Abstract
In the past decade, developing countries have been experiencing rapid land use and land cover changes, including deforestation and cultivation of previously forested land. However, little is known about the impact of deforestation and land-use changes on the life history of malaria vectors and their effects on malaria transmission. This study examined the effects of deforestation and crop cultivation on the adult survivorship of major malaria mosquitoes, Anopheles sinensis and An. minimus in the China-Myanmar border region. We examined three conditions: indoor, forested, and banana plantation. Mean survival time of An. sinensis in banana plantation environment was significantly longer than those in forested environment, and mosquitoes exhibited the longest longevity in the indoor environment. This pattern held for both males and females, and also for An. minimus. To further test the effect of temperature on mosquito survival, we used two study sites with different elevation and ambient temperatures. Significantly higher survivorship of both species was found in sites with lower elevation and higher ambient temperature. Increased vector survival in the deforested area could have an important impact on malaria transmission in Southeast Asia. Understanding how deforestation impacts vector survivorship can help combat malaria transmission.
Introduction
Malaria is a significant public health problem affecting predominantly vulnerable pregnant women and children in Africa [1–3]. Interestingly, in Southeast Asia, there has been a change in malaria epidemiology where the adult male population has borne a greater burden of disease [4, 5]. Although there is a strong correlation between malaria and poverty [6–9], malaria is both a cause and a consequence of poverty. Malaria’s devastating effects have historically been observed in countries of the Greater Mekong Subregion (GMS). Since the inception of the World Health Organization’s Mekong Malaria Program a decade ago, the malaria situation in the GMS has been greatly improved, reflected by the continuous decline in annual malaria incidence and deaths [1, 10]. However, as all countries within the GMS are moving towards malaria elimination, significant challenges remain, particularly in Myanmar, where the regional malaria burden is the heaviest [10]. Malaria epidemiology in this region has several characteristics, including being an epicenter of antimalarial drug resistance [10–13], high malaria transmission in the forested fringe areas [4, 14] and in the remote border region [15–17], and cross-border malaria introduction due to human movement [11]. For example, over 90% of the imported falciparum malaria in China was introduced across the China-Myanmar border [18, 19]. As the GMS countries aim at malaria elimination by 2030 [20], it is crucial to examine the current situations of malaria epidemiology and vector biology in the high-risk border region so that optimal elimination strategies can be developed.
Vector control has historically been the most effective method to reduce malaria transmission. It remains the most important tool available, but its effectiveness relies on a thorough understanding of vector biology and their interactions with the environment. Due to human population expansion and an increasing demand for food supply, deforestation has become a very serious problem in the China-Myanmar border area [21–23]. Consequently, previously forested areas are often converted to lands for subsistence and cash crops. The changes in the environmental conditions in the area have been shown to alter malaria vector species composition [24–27] and in turn, malaria transmission. Different vector species vary in their feeding behaviors and vector competence [28–30]. However, the ecological mechanisms underlying malaria vector species succession are not well understood.
The objective of the present study is to determine the effects of land use and land cover on the adult survivorship of major malaria vector species in the China-Myanmar border area using the life-table experiments. We found that mosquito survivorship was strongly influenced by the microclimatic conditions such as ambient temperature, which is directly affected by land use and land cover. This information is useful for predicting the impact of environmental and climate changes on vectorial capacity.
Materials and Methods
Ethics Statement
No specific permits were required for the described field studies. For mosquito collection in banana fields, oral consent was obtained from field owners in each location. These locations were not protected land, and the field studies did not involve endangered or protected species. The use of mice in mosquito blood-feeding was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All of the animals were handled according to approved institutional animal care and use committee (IACUC) protocols (#2008–2774) of University of California at Irvine.
Study Sites
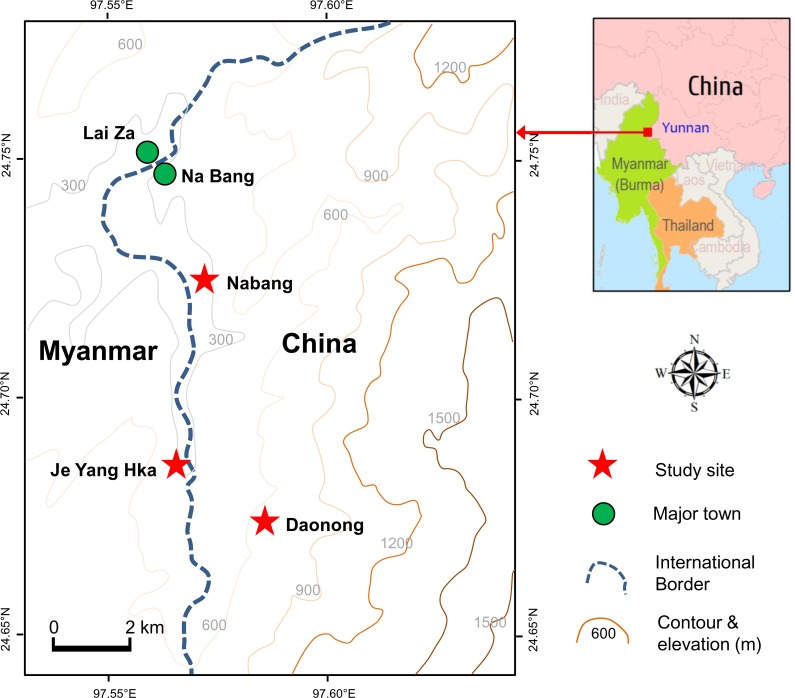
The study was conducted in the China-Myanmar border region (24°44´12"N, 97°36´9"E). The study sites were located in Kachin State, Myanmar and Yingjiang County of Yunnan Province, China (Fig 1). The study area covered an area of about 100 km2, including two villages (Nabang and Daonong) in China and one site near the town of Laiza, Kachin State in Myanmar. The two sites in China differ in elevation with Nabang at 240 m and Daonong at 660 m above sea level. The site in Myanmar, Je Yang Hka, is about 5 km from Nabang with an elevation of ~200 m above sea level. The study area is a hilly area, with mountains being covered mainly by forest or maize/banana plantations after deforestation and the valley areas being covered by banana plantations. Rubber, black pepper, and banana farming are the predominant agricultural activities, and food crops of mostly maize are cultivated at a very small scale. The average annual rainfall in the past 30 years was 1,464 mm and average monthly ambient temperature was 19.3°C. The mosquito larvae habitats in the study area included ponds, puddles, swamps, and other sources of stagnant aquatic habitats.
Fig 1. Study area and sampling sites in the China-Myanmar border region.
Effects of Land Use and Land Cover on Mosquito Survival
We examined three land cover scenarios: banana planation, forest, and indoor environment within typical local houses. The study was conducted from May to July in 2014, during the peak malaria transmission season in this area [31, 32]. The peak vector abundance was occurred from April to July each year [33, 34]. A forested area is defined as an area with >60% tree canopy coverage measured by ground shade area, and the vegetation was mainly subtropical evergreen broad-leaf rainforest with some deciduous trees in the canopy layer. The banana plantation was an area of banana plants planted two years prior to this study, and the canopy coverage was about 40% measured by ground shade area. Typical local houses have mixed brick and wood/bamboo structures, with brick/concrete walls on the ground floor and wood/bamboo walls on the elevated floor. Windows are usually not screened. Residents usually spend the night upstairs but may take naps downstairs during noon time. The major vector mosquito species are Anopheles minimus (forested area) and Anopheles sinensis (deforested area).
Approximately 5,000 Anopheline mosquito larvae were collected from local habitats and reared to adults in an insectary located in Nabang. To avoid using mosquitoes from one single female, we collected no more than 50 third- to fourth-instar larvae per habitat and reared them to adults under the same conditions. Emerged adults were identified to species or species complex using published morphological keys of Dong [35]. Species-specific polymerase chain reactions (PCR) were used for confirmatory identification of the species for a subset of adult mosquitoes [36–39]. Newly emerged adults were used for life-table studies. Briefly, 50 female and 50 male adult mosquitoes within 24 h post-emergence were placed in a cylindrical cage of 20 cm in diameter and 30 cm in height. The cage was covered with nylon mesh to prevent the escaping of mosquitoes. Four replicates were used for each of the three environments. In the forested environment and banana plantation, the cages were suspended under a tree or banana leaf, 2 m above the ground. In the indoor environment, mosquito cages were hung in the middle of the living room, also 2 m above the ground. A plastic cap filled with water was hung directly above the cages to prevent ants from entering the cages. Plastic covers were placed on the top of the cages to protect cages from rains. Mosquitoes were provided with 10% sucrose and one mouse in each cage was used to blood feed mosquitoes for approximately 30 minutes every morning. The cages were examined daily for the numbers of surviving and dead mosquitoes, and dead mosquitoes were then removed. HOBO data loggers (Onset Computer Corp., Bourne, MA) were placed inside the cages to record hourly temperature, relative humidity, and light intensity every min during the entire duration of the experiment. The HOBO data logger is a compact, battery-powered device equipped with an internal microprocessor, data storage, and one or more sensors, which can be used to track environmental temperature, relative humidity and light intensity. Life-table studies were conducted for An. sinensis and An. minimus, the two predominant malaria vector species in the study area.
Anopheles Mosquito Life-Table Experiments in High- and Low-Elevation Area
To further confirm the effects of microclimate conditions on mosquito survivorship, we conducted life-table studies in an indoor environment at two sites differing in elevation and in indoor air temperature. The study was conducted in Nabang (elevation 240 m) and Daonong (elevation 660 m) villages, from April to June in 2012. The same procedures described above were used for larval sample collection, mosquito rearing, cage placement conditions, and blood feeding to determine daily survivorship. Four replicates were used in each village. HOBO data loggers were placed inside the same houses to measure hourly indoor temperature, relative humidity, and light intensity at the two study sites for the entire duration of the experiment.
Statistical Analysis
Data were analyzed to address the following questions: 1) Do land use and land cover significantly affect mosquito survivorship? We addressed this question using Kaplan-Meier survival analysis [40] to determine the variation in daily survivorship among mosquitoes placed in different land use and land cover types, or between two sites of different elevations. 2) Do land use and land cover significantly affect the microclimatic conditions of local niches where adult mosquitoes were tested for survivorship? Daily average, minimum, and maximum temperatures and relative humidity were calculated from the hourly record. Analysis of variance (ANOVA) with repeated measures was used to determine the differences in these microclimatic variables across different land use and land cover types. The post hoc, Tukey’s honestly significant difference (HSD) test was used to determine which groups significantly differed from each other. Tukey’s HSD procedure was developed specifically to account for multiple comparisons and maintains an experiment-wise error rate at the specified level [41]. 3) Do mosquito species (An. sinensis and An. minimus) differ in their response to the microclimatic conditions in survivorship? Kaplan-Meier survival analysis was used to compare the two mosquito species under the same environmental conditions. A log-rank test was used to determine the significance of difference between two survival curves. All analyses were conducted using JMP statistical software [42].
Results
Community Structure of Anopheles Mosquitoes
Among the 7,736 adult Anopheles mosquitoes emerging from the larval collections, a total of 18 species were identified (Table 1). The most abundant species (complex) was An. sinensis (50.5%), followed by An. minimus (21.5%), An. barbirostris (10.8%) and An. maculatus (6.3%). Morphological identification of the mosquitoes was confirmed by species-specific PCR. The subsequent life-table studies were conducted with the two most abundant malaria vector species, An. sinensis and An. minimus.
Table 1. Composition of Anopheles adult mosquito rearing from larvae collected in China-Myanmar border region.
| Species | Female | Male | Total | Percentage (%) |
|---|---|---|---|---|
| Anopheles sinensis | 2,091 | 1,815 | 3,906 | 50.49 |
| Anopheles minimus | 894 | 770 | 1,664 | 21.51 |
| Anopheles barbirostris | 477 | 360 | 837 | 10.82 |
| Anopheles maculatus | 300 | 186 | 486 | 6.28 |
| Anopheles splendidus | 168 | 69 | 237 | 3.06 |
| Anopheles peditaeniatus | 87 | 96 | 183 | 2.37 |
| Anopheles vagus | 42 | 56 | 98 | 1.27 |
| Anopheles crawfordi | 42 | 36 | 78 | 1.01 |
| Anopheles kochi | 42 | 33 | 75 | 0.97 |
| Anopheles culicifacies | 25 | 21 | 46 | 0.59 |
| Anopheles jetporiensis | 20 | 15 | 35 | 0.45 |
| Anopheles barbumbrosus | 21 | 10 | 31 | 0.40 |
| Anopheles tessellatus | 14 | 6 | 20 | 0.26 |
| Anopheles hyrcanus | 10 | 8 | 18 | 0.23 |
| Anopheles annularis | 6 | 6 | 12 | 0.16 |
| Anopheles annularis | 3 | 1 | 4 | 0.05 |
| Anopheles messeae | 2 | 1 | 3 | 0.04 |
| Anopheles lesteri | 1 | 2 | 3 | 0.04 |
| Total | 4,245 | 3,491 | 7,736 | 100 |
Effects of Land Use and Land Cover on Mosquito Survivorship
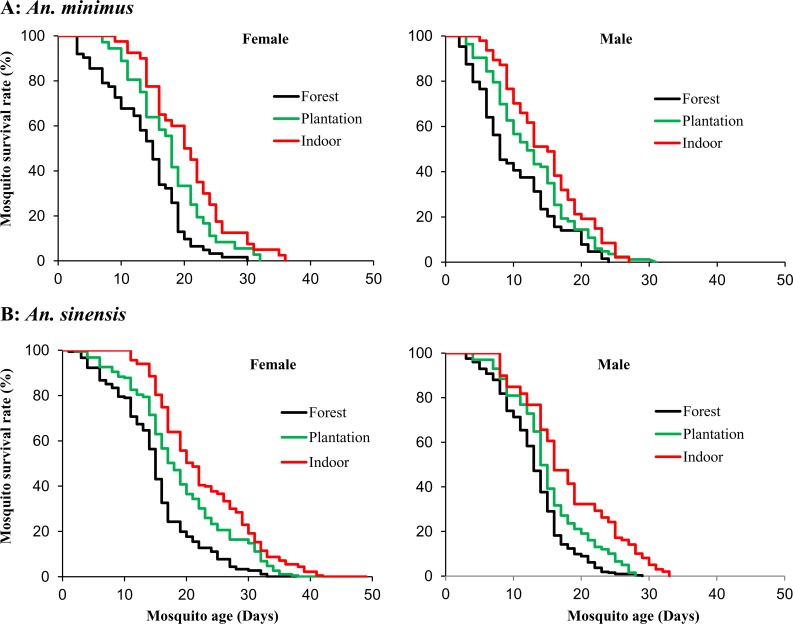
Survivorship of adults reared from wild-caught larvae and pupae was examined in three different environments: indoor, plantation, and forest (S1 Table). Female mosquitoes placed indoors survived significantly longer than those in banana plantation and forest for both An. minimus (F2,9 = 13.5, P < 0.0014) and An. sinensis (F2,9 = 33.6, P < 0.0001) (Fig 2). The mean survival duration of female An. minimus mosquitoes were 21.6, 18.8 and 14.8 days in indoor, banana plantation and forest, respectively (Table 2). A similar result was found in female An. sinensis mosquitoes in different land use and land cover settings. Male mosquitoes lived for a significantly shorter period of time than females for both An. minimus and An. sinensis, but the pattern of survivorship in indoor, banana plantation, and forest environment was the same as the females (Fig 2). The daily survival rate ranged from 0.88 to 0.91 for females and 0.84 to 0.89 for males (Table 2).
Fig 2. Kaplan-Meier survival analysis.
Impact of land use and land cover on survivorship of Anopheles minimus (top panel) and An. sinensis (bottom panel) adult mosquitoes. Three land use and land cover types were tested: indoor, banana planation and forest.
Table 2. Comparisons of mean and median survivorship of Anopheles minimus and An. sinensis adults in different land use and land cover conditions.
Values are shown as mean ± standard deviation.
| Species | An. minimus | An. sinensis | ||||
|---|---|---|---|---|---|---|
| Study site | Forest | Plantation | Indoor | Forest | Plantation | Indoor |
| Female survival time (days) | ||||||
| Mean±Standard deviation | 14.8±1.1A* | 18.8±1.5 A | 21.6±2.6 B | 15.0±1.3A | 19.0±1.3 B | 22.7±1.4 C |
| Median (95% confidence interval) | 16 (14–17) | 19 (15–20) | 21 (18–23) | 15 (14–16) | 18 (16–19) | 21 (19–22) |
| Male survival time (days) | ||||||
| Mean±Standard deviation | 11.2±1.3 A | 13.8±1.9 AB | 15.9±1.5 B | 14.1±1.6 A | 16.2±1.7 AB | 19.1±2.4 B |
| Median (95% confidence interval) | 9 (8–12) | 13 (11–16) | 16 (13–18) | 14 (14–15) | 15 (15–16) | 17 (17–20) |
| Female daily survival rate (%) | ||||||
| Mean±Standard deviation | 88.2±14.6 A | 90.2±13.0 a | 91.1±13.2 A | 87.9±13.9 A | 88.1±14.9 A | 91.4±10.5 A |
| Male daily survival rate (%) | ||||||
| Mean±Standard deviation | 85.6±16.2 A | 87.1±13.3 a | 88.7±16.3 A | 83.6±16.9 A | 87.4±14.3 A | 89.4±12.0 A |
* Values marked by different letters (A, B, and C) within the same row, for the same species, are significantly different at a level of 0.05 (Tukey-Kramer HSD test).
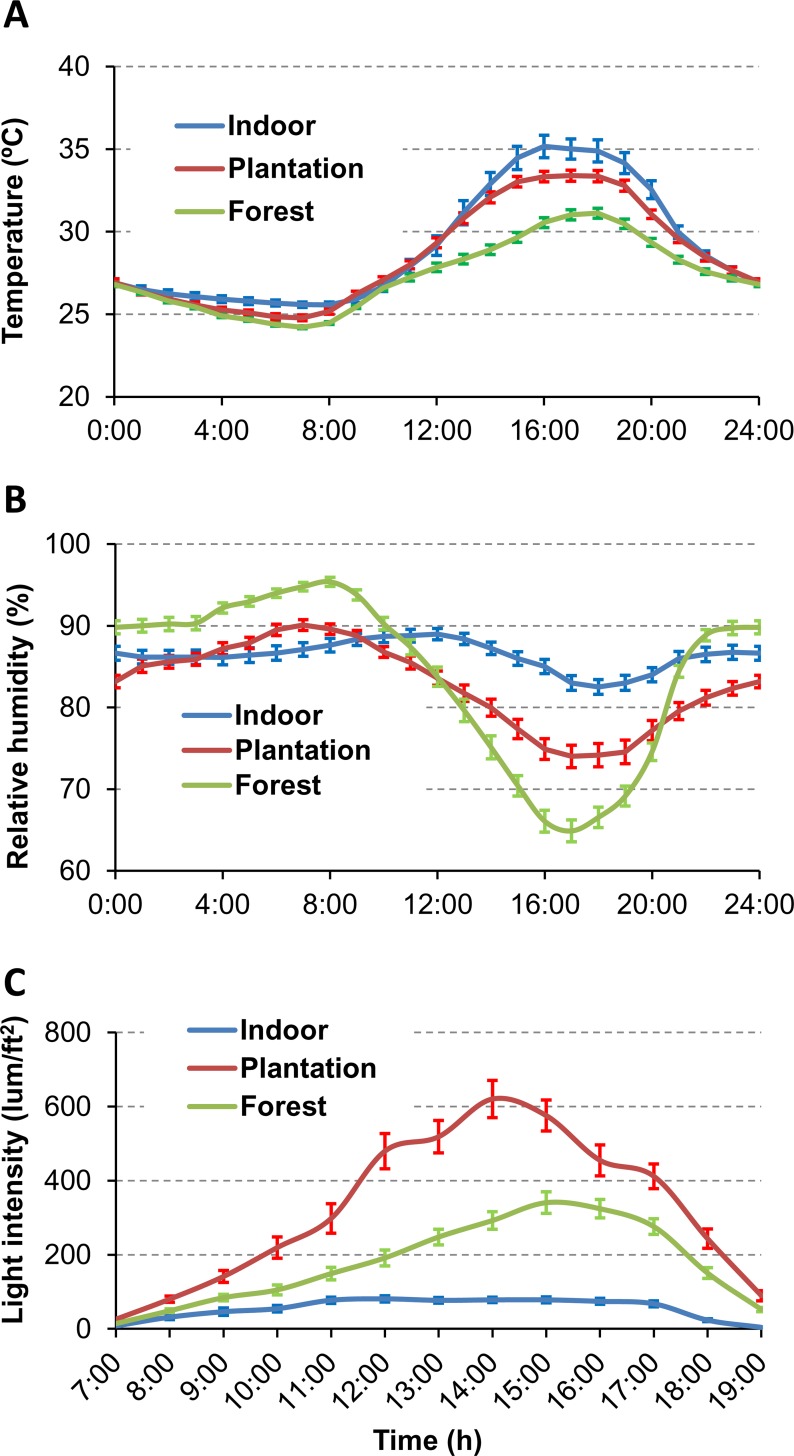
Effects of Land Use and Land Cover on Microclimatic Conditions
During the experiment period, the average ambient temperature in the forest was significantly lower than in the indoor environment (27.1 vs. 29.2°C, P < 0.0241) (Table 3), and the forest exhibited larger temperature fluctuations than those found in the indoor environment, as evidenced by larger differences between maximum and minimum temperature (Fig 3). The indoor environment exhibited the lowest light intensity. The relative humidity was high (>80%) and did not differ among these three land use and land cover conditions.
Table 3. Microclimate condition of mosquito niches tested in different land use and land cover conditions from May to July in 2014, in China-Myanmar border region.
Values are shown as mean ± standard deviation.
| Environment | Forest | Plantation | Indoor | F | P |
|---|---|---|---|---|---|
| Mean temperature (°C) | 27.1±2.1A* | 28.1±3.0 AB | 29.2±2.6 B | 3.94 | 0.0241 |
| Mean relative humidity (%) | 83.9±8.7 A | 82.7±5.3 A | 86.3±2.8 A | 1.78 | 0.1763 |
| Mean light intensity (lum/ft2) | 175.0±112.0 A | 319.8±202.6 B | 53.8±28.4 C | 12.7 | <0.0001 |
| Mean maximum temperature (°C) | 31.8±4.0 A | 30.1±2.2 AB | 29.9±1.2 C | 3.71 | 0.0294 |
| Mean minimum temperature (°C) | 23.8±1.5 A | 24.3±0.4 B | 26.9±0.6 B | 70.0 | <0.0001 |
| Mean maximum relative humidity (%) | 99.7±0.8 A | 93.7±2.0 B | 92.6±0.8 B | 197.3 | <0.0001 |
| Mean minimum relative humidity (%) | 54.9±13.5 A | 71.1±6.2 B | 77.4±1.5 C | 43.2 | <0.0001 |
| Mean maximum light intensity (lum/ft2) | 238.8±144.7 A | 524.1±61.5 B | 66.1±29.5 C | 14.1 | 0.0017 |
| Mean minimum light intensity (lum/ft2) | 18.7±5.3 A | 22.5±2.9 A | 4.2±2.5 B | 25.4 | 0.0002 |
* Values marked by different letters (A, B, and C) within the same row are significantly different at a level of 0.05 (Tukey-Kramer HSD test).
Fig 3. Daily dynamics of microclimatic conditions.
Dynamics of hourly indoor temperature (A), relative humidity (B) and light intensity (C) under three land use and land cover conditions.
Effects of Niche Temperature on Mosquito Survivorship
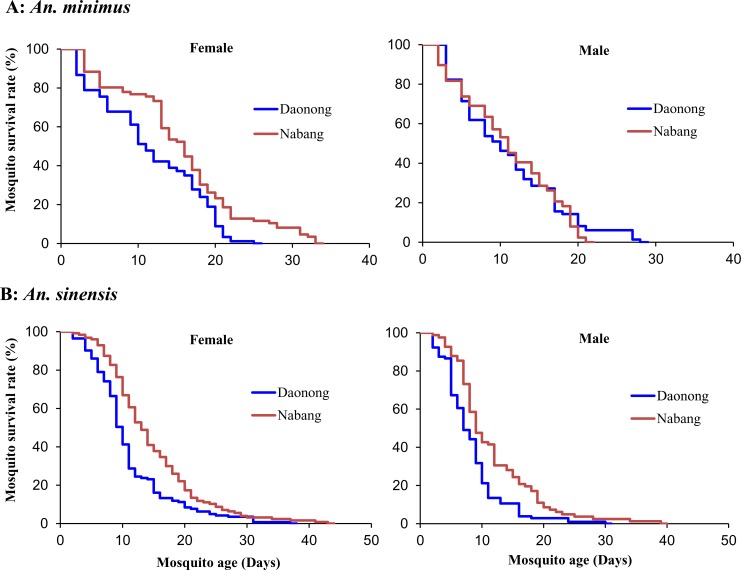
To determine the impact of temperature on mosquito survival while maintaining similar light intensity, we examined mosquito survivorship in the indoor environment in two sites differing in elevation and air temperature only (S2 Table). The HOBO data loggers confirmed that the mean indoor temperature at the low-elevation site (Nabang) was 3.1°C higher than that at the high-elevation site (Daonong) (29.3 vs. 26.2, F1,46 = 29.5, P <0.0001; Table 4). There was no significant difference in the average indoor relative humidity between the two sites (Table 4). We found that female mosquitoes placed in houses at the low-elevation site (Nabang village) survived significantly longer than those at the high-elevation site (Daonong village) for both An. minimus (χ2 = 7.32, df = 1, P <0.01) and An. sinensis (χ2 = 13.9, df = 1, P <0.001) (Fig 4). For An. minimus, the mean longevity was 15.5 days in Nabang and 11.7 days in Daonong. For An. sinensis, the mean longevity was 14.7 days in Nabang and 11.1 days (female) in Daonong (Table 5). The same trend held for males although they died significantly faster than females (Table 5).
Table 4. Indoor climate condition in sites with different elevations in China-Myanmar border region.
Values are shown as mean ± standard deviation.
| Study site | Daonong (660 m) | Nabang (240 m) | F | P |
|---|---|---|---|---|
| Mean temperature (°C) | 26.2±1.5 A* | 29.3±2.3 B | 29.5 | <0.0001 |
| Mean relative humidity (%) | 78.6±3.9 A | 77.6±7.6 B | 0.36 | 0.5491 |
| Mean maximum temperature (°C) | 31.1±3.2 A | 34.7±2.7 B | 16.2 | 0.0002 |
| Mean minimum temperature (°C) | 23.2±0.4 A | 25.8±0.8 B | 197.9 | <0.0001 |
| Mean maximum relative humidity (%) | 93.2±1.8 A | 92.4±2.3 A | 1.7 | 0.1899 |
| Mean minimum relative humidity (%) | 48.3±7.9 A | 51.6±11.8 A | 1.3 | 0.2631 |
* Values marked by different letters within the same row are significantly different at a level of 0.05 (Tukey-Kramer HSD test).
Fig 4. Life table survival analysis.
Survivorship of Anopheles minimus (A) and An. sinensis (B) in two study sites differing in ambient temperature along the China-Myanmar border region.
Table 5. Mean and median survivorship of Anopheles minimus and An. sinensis adults in indoor conditions in two sites with different elevations.
Values are shown as mean ± standard deviation.
| Species | An. minimus | An. sinensis | ||
|---|---|---|---|---|
| Study site | Daonong (660 m) | Nabang (240 m) | Daonong (660 m) | Nabang (240 m) |
| Female survival time (days) | ||||
| Mean ± Standard deviation | 11.7 ± 1.7 A* | 15.5 ± 0.7 B | 11.1 ± 1.1 A | 14.7 ± 0.7 B |
| Median (95% confidence interval) | 11 (10–12) | 16 (14–17) | 10 (9–11) | 13 (12–14) |
| Male survival time (days) | ||||
| Mean ± Standard deviation | 11.0 ± 0.8 A | 11.1 ± 0.4 B | 8.7 ± 1.0 A | 11.7 ± 1.3 B |
| Median (95% confidence interval) | 10 (8–12) | 11 (9–12) | 7 (7–9) | 9 (8–12) |
| Female daily survival rate (%) | ||||
| Mean ± Standard deviation | 85.3 ± 19.0 A | 91.9 ± 8.7 A | 89.5 ± 12.1A | 85.0 ± 17.5 A |
| Male daily survival rate (%) | ||||
| Mean ± Standard deviation | 89.1 ± 15.1 A | 90.2 ± 7.8 A | 87.5 ± 18.5 A | 89.7 ± 12.1 A |
* Values marked by different letters within the same row, for the same species, are significantly different at a level of 0.05 (Tukey-Kramer HSD test).
Discussion
The present study identified a significant effect of land use and land cover on vector survivorship. Mosquitoes placed under indoor environment exhibited significantly higher survivorship and longevity than banana plantation and forested environment. When mosquitoes were placed indoors in two sites differing in elevation, mosquitoes exhibited higher survivorship in sites with lower elevation. The effects of land use and land cover on mosquito survivorship likely resulted from differing microclimatic conditions among the habitats where adult mosquitoes were placed. Significantly higher mosquito survivorship was found in an indoor environment where mean daily temperature was 2°C higher than in the forested environment. This result on the impact of land use and land cover on mosquito survivorship was consistent with other studies on An. arabiensis and An. gambiae in African highlands [43, 44], and An. darlingi in the Peruvian Amazon [45].
The findings from this study have important implications for understanding malaria transmission and vector control in changing ecosystem. The developing world has been experiencing rapid land use and land cover changes. Deforestation is a major component of land use and land cover changes. Increased survivorship of adult mosquitoes in the indoor environment in deforested areas, as demonstrated in the present study, suggests that Indoor Residual Spraying (IRS) and Insecticide-Treated Nets (ITNs) should be used for vector control to prevent indoor malaria transmission. In addition, deforestation could alter the microclimatic conditions of aquatic habitats and subsequently enhanced survival and development of larval mosquitoes as demonstrated in An. gambiae and An. arabiensis in Africa [43, 46–48]. Because vector survivorship and vector density are important components of vectorial capacity, deforested agricultural areas could exhibit dramatically higher vectorial capacity than forested areas. Therefore, deforested agricultural area can increase the risk of malaria transmission.
There are several limitations in our study. First, although it is a conventional method, microcosm rearing of mosquitoes in cages for determination of vector survivorship was in a confined condition. In field conditions, mosquitoes could hide and rest in moisture and dry habitats with microclimate conditions that are different from our cage condition. Because it is not feasible to track the mosquitoes under field conditions, determination of vector survivorship under field conditions has been indirect based on biomarkers such as ovarian structural evaluation [49], fluorescent pigment pteridine concentration [50], cuticular hydrocarbon [51, 52], and gene expression [53]. These methods have significant limitation in estimation reliability such as the age of mosquitoes beyond certain period cannot be identified [54, 55], and sensitive to blood feeding and other physiological changes [56]. Our microcosm rearing of mosquitoes is the most direct measurement of mosquito survivorship. Second, we fed mouse blood and sucrose sugar in our experiments. The food source to adult mosquitoes may affect survivorship as An. minimus prefers biting human [57–59]. Because all mosquitoes were reared under the same food condition, the results on the impact of land use and land cover should be valid.
It is important to assess the impact of land use and land cover on vector-borne disease transmission when an economic development plan that significantly alters land use and land cover is being formulated. This study suggested that deforestation is the worst scenario, re-cultivation with banana plantation or other economically valuable trees such as rubber trees could boost incomes and reduce malaria transmission risk at the same time. Therefore, government policy should encourage local farmers to re-cultivate on deforested land. The estimated daily survival rate for An. sinensis and An. minimus under different land use and land covers provides a valuable parameter in modeling vector population dynamics and malaria transmission risk.
Supporting Information
(XLSX)
(XLSX)
Acknowledgments
The authors thank the staff at the field station of Southeast Asia ICEMR in Yunnan, China for their assistance in laboratory management, and three anonymous reviewers for critical review and valuable suggestions, and Amruta Dixit for her editorial assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work is supported by grants (to GY) from the National Institutes of Health (U19 AI089672, D43TW009527, and R01 AI083202). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. A strategic framework for malaria prevention and control during pregnancy in the Africa Region. Tech. Rep. AFR/MAL/04/01, World Health Organization, Geneva, Switzerland, 2004.
- 2.UNICEF RBM. Malaria: A major cause of child death and poverty in Africa. UNICEF; 2004. [Google Scholar]
- 3.WHO. World Malaria Report 2013. Geneva: World Health Organisation; Available: http://www.who.int/malaria/publications/world_malaria_report_2013/en/, 2013. [Google Scholar]
- 4.Dysoley L, Kaneko A, Eto H, Mita T, Socheat D, Borkman A, et al. Changing patterns of forest malaria among the mobile adult male population in Chumkiri District, Cambodia. Acta Trop. 2008;106(3):207–12. Epub 2008/05/13. 10.1016/j.actatropica.2007.01.007 . [DOI] [PubMed] [Google Scholar]
- 5.Abeyasinghe RR, Galappaththy GNL, Smith Gueye C, Kahn JG, Feachem RGA. Malaria control and elimination in Sri Lanka: Documenting progress and success factors in a conflict setting. PLoS ONE. 2012;7(8):e43162 10.1371/journal.pone.0043162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teklehaimanot, Paola Mejia A. Malaria and poverty. Annals of the New York Academy of Sciences. 2008;1136(1):32–7. 10.1196/annals.1425.037 [DOI] [PubMed] [Google Scholar]
- 7.Yusuf O, Adeoye B, Oladepo O, Peters D, Bishai D. Poverty and fever vulnerability in Nigeria: a multilevel analysis. Malaria Journal. 2010;9(1):235 10.1186/1475-2875-9-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricci F. Social implications of malaria and their relationships with poverty. Mediterr J Hematol Infect Dis, [Sl]. 2012;4(1):e2012048 Epub 2012-01-15. 10.4084/mjhid.2012.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tusting LS, Willey B, Lucas H, Thompson J, Kafy HT, Smith R, et al. Socioeconomic development as an intervention against malaria: a systematic review and meta-analysis. Lancet. 2013;382(9896):963–72. Epub 2013/06/25. 10.1016/s0140-6736(13)60851-x . [DOI] [PubMed] [Google Scholar]
- 10.Cui L, Yan G, Sattabongkot J, Cao Y, Chen B, Chen X, et al. Malaria in the Greater Mekong Subregion: heterogeneity and complexity. Acta Trop. 2012;121(3):227–39. 10.1016/j.actatropica.2011.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhumiratana A, Intarapuk A, Sorosjinda-Nunthawarasilp P, Maneekan P, Koyadun S. Border malaria associated with multidrug resistance on Thailand-Myanmar and Thailand-Cambodia borders: transmission dynamic, vulnerability, and surveillance. BioMed Research International. 2013;2013:13 10.1155/2013/363417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verdrager J. Epidemiology of the emergence and spread of drug-resistant falciparum malaria in South-East Asia and Australasia. J Trop Med Hyg. 1986;89(6):277–89. Epub 1986/12/01. . [PubMed] [Google Scholar]
- 13.Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379(9830):1960–6. Epub 2012/04/10. 10.1016/s0140-6736(12)60484-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delacollette C, D'Souza C, Christophel E, Thimasarn K, Abdur R, Bell D, et al. Malaria trends and challenges in the Greater Mekong Subregion. Southeast Asian J Trop Med Public Health. 2009;40(4):674–91. Epub 2009/10/22. . [PubMed] [Google Scholar]
- 15.WHO Regional Office for South-East Asia. Malaria in the Greater Mekong Subregion: Regional and Country Profiles. World Health Organization, New Delhi, India: 2010. [Google Scholar]
- 16.Huang F, Tang L, Yang H, Zhou S, Liu H, Li J, et al. Molecular epidemiology of drug resistance markers of Plasmodium falciparum in Yunnan Province, China. Malaria Journal. 2012;11(1):243 10.1186/1475-2875-11-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown T, Smith L, Oo EK, Shawng K, Lee T, Sullivan D, et al. Molecular surveillance for drug-resistant Plasmodium falciparum in clinical and subclinical populations from three border regions of Burma/Myanmar: cross-sectional data and a systematic review of resistance studies. Malaria Journal. 2012;11(1):333 10.1186/1475-2875-11-333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Liu H. Border malaria in Yunnan, China. Southeast Asian J Trop Med Public Health. 1997;28(3):456–9. [PubMed] [Google Scholar]
- 19.Lin H, Lu L, Tian L, Zhou S, Wu H, Bi Y, et al. Spatial and temporal distribution of falciparum malaria in China. Malaria Journal. 2009;8(1):130 10.1186/1475-2875-8-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. Eliminating malaria from the Greater Mekong subregion. the Malaria Policy Advisory Committee (MPAC), http://www.who.int/malaria/areas/greater_mekong/malaria-elimination-strategy/en/: 2015.
- 21.Mon MS, Mizoue N, Htun NZ, Kajisa T, Yoshida S. Factors affecting deforestation and forest degradation in selectively logged production forest: A case study in Myanmar. Forest Ecology and Management. 2012;267(0):190–8. 10.1016/j.foreco.2011.11.036. [DOI] [Google Scholar]
- 22.UK Essays. Causes and effects of deforestation in myanmar environmental sciences essay [Internet]. November 2013. Available: http://www.ukessays.com/essays/environmental-sciences/causes-and-effects-of-deforestation-in-myanmar-environmental-sciences-essay.php?cref=1: 2013. Accessed 7 July 2015.
- 23.Leimgruber P, Kelly DS, Steininger MK, Brunner J, Müller T, Songer M. Forest cover change patterns in Myanmar (Burma) 1990–2000. Environmental Conservation. 2005;32(04):356–64. 10.1017/S0376892905002493 [DOI] [Google Scholar]
- 24.Suwonkerd W, Ritthison W, Thuy Ngo C, Tainchum K, BM J., Chareonviriyaphap T. Vector Biology and Malaria Transmission in Southeast Asia, Anopheles mosquitoes—New insights into malaria vectors. Manguin S, editor. InTech; Available: http://www.intechopen.com/books/anopheles-mosquitoes-new-insights-into-malaria-vectors/vector-biology-and-malaria-transmission-in-southeast-asia2013. [Google Scholar]
- 25.Dhimal M, Ahrens B, Kuch U. Species composition, seasonal occurrence, habitat preference and altitudinal distribution of malaria and other disease vectors in eastern Nepal. Parasit Vectors. 2014;7:540 Epub 2014/11/29. 10.1186/s13071-014-0540-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mwangangi J, Mbogo C, Orindi B, Muturi E, Midega J, Nzovu J, et al. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malaria Journal. 2013;12(1):13 10.1186/1475-2875-12-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tainchum K, Ritthison W, Chuaycharoensuk T, Bangs MJ, Manguin S, Chareonviriyaphap T. Diversity of Anopheles species and trophic behavior of putative malaria vectors in two malaria endemic areas of northwestern Thailand. Journal of Vector Ecology. 2014;39(2):424–36. 10.1111/jvec.12118 [DOI] [PubMed] [Google Scholar]
- 28.Patz JA, Graczyk TK, Geller N, Vittor AY. Effects of environmental change on emerging parasitic diseases. International Journal for Parasitology. 2000;30(12–13):1395–405. 10.1016/S0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 29.Ramirez JL, Garver LS, Dimopoulos G. Challenges and Approaches for Mosquito Targeted Malaria Control. Current molecular medicine. 2009;9(2):116–30. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kar N, Kumar A, Singh O, Carlton J, Nanda N. A review of malaria transmission dynamics in forest ecosystems. Parasites & Vectors. 2014;7(1):265 10.1186/1756-3305-7-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Yang L, Jiang T, Zhang B, Wang S, Wu X, et al. Effects of a malaria elimination program: a retrospective study of 623 cases from 2008 to 2013 in a Chinese county hospital near the China—Myanmar border. Emerg Microbes Infect. 2016;5:e6 10.1038/emi.2016.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Li S, Cheng Z, Xiao N, Cotter C, Hwang J, et al. Transmission Risk from Imported Plasmodium vivax Malaria in the China-Myanmar Border Region. Emerg Infect Dis. 2015;21(10):1861–4. Epub 2015/09/25. 10.3201/eid2110.150679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu G, Yan G, Zhang N, Zhong D, Wang Y, He Z, et al. The Anopheles community and the role of Anopheles minimus on malaria transmission on the China-Myanmar border. Parasit Vectors. 2013;6(1):264 Epub 2013/09/17. 10.1186/1756-3305-6-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Zhong D, Cui L, Lee MC, Yang Z, Yan G, et al. Population dynamics and community structure of Anopheles mosquitoes along the China-Myanmar border. Parasit Vectors. 2015;8:445 Epub 2015/09/05. 10.1186/s13071-015-1057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong X. The Mosquito Fauna of Yunnan (Volumes one). Dong X, editor. Kunming: Yunnan Publishing Group Corporation, Yunnan Science & Technology Press; 2010. 394 p. [Google Scholar]
- 36.Garros C, Koekemoer LL, Coetzee M, Coosemans M, Manguin S. A single multiplex assay to identify major malaria vectors within the African Anopheles funestus and the oriental An. minimus groups. Am J Trop Med Hyg. 2004;70(6):583–90. [PubMed] [Google Scholar]
- 37.Joshi D, Park MH, Saeung A, Choochote W, Min GS. Multiplex assay to identify Korean vectors of malaria. Molecular Ecology Resources. 2010;10(4):748–50. 10.1111/j.1755-0998.2010.02835.x [DOI] [PubMed] [Google Scholar]
- 38.Corbel V, Stankiewicz M, Bonnet J, Grolleau F, Hougard JM, Lapied B. Synergism between insecticides permethrin and propoxur occurs through activation of presynaptic muscarinic negative feedback of acetylcholine release in the insect central nervous system. Neurotoxicology. 2006;27(4):508–19. [DOI] [PubMed] [Google Scholar]
- 39.Walton C, Somboon P, O'Loughlin SM, Zhang S, Harbach RE, Linton YM, et al. Genetic diversity and molecular identification of mosquito species in the Anopheles maculatus group using the ITS2 region of rDNA. Infect Genet Evol. 2007;7(1):93–102. [DOI] [PubMed] [Google Scholar]
- 40.Goel MK, Khanna P, Kishore J. Understanding survival analysis: Kaplan-Meier estimate. International Journal of Ayurveda Research. 2010;1(4):274–8. 10.4103/0974-7788.76794 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montgomery DC. Design and Analysis of Experiments: eighth edition, Wiley, section 3.5.7; 2013. [Google Scholar]
- 42.JMP. Statistical software Version 9. SAS Institute Inc., Cary, NC, 1989–2007. Available: http://www.jmp.com/software/; 2007. [Google Scholar]
- 43.Afrane YA, Zhou G, Lawson BW, Githeko AK, Yan G. Effects of microclimatic changes caused by deforestation on the survivorship and reproductive fitness of Anopheles gambiae in western Kenya highlands. Am J Trop Med Hyg. 2006;74(5):772–8. Epub 2006/05/12. . [PubMed] [Google Scholar]
- 44.Afrane YA, Zhou G, Lawson BW, Githeko AK, Yan G. Life-table analysis of Anopheles arabiensis in western Kenya highlands: effects of land covers on larval and adult survivorship. Am J Trop Med Hyg. 2007;77(4):660–6. PubMed Central PMCID: PMC17978067. [PubMed] [Google Scholar]
- 45.Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, et al. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. The American Journal of Tropical Medicine and Hygiene. 2006;74(1):3–11. [PubMed] [Google Scholar]
- 46.Afrane YA, Githeko AK, Yan G. The ecology of Anopheles mosquitoes under climate change: Case studies from the effects of environmental changes in east Africa highlands. Annals of the New York Academy of Sciences. 2012;1249:204–10. 10.1111/j.1749-6632.2011.06432.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wamae PM, Githeko AK, Menya DM, Takken W. Shading by Napier grass reduces malaria vector larvae in natural habitats in western Kenya highlands. Ecohealth. 2010;7(4):485–97. 10.1007/s10393-010-0321-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imbahale S, Paaijmans K, Mukabana W, van Lammeren R, Githeko A, Takken W. A longitudinal study on Anopheles mosquito larval abundance in distinct geographical and environmental settings in western Kenya. Malaria Journal. 2011;10(1):81 10.1186/1475-2875-10-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayes EJ, Wall R. Age-grading adult insects: a review of techniques. Physiological Entomology. 1999;24(1):1–10. 10.1046/j.1365-3032.1999.00104.x [DOI] [Google Scholar]
- 50.Wu D, Lehane MJ. Pteridine fluorescence for age determination of Anopheles mosquitoes. Med Vet Entomol. 1999;13(1):48–52. Epub 1999/04/09. . [DOI] [PubMed] [Google Scholar]
- 51.Caputo B, Dani FR, Horne GL, Petrarca V, Turillazzi S, Coluzzi M, et al. Identification and composition of cuticular hydrocarbons of the major Afrotropical malaria vector Anopheles gambiae s.s. (Diptera: Culicidae): analysis of sexual dimorphism and age-related changes. J Mass Spectrom. 2005;40(12):1595–604. Epub 2005/12/02. 10.1002/jms.961 . [DOI] [PubMed] [Google Scholar]
- 52.Brei B, Edman JD, Gerade B, Clark JM. Relative abundance of two cuticular hydrocarbons indicates whether a mosquito is old enough to transmit malaria parasites. J Med Entomol. 2004;41(4):807–9. Epub 2004/08/18. . [DOI] [PubMed] [Google Scholar]
- 53.Wang M-H, Marinotti O, James AA, Walker E, Githure J, Yan G. Genome-wide patterns of gene expression during aging in the African malaria vector Anopheles gambiae. PLoS ONE. 2010;5(10):e13359 10.1371/journal.pone.0013359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tyndale-Biscoe M. Age-grading methods in adult insects: a review. Bulletin of Entomological Research. 1984;74(03):341–77. 10.1017/S0007485300015637 [DOI] [Google Scholar]
- 55.Huho BJ, Ng'habi KR, Killeen GF, Nkwengulila G, Knols BG, Ferguson HM. A reliable morphological method to assess the age of male Anopheles gambiae. Malar J. 2006;5:62 Epub 2006/07/29. 10.1186/1475-2875-5-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerade BB, Lee SH, Scott TW, Edman JD, Harrington LC, Kitthawee S, et al. Field validation of Aedes aegypti (Diptera: Culicidae) age estimation by analysis of cuticular hydrocarbons. J Med Entomol. 2004;41(2):231–8. Epub 2004/04/06. . [DOI] [PubMed] [Google Scholar]
- 57.Rwegoshora RT, Sharpe RG, Baisley KJ, Kittayapong P. Biting behavior and seasonal variation in the abundance of Anopheles minimus species A and C in Thailand. Southeast Asian J Trop Med Public Health. 2002;33(4):694–701. Epub 2003/05/22. . [PubMed] [Google Scholar]
- 58.Nutsathapana S, Sawasdiwongphorn P, Chiprarop V, Cullen JR. The behavior of Anopheles minimus Theobald (Diptera: Culicidae) subjected to differing levels of DDT selection pressure in northern Thailand. Bull Entomol Res. 1986;76 10.1017/s0007485300014772 [DOI] [Google Scholar]
- 59.Sungvornyothin S, Muenvorn V, Garros C, Prabaripai A, Bangs MJ, Manguin S, et al. Trophic behavior and biting activity of the two sibling species of the Anopheles minimus complex in western Thailand. J Vector Ecol. 2006;31 10.3376/1081-1710(2006)31[252:tbabao]2.0.co;2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.