, Meng NJ, Li K, Gennaro V, Lepore AC, Trotti D, Pasinelli P. Inhibiting drug efflux transporters improves efficacy of ALS therapeutics. Annals of clinical and translational neurology. 2014;1:996–1005. doi: 10.1002/acn3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean YY, Lercher LD, Dreyfus CF. Glutamate elicits release of BDNF from basal forebrain astrocytes in a process dependent on metabotropic receptors and the PLC pathway. Neuron glia biology. 2008;4:35–42. doi: 10.1017/S1740925X09000052. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W. Glial cell line-derived neurotrophic factor does not enter normal mouse brain. Neurosci Lett. 2003;340:239–241. doi: 10.1016/s0304-3940(03)00007-7. [DOI] [PubMed] [Google Scholar]
- Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996a;347:1425–1431. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- Lacomblez L, Bensimon G, Leigh PN, Guillet P, Powe L, Durrleman S, Delumeau JC, Meininger V. A confirmatory dose-ranging study of riluzole in ALS. ALS/Riluzole Study Group-II. Neurology. 1996b;47:S242–250. doi: 10.1212/wnl.47.6_suppl_4.242s. [DOI] [PubMed] [Google Scholar]
- Li L, Oppenheim RW, Lei M, Houenou LJ. Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. J Neurobiol. 1994;25:759–766. doi: 10.1002/neu.480250702. [DOI] [PubMed] [Google Scholar]
- Meininger V, Lacomblez L, Salachas F. What has changed with riluzole? Journal of neurology. 2000;247(Suppl 6) VI/19-22. [PubMed] [Google Scholar]
- Milane A, Fernandez C, Dupuis L, Buyse M, Loeffler JP, Farinotti R, Meininger V, Bensimon G. P-glycoprotein expression and function are increased in an animal model of amyotrophic lateral sclerosis. Neurosci Lett. 2010;472:166–170. doi: 10.1016/j.neulet.2010.01.078. [DOI] [PubMed] [Google Scholar]
- Milane A, Fernandez C, Vautier S, Bensimon G, Meininger V, Farinotti R. Minocycline and riluzole brain disposition: interactions with p-glycoprotein at the blood-brain barrier. J Neurochem. 2007;103:164–173. doi: 10.1111/j.1471-4159.2007.04772.x. [DOI] [PubMed] [Google Scholar]
- Milane A, Vautier S, Chacun H, Meininger V, Bensimon G, Farinotti R, Fernandez C. Interactions between riluzole and ABCG2/BCRP transporter. Neurosci Lett. 2009;452:12–16. doi: 10.1016/j.neulet.2008.12.061. [DOI] [PubMed] [Google Scholar]
- Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst Rev. 2012;3:CD001447. doi: 10.1002/14651858.CD001447.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta I, Ohta M, Ohta K, Nishimura M, Mizuta E, Kuno S. Riluzole stimulates nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor synthesis in cultured mouse astrocytes. Neuroscience letters. 2001;310:117–120. doi: 10.1016/s0304-3940(01)02098-5. [DOI] [PubMed] [Google Scholar]
- Nogradi A, Szabo A, Pinter S, Vrbova G. Delayed riluzole treatment is able to rescue injured rat spinal motoneurons. Neuroscience. 2007;144:431–438. doi: 10.1016/j.neuroscience.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Nogradi A, Vrbova G. The effect of riluzole treatment in rats on the survival of injured adult and grafted embryonic motoneurons. Eur J Neurosci. 2001;13:113–118. doi: 10.1046/j.0953-816x.2000.01362.x. [DOI] [PubMed] [Google Scholar]
- Orrell RW. Motor neuron disease: systematic reviews of treatment for ALS and SMA. British medical bulletin. 2010;93:145–159. doi: 10.1093/bmb/ldp049. [DOI] [PubMed] [Google Scholar]
- Peluffo H, Estévez AG, Barbeito L, Stutzmann JM. Riluzole promotes survival of rat motoneurons in vitro by stimulating trophic activity produced by spinal astrocyte monolayers. Neurosci Lett. 1997;228:207–211. doi: 10.1016/s0304-3940(97)00384-4. [DOI] [PubMed] [Google Scholar]
- Pennica D, Arce V, Swanson TA, Vejsada R, Pollock RA, Armanini M, Dudley K, Phillips HS, Rosenthal A, Kato AC, Henderson CE. Cardiotrophin-1, a cytokine present in embryonic muscle, supports long-term survival of spinal motoneurons. Neuron. 1996;17:63–74. doi: 10.1016/s0896-6273(00)80281-0. [DOI] [PubMed] [Google Scholar]
- Pettmann B, Henderson CE. Neuronal cell death. Neuron. 1998;20:633–647. doi: 10.1016/s0896-6273(00)81004-1. [DOI] [PubMed] [Google Scholar]
- Raoul C, Henderson CE, Pettmann B. Programmed cell death of embryonic motoneurons triggered through the Fas death receptor. J Cell Biol. 1999;147:1049–1062. doi: 10.1083/jcb.147.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Kuncl RW. Neuroprotective strategies in a model of chronic glutamate-mediated motor neuron toxicity. J Neurochem. 1995;65:643–651. doi: 10.1046/j.1471-4159.1995.65020643.x. [DOI] [PubMed] [Google Scholar]
- Saneto RP, Vellis JD. Neuronal and glial cells: cell culture of the central nervous system. In: Turner AJ, Brachelard HS, editors. Neurochemistry a practical approach. Washington, D.C.; IRL Press Oxford: 1987. pp. 27–63. [Google Scholar]
- Schaar DG, Sieber BA, Dreyfus CF, Black IB. Regional and cell-specific expression of GDNF in rat brain. Exp Neurol. 1993;124:368–371. doi: 10.1006/exnr.1993.1207. [DOI] [PubMed] [Google Scholar]
- Schnaar RI, Schaffner AE. Separation of cell types from embryonic chicken and rat spinal cord: characterization of motoneuron-enriched fractions. J Neurosci. 1981;1:204–217. doi: 10.1523/JNEUROSCI.01-02-00204.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaxton C, Bott M, Walker B, Sparrow NA, Lambert S, Fernandez-Valle C. Schwannomin/merlin promotes Schwann cell elongation and influences myelin segment length. Mol Cell Neurosci. 2011;47:1–9. doi: 10.1016/j.mcn.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S, Lin CS, Cheah BC, Murray J, Menon P, Krishnan AV, Kiernan MC. Riluzole exerts central and peripheral modulating effects in amyotrophic lateral sclerosis. Brain. 2013;136:1361–1370. doi: 10.1093/brain/awt085. [DOI] [PubMed] [Google Scholar]
- Wilhelm JC, Xu M, Cucoranu D, Chmielewski S, Holmes T, Lau KS, Bassell GJ, English AW. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J Neurosci. 2012;32:5002–5009. doi: 10.1523/JNEUROSCI.1411-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Rosen KM, Hedstrom K, Rey O, Guha S, Hart C, Corfas G. Nerve injury induces glial cell line-derived neurotrophic factor (GDNF) expression in Schwann cells through purinergic signaling and the PKC-PKD pathway. Glia. 2013;61:1029–1040. doi: 10.1002/glia.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.