Abstract
Background
Although patients with minimally invasive follicular thyroid carcinoma (MIFTC) generally have an excellent prognosis, distant metastasis occurs in some patients. Risk factors for distant metastasis have been reported, none has been found to be conclusive. This study evaluated risk factors for distant metastasis, including protein markers, in patients with MIFTC.
Methods
A review of patient records identified 259 patients who underwent surgery at Asan Medical Center from 1996 to 2010 and were subsequently diagnosed with MIFTC. After review of pathological slides, 120 patients with paraffin blocks suited for tissue microarrays (TMA) were included in this study. Immunohistochemical stain of TMA slides was performed by protein markers; β-catenin, C-MET, CK19, estrogen receptor (ER) α, ER β, HBME-1, MMP2, PPAR γ and progesterone receptor.
Results
120 patients included 28 males (23.3%) and 92 females (76.7%), of mean age 41.5±10.8 years (range, 13–74 years). Eight patients (6.7%) had distant metastases during follow-up. Univariate analysis showed that age (≥45 years), male sex, and extensive vascular invasion (≥4 foci) were associated with distant metastasis. Multivariate regression analysis showed that extensive vascular invasion was the only independent risk factor for distant metastasis (p = 0.012). Although no protein markers on TMA analysis were directly related to distant metastasis of MIFTC, CK19 expression was more frequent in patients with than without extensive vascular invasion (p = 0.036).
Conclusion
Extensive vascular invasion was the only independent risk factor for distant metastasis of MIFTC. No proteins markers were directly related to distant metastasis, but CK19 was associated with extensive vascular invasion.
Introduction
Follicular thyroid carcinoma (FTC) is the second most common type of thyroid cancer, being present in 10–15% of patients with thyroid cancer. FTC can be histologically classified into two categories: minimally and widely invasive FTC. Minimally invasive FTC (MIFTC) is a grossly encapsulated solitary tumor with limited capsular and/or vascular invasion, whereas widely invasive FTC (WIFTC) is characterized by widespread infiltration of adjacent thyroid tissue and/or blood vessels by World Health Organization (WHO) classification. [1] Patients with MIFTC have an excellent prognosis because distant metastasis is very rare; by contrast, distant metastasis is observed in 10–30% of patients with WIFTC. [2, 3]
MIFTC is confirmed on pathological examination only after diagnostic hemithyroidectomy and it is difficult to preoperatively determine whether patients require complete thyroidectomy. Because of its excellent prognosis, it is generally known that MIFTCs do not need completion thyroidectomy. [2, 4, 5] Some patients at risk of developing distant metastasis, however, require complete thyroidectomy and radioactive iodine (RAI) ablation. [6–9]
Studies have suggested that age, sex, tumor size, and/or vascular invasion are clinicopathological risk factors for distant metastasis of MIFTC. Those studies, however, had limitations, including the small numbers of patients with distant metastases. Thus, to date, risk factors for distant metastasis remain unclear and there has been a growing interest to discover risk factors of distant metastasis through molecular biological research.
The expression of certain proteins may be related to distant metastases of MIFTC. Identification of specific biomarkers may allow identifying patients at risk for distant metastasis and in need of complete thyroidectomy and RAI ablation. Protein markers may be assessed by immunohistochemical (IHC) analysis of tissue microarrays (TMA), which is performed using the operative specimen and is cheap and convenient. This study was designed to evaluate clinicopathological risk factors for distant metastasis in patients with MIFTC and to determine protein biomarkers associated with patient prognosis.
Material and Methods
Tumor samples and patient data
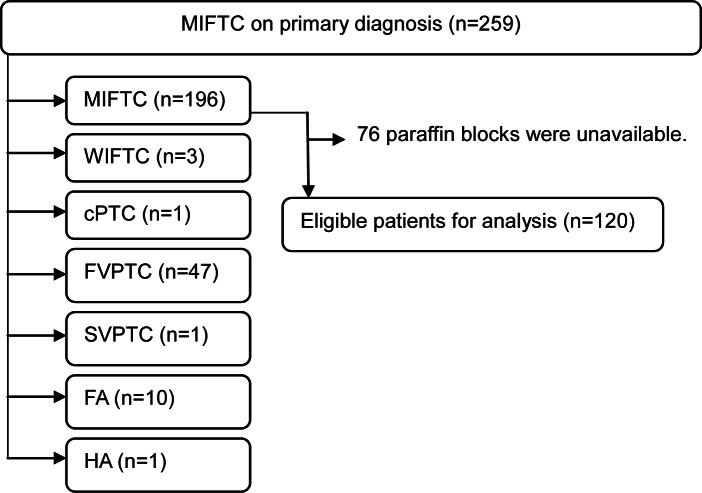
From February 1996 to December 2007, 259 patients were post-surgically diagnosed with MIFTC at Asan Medical Center. Hematoxylin and eosin (H&E) stained sections of these MIFTCs were reviewed according to WHO criteria by an experienced pathologist (DE Song). [1] 196 patients were confirmed as having MIFTC. Representative formalin-fixed and paraffin-embedded blocks were selected. Paraffin blocks for TMA were unavailable for 76 of these patients. Thus, 120 patients were included in this study (Fig 1).
Fig 1. The algorithm of selection for eligible patients in this study. All H&E slides were reviewed by an experienced pathologist (DE Song).
FA, follicular adenoma; cPTC, classical papillary thyroid carcinoma; FVPTC, follicular variant papillary thyroid carcinoma; HA, Hurthle cell adenoma; SVPTC; solid variant papillary thyroid carcinoma; WIFTC, widely invasive follicular thyroid carcinoma.
The medical records of these patients were retrospectively reviewed, and their clinicopathological characteristics and outcome data were recorded. Written informed consent was not obtained from participants for their clinical records and tissues to be used in this study. Patient identifiers were removed from all samples and all the data were de-identified prior to analysis by a researcher who was irrelevant to this study. All experiments were conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the Institutional Review Board of Asan Medical Center.
Construction of TMAs
Core tissue biopsies (2mm in diameter; three cores per patient) were taken from each paraffin block and arranged in new recipient paraffin blocks using TMArrayer (Beecher Instruments, Sun Prairie, WI, USA). One core was taken from the center of the tumor, and the other two cores were taken from the tumor borders. An additional core was taken from an area of normal thyroid as a control. Each block consisted of 54 cores, and a total of ten TMA blocks were produced. Sections from the TMA blocks were used for IHC stains in order to observe the expression of protein markers.
Selection of candidate proteins and IHC analysis
Based on review of the literature, protein markers associated with tumor development, progression, and metastasis were selected, especially those previously identified in thyroid carcinoma. Antibodies of protein markers used in this study are summarized in Table 1.
Table 1. Antibodies and conditions used in the study.
| Antibody | Clone | Manufacturer | Dilution |
|---|---|---|---|
| β-catenin | Polyclonal | Pharmingen, NJ | 1: 500 |
| C-MET | SP44 | Ventana, Tusan | 1: 4 |
| CK19 | A53-B/A2.26 | Cell Marque, CA | 1: 100 |
| ER α | Polyclonal | Santacruz, Ca | 1: 400 |
| ER β | Polyclonal | Santacruz, Ca | 1: 400 |
| HBME-1 | Polyclonal | DAKO, Denmark | 1: 200 |
| MMP2 | Polyclonal | Santacruz, Ca | 1: 10 |
| PPAR γ | Polyclonal | Santacruz, Ca | 1: 20 |
| PR | 16 | NOVO, UK | 1: 200 |
ER, estrogen receptor; PR, progesterone receptor
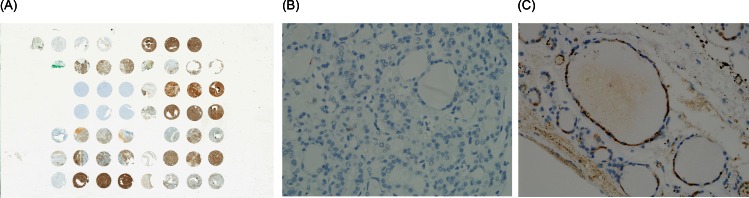
IHC was performed using 4μm sections and the appropriate primary antibodies, with the slides being analyzed by an experienced pathologist (DE Song). Immunoreactivity was defined as positive if >10% of tumor cells showed unequivocal positive staining. All results were evaluated relative to a negative control from the same tissue sample (Fig 2).
Fig 2. CK expression in minimally invasive follicular thyroid carcinoma.
(A) Overview image of TMA slide stained by CK19, (B) negative staining and (C) positive staining for CK19 (immunohistochemistry, original magnification ×200).
Validation
Although TMA can analyze many samples simultaneously, as well as analyze many types of markers, TMA has a disadvantage in that it represents a small part of each tumor. A validation test was performed to compensate for this disadvantage. Unstained slides produced from the original paraffin blocks of the entire tumors of all 120 MIFTCs were stained again. For validation staining, we only used antibodies of protein markers which showed relationship with MIFTC in TMA analysis. These slides were analyzed by the same experienced pathologist (DE Song), with immunoreactivity defined as above.
Statistical analysis
Patients were classified into two groups, those with and without distant metastasis. Clinicopathological characteristics and expression of protein markers were compared in these two groups. In addition, the correlations between clinicopathological characteristics and expression of protein markers were analyzed.
Risk factors for recurrence were evaluated by univariate and multivariate Cox regression analysis. Recurrence-free survival was assessed by the Kaplan–Meier method and compared by the log rank test. The correlations between frequencies of expression of protein markers and the risk factors were analyzed by the χ2test or Fisher’s exact test. P values <0.05 were considered statistically significant. All statistical analyses were performed using the SPSS program, version 21.0 (SPSS Inc, Chicago, IL, USA)
Results
Clinicopathological characteristics related to distant metastasis
Of the 120 patients, eight (6.7%) developed distant metastasis during the follow-up period. The median follow-up period was 99.5 months (range, 13–222) and the median time to distant metastasis was 34.5 months (range, 13–133 months). The clinicopathological characteristics of these patients are summarized in Table 2.
Table 2. Clinicopathologic characteristics of patients with distant metastasis.
| No. | Age (year) | Sex | Surgery | Tumor Size (cm) | Capsular invasion (foci) | Angioinvasion (foci) | Disease-free survival (months) | Distant metastasis | Treatment for metastasis | Status |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 | Female | Hemithyroidectomy | 4.5 | 0 | 10 | 100 | Lung, bone | RAI, Surgery | Dead |
| 2 | 54 | Female | Total thyroidectomy | 5.0 | 5 | 6 | 127 | Lung, bone | RAI | Alive |
| 3 | 57 | Male | Total thyroidectomy | 9.3 | 4 | 10 | 21 | Lung, bone | RAI | Dead |
| 4 | 51 | Male | Hemithyroidectomy | 0.8 | 0 | 2 | 25 | Lung | RAI | Alive |
| 5 | 47 | Female | Hemithyroidectomy | 2.7 | 1 | 0 | 133 | Bone | RAI | Alive |
| 6 | 53 | Male | Total thyroidectomy | 4 | 0 | 4 | 96 | Lung | No | Alive |
| 7 | 61 | Male | Total thyroidectomy | 5.2 | 1 | 4 | 19 | Bone | RAI | Alive |
| 8 | 27 | Male | Total thyroidectomy | 6.0 | 2 | 10 | 20 | Bone | RAI | Alive |
RAI, radioactive iodine
Age (≥45 years), male sex, tumor size ≥4.0 cm, undergoing total thyroidectomy, and extensive (≥4 foci) capsular or vascular invasion were more frequent in patients who did than did not develop distant metastasis. Univariate analysis showed that age (≥45 years; odds ratio [OR] = 5.07, 95% confidence interval [CI] = 1.01–25.43, p = 0.048), male sex (OR = 4.44, 95% CI = 1.08–18.25, p = 0.039), and extensive vascular invasion (OR = 6.86, 95% CI = 1.64–28.74, p = 0.008) were significant risk factors for distant metastasis of MIFTC. Multivariate analysis showed that extensive vascular invasion was the only independent risk factor related to distant metastasis (OR = 6.44, 95% CI = 1.50–27.69, p = 0.012) (Table 3).
Table 3. Univariate and multivariate analysis of risk factors for distant metastasis.
| Variables | No recurrence 112 (93.3%) | Distant metastasis 8 (6.7%) | Univariate | Multivariate* | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||||
| Sex | Female | 89 (79.5%) | 3 (37.5%) | Ref. | 0.039 | Ref. | 0.14 |
| Male | 23 (20.8%) | 5 (62.5%) | 4.44 (1.08–18.25) | 3.15 (0.68–14.63) | |||
| Age | < 45 years | 70 (62.5%) | 1 (12.5%) | Ref. | 0.048 | Ref. | 0.15 |
| ≥ 45 years | 42 (37.5%) | 7 (87.5%) | 5.07 (1.01–25.43) | 3.55 (0.62–20.22) | |||
| Surgical extent | Hemithyroidectomy | 66 (58.9%) | 3 (37.5%) | Ref. | 0.14 | NA | NA |
| Total thyroidectomy | 46 (41.1%) | 5 (62.5%) | 3.32 (0.67–16.49) | ||||
| Tumor size (cm) | < 4.0 cm | 63 (56.3%) | 2 (25.0%) | Ref. | 0.09 | NA | NA |
| ≥ 4.0 cm | 49 (43.8%) | 6 (75.0%) | 3.99 (0.80–19.85) | ||||
| Capsular invasion | < 4 foci | 103 (92.0%) | 5 (62.5%) | Ref. | 0.16 | NA | NA |
| ≥ 4 foci | 9 (8.0%) | 2 (37.5%) | 3.17 (0.64–15.74) | ||||
| Angioinvasion | < 4 foci | 105 (93.7%) | 2 (25.0%) | Ref. | 0.008 | Ref. | 0.012 |
| ≥ 4 foci | 7 (6.3%) | 6 (75.0%) | 6.86 (1.64–28.74) | 6.44 (1.50–27.69) | |||
*Multivariate analysis was performed using variables that showed statistically difference in univariate analysis.
CI, confidence interval; NA, not available; Ref., reference
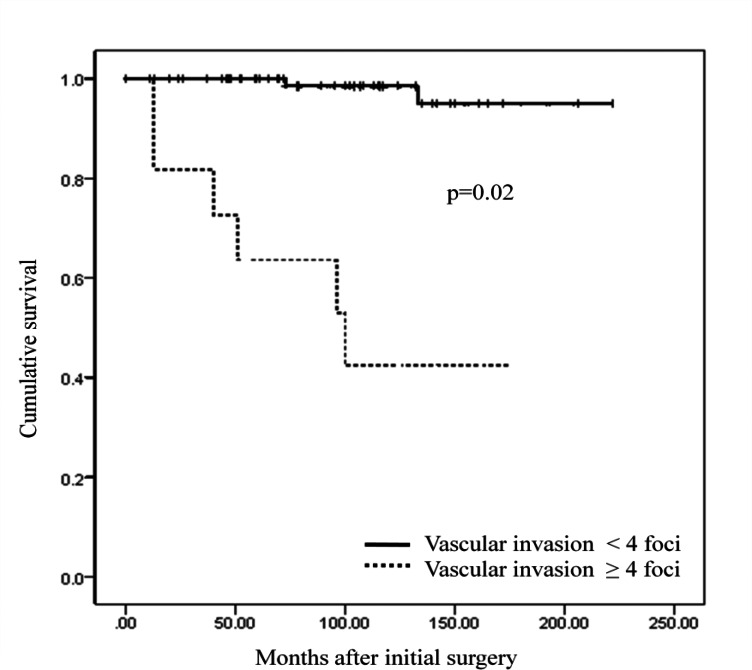
Recurrence-free survival (RFS) was significantly shorter in patients with than without extensive vascular invasion (p = 0.02) (Fig 3). 5-year and 10-year RFS rates were 63.6% and 42.4% in patients with extensive vascular invasion, while 95.0% and 98.5% in patients without extensive vascular invasion.
Fig 3. Comparison of recurrence free survival on basis of the presence of extensive vascular invasion.
Protein markers associated with distant metastasis on TMA analysis
None of the protein markers assessed showed significant differences in expression between patients with and without distant metastasis (Table 4). Several protein markers, however, correlated with clinicopathological risk factors (Table 5). β-catenin and estrogen receptor α were more frequently expressed in male patients. CK19 was more frequently detected in patients with extensive vascular invasion.
Table 4. Comparison of the expression of protein markers between the group with distant metastasis and without distant metastasis.
| No recurrence (n = 112) | Distant metastasis (n = 8) | p | |
|---|---|---|---|
| β-catenin | 5 (4.5): 107 (95.5) | 0 (0): 8 (100) | 0.7 |
| C-MET | 3 (2.7): 109 (97.3) | 0 (0): 8 (100) | 0.81 |
| CK19 | 41 (36.6): 71 (63.4) | 4 (50.0): 4 (50.0) | 0.35 |
| ER α | 4 (3.6): 108 (96.4) | 0 (0): 8 (100) | 0.76 |
| ER β | 42 (37.5): 70 (62.5) | 4 (50.0): 4 (50.0) | 0.36 |
| HBME-1 | 75 (67.0): 37 (33.0) | 6 (75.0): 2 (25.0) | 0.49 |
| MMP2 | 25 (22.3): 87 (77.7) | 2 (25.0): 6 (75.0) | 0.57 |
| PPAR γ | 32 (28.6): 80 (71.4) | 3 (37.5): 5 (62.5) | 0.43 |
| PR | 1 (0.9): 111 (99.1) | 0 (0): 8 (100) | 0.93 |
Data are described as positive (%): negative (%).
Table 5. Comparison of the expression of protein markers at TMA analysis according to clinicopathologic characteristics.
| Sex | p | Age (years) | p | Angioinvasion (foci) | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Male (n = 28) | Female (n = 92) | < 45 (n = 71) | ≥ 45 (n = 49) | < 4 (n = 107) | ≥ 4 (n = 13) | ||||
| β-catenin | 3 (10.7) | 2 (2.2) | 0.048 | 2 (2.8) | 3 (6.1) | 0.33 | 5 (4.7) | 0 (0) | 0.56 |
| C-MET | 2 (7.1) | 1 (1.1) | 0.14 | 0 (0) | 3 (6.1) | 0.035 | 3 (2.8) | 0 (0) | 0.71 |
| CK19 | 18 (64.3) | 57 (62.0) | 0.82 | 46 (64.8) | 29 (59.2) | 0.53 | 64 (59.8) | 12 (92.3) | 0.036 |
| ER α | 4 (14.3) | 0 (0) | 0.002 | 1 (1.4) | 3 (6.1) | 0.3 | 3 (2.8) | 1 (7.7) | 0.37 |
| ER β | 9 (32.1) | 37 (40.2) | 0.44 | 24 (33.8) | 22 (44.9) | 0.22 | 41 (38.3) | 5 (38.5) | 0.61 |
| HBME-1 | 17 (60.7) | 64 (69.6) | 0.38 | 50 (70.4) | 31 (63.3) | 0.41 | 72 (67.3) | 9 (69.2) | 0.58 |
| MMP2 | 8 (28.6) | 19 (20.7) | 0.38 | 15 (21.1) | 12 (24.5) | 0.67 | 22 (20.6) | 5 (38.5) | 0.14 |
| PPAR γ | 10 (35.7) | 25 (27.2) | 0.38 | 19 (26.8) | 16 (32.7) | 0.49 | 30 (28.0) | 5 (38.5) | 0.31 |
| PR | 0 (0) | 1 (1.1) | 0.77 | 1 (1.4) | 0 (0) | 0.59 | 1 (0.9) | 0 (0) | 0.89 |
Data are described as positive (%).
We only presented results with showing significant differences. The other clinicopathologic characteristics had no correlation with protein markers.
Validation IHC staining test
Validation tests failed to identify any correlation between protein marker expression and the development of distant metastasis or the presence of clinicopathological risk factors (data not shown). Also, no protein markers showed a significant correlation when we performed analysis for validation between the expression of protein markers and clinicopathologic risk factors. CK19 expression was detected more frequently in patients with extensive vascular invasion, but its level of significance was borderline (p = 0.061).
Discussion
The pathological diagnosis and treatment of FTC, especially MIFTC, is unclear due to the ambiguous criteria of the WHO classification. A review of all slides from patients initially diagnosed with MIFTC in our institution resulted in the reclassification of 63 of these patients as having other diagnoses, such as a follicular variant of papillary thyroid carcinoma or WIFTC. Although several proposals suggested that the criteria for FTC be revised, [2, 10, 11] no consensus has been reached on these criteria. All slides in this study were reviewed by a single experienced pathologist according to the WHO classification to prevent interobserver differences in diagnosis. The 120 patients confirmed as having MIFTC were included in this study.
This study found that extensive vascular invasion (≥4 foci) is an independent risk factor for distant metastasis in patients with MIFTC. Vascular invasion itself was reported to be a risk factor for distant metastasis of MIFTC, [2, 10, 12, 13] although other studies found that the mere presence of vascular invasion was not associated with prognosis. [5, 7, 8, 14] A review of 282 patients with MIFTC found that vascular invasion by four or more foci was associated with distant metastasis, suggesting the need for aggressive treatment, including mandatory complete thyroidectomy. [8] Our results are consistent with these earlier findings. The presence of vascular invasion itself was not a risk factor for distant metastasis (data not shown), while extensive vascular invasion was related to develop distant metastasis. Therefore we assumed that completion thyroidectomy followed by RAI therapy should be considered when extensive vascular invasion is present in MIFTC after diagnostic lobectomy.
Several other prognostic indices have been proposed for MIFTC. Many studies found that male sex, older age, and/or large tumor size were associated with poor prognosis. [2, 5–8, 13, 14] This study showed that age and sex were significantly associated with distant metastasis only on univariate analysis. Although these risk factors may affect prognosis, extensive vascular invasion was the most powerful risk factor. The results of multivariate analysis, however, were limited by the small number of patients in this study with distant metastasis.
This study was unable to identify protein markers directly related to distant metastasis in patients with MIFTC. Loss of estrogen receptor β and NRAS mutation have been associated with poor prognosis in patients with FTC, but these studies included patients with WIFTC. [15, 16] To date, no biomarkers have been found to be related to prognosis in patients with MIFTC. Although this study showed that CK19, estrogen receptor β, and PPAR-γ were differently expressed in patients with and without distant metastasis, the differences were not statistically significant.
Assessment of the correlation between expression of protein markers and clinicopathological characteristics showed that CK19 was more frequently expressed in patients who had MIFTC with extensive vascular invasion. In as much as extensive vascular invasion was an independent risk factor for distant metastasis, CK19 expression was indirectly associated with poorer prognosis in patients with MIFTC.
CK19 is a low molecular weight cytokeratin belonging to a subgroup of cytoskeletal proteins. Immunochemical detection of CK19 has been reported as diagnostic of PTC. [17, 18] Recently, CK19 expression was reported to adversely affect prognosis in patients with PTC, [19, 20] but the mechanism linking CK19 to the progression of thyroid carcinoma has not been determined. CK19 may be an indicator of stem cell capabilities and may indicate manifestations of undifferentiated characteristics in differentiated tumors. [21, 22] Moreover, CK19 may affect tumor metastasis by promoting cell mobility or extracellular degradation. [23] We assume that CK19 expression may be associated with the infiltration or migration of cancer cells and may correlate with the development of distant metastasis in patients with MIFTC. Expression of CK19, along with the presence of extensive vascular invasion, may be used to determine treatment strategies in patients with MIFTC invasion.
This study had several limitations. First, the number of patients who developed distant metastasis during the follow-up period was relatively small, it was difficult to obtain statistical determinations of the association between clinicopathological features and prognosis. Second, many paraffin blocks were in too poor condition to construct TMAs, making it difficult to evaluate the expression of protein markers in these sections from TMA blocks. Also, the TMA method itself is limited. A TMA cannot represent all of the features of an entire tumor, since each sample is taken from part of a tumor. To compensate for this drawback, TMAs were constructed by collecting three cores from each tumor, and validation tests were performed using the original paraffin blocks containing the entire tumors.
Conclusion
Extensive vascular invasion (≥4 foci) was an independent risk factor for distant metastasis in patients with MIFTC. Although no protein markers were directly related to distant metastasis, CK19 was more frequently expressed in patients with than without extensive vascular invasion.
Acknowledgments
This study was supported by a grant 2013–572 from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
Data Availability
All data for the paper are strictly prohibited from public opening by Institutional Review Board of Asan Medical Center. We cannot provide our data, because there is a law in Korea about use of medical data (The Protection & Use of Personal Information #13423), which does not permit to provide our data to other researchers or studies.
Funding Statement
This study was supported by a grant 2013-572 from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea. (http://ails.amc.seoul.kr). Ki-Wook Chung was received this funding.
References
- 1.DeLellis R, Lloyd R, Heitz P, Eng C. WHO Classification of Tumours, Pathology and Genetics of Tumours of Endocrine Organs. IARC Press, Lyon: 2004:73–6. [Google Scholar]
- 2.Lo CY, Chan WF, Lam KY, Wan KY. Follicular thyroid carcinoma: the role of histology and staging systems in predicting survival. Annals of surgery. 2005;242(5):708–15. Epub 2005/10/26. ; PubMed Central PMCID: PMCPmc1409851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito Y, Hirokawa M, Higashiyama T, Takamura Y, Miya A, Kobayashi K, et al. Prognosis and prognostic factors of follicular carcinoma in Japan: importance of postoperative pathological examination. World journal of surgery. 2007;31(7):1417–24. Epub 2007/05/31. 10.1007/s00268-007-9095-2 . [DOI] [PubMed] [Google Scholar]
- 4.Goffredo P, Cheung K, Roman SA, Sosa JA. Can minimally invasive follicular thyroid cancer be approached as a benign lesion?: a population-level analysis of survival among 1,200 patients. Annals of surgical oncology. 2013;20(3):767–72. Epub 2012/11/01. 10.1245/s10434-012-2697-4 . [DOI] [PubMed] [Google Scholar]
- 5.Sugino K, Kameyama K, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, et al. Does completion thyroidectomy improve the outcome of patients with minimally invasive follicular carcinoma of the thyroid? Annals of surgical oncology. 2014;21(9):2981–6. Epub 2014/04/29. 10.1245/s10434-014-3734-2 . [DOI] [PubMed] [Google Scholar]
- 6.Ban EJ, Andrabi A, Grodski S, Yeung M, McLean C, Serpell J. Follicular thyroid cancer: minimally invasive tumours can give rise to metastases. ANZ journal of surgery. 2012;82(3):136–9. Epub 2012/04/19. 10.1111/j.1445-2197.2011.05979.x . [DOI] [PubMed] [Google Scholar]
- 7.Sugino K, Kameyama K, Ito K, Nagahama M, Kitagawa W, Shibuya H, et al. Outcomes and prognostic factors of 251 patients with minimally invasive follicular thyroid carcinoma. Thyroid: official journal of the American Thyroid Association. 2012;22(8):798–804. Epub 2012/08/03. 10.1089/thy.2012.0051 . [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Hirokawa M, Masuoka H, Yabuta T, Kihara M, Higashiyama T, et al. Prognostic factors of minimally invasive follicular thyroid carcinoma: extensive vascular invasion significantly affects patient prognosis. Endocrine journal. 2013;60(5):637–42. Epub 2013/01/19. . [DOI] [PubMed] [Google Scholar]
- 9.Delbridge L, Parkyn R, Philips J, Barraclough B, Robinson B. Minimally invasive follicular thyroid carcinoma: completion thyroidectomy or not? ANZ journal of surgery. 2002;72(11):844–5. . [PubMed] [Google Scholar]
- 10.D'Avanzo A, Treseler P, Ituarte PH, Wong M, Streja L, Greenspan FS, et al. Follicular thyroid carcinoma: histology and prognosis. Cancer. 2004;100(6):1123–9. Epub 2004/03/17. 10.1002/cncr.20081 . [DOI] [PubMed] [Google Scholar]
- 11.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for follicular thyroid carcinoma: application to 171 consecutive patients treated in a tertiary referral centre. Endocrine-related cancer. 2007;14(1):29–42. 10.1677/erc.1.01284 . [DOI] [PubMed] [Google Scholar]
- 12.Collini P, Sampietro G, Pilotti S. Extensive vascular invasion is a marker of risk of relapse in encapsulated non-Hürthle cell follicular carcinoma of the thyroid gland: a clinicopathological study of 18 consecutive cases from a single institution with a 11-year median follow-up. Hisopathology. 2004;44(1):35–9. [DOI] [PubMed] [Google Scholar]
- 13.O'Neill CJ, Vaughan L, Learoyd DL, Sidhu SB, Delbridge LW, Sywak MS. Management of follicular thyroid carcinoma should be individualised based on degree of capsular and vascular invasion. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2011;37(2):181–5. Epub 2010/12/15. 10.1016/j.ejso.2010.11.005 . [DOI] [PubMed] [Google Scholar]
- 14.Ito Y, Miyauchi A, Tomoda C, Hirokawa M, Kobayashi K, Miya A. Prognostic significance of patient age in minimally and widely invasive follicular thyroid carcinoma: investigation of three age groups. Endocr J. 2014;61(3):265–71. . [DOI] [PubMed] [Google Scholar]
- 15.Jang EK, Song DE, Sim SY, Kwon H, Choi YM, Jeon MJ, et al. NRAS codon 61 mutation is associated with distant metastasis in patients with follicular thyroid carcinoma. Thyroid. 2014;24(8):1275–81. Epub 2014/05/14. 10.1089/thy.2014.0053 ; PubMed Central PMCID: PMCPmc4106375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heikkila A, Hagstrom J, Maenpaa H, Louhimo J, Siironen P, Heiskanen I, et al. Loss of estrogen receptor Beta expression in follicular thyroid carcinoma predicts poor outcome. Thyroid. 2013;23(4):456–65. 10.1089/thy.2012.0363 . [DOI] [PubMed] [Google Scholar]
- 17.Wiseman SM, Melck A, Masoudi H, Ghaidi F, Goldstein L, Gown A, et al. Molecular phenotyping of thyroid tumors identifies a marker panel for differentiated thyroid cancer diagnosis. Ann Surg Oncol. 2008;15(10):2811–26. 10.1245/s10434-008-0034-8 . [DOI] [PubMed] [Google Scholar]
- 18.de Matos PS, Ferreira AP, de Oliveira Facuri F, Assumpcao LV, Metze K, Ward LS. Usefulness of HBME-1, cytokeratin 19 and galectin-3 immunostaining in the diagnosis of thyroid malignancy. Histopathology. 2005;47(4):391–401. 10.1111/j.1365-2559.2005.02221.x . [DOI] [PubMed] [Google Scholar]
- 19.Calangiu CM, Simionescu CE, Stepan AE, Cernea D, Zavoi RE, Margaritescu C. The expression of CK19, vimentin and E-cadherin in differentiated thyroid carcinomas. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie. 2014;55(3):919–25. . [PubMed] [Google Scholar]
- 20.Liu Z, Li X, Shi L, Maimaiti Y, Chen T, Li Z, et al. Cytokeratin 19, thyroperoxidase, HBME-1 and galectin-3 in evaluation of aggressive behavior of papillary thyroid carcinoma. International journal of clinical and experimental medicine. 2014;7(8):2304–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitt AM, Anlauf M, Rousson V, Schmid S, Kofler A, Riniker F, et al. WHO 2004 criteria and CK19 are reliable prognostic markers in pancreatic endocrine tumors. The American journal of surgical pathology. 2007;31(11):1677–82. 10.1097/PAS.0b013e31805f675d . [DOI] [PubMed] [Google Scholar]
- 22.Li J, Goodyer CG, Fellows F, Wang R. Stem cell factor/c-Kit interactions regulate human islet-epithelial cluster proliferation and differentiation. The international journal of biochemistry & cell biology. 2006;38(5–6):961–72. Epub 2005/10/11. 10.1016/j.biocel.2005.08.014 . [DOI] [PubMed] [Google Scholar]
- 23.Ding SJ, Li Y, Tan YX, Jiang MR, Tian B, Liu YK, et al. From proteomic analysis to clinical significance: overexpression of cytokeratin 19 correlates with hepatocellular carcinoma metastasis. Molecular & cellular proteomics: MCP. 2004;3(1):73–81. 10.1074/mcp.M300094-MCP200 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data for the paper are strictly prohibited from public opening by Institutional Review Board of Asan Medical Center. We cannot provide our data, because there is a law in Korea about use of medical data (The Protection & Use of Personal Information #13423), which does not permit to provide our data to other researchers or studies.