Abstract
Aims
Although contact force (CF)-guided circumferential pulmonary vein isolation (CPVI) for paroxysmal atrial fibrillation (PAF) is useful, AF recurrence at long-term follow-up still remains to be resolved. The purpose of this study was to assess safety and efficacy of CF-guided CPVI and to compare residual conduction gaps during CPVI and long-term outcome between the conventional (non-CF-guided) and the CF-guided CPVI.
Methods and results
We studied the 50 consecutive PAF patients undergoing CPVI by a ThermoCool EZ Steer catheter (conventional group, mean age 61 ± 10 years) and the other 50 consecutive PAF patients by a ThermoCool SmartTouch catheter (CF group, 65 ± 11 years). The procedure parameters and residual conduction gaps during CPVI, and long-term outcome for 12 months were compared between the two groups. Circumferential pulmonary vein isolation was successfully accomplished without any major complications in both groups. Total procedure and total fluoroscopy times were both significantly shorter in the CF group than in the conventional group (160 ± 30 vs. 245 ± 61 min, P < 0.001, and 17 ± 8 vs. 54 ± 27 min, P < 0.001, respectively). Total number of residual conduction gaps was significantly less in the CF group than in the conventional group (2.7 ± 1.7 vs. 6.3 ± 2.7, P < 0.05). The AF recurrence-free rates after CPVI during 12-month follow-up were 96% (48/50) in the CF group and 82% (41/50) in the conventional group (P = 0.02 by log rank test). Multivariate Cox regression analysis further supported this finding.
Conclusion
Contact force-guided CPVI is safe and more effective in reducing not only the procedure time but also the AF recurrence than the conventional CPVI, possibly due to reduced residual conduction gaps during CPVI procedure.
Keywords: Atrial fibrillation, Ablation, Contact force, Circumferential pulmonary vein isolation, Residual conduction gap
What's new?
Incomplete pulmonary vein (PV) isolation (PVI) or PV reconnections after PVI have been shown to be responsible for recurrence of AF.
Recently developed new catheter system allows us to monitor CF of electrode to target tissue, which is a major determinant of lesion size.
In the present study, we showed that CF-guided CPVI using a ThermoCool SmartTouch catheter had a reduced number of residual conduction gaps and prevented AF recurrence at 12-month follow-up period compared with the conventional CPVI using a ThermoCool EZ Steer catheter.
The use of CF information during CPVI procedure and appropriate CF values to achieve complete lesion formation may contribute to prevention of AF recurrence after CPVI and help improve long-term outcome in patients with paroxysmal AF.
Introduction
Atrial fibrillation (AF) catheter ablation is useful for eliminating symptomatic paroxysmal AF (PAF) refractory or intolerant to antiarrhythmic drug therapy.1–3 Although pulmonary vein (PV) isolation (PVI) has been recognized as an essential procedure for AF ablation, recurrence of AF at long-term follow-up after PVI still remains to be resolved.4,5
Incomplete PVI or PV reconnections after PVI have been shown to be responsible for AF recurrence.6,7 Experimental study using a canine model showed that contact force (CF) of electrode to target tissue is a major determinant of lesion size.8 Recently developed catheter system practically enabled us to monitor this CF during PVI. Recent clinical studies using this new technology showed that the use of real-time CF information during PVI is useful in reducing PV reconnections and making sufficient lesion formation.9–11
We and others previously demonstrated that CF-guided circumferential PVI (CPVI) is effective in reducing residual conduction gaps requiring additional touch-up ablation and procedure time.9,12 Although the use of CF monitoring during CPVI is associated with prevention of AF recurrence at 12 months,13–17 the relationship of residual conduction gaps with long-term outcome in the CF-guided CPVI for PAF remains unclear. Thus, the purpose of this study was to assess the efficacy and safety of the CF-guided CPVI, and more importantly, to compare residual conduction gaps and long-term outcome between the conventional (non-CF-guided) and the CF-guided CPVI.
Methods
Study patients and anticoagulation therapy
The consecutive 100 PAF patients undergoing their first-ever ablation from January 2012 through August 2013 were enrolled. Of them, the first 50 patients underwent CPVI by a standard open-irrigated radiofrequency (RF) ablation catheter, ThermoCool EZ Steer catheter (Biosense Webster, Diamond Bar, CA, USA) (conventional group, mean age 61 ± 10 years, 19 females), while the latter 50 by a CF sensor-equipped open-irrigated RF ablation catheter, ThermoCool SmartTouch catheter (Biosense Webster) (CF group, mean age 65 ± 11 years, 20 females). The patients included in our previous study,12 in which they were randomly assigned to non-CF-guided and CF-guided CPVI and the results were compared between the two groups, were excluded from the present study. Also, those undergoing CPVI using a ThermoCool SF catheter (Biosense Webster) were excluded. The procedure parameters and long-term outcomes were compared between the conventional and the CF groups. All patients were administered an anticoagulant for at least 4 weeks before the study. Pre-procedural transthoracic and transoesophageal echocardiographies were performed in all patients to assess cardiac function, left atrial diameter, and mitral regurgitation grade, and to confirm the absence of left atrial thrombi. Warfarin was not interrupted before and after the CPVI procedure. Dabigatran, rivaroxaban, or apixaban was skipped only in the morning of the procedure day. These anticoagulants were continued for at least 6 months after ablation. The study protocol was approved by the Ethics Committee of our institution, and written informed consent was obtained from all patients before the study.
Cardiac catheterization
Cardiac catheterization and AF ablation procedure were performed as described previously.12,18,19 With patients under local anaesthesia, a 6 Fr double decapolar steerable catheter (BeeAT, Japan Lifeline Co, Tokyo, Japan) was inserted into the coronary sinus via the internal jugular vein. A 10 Fr SoundStar ultrasound catheter (Biosense Webster) was inserted into the right atrium via the right femoral vein, and anatomic mapping of the left atrium (LA) by CartoSound module equipped in a CARTO3 system (Biosense Webster) was performed. After transseptal puncture was performed, 5000 units of heparin was injected into the LA, followed by repetitive injection of 1000–2000 units of heparin to maintain an activated clotting time more than 300 s during the procedure. Two 8.5 Fr long sheaths (Daig SL1, St. Jude Medical, St. Paul, MN, USA) were inserted into the LA. By asking the patient to swallow contrast medium, anatomic position of the oesophagus was confirmed.
Integration of CartoSound image of the left atrium with computed tomography image
Intracardiac echography (ICE) images were displayed through the CartoSound module using an Acuson X300PE echocardiography system (Siemens Medical Solutions USA, Mountain View, CA, USA). Intracardiac echography plane LA images were obtained at the end-tidal position and 50% of the R-R interval before transseptal puncture as described previously.19 The range of the contours sampled was 6–9 between the ostia of the right PV and left PV, and these contours were registered as the LA ICE image. After visual alignment, the two images were integrated with the installed surface registration program.
Atrial fibrillation ablation
Circumferential pulmonary vein isolation was done in the integrated LA image using a ThermoCool EZ Steer catheter in the conventional group and a ThermoCool SmartTouch catheter in the CF group by the two operators (M.K. and K.O.) having experience of AF ablation in >200 cases. The ablation catheter was advanced into the LA via the long sheath, which was then pulled back to the right atrium in order to reduce systemic thromboembolic risk. Radiofrequency energy was delivered at 30 W in the anterior aspect of the CPVI line and 25 W in the posterior aspect using a Stockert 70 RF generator (Biosense Webster). Although RF energy was being delivered, the catheter tip was dragged by ∼2 mm every 20–25 s. When RF energy needed to be delivered on the oesophagus, it was reduced to 20 W, and the maximal application time was set to 20 s. The endpoint of CPVI was elimination of all PV potentials recorded by a circular catheter (Lasso Nav, Biosense Webster) placed at the ostium of the PV, and PV-to-LA block during pacing from 10 pairs of the circular catheter at 10-V output with 1-ms pulse width.
Analysis of residual conduction gaps
After completion of CPVI in both sides, it was again assessed if PV reconnection was present or not in all PVs. Thus, PV reconnection was assessed 20–30 min after completing CPVI, and when it was present, touch-up ablation was added.
As described previously, CPVI line was divided into eight segments in the left and right PVs.12 After encircling linear RF ablation was done in each of the left and right PVs, a circular catheter was placed at the ostium of the PV, and the presence of LA-to-PV connection indicated by the PV electrogram(s) was assessed. When the signal was detected, that is, there was a gap(s) in the encircling linear ablation line, touch-up ablation targeting the earliest electrogram site was performed until elimination of the electrical signal. The number of residual conduction gaps in each segment requiring touch-up ablation to complete CPVI after encircling linear ablation was compared between the two groups.
Follow-up
All patients were seen in the clinic at 1 and 3 months after CPVI and every 3 months thereafter until 12 months. The blanking period was defined as 3 months after CPVI. Any asymptomatic or symptomatic atrial tachyarrhythmia lasting more than 30 s including recurrent AF was detected by either a 12-lead electrocardiogram (ECG) or a 24-h Holter ECG at every visit to the clinic. Patients with symptomatic tachycardia episodes lasting more than 30 s without ECG documentation were also counted as a recurrence.
Statistical analysis
Data were expressed as mean ± standard deviation or n (%). Statistical analysis of the variables in the two groups was made by Fisher's exact test or χ2 test for categorical variables, and by a two-tailed unpaired t-test for continuous variables. Kaplan–Meier analysis was performed to compare long-term outcome between the two groups. Univariate and multivariate Cox regression analyses were performed to determine significant risks for recurrence of atrial tachyarrhythmia or AF. Data were analysed by SPSS software (version 22.0, IBM Corporation, NY, USA). A P-value of <0.05 was considered significant.
Results
Patient profiles
The clinical characteristics of the patients are summarized in Table 1. No significant differences in gender, risk factors, left ventricular ejection fraction, and left atrial diameter were found between the conventional and the CF groups. Mitral regurgitation grade was mostly none or mild in both groups and did not differ between the two groups. There was a trend for the CF group to be older, which was likely reflected by higher CHADS2 and CHA2DS2-VASc scores in the CF group, although these scores did not differ statistically between the two groups.
Table 1.
Comparison of patient profiles between the conventional and the CF groups
| Variable | Conventional group (n = 50) | CF group (n = 50) | P-Value |
|---|---|---|---|
| Age (years) | 61 ± 10 | 65 ± 11 | 0.05 |
| Female gender | 19 (38) | 20 (40) | 0.84 |
| Heart failure | 3 (6) | 6 (12) | 0.29 |
| Hypertension | 26 (52) | 32 (64) | 0.22 |
| Diabetes mellitus | 8 (16) | 5 (10) | 0.37 |
| Prior stroke or TIA | 4 (8) | 7 (14) | 0.34 |
| Vascular disease | 2 (4) | 0 (0) | 0.15 |
| CHADS2 score | 1.0 ± 1.1 | 1.3 ± 1.1 | 0.09 |
| CHA2DS2-VASc score | 1.8 ± 1.4 | 2.3 ± 1.8 | 0.08 |
| LVEF (%) | 65 ± 7 | 65 ± 10 | 0.21 |
| LAD (mm) | 38 ± 6 | 37 ± 7 | 0.30 |
| MR grade | 0.08 | ||
| None | 20 (40) | 12 (24) | |
| Mild | 30 (60) | 37 (74) | |
| Moderate | 0 (0) | 1 (2) | |
| Severe | 0 (0) | 0 (0) |
Data are given as mean ± standard deviation or number (%).
TIA, transient ischaemic attack; LVEF, left ventricular ejection fraction; LAD, left atrial diameter; MR, mitral regurgitation.
Procedural characteristics
The procedural characteristics are summarized in Table 2. Total procedure and total fluoroscopy times were both significantly shorter in the CF group than in the conventional group (160 ± 30 vs. 245 ± 61 min, P < 0.001, and 17 ± 8 vs. 54 ± 27 min, P < 0.001, respectively). Procedure and fluoroscopy times for CPVI, which were defined as times from initial RF application to completion of CPVI, were also both significantly shorter in the CF group than in the conventional group (62 ± 3 vs. 116 ± 6 min, P < 0.001, and 1 ± 0.2 vs. 12 ± 12 min, P < 0.001, respectively).
Table 2.
Comparisons of procedural characteristics and antiarrhythmic medication between the conventional and the CF groups
| Variable | Conventional group (n = 50) | CF group (n = 50) | P-Value |
|---|---|---|---|
| Total procedure time (min) | 245 ± 61 | 160 ± 30 | <0.001 |
| Total fluoroscopy time (min) | 54 ± 27 | 17 ± 8 | <0.001 |
| Additional linear ablation in LA | 9 (18) | 4 (8) | 0.23 |
| Roof line | 7 (14) | 4 (8) | 0.34 |
| Bottom line | 2 (4) | 3 (6) | 0.65 |
| Mitral isthmus line | 6 (12) | 1 (2) | 0.05 |
| Superior vena cava isolation | 8 (16) | 2 (4) | 0.09 |
| Antiarrhythmic medication after the procedure (none/class I AAA/AMD/bepridil) | 31/10/9/1 | 41/4/5/0 | 0.13 |
Data are given as mean ± standard deviation or number (%).
AAA, antiarrhythmic agent; AMD, amiodarone; LA, left atrium.
The bidirectional LA-PVs blocks after CPVI were successfully completed in all the cases. Additional linear ablation in LA was performed in 13 patients, who had no AF termination after CPVI and consequently had a cardioversion: 9 in the conventional group and 4 in the CF group (P = 0.23). As shown in Table 2, either roof, bottom, or mitral isthmus line was created. In the conventional group, two patients had both roof and mitral isthmus lines, and another two patients had all three additional linear ablations. The other five patients had one additional linear ablation. In the CF group, three patients had both roof and bottom lines and the other one both roof and mitral isthmus lines. No significant differences in additional linear ablation were found between the two groups. Superior vena cava (SVC) isolation in patients with SVC foci was performed in 10 patients: 8 in the conventional group and 2 in the CF group (P = 0.09). Bidirectional block in the SVC was created in all of the 10 patients. There were no major complications related to CPVI in all study patients. Regarding the use of antiarrhythmic agents after the procedure, 31 patients in the conventional group and 41 in the CF group had no drugs, although it did not differ statistically (Table 2, P = 0.13).
Residual conduction gaps
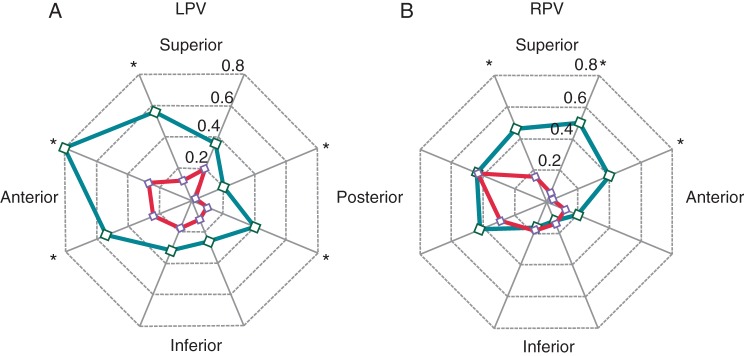
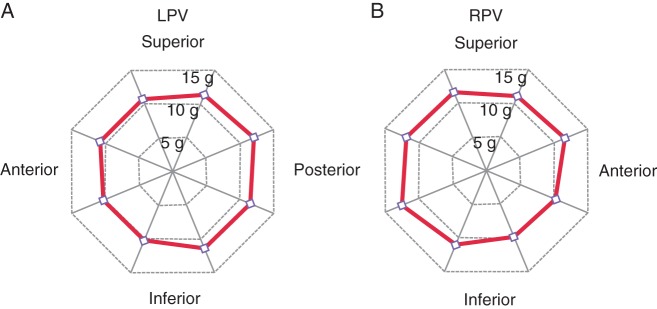
Figure 1 shows the number of residual conduction gaps requiring touch-up ablation after creating an ablation line around the left and right ipsilateral PVs. Total number of gaps in the conventional and CF groups were 6.3 ± 2.7 and 2.7 ± 1.7, respectively (P < 0.05). The number of gaps around left superior PV (LSPV), left inferior PV (LIPV), and right superior PV (RSPV) was significantly smaller in the CF group than in the conventional group, but not around right inferior PV (RIPV). Figure 2 shows the detailed distribution of residual conduction gaps in the eight segments of the left and right PVs for the conventional and CF groups. The number of gaps at the left PV was significantly smaller in the CF group than in the conventional group in all eight segments except in the posterosuperior aspect of LSPV and the anteroinferior and posteroinferior aspects of LIPV (Figure 2A). At the right PV, on the other hand, only anterosuperior aspect of RSPV in the CF group had a significantly smaller number of gaps than in the conventional group (Figure 2B). Figure 3A shows the mean CF values in the eight segments of the left and right PVs for the CF group, all of which were between 10 and 15 g.
Figure 1.
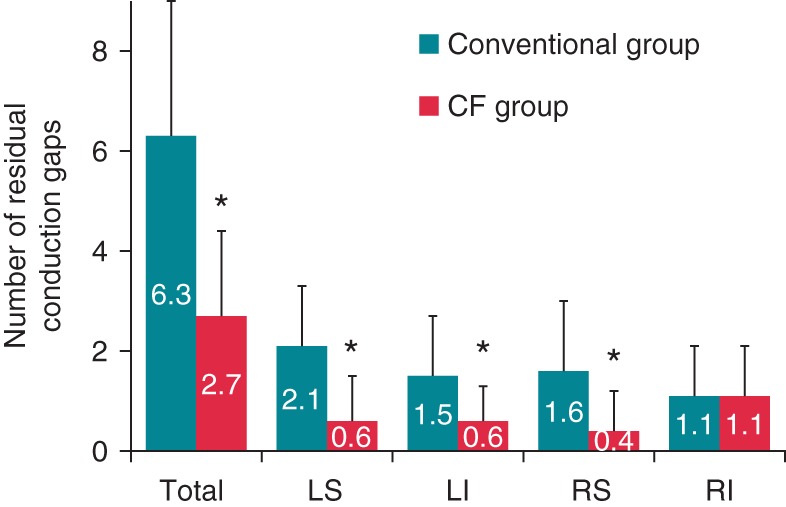
The number of residual conduction gaps requiring touch-up ablation in the conventional and CF groups. LS, left superior pulmonary vein; LI, left inferior pulmonary vein; RS, right superior pulmonary vein; RI, right inferior pulmonary vein. *P < 0.05.
Figure 2.
The distribution of residual conduction gaps in the eight segments of the left pulmonary vein (LPV) (A) and right pulmonary vein (RPV) (B). Data for the conventional and CF groups are shown in green and red, respectively. *P < 0.05.
Figure 3.
The mean contact force in the eight segments of the left pulmonary vein (LPV) (A) and right pulmonary vein (RPV) (B). All were kept between 10 and 15 g.
Long-term outcome
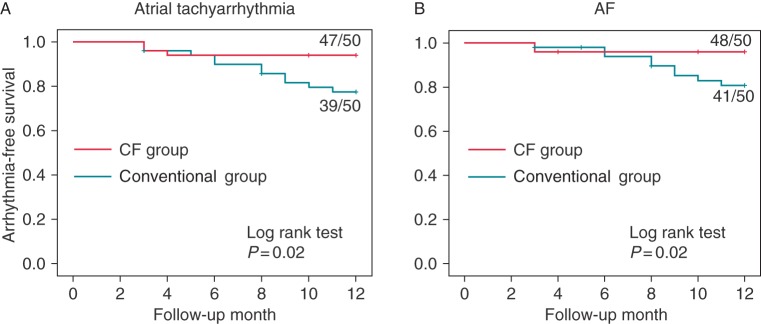
All patients were followed-up for 12 months. At the end of follow-up period, 39 patients (78%) in the conventional group and 47 patients (94%) in the CF group remained free from any atrial tachyarrhythmia including recurrent AF, and their difference was significant (P = 0.02 by log rank test, Figure 4A). Forty-one patients (82%) in the conventional group and 48 patients (96%) in the CF group were free from recurrent AF, and similarly their difference was significant (P = 0.02 by log rank test, Figure 4B).
Figure 4.
Arrhythmia-free survival curves for atrial tachyarrhythmia (A) and AF (B) evaluated by Kaplan–Meier analysis.
Circumferential pulmonary vein isolation was done by the two operators (M.K. and K.O.). In the conventional group, M.K. performed CPVI in 17 patients and K.O. in 33, whereas in the CF group, M.K. in 31 and K.O. in 19. There were no differences in event-free survival curves between the two operators at the end of follow-up period both in the conventional and in the CF groups.
Multivariate Cox regression analysis further showed that the use of CF was a significant determinant for preventing recurrence of atrial tachyarrhythmia [hazard ratio (HR) 0.19, 95% confidence interval (95% CI) 0.04–0.66, P = 0.007] (Table 3A) or AF (HR 0.23, 95% CI 0.05–0.83, P = 0.02) (Table 3B).
Table 3.
Risks for recurrence of atrial tachyarrhythmia and AF (n = 100)
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| (A) Analysis for atrial tachyarrhythmia | ||||||
| Use of CF | 0.26 | 0.06–0.82 | 0.02 | 0.19 | 0.04–0.66 | 0.007 |
| CHA2DS2-VASc score | 1.17 | 0.84–1.53 | 0.32 | 1.29 | 0.90–1.80 | 0.16 |
| LVEF | 1.04 | 0.97–1.11 | 0.33 | 1.07 | 0.98–1.16 | 0.11 |
| LAD | 1.03 | 0.95–1.11 | 0.48 | 1.01 | 0.91–1.11 | 0.85 |
| (B) Analysis for AF | ||||||
| Use of CF | 0.31 | 0.07–1.04 | 0.06 | 0.23 | 0.05–0.83 | 0.02 |
| CHA2DS2-VASc score | 1.17 | 0.81–1.56 | 0.37 | 1.32 | 0.88–1.88 | 0.17 |
| LVEF | 1.03 | 0.96–1.12 | 0.44 | 1.06 | 0.97–1.16 | 0.23 |
| LAD | 1.01 | 0.92–1.10 | 0.80 | 0.99 | 0.86–1.09 | 0.79 |
Adjustment for multivariate analysis was performed with all variables presented in this table.
HR, hazard ratio; CI, confidence interval; CF, contact force; AF, atrial fibrillation; LVEF, left ventricular ejection fraction; LAD, left atrial diameter.
Discussion
Major findings
In the present study, we found that the CF-guided CPVI using a ThermoCool SmartTouch catheter had a reduced number of residual conduction gaps compared with the conventional CPVI using a ThermoCool EZ Steer catheter, which in turn may contribute to the decrease in total procedure and total fluoroscopy times in the CF-guided CPVI. Most importantly, patients with the CF-guided CPVI showed a favourable long-term outcome compared with those with the conventional CPVI. Also no major complications related to CPVI were found in the CF-guided CPVI. These findings indicate that the CF-guided CPVI is safe and more effective in reducing not only the procedure time but also the recurrence of atrial tachyarrhythmias and/or AF than the conventional CPVI, possibly due to reduced residual conduction gaps during CPVI procedure.
Reduced residual conduction gaps in the contact force-guided circumferential pulmonary vein isolation
Atrial fibrillation recurrence after PVI is highly correlated with PV reconnections.6 The use of CF information during PVI has been shown to be useful in reducing PV reconnections and procedure time.9–11,20 These findings are supported by the present study showing that the total number of conduction gaps was significantly lower, and procedure and fluoroscopy times were also significantly shorter in the CF group than in the conventional group. Greater lesion durability, sufficient time to produce a complete lesion, and avoiding ablation at suboptimal force seem to be potential mechanisms for less residual conduction gaps in CF-guided ablation.
It should be noted that the number of conduction gaps in RIPV is similar between the CF and the conventional groups, whereas in the other regions, it is significantly less in the CF group than in the conventional group (Figure 1). Since mean CF values in all regions were maintained to 10–15 g in the CF group, this finding suggests that sufficient CF to prevent PV reconnections may have been obtained without CF information only in RIPV, whereas not in all other regions. Indeed, we previously showed that CF at 10–15 g was obtained only in RIPV without CF information during CPVI procedure.12 Thus, operators may have been able to get sufficient CF in RIPV without CF information. Furthermore, recent study by Sotomi et al.21 showed that there is a regional difference in optimal CF values to prevent PV reconnections: a lower CF (10–15 g) is enough in the posteroinferior segments, but a higher CF (17–22 g) is required in the other segments. This may also be partly associated with site difference in residual conduction gaps found in the present study.
Favourable outcome in the contact force-guided circumferential pulmonary vein isolation
Efficacy of CF-guided CPVI has been demonstrated by several recent studies showing that the use of CF information during CPVI procedure is associated with improved long-term freedom from atrial tachyarrhythmias at 12 months compared with a conventional CPVI without CF information.13,14 Andrade et al.22 also showed that the use of real-time CF guidance reduces occurrence of dormant conduction, and consequently prevents AF recurrence significantly at 12-month follow-up compared with a standard CPVI without CF guidance. In our previous randomized study, although CF-guided CPVI seemed to show a preferable outcome compared with non-CF-guided CPVI, the difference between the two groups did not reach a statistical significance.12 This was because of the small number of the study patients (the number of PAF patients was 14 in CF-guided group and 13 in non-CF-guided group) and short follow-up period (6 months). The present study compared the 12-month outcomes of the 50 patients with conventional CPVI with those of the other 50 with CF-guided CPVI, and showed significantly reduced AF recurrence in the CF group, supporting the benefit of the CF use on long-term outcome. Multivariate Cox regression analysis also supports this finding. Furthermore, a potential link of reduced residual conduction gaps with prevention of AF recurrence was demonstrated in the present study.
Recent retrospective multicentre study by Jarman et al.16 also demonstrated improved arrhythmia-free outcomes at 12 months in PAF patients with CF-guided CPVI, and reduced fluoroscopy time during the procedure. Although they further showed that mitral regurgitation was an independent predictor for AF recurrence, the present study found no difference in mitral regurgitation grade between the conventional and the CF groups (Table 1).
Recent prospective, single-centre study demonstrated that a lower incidence of AF recurrence was observed in PAF patients with high CF values (≥22 g) compared with those with low CF values (<22 g) during a mean follow-up of 19 months (4.0 vs. 20.0%).23 The TOCCATA study also showed that PAF patients with high CF values (>20 g) experienced relatively lower incidence of AF recurrence (20%), whereas AF recurrence occurred in all patients with low CF values (<10 g) at 12-month follow-up.24 Also higher incidence of PV reconnections was observed in PAF patients with low CF values (<10 g).25 All these studies suggest that low CF values, especially <10 g, are associated with PV reconnections and AF recurrence. The CF values targeted in the present study (10–15 g) are based on our previous study showing that there is no difference in the number of residual conduction gaps between the CF values from 10 to <15 g (10 ≤ CF < 15 g) and ≥15 g.12 The present study confirmed that this range of CF values (10–15 g) is sufficient to prevent AF recurrence at 12 months. The other studies further showed that a mean of CF values was 17.9 g21 and 13–17 g at each site22 for favourable long-term outcome. Although 10–20 g may be a preferred CF value to achieve favourable outcome, other factors such as stability and force time integral (FTI) may also be associated with it. Indeed, recent SMART-AF Trial showed that stable CF during CPVI is associated with reduced AF recurrence at 12-month follow-up.15 Further studies are required to clarify optimal CF values and CF-related parameters for preventing AF recurrence.
Study limitations
There are some limitations. First, this is not a randomized study comparing the long-term outcome between the conventional and the CF groups. We compared the results between the first successive 50-patient group undergoing CPVI without CF information and the latter successive 50-patient group undergoing CPVI with CF information. We designed the present study in this way, because a CF sensor-equipped ThermoCool SmartTouch catheter became available in Japan at the mid-study period (October 2012), and our previous randomized study evaluating the usefulness of a CF catheter, the participants of which were excluded from the present study, was performed from November 2012 to March 2013.12 Also the improvement of the operator's catheter manipulation with accumulating experience with CF-guided CPVI may have favourably affected the outcomes in the CF groups, as we previously described.12 Secondly, the patients in the CF group tended to be older (P = 0.05). Nevertheless, the CF group showed favourable outcomes, indicating its minimum influence on the study. Thirdly, we performed 24-h Holter ECG recording at 3, 6, 9, and 12 months after CPVI to detect any asymptomatic AF recurrence or atrial tachyarrhythmias. Since this modality has a limitation in detecting the actual rate of recurrence, continuous monitoring using an implantable device or others during follow-up period would allow us to detect asymptomatic arrhythmias. Finally, data regarding FTI and catheter stability evaluated by the VisiTag module were not measured in the present study. Appropriate real-time use of these information during CPVI may potentially contribute to the improvement of ablation procedure and long-term outcome. Further studies using these tools are awaited.
Conclusions
The CF-guided AF ablation using a ThermoCool SmartTouch catheter is safe and more effective in reducing procedure time and preventing AF recurrence during long-term period. These beneficial effects of the CF-guided CPVI are likely due to reduced residual conduction gaps, possibly through achieving complete lesion formation.
Conflict of interest: K.O. and M.K. have received speaker honoraria from Johnson & Johnson K.K. S.S. and D.H. have received research grant support from Johnson & Johnson K.K. and Medtronic Japan Co., Ltd. The other authors have no relevant disclosures.
References
- 1. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:e199–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Europace 2012;14:1385–413. [DOI] [PubMed] [Google Scholar]
- 4. Bertaglia E, Tondo C, De Simone A, Zoppo F, Mantica M, Turco P, et al. Does catheter ablation cure atrial fibrillation? Single-procedure outcome of drug-refractory atrial fibrillation ablation: a 6-year multicentre experience. Europace 2010;12:181–7. [DOI] [PubMed] [Google Scholar]
- 5. Medi C, Sparks PB, Morton JB, Kistler PM, Halloran K, Rosso R, et al. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: results from long-term follow-up. J Cardiovasc Electrophysiol 2011;22:137–41. [DOI] [PubMed] [Google Scholar]
- 6. Cappato R, Negroni S, Pecora D, Bentivegna S, Lupo PP, Carolei A, et al. Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation 2003;108:1599–604. [DOI] [PubMed] [Google Scholar]
- 7. Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FT, et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation 2005;111:127–35. [DOI] [PubMed] [Google Scholar]
- 8. Yokoyama K, Nakagawa H, Shah DC, Lambert H, Leo G, Aeby N, et al. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol 2008;1:354–62. [DOI] [PubMed] [Google Scholar]
- 9. Martinek M, Lemes C, Sigmund E, Derndorfer M, Aichinger J, Winter S, et al. Clinical impact of an open-irrigated radiofrequency catheter with direct force measurement on atrial fibrillation ablation. Pacing Clin Electrophysiol 2012;35:1312–8. [DOI] [PubMed] [Google Scholar]
- 10. Stabile G, Solimene F, Calò L, Anselmino M, Castro A, Pratola C, et al. Catheter-tissue contact force for pulmonary veins isolation: a pilot multicentre study on effect on procedure and fluoroscopy time. Europace 2014;16:335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haldar S, Jarman JW, Panikker S, Jones DG, Salukhe T, Gupta D, et al. Contact force sensing technology identifies sites of inadequate contact and reduces acute pulmonary vein reconnection: a prospective case control study. Int J Cardiol 2013;168:1160–6. [DOI] [PubMed] [Google Scholar]
- 12. Kimura M, Sasaki S, Owada S, Horiuchi D, Sasaki K, Itoh T, et al. Comparison of lesion formation between contact force-guided and non-guided circumferential pulmonary vein isolation: a prospective, randomized study. Heart Rhythm 2014;11:984–91. [DOI] [PubMed] [Google Scholar]
- 13. Wutzler A, Huemer M, Parwani AS, Blaschke F, Haverkamp W, Boldt LH. Contact force mapping during catheter ablation for atrial fibrillation: procedural data and one-year follow-up. Arch Med Sci 2014;10:266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marijon E, Fazaa S, Narayanan K, Guy-Moyat B, Bouzeman A, Providencia R, et al. Real-time contact force sensing for pulmonary vein isolation in the setting of paroxysmal atrial fibrillation: procedural and 1-year results. J Cardiovasc Electrophysiol 2014;25:130–7. [DOI] [PubMed] [Google Scholar]
- 15. Natale A, Reddy VY, Monir G, Wilber DJ, Lindsay BD, McElderry HT, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014;64:647–56. [DOI] [PubMed] [Google Scholar]
- 16. Jarman JW, Panikker S, DAS M, Wynn GJ, Ullah W, Kontogeorgis A, et al. Relationship between contact force sensing technology and medium-term outcome of atrial fibrillation ablation: a multicenter study of 600 patients. J Cardiovasc Electrophysiol 2015;26:378–84. [DOI] [PubMed] [Google Scholar]
- 17. Kautzner J, Neuzil P, Lambert H, Peichl P, Petru J, Cihak R, et al. EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace 2015;17:1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Itoh T, Sasaki S, Kimura M, Owada S, Horiuchi D, Sasaki K, et al. Three-dimensional cardiac image integration of electroanatomical mapping of only left atrial posterior wall with CT image to guide circumferential pulmonary vein ablation. J Interv Card Electrophysiol 2010;29:167–73. [DOI] [PubMed] [Google Scholar]
- 19. Kimura M, Sasaki S, Owada S, Horiuchi D, Sasaki K, Itoh T, et al. Validation of accuracy of three-dimensional left atrial CartoSoundTM and CT image integration: influence of respiratory phase and cardiac cycle. J Cardiovasc Electrophysiol 2013;24:1002–7. [DOI] [PubMed] [Google Scholar]
- 20. le Polain de Waroux JB, Weerasooriya R, Anvardeen K, Barbraud C, Marchandise S, De Meester C, et al. Low contact force and force-time integral predict early recovery and dormant conduction revealed by adenosine after pulmonary vein isolation. Europace 2015;17:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sotomi Y, Kikkawa T, Inoue K, Tanaka K, Toyoshima Y, Oka T, et al. Regional difference of optimal contact force to prevent acute pulmonary vein reconnection during radiofrequency catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2014;25:941–7. [DOI] [PubMed] [Google Scholar]
- 22. Andrade JG, Monir G, Pollak SJ, Khairy P, Dubuc M, Roy D, et al. Pulmonary vein isolation using “contact force” ablation: the effect on dormant conduction and long-term freedom from recurrent atrial fibrillation–a prospective study. Heart Rhythm 2014;11:1919–24. [DOI] [PubMed] [Google Scholar]
- 23. Providência R, Marijon E, Combes S, Bouzeman A, Jourda F, Khoueiry Z, et al. Higher contact-force values associated with better mid-term outcome of paroxysmal atrial fibrillation ablation using the SmartTouch™ catheter. Europace 2015;17:56–63. [DOI] [PubMed] [Google Scholar]
- 24. Reddy VY, Shah D, Kautzner J, Schmidt B, Saoudi N, Herrera C, et al. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm 2012;9:1789–95. [DOI] [PubMed] [Google Scholar]
- 25. Park CI, Lehrmann H, Keyl C, Weber R, Schiebeling J, Allgeier J, et al. Mechanisms of pulmonary vein reconnection after radiofrequency ablation of atrial fibrillation: the deterministic role of contact force and interlesion distance. J Cardiovasc Electrophysiol 2014;25:701–8. [DOI] [PubMed] [Google Scholar]