Abstract
Background
To evaluate the feasibility of executing a randomized controlled trial of progressive resistance training (PRT) in women with polycystic ovary syndrome (PCOS).
Methods
Women with PCOS were randomized to an experimental (PRT) group or a no-exercise (usual care) control group. The PRT group was prescribed two supervised and two unsupervised (home-based) training sessions per week for 12 weeks. Feasibility outcomes included recruitment and attrition, adherence, adverse events, and completion of assessments. Secondary outcomes, collected pre and post intervention, included a range of pertinent physiological, functional and psychological measures.
Results
Fifteen participants were randomised into the PRT group (n = 8) or control group (n = 7); five women (n = 2 in PRT group and n = 3 in control group) withdrew from the study. The most successful recruitment sources were Facebook (40 %) and online advertisement (27 %), while least successful methods were referrals by clinicians, colleagues and flyers. In the PRT group, attendance to supervised sessions was higher (95 %; standard deviation ±6 %) compared to unsupervised sessions (51 %; standard deviation ±28 %). No adverse events were attributed to PRT. Change in menstrual cycle status was not significantly different between groups over time (p = 0.503). However, the PRT group significantly increased body weight (p = 0.01), BMI (p = 0.04), lean mass (p = 0.01), fat-free mass (p = 0.005) and lower body strength (p = 0.03), while reducing waist circumference (p = 0.03) and HbA1c (p = 0.033) versus the control group. The PRT group also significantly improved across several domains of disease-specific and general health-related quality of life, depression, anxiety and exercise self-efficacy.
Conclusion
A randomized controlled trial of PRT in PCOS would be feasible, and this mode of exercise may elicit a therapeutic effect on clinically important outcomes in this cohort. The success of a large-scale trial required to confirm these findings would be contingent on addressing the feasibility hurdles identified in this study with respect to recruitment, attrition, compliance, and collection of standardized clinical data.
Trial registration
Australia New Zealand Clinical Trials Registry; ACTRN12614000517673 Registered 15 May 2014.
Keywords: Weight training, Exercise, Quality of life, Menstrual cyclicity, Psychological health
Background
Polycystic ovary syndrome (PCOS) is a common endocrine disease that affects 9-18 % of women [1]. Hyperandrogenism, menstrual irregularity and polycystic ovaries define the condition [2], and common features include insulin resistance, hirsutism, acne, alopecia, and markers of cardiometabolic disease risk (e.g. android obesity, inflammation, dyslipidemia, etc.) [3, 4]. PCOS is a leading cause of oligo/anovulation and oligo/amenorrhea, infertility, and miscarriages [5, 6]. Depression [7], anxiety [8], body image difficulties [9] and low health-related quality of life (HRQoL) [10] are common in this population.
Clinical trials in PCOS have shown that aerobic training (e.g. cycling, walking) or high-intensity interval training prescribed for 12-24 weeks can significantly improve important clinical outcomes, including insulin sensitivity, body fat percentage, total and LDL-cholesterol, c-reactive protein (CRP), and psychological outcomes (e.g. HRQoL and depression) [11–17]. Such studies have informed clinical practice guidelines, which recommend that women with PCOS engage in ≥90 min of aerobic training weekly [18].
Progressive resistance training (PRT) is the most potent exercise modality for improving skeletal muscle mass and quality [19, 20]. Notably, studies have consistently shown that PRT can counteract metabolic diseases, including insulin resistance [20–23]. This is a key consideration for women with PCOS given that insulin resistance is implicated in the etiology of the disease [24]. The myogenic adaptations induced by PRT are often accompanied by a range of physiological (metabolic), functional and psychological adaptations that may be clinically important in this cohort [19]. PRT is currently recommended within exercise prescription guidelines for healthy adults and those with other major chronic diseases, including type 2 diabetes [25–31]. However, PRT is not currently recommended within clinical practice guidelines for PCOS [18].
Only two studies to date have investigated the isolated effect of PRT in women with PCOS [32–34]. These studies have shown that chronic PRT (10-16 weeks) can significantly improve several clinically important outcomes in this cohort, including body composition, circulating androgens, blood glucose, sexual dysfunction, depression and anxiety [32–34]. However, there is currently a need for additional research on the safety and efficacy of PRT in PCOS utilizing robust study designs. Therefore, the purpose of the present pilot study was to evaluate the feasibility of a executing a randomized controlled trial (RCT) of PRT in women with PCOS to inform the development of a large-scale clinical trial.
Methods
Study design
This study randomized participants into an experimental (PRT) group or a no-exercise (usual care) control group. Randomization assignments were generated via www.randomization.com by an investigator not involved in data collection, and given to participants in sealed envelopes upon the completion of baseline testing. The Human Research Ethics Committee at Western Sydney University (reference H10448) approved all procedures, and written informed consent was obtained from all participants.
Participants and recruitment
Eligibility Criteria: (1) Age 18-42 years with a diagnosis of PCOS (confirmed via the participant’s general practitioner or specialist); (2) not currently participating in PRT; (3) not pregnant nor breastfeeding; (4) no history of cardiovascular, kidney, respiratory disease, uncontrolled hypertension or cancer; (5) no use of cigarettes for >6 months; (6) no acute or chronic medical condition that would make assessment and interventions potentially hazardous or any of the outcomes impossible to assess; (7) cognition and English language sufficient to understand research procedures and provide informed consent; and (8) willingness to be randomised and undergo study protocols. Participants were recruited by the lead investigator (L.V.) from February 20 to September 21, 2014 (~7.5 months.). Recruitment was done via flyer advertisements, social media advertisement (i.e. Facebook), free online advertisement (i.e. Gumtree), referrals from local clinicians, word of mouth, referrals by a research colleague who had conducted PCOS research, and a database from a previous PCOS study on the use of herbal medicine.
Feasibility outcomes
Recruitment and attrition
Recruitment (or referral) source data was obtained by asking each potential participant upon initial contact how she first became aware of the study. One source was recorded per potential participant. Recruitment rate was computed by dividing the number of women randomized by the recruitment period (~7.5 months). Participant attrition was monitored throughout the study, and reasons for attrition were sought from all participants who withdrew.
Adherence
Adherence to the PRT intervention, including supervised and home-based components, was recorded using log-books and computed as the number of sessions attended divided by the number of sessions offered, multiplied by 100 %.
Adverse events
Adverse events in both groups were recorded using a structured questionnaire administered weekly, in person in the PRT group and via telephone/email in the control group.
Completion of assessments
The clinical outcomes assessments completed at baseline (week 0) and follow-up (week 13), and weekly status checks to monitor adverse events, were recorded for each participant by the lead investigator (L.V) at the university campus and/or a private clinic.
Clinical outcomes
Clinical outcomes were assessed prior to and following the 12-week intervention period (week 0 and 13). Week 13 testing was completed >72 h after the final exercise session in the PRT group.
Physiological outcomes
Participants retrospectively reported on the duration of their last menstrual period at baseline (week 0) and that of their most recent period at follow-up (week 13). During the study, menstrual cyclicity was self-monitored by participants using a standardized menstrual diary. Menstrual cycle was categorized as amenorrhea (no period for >199 days) [35], oligomenorrhea (cycle duration of 35 to 199 days) [35] or normal cycle (cycle duration of 23 to 35 days) [36]. Improvement or worsening of menstrual cyclicity was defined as a change in the menstrual cycle category from week 0 to week 13.
Height and weight were measured using calibrated equipment and standard procedures [37]; body mass index (BMI) was derived from these measures. Waist circumference was measured at the level of the umbilicus [38]. Body composition was analyzed using dual-energy X-ray absorptiometry (DEXA) as previously described [39]; measurements were obtained for fat mass, lean (muscle) mass, fat-free mass and body fat percentage.
Blood samples were obtained and analyzed through a private pathology lab (Douglas Hanly Moir) the morning following an 8-h overnight fast. Biochemical assays were obtained for: glycosylated haemoglobin (HbA1c), insulin, glucose, high-sensitivity C-reactive protein (CRP), testosterone, and sex-hormone binding globulin (SHBG) using standard assays (coefficient of variation (CV): 0.5 % to 6 %). Free androgen index was calculated by [testosterone (mmol/litre) / SHBG (mmol/litre) x 100], and HOMA-2 was calculated by an online computer model available at www.OCDEM.ox.ac.uk.
Functional outcomes
Upper and lower body maximum isometric strength was assessed using triceps extension and knee extension positions, respectively, using a portable electronic dynamometer (Chatillon DFX-II, AMETEK, Paoli, PA; CV 9.4 %) as previously described [40].
Psychological outcomes
Disease-specific HRQoL was evaluated using the Polycystic Ovary Syndrome Questionnaire (PCOSQ), a 26-item scale which assesses 5 domains: emotions, body hair, weight, infertility problems and menstrual problems [41]. Domain scores can range from 1 to 7 with higher scores representing higher quality of life. The Medical Outcomes Trust Short Form-36 (SF-36) was used to evaluate eight domains of general HRQoL (i.e. Physical Functioning, Role Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role Emotional and Mental Health) [42]. Higher scores, ranging from 0-100, denote higher HRQoL. The Depression, Anxiety and Stress Scale 21 (DASS-21) was used to evaluate symptoms of (1) depression (i.e. hopelessness, low self-esteem, and low positive affect) (2) anxiety (i.e. autonomic arousal, musculoskeletal symptoms, situational anxiety and subjective experience of anxious arousal) and (3) stress (i.e. tension, agitation, and negative affect). Higher scores in each scale denote more severe symptoms. The Exercise Self-Efficacy Scale was used to measures the self-efficacy of individuals to undertake physical exercise [43]. Scores can range from 10 to 40 with higher scores indicating higher self-efficacy.
Intervention
Participants in the PRT group were prescribed two supervised training sessions per week on non-consecutive days (i.e. Monday, Wednesday or Friday) for 12 weeks at the university campus. The PRT group also performed two home-based (unsupervised) exercise sessions consisting of lower-intensity calisthenics to facilitate habitual movement and behaviour change [44]. Supervised sessions lasted for approximately 60 min, and included a standardized (5 min) warm-up and cool-down on exercise cycle or treadmill. PRT exercises included lat pulldown, leg curl, seated row, leg press, calf raise, chest press, split squat, shoulder press, biceps curl, triceps extension and abdominal curl. All sets (except abdominal curl) were performed to neuromuscular fatigue, i.e. 8-12 repetitions maximum; loads were increased with strength gains. Two sets of each exercise were prescribed in the first 2 weeks of training. From week 3, all exercises except split squats and shoulder press were progressed to 3 sets.
The home-based calisthenics exercises were undertaken on non-PRT days and included lying external hip rotations (‘clam shells’), side leg raises, push-ups on knees, wall squats, oblique curls, core stabilization exercises (‘bird dog’ and abdominal hollowing), performed for 3 sets x 10 repetitions each. Participants received a different set of callisthenic home-based exercises every four weeks. Participants were asked to record the number of repetitions of each exercise performed in a log book. This record was collected weekly.
Control
Participants in the control group did not receive any PRT intervention and were instructed to continue with their current lifestyle and usual healthcare and medical treatments.
Statistical analyses
All available data were included, regardless of patient compliance to the intervention in an intention-to-treat analysis, performed using the Statistical Package for the Social Sciences (IBM© SPSS©, Version 22.0). Data from patients unavailable for follow-up assessments at week 12 were carried forward from baseline. Baseline characteristics were compared using t-tests and chi-square, as appropriate. Within group changes over time were evaluated by paired t-tests. Group x time effects were determined by analysis of covariance of the post-treatment score controlling for the baseline score for all continuous variables, and by chi square for change (improvement or worsening) of menstrual cyclicity. Furthermore, sensitivity analyses were completed without imputation of missing data, and using parametric and bootstrap method (10,000 replicates of the original sample size) to estimate the effect sizes, and the 95 % confidence interval (CI). A p value of <0.05 was considered as indicative of statistical significance.
Results
Feasibility outcomes
Recruitment and attrition
Seventy-eight women contacted the principal investigator regarding participation and received information about the study (Fig. 1). Fourteen women were not reviewed due to their lack of further contact. Sixty-four women were reviewed for eligibility. Of these women, 25 were deemed ineligible primarily due to living outside the local region (n = 22) (20 of these women were referred by Facebook). Thirty-nine women met the eligibility criteria for the study and 20 of them refused participation with the main reason provided being ‘lack of time’ (n = 16). Nineteen (n = 19) women consented and four (n = 4) did not complete baseline testing due to other commitments (n = 1) or refusal to be randomized to a no-exercise control group (n = 3). Fifteen women (n = 15) completed baseline testing and were randomized into the PRT group (n = 8) or the control group (n = 7) resulting in a randomization-to-screening percentage of 23 % (15 randomized / 64 reviewed). Of these women, the main sources of recruitment were Facebook (n = 6, 40 %) and online advertisement (via Gumtree) (n = 4, 26.6 %), followed by referrals by local clinicians (n = 3, 20 %), a research colleague (n = 1, 6.7 %) and flyers (n = 1, 6.7 %). Two women in the PRT group and three women in the control group withdrew from the study and were unavailable for follow-up testing, with reasons presented in Fig. 1. Two women (one per group) became pregnant during the trial and were excluded from analyses due to the confounding effect of pregnancy on the clinical outcomes. Thirteen women were included in the statistical analyses. Recruitment rate averaged two women per month (15 participants / 7.5 months).
Fig. 1.
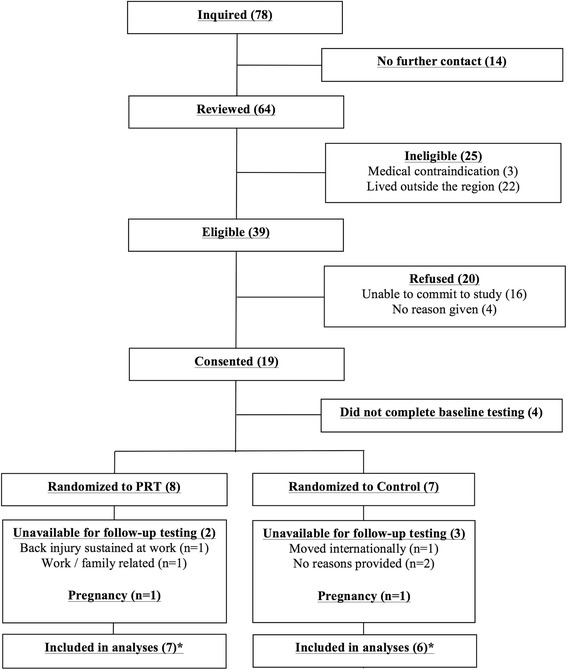
Participant flow chart. *Baseline data carried forward for two participants in the PRT and three participants in the control group that did not complete follow-up testing. Data for one pregnancy in each group excluded from the analyses
Adherence to PRT
Adherence to training inclusive of the two participants in the PRT group who withdrew from training (Fig. 1) was 76 % ± 13 % for supervised training, 43 % ± 26 % for home-based (unsupervised) calisthenics training, and 60 % ± 10 % overall. Excluding these two participants, attendance was 95 % ± 6 % for supervised training, 51 % ± 28 % for unsupervised training and 73 % ± 6 % overall.
Adverse events
One participant in the PRT group sustained an ankle sprain (week 10) from a fall outside of training. This participant continued to train with two adjustments of the program to accommodate the injury (i.e. leg press exercise was performed unilaterally using the non-affected leg and knee extensions were substituted for the split squat). Another participant in the PRT group sustained a lower back injury at work in week 4, which resulted in withdrawal and inability to complete follow-up testing (Fig. 1). No adverse events were attributed to PRT. No adverse events were reported by any participant in the control group.
Completion of assessments
All participants in the PRT group (n = 5) and control group (n = 3) available for follow-up testing completed all clinical outcomes assessments (Fig. 1). All weekly status checks (100 %) were completed in person by the PRT group (n = 5), while the control group (via telephone/email) completed only 58 %.
Baseline characteristics
There were no significant differences between groups at baseline, according to the descriptive characteristics presented in Table 1. However, trends were noted for waist circumference (p = 0.06) and hip circumference (p = 0.10). The age and BMI of participants ranged from 18-40 years and 18.3 kg/m2 to 54.6 kg/m2, respectively. In the total cohort, six participants had oligomenorrhea, four had amenorrhea, and three had a regular cycle at baseline. The most common prescription medication was metformin (n = 4 in the PRT group and n = 1 in the control group) and one participant in each group was prescribed an oral contraceptive.
Table 1.
Baseline characteristics of the total cohort and groups
| Characteristic | Total Cohort (n = 13) | PRT Group (n = 7) | Control Group (n = 6) |
|---|---|---|---|
| Age (y) | 27 (5) | 26 (7) | 29 (3) |
| Height (cm) | 163.8 (9.3) | 168.2 (4.6) | 158.7 (11.1) |
| Body weight (kg) | 102.9 (35.0) | 117.4 (36.6) | 86 (27.0) |
| Body mass index (kg/m2) | 37.8 (11.4) | 41.3 (12.5) | 34.0 (9.4) |
| Waist circumference (cm) | 110.8 (27.2) | 123.6 (29.0) | 96.0 (16.3) |
| Hip circumference (cm) | 124.1 (22.1) | 133.5 (22.7) | 113.0 (17.0) |
| Waist-to-hip ratio | 0.88 (0.09) | 0.92 (0.08) | 0.85 (0.10) |
| Systolic blood pressure (mmHg) | 116 (9) | 114 (8) | 118 (10) |
| Diastolic blood pressure (mmHg) | 76 (6) | 76 (7) | 76 (6) |
| Menstrual cycle status (n): | |||
| Normal cycle | 3 | 2 | 1 |
| Oligomenorrhea | 6 | 2 | 4 |
| Amenorrhea | 4 | 3 | 1 |
Data reported as mean (standard deviation) except menstrual cycle status (n)
Clinical outcomes
Physiological outcomes
Menstrual cyclicity improved from baseline in three women (one in the PRT group and two in the control group). Menstrual cyclicity worsened in one woman in the PRT group and remained unchanged in the other nine women in the study. Change in menstrual cycle status was not significantly different between groups (p = 0.503).
The PRT group reported a significant increase in body weight (p = 0.01) and BMI (p = 0.04) compared to the control group (Table 2). There was also a significant reduction in waist circumference (p = 0.03) and a significant increase in lean mass (p = 0.01) and fat-free mass (p = 0.005), indicating that the weight gain was due to muscle hypertrophy. There were no differences in fat mass or percent body fat between groups over time.
Table 2.
Summary of within and between group changes on clinical outcomes
| Outcome Measure | PRT (n = 7) | Control (n = 6) | P (between groups) | Effect Size (95 % CI)a | ||||
|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 12 | P | Week 0 | Week 12 | P | |||
| Physiological outcomes | ||||||||
| Body weight (kg) | 117.4 (36.6) | 118.9 (35.4) | 0.06 | 86.0 (26.7) | 86.0 (26.8) | 0.77 | 0.01 | 0.49 [0.61,3.83] |
| Body mass index (kg/m2) | 41.3 (12.5) | 41.7 (12.1) | 0.12 | 33.8 (9.4) | 33.8 (9.4) | N/A | 0.04 | 0.37[0.04,1.20] |
| Waist Circumference (cm) | 123.6 (29.0) | 121.5 (29.1) | 0.04 | 95.9 (16.3) | 96.6 (17.2) | 0.20 | 0.03 | 0.40b |
| Fat mass (kg) | 58.6 (23.3) | 59.0 (22.7) | 0.50 | 39.6 (18.3) | 40.6 (19.2) | 0.24 | 0.59 | 0.03[-2.96,1.79] |
| Lean mass (kg) | 54.7 (12.5) | 56.0 (11.3) | 0.16 | 43.9 (10.4) | 43.0 (10.0) | 0.23 | 0.01 | 0.49b |
| Fat-free mass (kg) | 57.4 (13.6) | 58.8 (11.9) | 0.13 | 46.8 (10.8) | 46.0 (10.4) | 0.26 | 0.005 | 0.57b |
| Percent body fat (%) | 49.7 (8.4) | 49.6 (7.6) | 0.84 | 45.2 (10.7) | 46.1 (11.2) | 0.22 | 0.36 | 0.08[-2.66,1.06] |
| HbA1c (%) | 5.3 (0.4) | 5.1 (0.3) | 0.037 | 5.1 (0.3) | 5.2 (0.4) | 0.24 | 0.03 | 0.39b |
| Fasting insulin (mU/L) | 21 (11) | 20 (12) | 0.49 | 9 (5) | 10 (6) | 0.18 | 0.24 | 0.14[-5.50,1.55] |
| Fasting glucose (mmol/L) | 4.7 (0.8) | 4.9 (0.7) | 0.03 | 4.8 (0.4) | 4.9 (0.4) | 0.71 | 0.18 | 0.17[-0.08,0.40] |
| HOMA-2 | 2.62 (1.33) | 2.56 (1.43) | 0.72 | 1.19 (0.64) | 1.24 (0.71) | 0.19 | 0.27 | 0.11[-0.65,0.20] |
| hsCRP (mg/L) | 8.9 (10.7) | 8.0 (10.8) | 0.38 | 3.6 (5.1) | 3.9 (5.4) | 0.12 | 0.36 | 0.09[-3.57,1.41] |
| Testosterone (nmol/L) | 1.5 (0.3) | 1.7 (0.5) | 0.38 | 1.7 (0.4) | 1.8 (0.3) | 0.11 | 0.91 | 0.00[-0.44,0.49] |
| SHBG (nmol/L) | 32 (26) | 27 (22) | 0.39 | 47 (25) | 47 (25) | 0.36 | 0.25 | 0.13[-22.58,6.50] |
| Free androgen index (%) | 8.6 (7.3) | 9.8 (6.6) | 0.26 | 4.5 (2.7) | 4.7 (2.7) | 0.11 | 0.18 | 0.17[-0.82,3.83] |
| Functional outcomes | ||||||||
| Upper body strength | 190.2 (42.5) | 211.5 (52.1) | 0.10 | 141.3 (53.0) | 134 (51.0) | 0.12 | 0.06 | 0.33b |
| Lower body strength | 258.7 (67.2) | 313.2 (90.3) | 0.04 | 221.8 (59.5) | 214.8 (64.9) | 0.08 | 0.03 | 0.45b |
| Psychological outcomes | ||||||||
| PCOSQ | ||||||||
| Emotions | 4.4 (0.6) | 5.1 (0.5) | 0.02 | 3.2 (1.0) | 3.1 (0.9) | 0.13 | 0.003 | 0.60[0.53,1.95] |
| Body hair | 4.9 (2.0) | 4.5 (1.5) | 0.37 | 4.2 (1.7) | 4.2 (1.9) | 1.0 | 0.58 | 0.03[-1.22,0.71] |
| Weight | 3.3(1.7) | 4.1(1.7) | 0.06 | 2.4 (1.5) | 2.3 (1.5) | 0.70 | 0.04 | 0.35[0.03,1.796] |
| Infertility problems | 0.9 (0.4) | 3.5 (0.9) | 0.01 | 1.1 (0.6) | 1.9 (1.2) | 0.11 | 0.03 | 0.41b |
| Menstrual problems | 4.1 (0.8) | 3.9 (0.9) | 0.52 | 3.0 (1.4) | 3.0 (1.4) | N/A | 0.92 | 0.00[-1.06,0.96] |
| SF-36 | ||||||||
| Physical Functioning | 83.0 (10.4) | 89.3 (12.4) | 0.05 | 94.2 (5.8) | 90.8 (10.7) | 0.18 | 0.02 | 0.42b |
| Role Physical | 21.4 (4.9) | 22.3 (3.3) | 0.36 | 17.7 (7.3) | 18.8 (7.9) | 0.70 | 0.70 | 0.02[-4.58,6.58] |
| Bodily Pain | 67.3 (20.0) | 65.3 (18.0) | 0.55 | 45.2 (28.3) | 48.8 (22.0) | 0.36 | 0.87 | 0.00[-10.46,9.00] |
| General Health | 49.0 (22.3) | 54.3 (25.2) | 0.27 | 53.3 (14.4) | 49.2 (10.7) | 0.19 | 0.14 | 0.20[-3.73,22.58] |
| Vitality | 47.3 (26.2) | 56.2 (21.3) | 0.12 | 35.4 (26.4) | 30.2 (22.9) | 0.29 | 0.02 | 0.45b |
| Social Functioning | 75.0 (14.4) | 89.3 (15.2) | 0.08 | 60.4 (18.4) | 52.1 (9.4) | 0.24 | 0.002 | 0.64b |
| Role Emotional | 18.0 (5.8) | 23.0 (4.1) | 0.10 | 9.7 (8.2) | 8.3 (7.5) | 0.36 | 0.009 | 0.51b |
| Mental Health | 71.4 (22.6) | 84.2 (17.2) | 0.06 | 51.7 (20.7) | 46.7 (20.7) | 0.31 | 0.009 | 0.52b |
| DASS-21 | ||||||||
| Depression | 10.8 (6.8) | 5.4 (5.4) | 0.050 | 12.7 (9.6) | 14.7 (9.0) | 0.20 | 0.01 | 0.50b |
| Anxiety | 10.3 (5.6) | 7.4 (7.0) | 0.082 | 12.3 (11.2) | 14.3 (10.8) | 0.11 | 0.03 | 0.41b |
| Stress | 12.6 (7.9) | 9.7 (9.3) | 0.220 | 21.7 (10.9) | 23.0 (11.2) | 0.33 | 0.17 | 0.18 [-11.11,2.25] |
| Exercise Self Efficacy Scale | 29.4 (4.7) | 31.3 (4.2) | 0.095 | 32.5 (5.3) | 30.0 (4.0) | 0.10 | 0.04 | 0.37b |
a = parametric between groups effect sizes and their confidence intervals
b = sample size too small to estimate the confidence intervals
Data reported as mean (standard deviation). Abbreviations: HbA1c hemoglobin A1c, HOMA-2 Homeostatic model of assessment 2, CRP c-reactive protein, SHBG sex hormone binding globulin, PCOSQ Polycystic Ovary Syndrome Questionnaire, SF-36 Medical Outcomes Trust Short Form-36, DASS-21 Depression, Anxiety and Stress Scale 21, Effect size = Cohen’s d
The PRT group reported a significant reduction in HbA1c over time compared to the control group (p = 0.03). Unexpectedly, within group analysis revealed that the PRT group reported a significant increase in fasting glucose (p = 0.03). No other physiological adaptations to PRT were noted.
Functional outcomes
The PRT group experienced a trend toward increased upper body strength (p = 0.06) and a significant increase in lower body strength (p = 0.03) compared to the control group.
Psychological outcomes
The PRT group significantly improved three of five PCOSQ domain scores compared to the control group: emotions (p = 0.003), weight (p = 0.04) and infertility problems (p = 0.03) versus the control group. In addition, the PRT group reported a significantly improvement in five of eight HRQoL (SF-36) domain scores (physical functioning [p = 0.02], vitality [p = 0.02], social functioning [p = 0.002], role emotional [p = 0.009] and mental health [p = 0.009)], two DASS-21 domains (depression [p = 0.01] and anxiety [p = 0.03]), and exercise self-efficacy (p = 0.04) compared to the control group. No other psychological group x time effects were noted.
Post hoc analyses
To determine the effect of higher adherence on the PRT group, post hoc analyses were performed using data from participants who completed >75 % of the supervised sessions (and >60 % of the total training sessions, i.e. supervised plus home-based) (n = 5). The outcomes of these analyses did not differ from the primary analyses.
Sensitivity analyses
Group x time analyses were performed without imputation of missing data from participants that did not undergo final assessment (i.e. per protocol). The outcomes of these analyses did not differ with the primary analyses, except that group x time interaction effects for the following outcomes became non-significant: body weight (p = 0.07), BMI (p = 0.15), and lean mass (p = 0.07). Using both parametric and bootstrap method, our result indicated that the confidence intervals of effect sizes cross each other, indicating that the two methods did not differ statistically and hence, the effect sizes and their CI’s for only the parametric method was reported.
Discussion
This pilot study evaluated the feasibility of a PRT intervention in women with PCOS within an RCT to inform the development of a robust clinical trial. Our findings suggest that it is feasible to investigate PRT in women with PCOS, and that this anabolic intervention may induce a number of clinically important adaptations.
The study enrolled approximately two participants per month over 7.5 months. This rate of recruitment is similar to studies prescribing aerobic training in women with PCOS (0.5 to 3 women per month) [45–49]. However, the duration of recruitment in these studies was longer (8-40 months) resulting in larger sample sizes (n = 20 to n = 124) [45–49]. Other studies have not stated the duration of recruitment [14–16, 50–52]. Clearly, participant recruitment is a challenging aspect of clinical research [53], and strategies for enhancing recruitment are therefore important. Our most successful recruitment methods were the social media platform Facebook (n = 6, 40 %) and online advertisement via Gumtree (n = 4, 26.6 %). The least successful strategies included referral by local clinicians (n = 3, 20 %), research colleagues (n = 1, 6.7 %) and flyers (n = 1, 6.7 %). Having the support of local clinicians is essential to fostering participant recruitment in clinical trials [54]. However, clinicians are often busy with their own professional duties and may not be able to offer the level of support required [55]. To foster recruitment via clinicians, it may be necessary to engage in regular meetings and/or create more streamlined pathways for referral.
Although Facebook was our most effective recruitment method, it also resulted in a large number of ineligible women contacting the principal investigator. Future studies conducted within small geographic regions should consider more targeted methods of advertising via Facebook (i.e. paid advertisement to more effectively reach the local community). Alternatively, studies conducted across multiple geographical sites or countries may benefit from using Facebook for wider-scale recruitment. No previous study of exercise in PCOS has noted the use of Facebook as a recruitment method [14, 16, 45, 48–51].
Our participant attrition rate was 38 % overall (i.e. 29 % (2/7) in the PRT group and 50 % (3/6) in the control group). Previous studies of exercise training in PCOS have reported participant attrition rates ranging from 25 % to 45 % [14, 45, 47–49, 51], while other studies have not provided these data [46, 48, 52]. In the present study, two women in the control group were lost immediately after randomization, while three other women consented and withdrew from the study given their concerns about randomization. It has been suggested that participants who are randomized to a no-treatment control group, or a treatment group contrary to their desire, may not accept randomization and refuse participation [56]. Strategies to minimize participant attrition may include the use of a placebo-control (i.e. unloaded or non-progressive training) or a waitlist control group. Moreover, future studies could opt to investigate the additive effect of PRT to an exercise intervention prescribed according to current guidelines which emphasize aerobic training [17, 18], i.e. by comparing a group receiving PRT plus aerobic training to a group receiving aerobic training only.
Attendance to supervised training sessions in the present study was high (95 ± 6 %). High attendance rates to supervised training (>80 %) have been noted in many trials of aerobic training in PCOS [16, 46, 52]. By comparison, attendance to the home-based sessions in the present study was lower (51 %). A previous PCOS study which prescribed unsupervised exercise (i.e. walking) also demonstrated a lower compliance rate (43 %) [48]. Studies in other cohorts have reported both high [57] and low compliance to home-based training [58]. Potential reasons for low compliance to home-based exercise prescriptions may include lack of motivation or time [59]. Future studies should consider possible strategies to foster adherence to unsupervised training, including SMS reminders, effective time management strategies (e.g. diarizing sessions into a routine schedule), motivational cues and rewards [60–62], and other behavior strategies [63–65].
All reported adverse events were not related to the study intervention indicating that PRT is safe intervention for women with PCOS. This finding is consistent with the evidence base in other chronically diseased cohorts, including type 2 diabetes [66–70].
In general, the completion of clinical outcomes assessments was satisfactory in the present study. However, the administration of weekly status checks via email and telephone to monitor adverse events in the control group proved problematic with only 58 % completed. This may be improved by arranging weekly visits with participants, if feasible.
The PRT intervention applied in the present study yielded a number of clinically important adaptations that may be associated with better disease management and treatment of the underlying pathology. No significant change in menstrual cyclicity was noted in the PRT or control group. However, studies of aerobic training [47, 52] and dietary and exercise intervention, including those involving aerobic plus PRT [51] have shown improved menstrual cyclicity using similar methodology (i.e. self-reported menstrual diary). There is reason to hypothesize that PRT can improve menstrual cyclicity in women with PCOS [24]. Therefore, future studies should continue to investigate this endpoint in women with oligo/amenorrhea, including the dosages of PRT required to induce adaptation. Such investigations require vigilant monitoring of menstrual cycles (i.e. date of last menstrual period in women with amenorrhea and documenting menstrual cycle length in women with oligomenorrhea and regular cycles) and reporting of confounding variables including physical activity and dietary habits.
PRT may counteract the etiology of PCOS through its effect on body composition [24]. Improvements in skeletal muscle size and quality secondary to PRT in type 2 diabetes have been accompanied by reductions in visceral fat [67, 71] and improvements in insulin sensitivity and glucoregulation [67, 71–73]. Increased insulin sensitivity and glucoregulation, in turn, may downregulate androgen synthesis and hyperandrogenemia in women with PCOS, which could counteract the disease process (i.e. premature growth arrest of follicles) and menstrual irregularity [24]. The anthropometric changes experienced by the PRT group in the present study, including the reduction in waist circumference (p = 0.03) and increase in fat-free mass (p = 0.005) were accompanied by an improvement in HbA1c (p = 0.031) versus the control group, indicating parallel improvement of body composition and glucoregulation. However, the PRT group experienced no change in HOMA-2, several key endocrine markers (i.e. free androgen index [p = 0.18], testosterone [p = 0.91], SHBG [p = 0.246]) or other key haemotological markers associated with PCOS (i.e. CRP, fasting insulin) versus the control group (Table 2). These physiological data are difficult to interpret within a small-scale study. Future studies are required to investigate these adaptations, including the determination of dose-response effects for each outcome. These studies would also benefit from using more sensitive measures of insulin resistance, including the euglycaemic-hyperinsulaemic clamp [74], and gold-standard assessment of steroids involving liquid chromatography-tandem mass spectrometry [75] with the standardisation of assessments to a particular phase of the menstrual cycle (i.e. day 2-5 of menstrual cycle), if required.
Unexpectedly, within group analyses showed that the PRT group increased fasting glucose over time (p = 0.03). This finding may be reflective of an acute effect, i.e. in response to psychological stress or lack of sleep. Previous studies in PCOS, type 2 diabetes and/or obesity that prescribed aerobic, mixed training, and/or diet plus exercise have reported significant reductions in fasting insulin and fasting glucose [46, 47, 52, 76–79] and therefore this outcome requires further exploration in PCOS.
The PRT group demonstrated a trend toward increased upper body strength (p = 0.06) and a significant rise in lower body strength (p = 0.03) compared to the control group. PRT is the modality of choice for eliciting strength gains [80, 81] and these adaptations are typically accompanied by improvements in performance-based tests and psychological attributes, including HRQoL [40, 82, 83]. Women with PCOS have been shown to have greater muscular strength than their healthy peers [84] and future studies are required to determine the clinical importance of strength adaptation in this cohort.
The PRT group improved three of five PCOSQ domains versus the control group: emotions (p = 0.003), weight (p = 0.04) and infertility problems (p = 0.03). In addition, the PRT group significantly improved five of eight HRQoL (SF-36) domain scores (physical functioning [p = 0.02], vitality [p = 0.02], social functioning [p = 0.002], role emotional [p = 0.009] and mental health [p = 0.009]), DASS-21 domains for depression (p = 0.01) and anxiety (p = 0.03), and exercise self-efficacy (p = 0.035) versus the control group. The psychological benefits of exercise or physical activity in women with PCOS have been noted previously and are clinically relevant [85, 86]. Future studies involving placebo-control groups are required to determine if these psychological adaptations can be attributed solely to the PRT, or are influenced by the social interaction with trainers and/or other participants.
Strengths of this study include the collection of feasibility outcomes, and the inclusion of important clinical outcomes. Limitations of the study included the small sample size (n = 15) and lack of monitoring and controlling for key confounding variables such as physical activity and diet.
Conclusion
In summary, a randomized clinical trial of PRT in PCOS would be feasible to conduct, and this mode of exercise may elicit a therapeutic effect on a range of pertinent outcomes in this cohort. A suitably powered clinical trial is required to confirm these findings and answer novel research questions in relation to prescribing PRT as a therapeutic intervention in PCOS. The success of a large-scale trial required to confirm these findings would be contingent on addressing the feasibility hurdles identified in this study with respect to recruitment, attrition, compliance, and collection of standardized clinical data.
Ethics approval and consent to participate
The Human Research Ethics Committee at Western Sydney University (reference H10448) approved all procedures, and written informed consent was obtained from all participants.
Availability of data and materials
The data and materials can be made available on request via contacting the corresponding author.
Acknowledgements
We sincerely thank the staff at Western Sydney University Penrith gym for their support of this study, and Catherine Phillips, Sean Raftry, Kurt Fittler and Susan Arentz in providing technical support for the study.
Funding
This research was supported by higher degree research funding provided by Western Sydney University.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CRP
C-reactive protein
- DASS-21
depression, anxiety and stress scale 21
- DEXA
dual-energy X-ray absorptiometry
- HbA1c
glycosylated haemoglobin
- HRQoL
health-related quality of life
- PCOS
polycystic ovary syndrome
- PCOSQ
polycystic ovary syndrome questionnaire
- PRT
progressive resistance training
- RCT
randomized controlled trial
- SF-36
medical outcomes trust short form-36
- SHBG
sex-hormone binding globulin
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LV conceived and designed the study, was involved in the acquisition of data, delivery of intervention and assessments, and drafted the manuscript. BSC provided consultation regarding the design of the study and statistical analysis, and contributed to drafting the manuscript. SS and CS provided clinical and research expertise, interpretation of results, and drafted the manuscript. KA provided statistical expertise, and contributed to drafting of the manuscript. All authors have read and approved the final manuscript.
References
- 1.March WA, Moore VM, Willson KJ, Phillips DIW, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Work-shop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. [DOI] [PubMed]
- 3.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352(12):1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 4.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. 2007. [DOI] [PubMed] [Google Scholar]
- 5.Giudice LC. Endometrium in PCOS: implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab. 2006;20(2):235–244. doi: 10.1016/j.beem.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Gorry A, White DM, Franks S. Infertility in polycystic ovary syndrome. Endocrine. 2006;30(1):27–33. doi: 10.1385/ENDO:30:1:27. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya SM, Jha A. Prevalence and risk of depressive disorders in women with polycystic ovary syndrome (PCOS) Fertil Steril. 2010;94(1):357–359. doi: 10.1016/j.fertnstert.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Deeks AA, Gibson-Helm ME, Teede HJ. Anxiety and depression in polycystic ovary syndrome: a comprehensive investigation. Fertil Steril. 2010;93(7):2421–2423. doi: 10.1016/j.fertnstert.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Himelein MJ, Thatcher SS. Depression and body image among women with polycystic ovary syndrome. J Health Psychol. 2006;11(4):613–625. doi: 10.1177/1359105306065021. [DOI] [PubMed] [Google Scholar]
- 10.Barnard L, Ferriday D, Guenther N, Strauss B, Balen A, Dye L. Quality of life and psychological well being in polycystic ovary syndrome. Hum Reprod. 2007;22(8):2279–2286. doi: 10.1093/humrep/dem108. [DOI] [PubMed] [Google Scholar]
- 11.Abazar E, Taghian F, Mardanian F, Forozandeh D. Effects of aerobic exercise on plasma lipoproteins in overweight and obese women with polycystic ovary syndrome. Adv Biomed Res. 2015;4:68. doi: 10.4103/2277-9175.153892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haqq L, McFarlane J, Dieberg G, Smart N. The effect of lifestyle intervention on body composition, glycaemic control and cardio-respiratory fitness in women with polycystic ovarian syndrome: a systematic review and meta-analysis. Int J Sport Nutr Exerc Metab. 2014;25(6):533-40. [DOI] [PubMed]
- 13.Harrison CL, Lombard CB, Moran LJ, Teede HJ. Exercise therapy in polycystic ovary syndrome: a systematic review. Hum Reprod Update. 2011;17(2):171–183. doi: 10.1093/humupd/dmq045. [DOI] [PubMed] [Google Scholar]
- 14.Harrison CL, Stepto NK, Hutchison SK, Teede HJ. The impact of intensified exercise training on insulin resistance and fitness in overweight and obese women with and without polycystic ovary syndrome. Clin Endocrinol (Oxf) 2012;76(3):351–357. doi: 10.1111/j.1365-2265.2011.04160.x. [DOI] [PubMed] [Google Scholar]
- 15.Hutchison S, Teede H, Rachoń D, Harrison C, Strauss B, Stepto N. Effect of exercise training on insulin sensitivity, mitochondria and computed tomography muscle attenuation in overweight women with and without polycystic ovary syndrome. Diabetologia. 2012;55(5):1424–1434. doi: 10.1007/s00125-011-2442-8. [DOI] [PubMed] [Google Scholar]
- 16.Hutchison SK, Stepto NK, Harrison CL, Moran LJ, Strauss BJ, Teede HJ. Effects of exercise on insulin resistance and body composition in overweight and obese women with and without polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96(1):E48–E56. doi: 10.1210/jc.2010-0828. [DOI] [PubMed] [Google Scholar]
- 17.Thomson RL, Buckley JD, Brinkworth GD. Exercise for the treatment and management of overweight women with polycystic ovary syndrome: a review of the literature. Obes Rev. 2011;12(5):e202–e210. doi: 10.1111/j.1467-789X.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- 18.Alliance PA. Evidence Based Guidelines for Assessment and Management of Polycystic Ovary Syndrome. Melbourne: Jean Hailes Foundation for Women’s Health on behalf of the PCOS Australian Alliance; 2011. [Google Scholar]
- 19.Ciccolo J, Kraemer WJ. Resistance Training for the Prevention and Treatment of Chronic Disease. Boca Raton: CRC Press; 2013.
- 20.Tresierras M, Balady G. Resistance training in the treatment of diabetes and obesity: mechanisms and outcomes. J Cardiopulm Rehabil Prev. 2009;29(2):67–75. doi: 10.1097/HCR.0b013e318199ff69. [DOI] [PubMed] [Google Scholar]
- 21.Strasser B, Schobersberger W. Evidence for resistance training as a treatment therapy in obesity. J Obes 2011. doi:10.1155/2011/482564. [DOI] [PMC free article] [PubMed]
- 22.Strasser B, Siebert U, Schobersberger W. Resistance training in the treatment of the metabolic syndrome. Sports Med. 2010;40(5):397–415. doi: 10.2165/11531380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Sigal R, Kenny G, Boulé N, Wells G, Prud'homme D, Fortier M, Reid R, Tulloch H, Coyle D, Phillips P, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes. Ann Intern Med. 2007;147:357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 24.Cheema BS, Vizza L, Swaraj S. Progressive resistance training in polycystic ovary syndrome: can pumping iron improve clinical outcomes? Sports Med. 2014;44(9):1197-1207. [DOI] [PubMed]
- 25.Hordern MD, Dunstan DW, Prins JB, Baker MK, Singh MAF, Coombes JS. Exercise prescription for patients with type 2 diabetes and pre-diabetes: a position statement from Exercise and Sport Science Australia. J Sci Med Sport. 2012;15(1):25–31. doi: 10.1016/j.jsams.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Sharman JE, Stowasser M. Australian association for exercise and sports science position statement on exercise and hypertension. J Sci Med Sport. 2009;12(2):252–257. doi: 10.1016/j.jsams.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Hayes S, Spence R, Galvao D, Newton R. Australian Association of Exercise and Sport Science position stand: Optimising cancer outcomes through exercise. J Sci Med Sport. 2009;12:428–434. doi: 10.1016/j.jsams.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Donnelly J, Blair S, Jakicic J, Manore M, Rankin J, Smith B. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 29.Selig SE, Levinger I, Williams AD, Smart N, Holland DJ, Maiorana A, Green DJ, Hare DL. Exercise & Sports Science Australia Position Statement on exercise training and chronic heart failure. J Sci Med Sport. 2010;13(3):288–294. doi: 10.1016/j.jsams.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147–167. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraemer W, Adams K, Cafarelli E, Dudley G, Dooly C, Feigenbaum M, Fleck S, Franklin B, Fry A, Hoffman J, et al. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34(2):364–380. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 32.Almenning I, Rieber-Mohn A, Lundgren KM, Shetelig Løvvik T, Garnæs KK, Moholdt T. Effects of high intensity interval training and strength training on metabolic, cardiovascular and hormonal outcomes in women with polycystic ovary syndrome: a pilot study. PLoS One. 2015;10(9):e0138793. doi: 10.1371/journal.pone.0138793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lara LAS, Ramos FKP, Kogure GS, Costa RS, Silva de Sá MF, Ferriani RA, Reis RM. Impact of Physical Resistance Training on the Sexual Function of Women with Polycystic Ovary Syndrome. J Sex Med. 2015;12(7):1584-1590. [DOI] [PubMed]
- 34.Miranda-Furtado CL, Ramos FKP, Kogure GS, Santana-Lemos BA, Ferriani RA, Calado RT, dos Reis RM. A nonrandomized trial of progressive resistance training intervention in women with polycystic ovary syndrome and its implications in telomere content. Reprod Sci. 2015;23(5):644-54. [DOI] [PubMed]
- 35.Fauser BCJM, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JSE, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38.e25. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 36.De Jonge CJ, Barratt CL. Assisted reproductive technology: accomplishments and new horizons. Cambridge: University Press; 2002. p. 274.
- 37.Thompson WR, Gordon NF, Pescatello LS. ACSM’s guidelines for exercise testing and prescription. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2010.
- 38.Wessel TR, Arant CB, Olson MB, Johnson BD, Reis SE, Sharaf BL, Shaw LJ, Handberg E, Sopko G, Kelsey SF. Relationship of physical fitness vs body mass index with coronary artery disease and cardiovascular events in women. JAMA. 2004;292(10):1179–1187. doi: 10.1001/jama.292.10.1179. [DOI] [PubMed] [Google Scholar]
- 39.Glickman SG, Marn CS, Supiano MA, Dengel DR. Validity and reliability of dual-energy X-ray absorptiometry for the assessment of abdominal adiposity. J Appl Physiol. 2004;97(2):509–514. doi: 10.1152/japplphysiol.01234.2003. [DOI] [PubMed] [Google Scholar]
- 40.Cheema B, Abas H, Smith B, O'Sullivan A, Chan M, Patwardhan A, Kelly J, Gillin A, Pang G, Lloyd B, et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18(5):1594–1601. doi: 10.1681/ASN.2006121329. [DOI] [PubMed] [Google Scholar]
- 41.Cronin L, Guyatt G, Griffith L, Wong E, Azziz R, Futterweit W, Cook D, Dunaif A. Development of a health-related quality-of-life questionnaire (PCOSQ) for women with polycystic ovary syndrome (PCOS) 1. J Clin Endocrinol Metab. 1998;83(6):1976–1987. doi: 10.1210/jcem.83.6.4990. [DOI] [PubMed] [Google Scholar]
- 42.Stewart AL, Greenfield S, Hays RD, Wells K, Rogers WH, Berry SD, McGlynn EA, Ware JE. Functional status and well-being of patients with chronic conditions: results from the Medical Outcomes Study. JAMA. 1989;262(7):907–913. doi: 10.1001/jama.1989.03430070055030. [DOI] [PubMed] [Google Scholar]
- 43.Kroll T, Kehn M, Ho P-S, Groah S. The SCI exercise self-efficacy scale (ESES): development and psychometric properties. Int J Behav Nutr Phys Act. 2007;4(1):34. doi: 10.1186/1479-5868-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perri MG, Martin AD, Leermakers EA, Sears SF, Notelovitz M. Effects of group-versus home-based exercise in the treatment of obesity. J Consult Clin Psychol. 1997;65(2):278. doi: 10.1037/0022-006X.65.2.278. [DOI] [PubMed] [Google Scholar]
- 45.Brown AJ, Setji TL, Sanders LL, Lowry KP, Otvos JD, Kraus WE, Svetkey PL. Effects of exercise on lipoprotein particles in women with polycystic ovary syndrome. Med Sci Sports Exerc. 2009;41(3):497–504. doi: 10.1249/MSS.0b013e31818c6c0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giallauria F, Palomba S, Maresca L, Vuolo L, Tafuri D, Lombardi G, Colao A, Vigorito C, Francesco O. Exercise training improves autonomic function and inflammatory pattern in women with polycystic ovary syndrome (PCOS) Clin Endocrinol (Oxf) 2008;69(5):792–798. doi: 10.1111/j.1365-2265.2008.03305.x. [DOI] [PubMed] [Google Scholar]
- 47.Palomba S, Giallauria F, Falbo A, Russo T, Oppedisano R, Tolino A, Colao A, Vigorito C, Zullo F, Orio F. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: a 24-week pilot study. Hum Reprod. 2008;23(3):642–650. doi: 10.1093/humrep/dem391. [DOI] [PubMed] [Google Scholar]
- 48.Randeva HS, Lewandowski KC, Drzewoski J, Brooke-Wavell K, O’Callaghan C, Czupryniak L, Hillhouse EW, Prelevic GM. Exercise decreases plasma total homocysteine in overweight young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(10):4496–4501. doi: 10.1210/jc.2001-012056. [DOI] [PubMed] [Google Scholar]
- 49.Stener-Victorin E, Jedel E, Janson PO, Sverrisdottir YB. Low-frequency electroacupuncture and physical exercise decrease high muscle sympathetic nerve activity in polycystic ovary syndrome. Am J Physiol Regul Integr Comp Physiol. 2009;297(2):R387–R395. doi: 10.1152/ajpregu.00197.2009. [DOI] [PubMed] [Google Scholar]
- 50.Bruner B, Chad K, Chizen D. Effects of exercise and nutritional counseling in women with polycystic ovary syndrome. Appl Physiol Nutr Metab. 2006;31(4):384–391. doi: 10.1139/h06-007. [DOI] [PubMed] [Google Scholar]
- 51.Thomson RL, Buckley JD, Noakes M, Clifton PM, Norman RJ, Brinkworth GD. The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(9):3373–3380. doi: 10.1210/jc.2008-0751. [DOI] [PubMed] [Google Scholar]
- 52.Vigorito C, Giallauria F, Palomba S, Cascella T, Manguso F, Lucci R, De Lorenzo A, Tafuri D, Lombardi G, Colao A, et al. Beneficial effects of a three-month structured exercise training program on cardiopulmonary functional capacity in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(4):1379–1384. doi: 10.1210/jc.2006-2794. [DOI] [PubMed] [Google Scholar]
- 53.Patel MX, Doku V, Tennakoon L. Challenges in recruitment of research participants. Adv Psychiatr Treat. 2003;9(3):229–238. doi: 10.1192/apt.9.3.229. [DOI] [Google Scholar]
- 54.Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, Entwistle V, Garcia J, Roberts I, Grant A et al. Recruitment to randomised trials: strategies for trial enrollment and participation study. The STEPS study. Health Technol Assess. 2007, 11(48):iii, ix-105. [DOI] [PubMed]
- 55.Blanton S, Morris DM, Prettyman MG, McCulloch K, Redmond S, Light KE, Wolf SL. Lessons learned in participant recruitment and retention: The EXCITE Trial. Phys Ther. 2006;86(11):1520–1533. doi: 10.2522/ptj.20060091. [DOI] [PubMed] [Google Scholar]
- 56.Smith CA, Pirotta M, Kilbreath S. A feasibility study to examine the role of acupuncture to reduce symptoms of lymphoedema after breast cancer: a randomised controlled trial. Acupunct Med. 2014;32(5):387–393. doi: 10.1136/acupmed-2014-010593. [DOI] [PubMed] [Google Scholar]
- 57.Nelson ME, Layne JE, Bernstein MJ, Nuernberger A, Castaneda C, Kaliton D, Hausdorff J, Judge JO, Buchner DM, Roubenoff R. The effects of multidimensional home-based exercise on functional performance in elderly people. J Gerontol A Biol Sci Med Sci. 2004;59(2):M154–M160. doi: 10.1093/gerona/59.2.M154. [DOI] [PubMed] [Google Scholar]
- 58.Mayoux-Benhamou MA, Roux C, Perraud A, Fermanian J, Rahali-Kachlouf H, Revel M. Predictors of compliance with a home-based exercise program added to usual medical care in preventing postmenopausal osteoporosis: an 18-month prospective study. Osteoporos Int. 2005;16(3):325–331. doi: 10.1007/s00198-004-1697-z. [DOI] [PubMed] [Google Scholar]
- 59.Johnson CA, Corrigan SA, Dubbert PM, Gramling SE. Perceived barriers to excercise and weight control practices in community women. Women Health. 1990;16(3-4):177–191. doi: 10.1300/J013v16n03_10. [DOI] [PubMed] [Google Scholar]
- 60.Chao D, Foy CG, Farmer D. Exercise adherence among older adults: challenges and strategies. Control Clin Trials. 2000;21(5, Supplement 1):S212–S217. doi: 10.1016/S0197-2456(00)00081-7. [DOI] [PubMed] [Google Scholar]
- 61.Dalle GR, Calugi S, Centis E, El GM, Marchesini G. Cognitive-behavioral strategies to increase the adherence to exercise in the management of obesity. J Obes. 2010;2011:348293. [DOI] [PMC free article] [PubMed]
- 62.Hillsdon M, Thorogood M. A systematic review of physical activity promotion strategies. Br J Sports Med. 1996;30(2):84–89. doi: 10.1136/bjsm.30.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kozica SL, Lombard CB, Ilic D, Ng S, Harrison CL, Teede HJ. Acceptability of delivery modes for lifestyle advice in a large scale randomised controlled obesity prevention trial. BMC Public Health. 2015;15:699. doi: 10.1186/s12889-015-1995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lombard CB, Deeks AA, Ball K, Jolley D, Teede HJ. Weight, physical activity and dietary behavior change in young mothers: short term results of the HeLP-her cluster randomized controlled trial. Nutr J. 2009;8:17–7. [DOI] [PMC free article] [PubMed]
- 65.Teede HJ, Misso ML, Deeks AA, Moran LJ, Stuckey BG, Wong JL, Norman RJ, Costello MF. Assessment and management of polycystic ovary syndrome: summary of an evidence-based guideline. Med J Aust. 2011;195(6):S65–112. doi: 10.5694/mja11.10915. [DOI] [PubMed] [Google Scholar]
- 66.Benson A, Torode M, Singh MF. The effect of high-intensity progressive resistance training on adiposity in children: a randomized controlled trial. Int J Obes (Lond) 2008;32(6):1016–1027. doi: 10.1038/ijo.2008.5. [DOI] [PubMed] [Google Scholar]
- 67.Dunstan DW, Daly RM, Owen N, Jolley D, de Courten M, Shaw J, Zimmet P. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25(10):1729–1736. doi: 10.2337/diacare.25.10.1729. [DOI] [PubMed] [Google Scholar]
- 68.Lemmey AB, Marcora SM, Chester K, Wilson S, Casanova F, Maddison PJ. Effects of high‐intensity resistance training in patients with rheumatoid arthritis: A randomized controlled trial. Arthritis Care Res. 2009;61(12):1726–1734. doi: 10.1002/art.24891. [DOI] [PubMed] [Google Scholar]
- 69.Seynnes O, Singh MAF, Hue O, Pras P, Legros P, Bernard PL. Physiological and functional responses to low-moderate versus high-intensity progressive resistance training in frail elders. J Gerontol A Biol Sci Med Sci. 2004;59(5):M503–M509. doi: 10.1093/gerona/59.5.M503. [DOI] [PubMed] [Google Scholar]
- 70.Singh NA, Quine S, Clemson LM, Williams EJ, Williamson DA, Stavrinos TM, Grady JN, Perry TJ, Lloyd BD, Smith EU. Effects of high-intensity progressive resistance training and targeted multidisciplinary treatment of frailty on mortality and nursing home admissions after hip fracture: a randomized controlled trial. J Am Med Dir Assoc. 2012;13(1):24–30. doi: 10.1016/j.jamda.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 71.Gordon B, Benson A, Bird S, et al. Resistance training improves metabolic health in type 2 diabetes: a systematic review. Diabetes Res Clin Pract. 2009;83:157–75. [DOI] [PubMed]
- 72.Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, Roubenoff R, Tucker KL, Nelson ME. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25(12):2335–2341. doi: 10.2337/diacare.25.12.2335. [DOI] [PubMed] [Google Scholar]
- 73.Ishii T, Yamakita T, Sato T, Tanaka S, Fujii S. Resistance training improves insulin sensitivity in NIDDM subjects without altering maximal oxygen uptake. Diabetes Care. 1998;21(8):1353–1355. doi: 10.2337/diacare.21.8.1353. [DOI] [PubMed] [Google Scholar]
- 74.Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, Teede HJ. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic–hyperinsulaemic clamp. Hum Reprod. 2013. [DOI] [PubMed]
- 75.Handelsman D, Wartofsky L. Requirement for mass spectrometry sex steroid assays in the Journal of Clinical Endocrinology and Metabolism. J Clin Endocrinol Metab. 2013;98(10):3971–3973. doi: 10.1210/jc.2013-3375. [DOI] [PubMed] [Google Scholar]
- 76.Brooks N, Layne JE, Gordon PL, Roubenoff R, Nelson ME, Castaneda-Sceppa C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int J Med Sci. 2007;4(1):19. doi: 10.7150/ijms.4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kadoglou NP, Iliadis F, Angelopoulou N, Perrea D, Ampatzidis G, Liapis CD, Alevizos M. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil. 2007;14(6):837–843. doi: 10.1097/HJR.0b013e3282efaf50. [DOI] [PubMed] [Google Scholar]
- 78.Kim ES, Im JA, Kim KC, Park JH, Suh SH, Kang ES, Kim SH, Jekal Y, Lee CW, Yoon YJ. Improved insulin sensitivity and adiponectin level after exercise training in obese Korean youth. Obesity. 2007;15(12):3023–3030. doi: 10.1038/oby.2007.360. [DOI] [PubMed] [Google Scholar]
- 79.Yokoyama H, Emoto M, Araki T, Fujiwara S, Motoyama K, Morioka T, Koyama H, Shoji T, Okuno Y, Nishizawa Y. Effect of aerobic exercise on plasma adiponectin levels and insulin resistance in type 2 diabetes. Diabetes Care. 2004;27(7):1756–1758. doi: 10.2337/diacare.27.7.1756. [DOI] [PubMed] [Google Scholar]
- 80.Braith RW, Stewart KJ. Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation. 2006;113(22):2642–2650. doi: 10.1161/CIRCULATIONAHA.105.584060. [DOI] [PubMed] [Google Scholar]
- 81.Kraemer WJ, Ratamess NA, French DN. Resistance training for health and performance. Curr Sports Med Rep. 2002;1(3):165–171. doi: 10.1249/00149619-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 82.Dunstan D, Puddey I, Beilin L, Burke V, Morton A, Stanton K. Effects of a short-term circuit weight training program on glycaemic control in NIDDM. Diabetes Res Clin Pract. 1998;40(1):53–61. doi: 10.1016/S0168-8227(98)00027-8. [DOI] [PubMed] [Google Scholar]
- 83.Ibañez J, Izquierdo M, Argüelles I, Forga L, Larrión JL, García-Unciti M, Idoate F, Gorostiaga EM. Twice-weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care. 2005;28(3):662–667. doi: 10.2337/diacare.28.3.662. [DOI] [PubMed] [Google Scholar]
- 84.Kogure GS, Silva RC, Picchi Ramos FK, Miranda-Furtado CL, Lara LA, Ferriani RA, Dos Reis RM. Women with polycystic ovary syndrome have greater muscle strength irrespective of body composition. Gynecol Endocrinol. 2015;31(3):237–242. doi: 10.3109/09513590.2014.982083. [DOI] [PubMed] [Google Scholar]
- 85.Banting LK, Gibson-Helm M, Polman R, Teede HJ, Stepto NK. Physical activity and mental health in women with Polycystic Ovary Syndrome. BMC Womens Health. 2014;14(1):1–9. doi: 10.1186/1472-6874-14-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Conte F, Banting L, Teede HJ, Stepto NK. Mental health and physical activity in women with polycystic ovary syndrome: a brief review. Sports Med. 2015;45(4):497–504. doi: 10.1007/s40279-014-0291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials can be made available on request via contacting the corresponding author.


