Abstract
Objectives
Knee trauma is a known cause of meniscal tear. However, meniscal pathology where the aetiology is often unclear is a frequent finding on knee magnetic resonance imaging (MRI).Our objective was to investigate potential risk factors for medial meniscal lesions or extrusion in middle-aged and elderly persons.
Methods
Prospective cohort study using population-based subjects from Birmingham, Alabama and Iowa City, Iowa, United States (the Multicenter Osteoarthritis Study (MOST)). We studied 644 men and women aged 50 to 79 years with or at high risk of knee osteoarthritis (Kellgren and Lawrence grade 0 to 2) but with normal medial meniscal status at baseline. We scored paired baseline and 30-month 1.0T knee MRIs for meniscal lesions and extrusion (pathology) and evaluated the following systemic, knee-specific, and compartment-specific potential risk factors: age, sex, body mass index, bony enlargement of finger joints, knee trauma, leg-length inequality, and knee alignment.
Results
Of 791 knees, 77 (9.7%) had medial meniscal pathology at 30-months follow-up. Sixty-one of these 77 knees (81%) had no report of trauma during follow-up. Including all potential risk factors in the multivariable model, the adjusted odds ratio (OR) for medial meniscal pathology was 4.14 (95% confidence interval 2.06, 8.31) for knee trauma during follow-up, 1.64 (1.00, 2.70) for ≥5 bony enlargements of finger joints (vs. ≤4), and 2.00 (1.18, 3.40) for varus alignment (vs. not varus) at baseline exam. Further, obesity was a risk factor for the development of meniscal extrusion, OR 3.04 (1.04, 8.93) but not for meniscal lesions, OR 1.15 (0.52, 2.54).
Conclusions
Apart from knee trauma, possible generalised osteoarthritis, expressed as multiple bony enlargements of finger joints, varus alignment, and obesity are risk factors for medial meniscal pathology.
Keywords: Menisci, tibial, Knee, Osteoarthritis, Magnetic Resonance Imaging, Risk Factor
In the tibiofemoral compartment of the knee there are two wedge-shaped discs of fibrocartilage, the medial and lateral meniscus. They provide important functions in absorbing shocks and distributing load over the surrounding joint cartilage.[1–3] When a meniscus is damaged or removed by surgery, there is a highly increased risk of developing knee osteoarthritis.[4–7] The knee is one of the most common sites of osteoarthritis, causing pain, reduced knee function, and disability to a large proportion of middle-aged and elderly persons.[8] Osteoarthritis is an increasingly important health concern in most developed countries and is according to WHO among the top 10 conditions in Europe with respect to burden on the society.[8]
The mechanism by which meniscal tear occurs is traditionally considered to be due to acute knee trauma.[9] However, meniscal lesions are frequent incidental findings in middle-aged and elderly persons on knee magnetic resonance imaging (MRI) with an overall prevalence ranging from about 19% in knees of women 50 to 59 years of age to 56% in knees of men 70 to 90 years of age.[10] These meniscal lesions are typically horizontal cleavage lesions or flap tears of the body or posterior horn of the medial meniscus with or without fibrillation and are often accompanied by meniscal extrusion (radial displacement of the meniscus outside the joint margin).[10–12] In the general population most of these meniscal pathologies do not per se cause symptoms[10], but as diminishing meniscal function is a strong risk factor for knee osteoarthritis and osteoarthritis progression[4, 13, 14], any such pathology may still be a key factor in several aspects, in particular in early-stage knee osteoarthritis. These findings are sometimes referred to as being of degenerative character, even if there is little evidence of their aetiology.[15] To date there have been no longitudinal studies of risk factors for such meniscal pathology. One reason for lack of studies is the difficulty to ascertain meniscal status at both baseline and follow-up. This requires repeated, expensive, and time-consuming imaging methods such as knee MRI. Hence, there is a strong rationale for the present study, where we use data from a large on-going cohort study. For risk factors, we focused on the effects of common systemic and biomechanical factors that are likely to precede and possibly cause meniscal pathology. Cartilage damage or bone marrow lesions were not evaluated as risk factors because they may often be a consequence of meniscal pathology.[13, 14, 16] Thus, using a prospective cohort study design with repeat knee MRI exams over 30 months our aim was to evaluate common demographic, systemic, but also certain biomechanical knee-specific potential risk factors that may be casually associated with meniscal pathology.
METHODS
Design overview
The Multicenter Osteoarthritis Study (MOST) is a large, prospective cohort study of individuals aged 50 to 79 years in which the primary goal was to identify risk factors for incident and progressive knee osteoarthritis.[17] Study subjects either had knee osteoarthritis at baseline or were at high risk of developing the disease. Factors considered to contribute to a high risk of knee osteoarthritis included being overweight or obese, having either knee pain, aching, or stiffness on most of the preceding 30 days, a prior knee injury that made it difficult to walk for at least one week, or previous knee surgery. Written informed consent was obtained before participation at each visit, as approved by the institutional review boards of the participating institutions.
Setting and participants –MOST parent study
All 3,026 subjects in MOST were recruited from two communities in the United States (Birmingham, Alabama and Iowa City, Iowa) through mass mailing of letters and study brochures, supplemented by media and community outreach campaigns. Recruitment was based on the presence of one or several risk factors for osteoarthritis as detailed above. Subjects were excluded if they screened positive for rheumatoid arthritis[18], had ankylosing spondylitis, psoriatic arthritis, chronic reactive arthritis, a severe medical condition that made continued participation in the study unlikely, bilateral knee replacement surgery, inability to walk without the help of another person or walker, or were planning to move out of the area during the next 3 years.
At the baseline clinic visits, subjects underwent weight-bearing posteroanterior knee radiography, using a fixed flexion protocol.[19, 20] One musculoskeletal radiologist and 1 of 2 rheumatologists graded all films according to the Kellgren and Lawrence scale[21]; discrepancies were adjudicated by a panel of 3 readers. Readers were blinded to MRI findings and clinical data. The two person interobserver reliability for determining Kellgren and Lawrence grade ranged from κ= 0.77 to 0.80. Subjects were also weighed and had their height measured at baseline.
Knee MRI scans and sampling
At baseline and 30-month follow-up, knee MRIs of all MOST participants who were willing and had no contraindications were obtained with a 1.0T MR system (OrthOne; ONI, Wilmington, MA) with a circumferential transmit–receive extremity coil. MRIs were performed using sagittal and axial fat-suppressed fast spin-echo proton density–weighted sequences (repetition time [TR] 5,800/2,500 msec, time to echo [TE] 35 msec, slice thickness 3 mm, field of view [FOV] 14 cm, matrix 288 × 192 pixels), and coronal STIR sequence (TR 7,820 msec, TE 15 msec, slice thickness 3 mm, FOV 14 cm, matrix 256 × 256 pixels).[22, 23] Two musculoskeletal radiologists blinded to clinical and radiographic data read the paired images separately with knowledge of time sequence.
Of participants studied at baseline, 90% had 30-month follow-up clinical visits (Fig. 1). The study sample selected for MRI readings has been previously described.[24] In this study we included all knees with Kellgren and Lawrence (KL) grade 0 to 2 at baseline, i.e., we elected not to include subjects with severe pre-existing radiographic osteoarthritis (KL grade 3 or 4) as pre-existing meniscal pathology is very frequent in these subjects. Further, we restricted our analyses to include only knees with normal medial meniscal integrity and no extrusion of the medial meniscus on MRI at baseline (n=791).We focused on medial meniscal pathology only because lateral meniscal pathology is rarer[10], and our multivariable analysis included mechanical knee alignment, which is as a compartment-specific risk factor.
Figure 1.
Study flow chart (please note that a person may contribute with one knee to the analysis while the other knee was excluded). MOST=Multicenter Osteoarthritis Study, MRI=magnetic resonance imaging
Meniscal outcome variable
Meniscal integrity on paired baseline and 30-month MRIs was assessed using the Whole-Organ MRI Score (WORMS) method.[25] Meniscal tear, maceration, and (or) destruction of the anterior horn, body segment, and the posterior horn, collectively here referred to as meniscal lesions, were assessed using a 5-item ordered scale, where 0 = intact, and 1 = minor radial or parrot-beak tear, 2 = nondisplaced tear, 3 = displaced tear or partial maceration or destruction, or 4 = complete maceration, or destruction (interobserver weighted κ= 0.80). The readers regarded an increased intrameniscal signal (often a linear signal within the meniscus) as a meniscal tear when it communicated with the inferior or superior margin, and (or) free edge of the meniscus on at least 2 slices.
Meniscal positioning was graded as 0 = no extrusion, or grade 1 or 2 (extrusion ≤50%, and extrusion ≥50%, respectively) from the midposterior coronal slice where the medial tibial spine was depicted to its maximum extent (interobserver weighted κ= 0.60). The point of reference for meniscal extrusion was the tibial plateau osteochondral junction at the joint margin (excluding osteophytes).
For this study, because meniscal lesions and extrusion are often related and have similar effects of increased risk for cartilage loss and bone marrow lesions[14, 16], and to ensure sufficient numbers with the outcome, we primarily combined the two constructs meniscal integrity and meniscal positioning to create a dichotomous outcome variable for the medial compartment: no meniscal pathology = intact meniscus and no meniscal extrusion at both baseline and the 30-month examination vs. new development of meniscal pathology = meniscal lesion or extrusion at 30 months but having had normal medial meniscal status at the baseline examination. However, we also evaluated meniscal lesions and meniscal extrusion, as two separate outcomes.
Exposure variables
At both 15 and 30-month follow-up, subjects were asked if they had injured their left or right knee (and which side it was) badly enough to limit their ability to walk for at least two days since the last study visit.
At the baseline clinic visit, examiners evaluated the finger joints for bony enlargement of study subjects’ both hands. These bony enlargements may indicate a predisposition for generalised osteoarthritis, and hypothetically also to a greater risk for degenerative meniscal pathology.[26–29] The examined joints were the first interphalangeal, distal interphalangeal (Heberden’s nodes), proximal interphalangeal (Bouchard’s nodes), and the first carpometacarpal joint (base of thumb). Using the median value of finger joints with bony enlargement (4 joints), we created a dichotomised exposure variable, 0–4 vs. 5 or more.
Full-limb radiographs of both legs for determination of mechanical axis and leg-length were obtained at baseline. The mechanical axis was defined as the angle formed by the intersection of a line from the centre of the head of the femur to the centre of the femoral notch in the knee, and a second line from the centre of the talus to the centre of the tibial spines in the knee (for interobserver agreement intraclass correlation coefficient was 0.99, P < 0.001). Based on prior work, we defined varus as < 179°.[30]
Leg-length inequality has recently been reported to be a risk factor for the development and progression of knee osteoarthritis and could hypothetically be related to increased ground-reaction forces.[31, 32] We defined leg length as the distance from the centre of the femoral head to the tibial mid-plafond point. The mid-plafond point is the most distal portion of the tibia directly over the talar dome and does not include the ankle joint. For leg-length inequality, intra- and interobserver intraclass correlation coefficients were 0.96 and 0.97, respectively (both P < 0.001). We defined clinically significant leg-length inequality as a difference by 1 cm or more, and created a 2-item categorical variable: leg-length inequality less than 1 cm, and leg-length inequality by 1 cm or more.
Statistical analysis
To evaluate the effect of potential risk factors for medial meniscal pathology, we calculated adjusted odds ratios using logistic regression. We used generalised estimating equations to account for correlation between two knees from the same subject. In the model we evaluated age, gender, body mass index, finger joints with bony enlargements, knee injury limiting the ability to walk for at least 2 days during follow-up, knee alignment, and leg-length inequality; all entered simultaneously. Further, race and clinical site (Alabama or Iowa) were adjusted for because MRI readings were matched by clinical site. We also performed a couple of sensitivity analysis evaluating the effect of additional adjustment for Kellgren and Lawrence grade and (or) with adjustment for MOST recruitment variables. As MOST is enriched with subjects with one or more risk factors for knee osteoarthritis, which may potentially bias the relative estimates of effect, we conditioned on study recruitment variables that were not already included in the model. These risk factors (the information obtained at the telephone screening interview), were: a self-report of previous knee injury (so badly that it was difficult to walk for at least one week), previous knee surgery, and the presence of knee pain, aching or stiffness on most days the last 30 days. All tests were performed using SAS for Windows, version 9.1 (SAS Institute, Cary, NC). P values less than or equal to 0.05 were considered statistically significant.
RESULTS
The study sample with normal medial meniscal status at baseline knee MRI consisted of 791 knees from 644 persons (64.6% women). The mean (SD) age of subjects was 60.0 (7.3) years with a mean (SD) body mass index of 29.5 (4.7) (Table 1). At baseline, the distribution of severity of tibiofemoral radiographic osteoarthritis, Kellgren and Lawrence grade, was 563 knees with grade 0, 143 knees with grade 1 and 112 knees with grade 2.
Table 1.
Study sample characteristics at baseline and information on knee injury during follow-up shown by the outcome.
| Medial meniscal pathology at 30- months follow-up |
||
|---|---|---|
| Yes | No | |
| Person specific characteristic* | N=77 | N=577 |
| Mean age ± SD, years | 60.2 ± 7.4 | 60.0 ± 7.3 |
| Women, n (%) | 43 (60) | 377 (65) |
| Mean body mass index ± SD, kg/m2 | 30.4 ± 5.2 | 29.4 ± 4.6 |
| Median number of bony enlargements of finger joints (min, max) |
5 (0, 18) | 3 (0, 20) |
| Knee-specific characteristic | N=77 | N=714 |
| Leg-length inequality†, n (%) | 11 (14) | 115 (16) |
| Mean alignment§ ± SD, degrees | 178.3 ± 2.5 | 179.5 ± 2.7 |
| Knee injury during follow-up‡, n (%) | 15 (31) | 33 (5) |
Missing values: bony enlargement of finger joints n=1, leg length inequality n=15, and knee alignment n=6.
Ten subjects are included in both columns as they have one knee with and the other knee without medial meniscal pathology at follow-up (the total number of unique study subjects is 644).
Leg-length inequality by 1 cm or more.
Values <179 are varus and >181 are valgus.
Knee injury leading to limited ability to walk for 2 days or more.
The analyses focused on medial meniscal pathology only. Of the 791 knees, 77 (9.7%) had such findings on MRI at the 30-month follow-up (Fig 2, 3, 4). Of those, 31 had both meniscal lesion (tear, destruction or maceration) and meniscal extrusion, 28 cases had meniscal lesions but no extrusion, and 18 cases had meniscal extrusion but no definite meniscal lesion. The lesions predominantly involved the posterior horn (88%), followed by the meniscus body (53%). None (0%) involved the anterior horn.
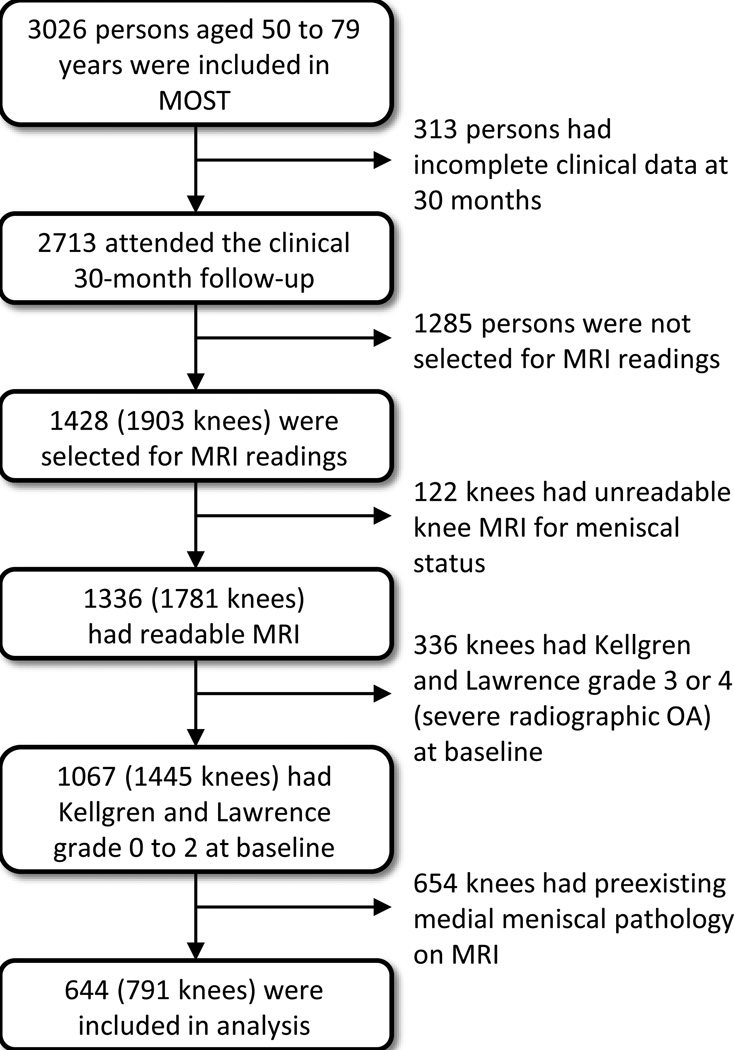
Figure 2. Meniscal tear.
A) Baseline sagittal fat-suppressed proton density-weighted 1.0T MRI shows normal triangular appearance of the posterior horn of the medial meniscus without tear or intramensical signal alterations. B) Follow-up image shows a meniscal tear reaching the superior and inferior surface of the posterior horn (arrow).
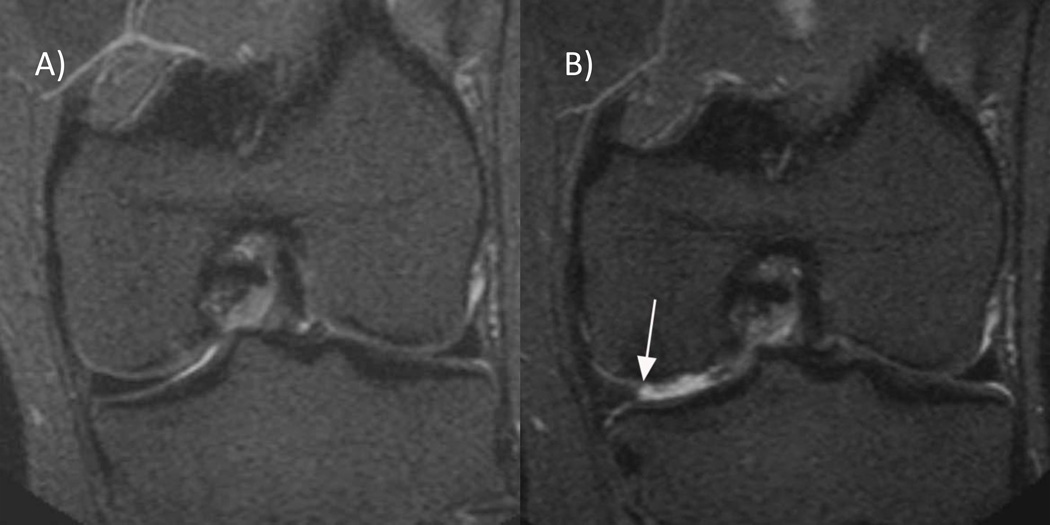
Figure 3. Partial meniscal maceration.
A) Baseline coronal 1.0T STIR MRI shows a normal body of the medial meniscus. B) 30 months follow-up image shows partial maceration of the meniscal body with an amputated triangular appearance (arrow).
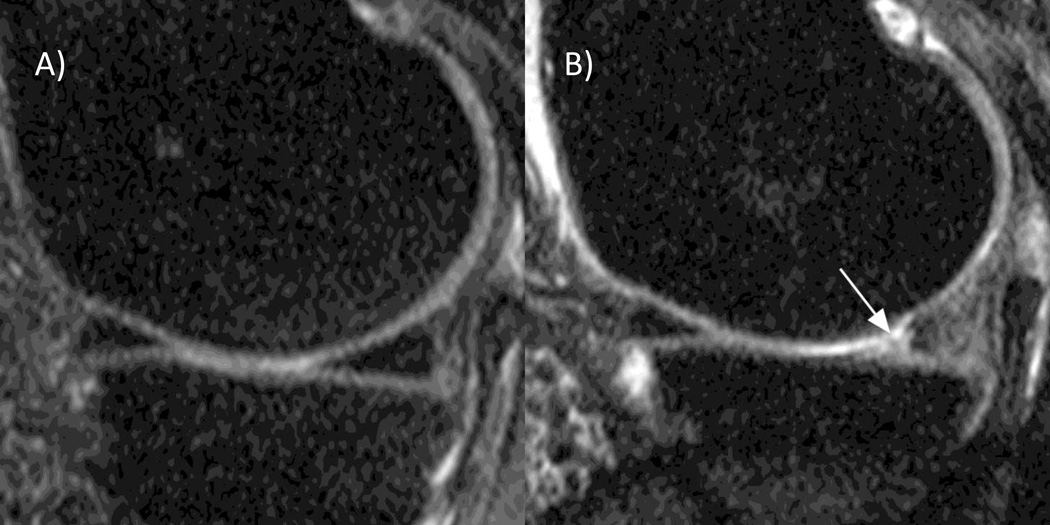
Figure 4. Meniscal extrusion.
A) Baseline coronal 1.0T STIR MRI depicts a normal position of the body of the medial meniscus in alignment with the tibial plateau. B) The 30-months follow-up image shows medial meniscal extrusion of 3 mm in regard to the tibial plateau (arrow).
Of those knees reported to have sustained an injury leading to reduced ability to walk for at least two days during the follow-up, the adjusted odds ratio for medial meniscal pathology was increased by over 4-fold, OR 4.14 (95% confidence interval [95% CI] 2.06, 8.31) (Table 2). Still, the majority, 62 of the 77 knees (81%) with meniscal pathology on the medial side, did not have a report of knee injury during follow-up.
Table 2.
Evaluation of potential risk factors for medial meniscal pathology on magnetic resonance imaging over 30 months in knees (n=791) of middle-aged and elderly persons.
| Risk factor | Crude* OR |
Adjusted† OR (95% CI) |
Fully Adjusted§ OR (95% CI) |
|---|---|---|---|
| Age, years | |||
| 50 to 55 | ref | ref | ref |
| 56 to 63 | 0.95 | 0.96 (0.53, 1.72) | 1.03 (0.55, 1.91) |
| 64 to 79 | 1.03 | 1.12 (0.62, 2.02) | 1.08 (0.57, 2.05) |
| Gender | |||
| male | ref | ref | ref |
| female | 0.71 | 0.70 (0.43, 1.15) | 0.80 (0.46, 1.40) |
| Body mass index, kg/m2 | |||
| <25 | ref | ref | ref |
| 25 to 29 | 0.99 | 0.94 (0.43, 2.02) | 0.96 (0.44, 2.09) |
| 30 or above | 1.69 | 1.62 (0.79, 3.33) | 1.52 (0.73, 3.16) |
| Bony enlargements of finger joints‡, n | |||
| 0 to 4 | ref | ref | ref |
| 5 or more | 1.61 | 1.66 (1.03, 2.68) | 1.64 (1.00, 2.70) |
| Knee injury during follow-up** | |||
| no | ref | ref | ref |
| yes | 4.84 | 4.67 (2.31, 9.42) | 4.14 (2.06, 8.31) |
| Knee alignment | |||
| not varus (≥179°) | ref | ref | ref |
| varus (<179°) | 2.20 | 2.09 (1.24, 3.51) | 2.00 (1.18, 3.40) |
| Leg-length inequality | |||
| less than 1 cm | ref | ref | ref |
| 1 cm or more | 0.84 | 0.80 (0.40, 1.61) | 0. 90 (0.45, 1.80) |
OR= odds ratio, 95% CI=95% confidence interval, ref = reference category
Adjusted for race and clinical site only (generalised estimating equations).
Adjusted for race, clinical site, age, sex, and body mass index only (generalised estimating equations).
The primary model, adjusted for all covariates in the table and race and clinical site (generalised estimating equations).
Test for trend using continuous predictor variable, P=0.28.
Knee injury leading to limited ability to walk for 2 days or more.
Keeping all potential risk factors in the model, including a report of knee injury or not, the estimate of risk was increased by about 60% if the subject had 5 or more bony enlargements of finger joints at baseline compared to 4 or less. Having a varus aligned knee was also associated with 100% increased estimate of risk compared to not being varus. Further, obesity (body mass index 30 or more) had an approximately a 50% increased risk for medial meniscal pathology in the knee compared with having body mass index 25 or less, although this was not statistically significant. Age, gender, and leg-length inequality were found not to substantially affect the risk of medial meniscal pathology over 30 months (Table 2).
Additional adjustment for Kellgren and Lawrence grade at baseline and (or) the MOST recruitment variables did not essentially alter the estimates of risk (data not shown).
The evaluation of risk factors for the development of meniscal lesions and extrusion as two separate outcomes yielded essentially the same overall picture as the model using the composite outcome with the exception of the effect of body mass index. Obesity was a significant risk factor for the development of medial meniscal extrusion, OR 3.04 (95% CI 1.04, 8.93), while it was not so for meniscal lesions, OR 1.15 (95%CI 0.52, 2.54) (Web appendix).
DISCUSSION
This prospective cohort study provides novel evidence in support of the hypothesis that meniscus pathology often is a result of both systemic effects and local biomechanical factors, not only a result of acute knee trauma. Importantly, while knee trauma, which was associated with approximately 4-fold increased risk, was the most notable risk factor for medial meniscal pathology in this study, still 81% of persons who developed such pathology did not report knee trauma during follow-up.
Of the systemic risk factors we evaluated, bony enlargements of finger joints can help identify subjects with generalised osteoarthritis and has strong genetic determinants.[26, 27] Furthermore, Heberden’s nodes have been associated with incidence and progression of knee osteoarthritis.[33] We found that having multiple bony enlargements of finger joints was associated with about 60% increased risk of developing meniscal pathology. In support of our findings, in subjects followed-up after knee meniscectomy, those with radiographic hand osteoarthritis more often had a degenerative type of meniscal tear at the index surgery.[28] Further, systemic effects on e.g., collateral ligaments and degeneration of meniscal attachments may predispose to meniscal extrusion.[34–36] The present longitudinal data support the hypothesis that the meniscal tissue is affected by degradation possibly related to an early-stage generalised osteoarthritis process.[28, 37]
Of the biomechanical risk factors that we evaluated, knee malalignment, which may be influenced by familiar factors[38], is an important compartment-specific risk factor for osteoarthritis progression[39, 40] and has been associated with meniscal pathology in knee osteoarthritis.[41] However, the role of malalignment in disease initiation remains controversial.[42, 43] We found that knees with varus malalignment at baseline, i.e., increased loading of the medial compartment, vs. not varus had about 100% increased risk of meniscal pathology in the same compartment. The finding corroborates an arthroscopy-series where medial meniscal tear was associated with varus alignment.[44] It is plausible that meniscal destruction and extrusion may also contribute directly to altered alignment of the knee. One challenge is the difficulty to tease out the effects of altered meniscus integrity and positioning from effects of e.g., bone attrition and cartilage loss.[41, 45]
Our study failed to detect any significant effect of leg-length inequality with respect to the development of meniscal pathology. The number of subjects with leg-length inequality was however low. Negative findings must in general be interpreted with caution due to the outcome being uncommon and low prevalence of certain risk factors. Importantly, the analyses evaluating meniscal lesions and meniscal extrusion as separate outcomes revealed that obesity seemed to be a stronger risk factor for extrusion than meniscal lesions. Results for the other risk factors were essentially the same for both meniscal lesions and extrusion (Web appendix).
Knee osteoarthritis is often a result of increased biomechanical loading in susceptible individuals and the pathological response of joint tissues to such abnormal biomechanical stress.[46] This study sheds further light on a plausible pathway by which knee malalignment and obesity could result in chronic overloading. Such overloading, coupled with degenerative meniscal matrix changes due to “osteoarthritis in the meniscus”, could lead to meniscal fatigue and rupture/extrusion. Once the meniscus loses its critical function in the knee joint, increased biomechanical loading patterns on joint cartilage may result in cartilage loss[13, 14], bone alterations including trabecular bone changes[47], increased bone mineral density[48], development of subchondral bone marrow lesions.[16], and increasing malalignment. The vicious cycle of knee osteoarthritis is in motion.
All of our exposure variables were observed independently of the MRI-based meniscal outcome variable minimising the risk of dependent errors or other bias. However, measurements of the outcome and certain exposure variables are still subject to measurement error, which may result in misclassification. This misclassification is expected to be non-differential, biasing estimates of effect toward the null. We do not know the true nature or severity of the knee injuries reported. Hence, we cannot exclude the possibility of recall bias, i.e., more frequent recollection of knee injury if having a painful knee. Further, there is one report of increased prevalence of meniscal tear in professional floor layers exposed to frequent kneeling suggesting that chronic overloading might be a risk factor.[49] We cannot exclude the possibility of residual confounding due to physically demanding occupational or recreational activities increasing the risk for both bony enlargements of the hands and meniscal pathology. However, knee injury may to a certain extent serve as a proxy for such possible activities and we controlled for that in our analyses. Our observation period of 30 months is a relatively short time perspective with respect to the development of degenerative changes of meniscal tissue. More time points and even longer follow-ups including future studies of the association with patient-relevant outcomes such as knee pain will provide further information on the natural course of meniscal pathology, its risk factors, and its impact in knee osteoarthritis. Further studies will also be required to study factors associated with meniscal pathology in younger individuals.
In conclusion, this prospective study provides important evidence of a combined systemic and local biomechanical effect on the risk of developing meniscal pathology in middle-aged and elderly persons. For medial meniscal pathology, generalised osteoarthritis expressed as the presence of multiple bony enlargements of finger joints and varus alignment were found to be risk factors in addition to knee injury.
Acknowledgments
We would like to thank all MOST staff and study participants at Birmingham, Alabama and Iowa City, Iowa, the UCSF MOST Coordinating Center, San Francisco, California (especially Charles McCulloch and Irina Tolstykh), and the staff at Boston University Clinical Epidemiology Research and Training Unit.
FUNDING
The Multicenter Osteoarthritis (MOST) Study is a cooperative epidemiological study of knee OA funded by the National Institute on Aging (NIA): Felson – 1 U01 AG18820; Torner – 1 U01 AG18832; Lewis – 1 U01 AG18947; Nevitt – 1 U01 AG19069. Dr Englund is supported by the Swedish Research Council, and the Faculty of Medicine, Lund University, Sweden.
Footnotes
POTENTIAL COMPETING INTERESTS
AG is shareholder of Boston Imaging Core Lab, LLC (BICL), Boston, Massachusetts, USA, a company providing radiological image assessment services, and Synarc Inc, and consultant to Merck Serono, Novartis, Genzyme, Facet Solutions and Stryker. FWR and MDC are shareholders of BICL. None of the other authors have declared any conflict of interest.
LICENCE STATEMENT
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Annals of the Rheumatic Diseases and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence (http://group.bmj.com/products/journals/instructions-for-authors/licence-forms).
REFERENCES
- 1.Fukubayashi T, Kurosawa H. The contact area and pressure distribution pattern of the knee A study of normal and osteoarthrotic knee joints. Acta Orthop Scand. 1980 Dec;51(6):871–879. doi: 10.3109/17453678008990887. [DOI] [PubMed] [Google Scholar]
- 2.Seedhom BB, Hargreaves DJ. Transmission of the load in the knee joint with special reference to the role of the meniscus. Part I+II. Eng Med. 1979;4:207–228. [Google Scholar]
- 3.Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975;(109):184–192. doi: 10.1097/00003086-197506000-00027. [DOI] [PubMed] [Google Scholar]
- 4.Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009 Mar;60(3):831–839. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum. 2004;50(9):2811–2819. doi: 10.1002/art.20489. [DOI] [PubMed] [Google Scholar]
- 6.Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30:164–170. [PubMed] [Google Scholar]
- 7.Jørgensen U, Sonne-Holm S, Lauridsen F, Rosenklint A. Long-term follow-up of meniscectomy in athletes A prospective longitudinal study. J Bone Joint Surg Br. 1987 Jan;69(1):80–83. doi: 10.1302/0301-620X.69B1.3818740. [DOI] [PubMed] [Google Scholar]
- 8.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006 May 27;367(9524):1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 9.Smillie IS. Injuries of the Knee Joint. 3. Baltimore: The Williams and Wilkins Co; 1962. Surgical Pathology of the Menisci; pp. 51–90. [Google Scholar]
- 10.Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008 Sep 11;359(11):1108–1115. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gale DR, Chaisson CE, Totterman SM, Schwartz RK, Gale ME, Felson D. Meniscal subluxation: association with osteoarthritis and joint space narrowing. Osteoarthritis Cartilage. 1999 Nov;7(6):526–532. doi: 10.1053/joca.1999.0256. [DOI] [PubMed] [Google Scholar]
- 12.Noble J, Hamblen DL. The pathology of the degenerate meniscus lesion. J Bone Joint Surg Br. 1975 May;57(2):180–186. [PubMed] [Google Scholar]
- 13.Berthiaume MJ, Raynauld JP, Martel-Pelletier J, Labonte F, Beaudoin G, Bloch DA, et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis. 2005 Apr;64(4):556–563. doi: 10.1136/ard.2004.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006 Mar;54(3):795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 15.Ding C, Martel-Pelletier J, Pelletier JP, Abram F, Raynauld JP, Cicuttini F, et al. Meniscal tear as an osteoarthritis risk factor in a largely non-osteoarthritic cohort: a cross-sectional study. J Rheumatol. 2007 Apr;34(4):776–784. [PubMed] [Google Scholar]
- 16.Englund M, Guermazi A, Roemer FW, Yang M, Zhang Y, Nevitt MC, et al. Meniscal pathology on MRI increases the risk for both incident and enlarging subchondral bone marrow lesions of the knee: the MOST Study. Ann Rheum Dis. Apr 26; doi: 10.1136/ard.2009.121681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felson DT, Nevitt MC. Epidemiologic studies for osteoarthritis: new versus conventional study design approaches. Rheum Dis Clin North Am. 2004 Nov;30(4):783–797. doi: 10.1016/j.rdc.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995 Jul;5(4):297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 19.Kothari M, Guermazi A, von Ingersleben G, Miaux Y, Sieffert M, Block JE, et al. Fixed-flexion radiography of the knee provides reproducible joint space width measurements in osteoarthritis. Eur Radiol. 2004 Sep;14(9):1568–1573. doi: 10.1007/s00330-004-2312-6. [DOI] [PubMed] [Google Scholar]
- 20.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003 Mar;32(3):128–132. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 21.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthritis. Ann Rheum Dis. 1957;16:494–501. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roemer FW, Guermazi A, Lynch JA, Peterfy CG, Nevitt MC, Webb N, et al. Short tau inversion recovery and proton density-weighted fat suppressed sequences for the evaluation of osteoarthritis of the knee with a 1.0 T dedicated extremity MRI: development of a time-efficient sequence protocol. Eur Radiol. 2005 May;15(5):978–987. doi: 10.1007/s00330-004-2608-6. [DOI] [PubMed] [Google Scholar]
- 23.Roemer FW, Lynch JA, Niu J, Zhang Y, Crema MD, Tolstykh I, et al. A comparison of dedicated 1.0 T extremity MRI vs large-bore 1.5 T MRI for semiquantitative whole organ assessment of osteoarthritis: the MOST study. Osteoarthritis Cartilage. Feb;18(2):168–174. doi: 10.1016/j.joca.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roemer FW, Guermazi A, Javaid MK, Lynch JA, Niu J, Zhang Y, et al. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: the MOST Study. A longitudinal multicentre study of knee osteoarthritis. Ann Rheum Dis. 2009 Sep;68(9):1461–1465. doi: 10.1136/ard.2008.096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004 Mar;12(3):177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Kellgren JH, Moore R. Generalised osteoarthritis and Heberden's nodes. Br Med J. 1952;i:181–184. doi: 10.1136/bmj.1.4751.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stecher RM. Heberden's nodes: heredity in hypertrophic arthritis of the finger joints. Am J Med Sci. 1941;210:801–809. [Google Scholar]
- 28.Englund M, Paradowski PT, Lohmander LS. Association of radiographic hand osteoarthritis with radiographic knee osteoarthritis after meniscectomy. Arthritis Rheum. 2004;50(2):469–475. doi: 10.1002/art.20035. [DOI] [PubMed] [Google Scholar]
- 29.Thaper A, Zhang W, Wright G, Doherty M. Relationship between Heberden's nodes and underlying radiographic changes of osteoarthritis. Ann Rheum Dis. 2005 Aug;64(8):1214–1216. doi: 10.1136/ard.2004.031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Englund M, Niu J, Guermazi A, Roemer FW, Hunter DJ, Lynch JA, et al. Effect of meniscal damage on the development of frequent knee pain, aching, or stiffness. Arthritis Rheum. 2007 Nov 29;56(12):4048–4054. doi: 10.1002/art.23071. [DOI] [PubMed] [Google Scholar]
- 31.Bhave A, Paley D, Herzenberg JE. Improvement in gait parameters after lengthening for the treatment of limb-length discrepancy. J Bone Joint Surg Am. 1999 Apr;81(4):529–534. doi: 10.2106/00004623-199904000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Harvey WF, Yang M, Cooke TD, Segal NA, Lane N, Lewis CE, et al. Association of leg-length inequality with knee osteoarthritis: a cohort study. Ann Intern Med. Mar 2;152(5):287–295. doi: 10.1059/0003-4819-152-5-201003020-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000 May;43(5):995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Blankenbaker DG, De Smet AA, Fine JP. Is intra-articular pathology associated with MCL edema on MR imaging of the non-traumatic knee? Skeletal Radiol. 2005 Aug;34(8):462–467. doi: 10.1007/s00256-005-0931-x. [DOI] [PubMed] [Google Scholar]
- 35.Tan AL, Toumi H, Benjamin M, Grainger AJ, Tanner SF, Emery P, et al. Combined high-resolution magnetic resonance imaging and histological examination to explore the role of ligaments and tendons in the phenotypic expression of early hand osteoarthritis. Ann Rheum Dis. 2006 Oct;65(10):1267–1272. doi: 10.1136/ard.2005.050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen DY, Propeck T, Kane SM, Godbee MT, Rall KL. MRI description of knee medial collateral ligament abnormalities in the absence of trauma: edema related to osteoarthritis and medial meniscal tears. Magn Reson Imaging. 2007 Feb;25(2):209–214. doi: 10.1016/j.mri.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a 16-year followup of meniscectomy. Arthritis Rheum. 2003;48(8):2178–2187. doi: 10.1002/art.11088. [DOI] [PubMed] [Google Scholar]
- 38.Tufan A, Meulenbelt I, Bijsterbosch J, Kroon HM, Bierma-Zeinstra SM, Nelissen RG, et al. Familial influence on tibiofemoral alignment. Ann Rheum Dis. Mar;69(3):542–545. doi: 10.1136/ard.2008.097873. [DOI] [PubMed] [Google Scholar]
- 39.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001 Jul 11;286(2):188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 40.Sharma L, Eckstein F, Song J, Guermazi A, Prasad P, Kapoor D, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008 Jun;58(6):1716–1726. doi: 10.1002/art.23462. [DOI] [PubMed] [Google Scholar]
- 41.Hunter DJ, Zhang Y, Niu J, Tu X, Amin S, Goggins J, et al. Structural factors associated with malalignment in knee osteoarthritis: the Boston osteoarthritis knee study. J Rheumatol. 2005 Nov;32(11):2192–2199. [PubMed] [Google Scholar]
- 42.Hunter DJ, Niu J, Felson DT, Harvey WF, Gross KD, McCree P, et al. Knee alignment does not predict incident osteoarthritis: the Framingham osteoarthritis study. Arthritis Rheum. 2007 Apr;56(4):1212–1218. doi: 10.1002/art.22508. [DOI] [PubMed] [Google Scholar]
- 43.Sharma L, Song J, Dunlop D, Felson D, Lewis CE, Segal N, et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis. Nov;69(11):1940–1945. doi: 10.1136/ard.2010.129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Habata T, Ishimura M, Ohgushi H, Tamai S, Fujisawa Y. Axial alignment of the lower limb in patients with isolated meniscal tear. J Orthop Sci. 1998;3(2):85–89. doi: 10.1007/s007760050026. [DOI] [PubMed] [Google Scholar]
- 45.Hunter DJ, Zhang YQ, Tu X, Lavalley M, Niu JB, Amin S, et al. Change in joint space width: hyaline articular cartilage loss or alteration in meniscus? Arthritis Rheum. 2006 Aug;54(8):2488–2495. doi: 10.1002/art.22016. [DOI] [PubMed] [Google Scholar]
- 46.Englund M. The role of biomechanics in the initiation and progression of OA of the knee. Best Pract Res Clin Rheumatol. 2010 Feb;24(1):39–46. doi: 10.1016/j.berh.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Podsiadlo P, Dahl L, Englund M, Lohmander LS, Stachowiak GW. Differences in trabecular bone texture between knees with and without radiographic osteoarthritis detected by fractal methods. Osteoarthritis Cartilage. 2008 Mar;16(3):323–329. doi: 10.1016/j.joca.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 48.Lo GH, Niu J, McLennan CE, Kiel DP, McLean RR, Guermazi A, et al. Meniscal damage associated with increased local subchondral bone mineral density: a Framingham study. Osteoarthritis Cartilage. 2008 Feb;16(2):261–267. doi: 10.1016/j.joca.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rytter S, Jensen LK, Bonde JP, Jurik AG, Egund N. Occupational kneeling and meniscal tears: a magnetic resonance imaging study in floor layers. J Rheumatol. 2009 Jul;36(7):1512–1519. doi: 10.3899/jrheum.081150. [DOI] [PubMed] [Google Scholar]