Abstract
Objective
To assess short- and long-term reproducibility of marrow adipose tissue (MAT) quantification by 1H-MR spectroscopy
Materials and Methods
Our study was IRB-approved and HIPAA compliant. Written informed consent was obtained. We studied 20 overweight/obese but otherwise healthy subjects (12 f, 8 m) with a mean age of 37±6 years. All subjects underwent proton magnetic resonance spectroscopy (1H-MRS) of the 4th lumbar vertebral body using a single-voxel point-resolved spatially localized spectroscopy sequence without water suppression at 3 Tesla. Measurements were repeated after 6 weeks and 6 months using identical scanning protocols. The following clinical parameters were collected: weight, BMI, exercise status, and trabecular bone mineral density (BMD) by quantitative computed tomography. Short-term (baseline - 6-weeks) and long-term (baseline - 6-month) reproducibility of MAT was assessed by coefficient of variance (CV), standard deviation (SD), and interclass correlation coefficients (ICC). Short- and long-term changes in clinical parameters were assessed by paired t-test.
Results
For short-term reproducibility between baseline and 6-week scans CV was 9.9%, SD was 0.08 and ICC was 0.97 (95% CI 0.94–099). For long-term reproducibility between baseline and 6 months scans CV was 12.0%, SD was 0.10, and ICC was 0.95 (95% CI 0.88 to 0.98). There was no significant short- or long-term change in clinical parameters (weight, BMI, exercise status, BMD) (p>0.2).
Conclusion
1H-MRS is a reproducible method for short- and long-term quantification of MAT. Our results can guide sample size calculations for interventional and longitudinal studies.
Keywords: Proton MR spectroscopy, marrow adipose tissue, reproducibility
Introduction
Recent studies have established an important link between bone and fat and explored the potential impact of marrow adipose tissue (MAT) in the pathogenesis of bone loss (1, 2). Bone strength is affected not only by bone mineral density (BMD) and bone microarchitecture (3), but also its micro-environment (4). MAT is increased in several osteoporotic states, such as advanced age, immobility, glucocorticoid use, and anorexia nervosa (1, 5), suggesting that MAT may contribute to impaired bone strength and increased fracture risk.
The non-invasive quantification of MAT using proton magnetic resonance spectroscopy (1H-MRS) has improved the feasibility of assessing MAT in vivo (5–10). Moreover, MAT content assessed by 1H-MRS in combination with BMD by DXA may be more valuable than either parameter alone in evaluating skeletal integrity (4, 11). Studies have demonstrated an inverse association between MAT and BMD in adults with obesity and anorexia nervosa (5, 12, 13) and MAT assessed by 1H-MRS has recently been incorporated as an endpoint in clinical trials (14, 15). The diagnostic value of this technique depends greatly upon the precision of the measurements and thus the reliability of any estimated change.
Most studies on 1H-MRS variability for MAT quantification only performed measurements within the same day after repositioning (5, 16) and there is limited data on longer-term reproducibility. These data are relevant for sample size calculations for longitudinal and interventional studies where MAT by 1H-MRS is used as a biomarker of skeletal integrity.
The purpose of our study was therefore to assess short- and long-term reproducibility of MAT quantification by 1H-MRS.
Materials and Methods
The study was approved by our Institutional Review Board and complied with Health Insurance Portability and Accountability Act guidelines. Written informed consent was obtained from all subjects after the nature of the procedures had been fully explained.
Subjects
The study group was comprised of 20 healthy overweight/obese premenopausal women and men of similar mean age who were part of placebo groups in two 6-month clinical trials (17, 18). Inclusion criteria were ages 18 to 45 years, BMI ≥ 25 kg/m2, and eumenorrhea in women. Exclusion criteria included hypothalamic or pituitary disorders, diabetes mellitus or other chronic illnesses, smoking, use of osteoporosis medication or medication that could influence bone metabolism, and contraindications to MRI such as the presence of a pacemaker or metallic implant. Subjects were instructed to maintain a stable diet and exercise regimen over the 6-month period.
Each participant underwent 1H-MRS for quantification of MAT content. Because MAT is known to be affected by BMI (5, 8), physical activity (19, 20), and trabecular BMD (7, 21), we assessed these parameters at time of 1H-MRS to ensure stability of these measures. Activity was assessed using the Paffenbarger questionnaire, a self-administered questionnaire that ascertains current levels of activity. Trabecular BMD of the 4th lumbar vertebral body was measured by quantitative computed tomography (QCT) as previously described (22). All studies were repeated after 6 weeks and after 6 months using identical protocols and equipment.
Clinical characteristics and MAT data have been previously reported in a subset of subjects (8, 12–14); however no data on MAT reproducibility have been described in any of the subjects.
1H-MR spectroscopy of bone marrow
All subjects underwent 1H-MRS of the 4th lumbar vertebral body to determine MAT content after an 8-hour overnight fast using a 3.0T MRI (Siemens Trio, Siemens Medical Systems, Erlangen, Germany). A body matrix phased array coil was positioned over the lumbar region. A tri-plane gradient echo localizer pulse sequences of the lumbar spine was obtained with echo time (TE) of 5 ms and repetition time (TR) of 15 ms, and slice thickness of 3mm to localize the L4 vertebral body. A voxel measuring 15 × 15 × 15 mm3 (3.4 mL) was placed within the L4 vertebral body. Single-voxel 1H-MRS was acquired using a point-resolved spatially localized spectroscopy (PRESS) pulse sequence without water suppression (TE: 30 ms, TR: 3,000 ms, 8 acquisitions, 1024 data points, receiver bandwidth: 2 KHz). For each voxel placement, automated optimization of gradient shimming was performed. Scan time for the localizer sequence and 1H-MRS including shimming was 4 min.
1H-MR Spectroscopy Data Analysis
Fitting of all 1H-MRS data was performed using LCModel (version 6.3-0K) (23). Data were transferred from the scanner to a Linux workstation and metabolite quantification was performed using eddy current correction and water scaling. A customized fitting algorithm for bone marrow analysis (LCModel “marrow”) provided estimates for 5 lipid peaks: methyl protons at 0.9 ppm (-CH3); methylene protons at 1.3 ppm [(-CH2-)n]; methylene protons β to carbonyl at 1.6 ppm (-CH2-O-CO-CH2-CH2-); allylic methylene protons at 2.0 ppm (-CH=CH-CH2); and olefinic protons at 5.3 ppm (-CH=CH-). Total marrow lipid content was determined by combining all lipid peaks (0.9, 1.3, 1.6, 2.0, and 5.3 ppm). Lipid resonances were scaled to unsuppressed water peak (4.7 ppm) and expressed in MAT fraction
Statistical Analysis
JMP Statistical Database Software (version 11.0; SAS Institute, Cary, NC) and MedCalc software (version 14; Mariakerke, Belgium) was used for statistical analyses. Short- and long-term reproducibility was assessed by coefficient of variance (CV), standard deviation (SD), and interclass correlation coefficient (ICC) with 95% confidence intervals (CI). Because bone marrow fat can be influenced by BMI, exercise status, and trabecular BMD, we assessed short- and long-term stability of these parameters by paired t-test. P < 0.05 was used to denote significance. Data are presented as mean ± SD.
Results
Baseline subject characteristics are shown in Table 1. The study group included 12 women and 8 men. The age of study participants ranged from 26 to 43 years, with a mean age of 37±6 years. BMI of study participants ranged from 25.1 to 46.7 kg/m2, with a mean BMI of 35.5±6.0 kg/m2.
Table 1.
Baseline characteristics
| Variable | Subjects (n=20) |
|---|---|
| Age (years) | 37±6 |
| Weight (kg) | 102.2±22.0 |
| BMI (kg/m2) | 35.5±6.0 |
| Vigorous activity (hours/week) | 5.6±7.6 |
| Moderate activity (hours/week) | 13.8±14.1 |
| L4 trabecular BMD (mg/cm3) | 149.5±26.3 |
| L4 marrow adipose tissue lipid/water ratio | 0.73±0.33 |
Data presented as mean ± SD. BMI: body mass index, BMD: bone mineral density
For short-term reproducibility between baseline and 6-week scans CV was 9.9%, SD was 0.08 and ICC was 0.97 (95% CI 0.94 to 0.99) (Figure 1). There was no significant change in weight (p=0.2), BMI (p=0.2), vigorous activity (p=0.9), moderate activity (p=0.8), or trabecular BMD (p=0.6) between the baseline and 6-week visits.
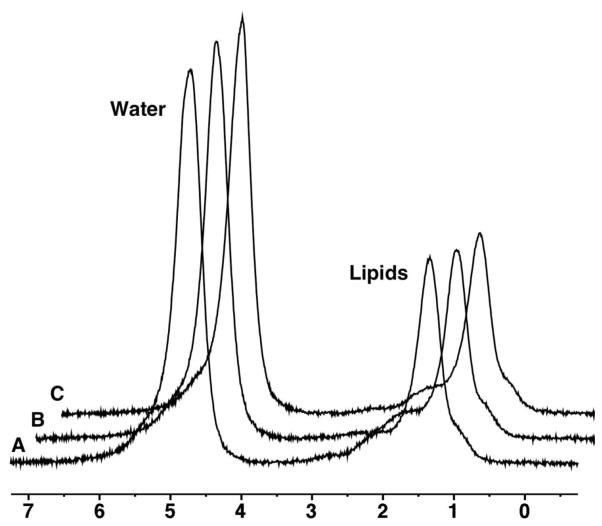
Figure 1.
1H-MRS of L4 in a 43 year-old woman obtained at baseline (A), 6 weeks (B), and 6 months (C). Marrow adipose tissue (MAT) content by 1H-MR spectroscopy (1H-MRS) shows low variation between scans (lipid to water ratio 0.54 at baseline, 0.52 at 6 weeks and 0.53 at 6 months). For purposes of visual comparison, the amplitudes of unsuppressed water are scaled identically.
For long-term reproducibility between baseline and 6-month scans CV was 12.3%, SD was 0.10, and ICC was 0.95 (95% CI 0.86 to 0.98) (Figure 1 and Table 2). There was no significant change in weight (p=0.2), BMI (p=0.2), vigorous activity (p=0.3), moderate activity (p=0.9), or trabecular BMD (p=0.3) between the baseline and 6-month visits.
Table 2.
Short- and long-term reproducibility of marrow adipose tissue (MAT) quantification by proton magnetic resonance spectroscopy (1H-MRS).
| Coefficient of Variation (CV) | Standard Deviation (SD) | Interclass Correlation Coefficient (ICC) (95% confidence interval) | |
|---|---|---|---|
| Baseline to 6-week reproducibility | 9.9% | 0.08 | 0.97 (0.94–0.99) |
| Baseline to 6-month reproducibility | 12.3% | 0.10 | 0.95 (0.86 to 0.98) |
Discussion
Recent studies suggest that marrow adipogenesis may play an important role in the pathogenesis of osteoporosis, and MAT quantification has received increasing attention as a potential biomarker for skeletal integrity (1, 24). In fact, targeting MAT may become a novel therapeutic approach for osteoporosis (1, 25). Therefore, there is a growing need for non-invasive and accurate quantification of MAT content.
1H-MRS is a non-invasive technique that is frequently used to assess MAT in vivo (5–10). The variability of MAT concentrations between imaging sessions is of consequence for longitudinal studies performed to investigate effects of pharmacological, dietary, or other interventions. As measurements may vary within a subject, knowledge of such differences is important to accurately determine the clinical significance of pathophysiological changes following interventions.
Prior studies have focused on the inter-day reproducibility of 1H-MRS for MAT quantification by repositioning subjects between scans. Li et al reported a CV of 1.7% of MAT by1H-MRS of the lumbar spine scanning 6 subjects twice on the same day after repositioning (16). We previously performed 1H-MRS of the lumbar spine in 5 subjects twice on the same day after repositioning and found a mean difference of 4.5% between measurements (5). In a different study using 1H-MRS of the femur in 3 volunteers scanned within 10 days we found a CV of 5% (26). Griffith et al performed 1H-MRS of the proximal femur in 36 subjects twice within one week and reported ICCs between 0.78 and 0.85 (27). However, there are no data on longer-term variability of MAT quantification. We therefore scanned a group of healthy subjects three times over a period of 6 months under controlled conditions using identical imaging protocols. Our study shows that reproducible MAT measurements can be obtained by 1H-MRS with acceptable short-term and long-term CVs and high ICCs.
In our study, the CVs of scans obtained after 6 weeks and 6 months were 9.9% and 12%, respectively, which is higher than the reported CVs from studies obtained when subjects were rescanned the same day after repositioning. However, our ICCs of 0.97 and 0.95 demonstrate better agreement than the reported values from scans performed after one week. Our results provide data by which study designs can be optimized and study sample size powered for the detection of changes in MAT during interventional and longitudinal trials.
Factors affecting the variability of 1H-MRS MAT quantification include natural biologic variations in metabolite concentrations and variations related to physical activity, diet, trabecular bone, or medication use, as well as non-biologic factors related to equipment instability, voxel placement, and patient repositioning. We attempted to control biological factors such as weight and exercise between scans intervals and performed all imaging after an overnight fast. In addition, we used identical 1H-MRS protocols with reproducible voxel localization to minimize imperfect relocalization as a substantial source of measurement variability. Nevertheless, our study had several limitations. First, our study group was comprised of overweight/obese but otherwise healthy volunteers and our data may not be able to be extrapolated to patients with osteoporosis or other marrow disorders. Second, we only performed MAT measurements of the L4 vertebral body and did not study MAT variability of other vertebral bodies or the femur.
In conclusion, 1H-MRS is a reproducible method for short- and long-term quantification of MAT. Our results can guide sample size calculations for interventional and longitudinal studies where assessments are repeated over time in different imaging sessions.
Acknowledgments
Funding: This research was supported by the National Institutes of Health grants R01 HL-077674, UL1 RR 025758, and K23 RR-23090.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 2.Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med. 2009;122:409–414. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 3.Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement. 2000;17:1–45. [PubMed] [Google Scholar]
- 4.Schellinger D, Lin CS, Hatipoglu HG, Fertikh D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol. 2001;22:1620–1627. [PMC free article] [PubMed] [Google Scholar]
- 5.Bredella MA, Fazeli PK, Miller KK, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:2129–2136. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum T, Yap SP, Karampinos DC, et al. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J Magn Reson Imaging. 2012;35:117–124. doi: 10.1002/jmri.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bredella MA, Daley SM, Kalra MK, Brown JK, Miller KK, Torriani M. Marrow Adipose Tissue Quantification of the Lumbar Spine by Using Dual-Energy CT and Single-Voxel H MR Spectroscopy: A Feasibility Study. Radiology. 2015:142876. doi: 10.1148/radiol.2015142876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bredella MA, Gill CM, Gerweck AV, et al. Ectopic and serum lipid levels are positively associated with bone marrow fat in obesity. Radiology. 2013;269:534–541. doi: 10.1148/radiol.13130375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffith JF, Yeung DK, Ma HT, Leung JC, Kwok TC, Leung PC. Bone marrow fat content in the elderly: a reversal of sex difference seen in younger subjects. J Magn Reson Imaging. 2012;36:225–230. doi: 10.1002/jmri.23619. [DOI] [PubMed] [Google Scholar]
- 10.Jaramillo D, Bedoya MA, Wang DJ, et al. Quantification of Bone Marrow Involvement in Treated Gaucher Disease With Proton MR Spectroscopy: Correlation With Bone Marrow MRI Scores and Clinical Status. AJR Am J Roentgenol. 2015;204:1296–1302. doi: 10.2214/AJR.14.13563. [DOI] [PubMed] [Google Scholar]
- 11.Schellinger D, Lin CS, Lim J, Hatipoglu HG, Pezzullo JC, Singer AJ. Bone marrow fat and bone mineral density on proton MR spectroscopy and dual-energy X-ray absorptiometry: their ratio as a new indicator of bone weakening. AJR Am J Roentgenol. 2004;183:1761–1765. doi: 10.2214/ajr.183.6.01831761. [DOI] [PubMed] [Google Scholar]
- 12.Bredella MA, Lin E, Gerweck AV, et al. Determinants of bone microarchitecture and mechanical properties in obese men. J Clin Endocrinol Metab. 2012;97:4115–4122. doi: 10.1210/jc.2012-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bredella MA, Torriani M, Ghomi RH, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 2011;19:49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bredella MA, Gerweck AV, Barber LA, et al. Effects of growth hormone administration for 6 months on bone turnover and bone marrow fat in obese premenopausal women. Bone. 2014;62:29–35. doi: 10.1016/j.bone.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schafer AL, Li X, Schwartz AV, et al. Changes in vertebral bone marrow fat and bone mass after gastric bypass surgery: A pilot study. Bone. 2015;74:140–145. doi: 10.1016/j.bone.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Kuo D, Schafer AL, et al. Quantification of vertebral bone marrow fat content using 3 Tesla MR spectroscopy: reproducibility, vertebral variation, and applications in osteoporosis. J Magn Reson Imaging. 2011;33:974–979. doi: 10.1002/jmri.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bredella MA, Gerweck AV, Lin E, et al. Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity. J Clin Endocrinol Metab. 2013;98:3864–3872. doi: 10.1210/jc.2013-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bredella MA, Lin E, Brick DJ, et al. Effects of GH in women with abdominal adiposity: a 6-month randomized, double-blind, placebo-controlled trial. Eur J Endocrinol. 2012;166:601–611. doi: 10.1530/EJE-11-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph RP, Casazza K, Durant NH. The effect of a 3-month moderate-intensity physical activity program on body composition in overweight and obese African American college females. Osteoporos Int. 2014;25:2485–2491. doi: 10.1007/s00198-014-2825-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singhal V, Maffazioli GD, Cano Sokoloff N, et al. Regional fat depots and their relationship to bone density and microarchitecture in young oligo-amenorrheic athletes. Bone. 2015;77:83–90. doi: 10.1016/j.bone.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machann J, Stefan N, Schick F. (1)H MR spectroscopy of skeletal muscle, liver and bone marrow. Eur J Radiol. 2008;67:275–284. doi: 10.1016/j.ejrad.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 22.Bredella MA, Torriani M, Ghomi RH, et al. Determinants of bone mineral density in obese premenopausal women. Bone. 2011;48:748–754. doi: 10.1016/j.bone.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 24.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int. 2008;19:1323–1330. doi: 10.1007/s00198-008-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bredella MA, Fazeli PK, Daley SM, et al. Marrow fat composition in anorexia nervosa. Bone. 2014;66:199–204. doi: 10.1016/j.bone.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffith JF, Yeung DK, Chow SK, Leung JC, Leung PC. Reproducibility of MR perfusion and (1)H spectroscopy of bone marrow. J Magn Reson Imaging. 2009;29:1438–1442. doi: 10.1002/jmri.21765. [DOI] [PubMed] [Google Scholar]