Abstract
Background
Brachial-ankle pulse wave velocity (PWV) is increasingly used for the measurement of arterial stiffness. In the present study, we quantified the interrelationship between brachial-ankle and carotid-femoral PWV in a workplace population, and investigated the associations with cardiovascular risk factors and carotid intima-media thickness (IMT).
Methods
Brachial-ankle and carotid-femoral PWV were measured using the Omron-Colin VP1000 and SphygmoCor devices, respectively. We investigated the interrelationship by the Pearson's correlation analysis and Bland-Altman plot, and performed sensitivity and specificity analyses.
Results
The 954 participants (mean ± standard deviation age 42.6 ± 14.2 years) included 630 (66.0%) men and 203 (21.3%) hypertensive patients. Brachial-ankle (13.4 ± 2.7 m/s) and carotid-femoral PWV (7.3 ± 1.6 m/s) were significantly correlated in all subjects (r = 0.75) as well as in men (r = 0.72) and women (r = 0.80) separately. For arterial stiffness defined as a carotid-femoral PWV of 10 m/s or higher, the sensitivity and specificity of brachial-ankle PWV of 16.7 m/s or higher were 72 and 94%, respectively. The area under the receiver operating characteristic curve was 0.953. In multiple stepwise regression, brachial-ankle and carotid-femoral PWV were significantly (p < 0.001) associated with age (partial r = 0.33 and 0.34, respectively) and systolic blood pressure (partial r = 0.71 and 0.66, respectively). In addition, brachial-ankle and carotid-femoral PWV were significantly (p < 0.001) associated with carotid IMT (r = 0.57 and 0.55, respectively) in unadjusted analysis, but not in analysis adjusted for cardiovascular risk factors (p ≥ 0.08).
Conclusions
Brachial-ankle and carotid-femoral PWV were closely correlated, and had similar determinants. Brachial-ankle PWV can behave as an ease-of-use alternative measure of arterial stiffness for assessing cardiovascular risk.
Key Words: Pulse wave velocity, Arterial stiffness, Age, Systolic blood pressure, Intima-media thickness
Introduction
With the applanation tonometry technique, pulse waves can be estimated from superficial arterial sites, such as the carotid and femoral arteries. Pulse wave velocity (PWV) can then be calculated by dividing the distance of the travel path of the pulse waves by the time difference between the pulse waves of the two arterial sites. Among several possible measurements of PWV between two superficial arterial sites, carotid-femoral PWV is considered as a standard measure of aortic stiffness [1]. There is evidence that carotid-femoral PWV predicts cardiovascular events and mortality in the general population [2,3] and in patients with various diseases [4,5,6,7,8], such as hypertension [5], diabetes mellitus [6] and end-stage renal disease [7,8]. Current hypertension guidelines recommend the use of carotid-femoral PWV for the assessment of arterial stiffness and cardiovascular risk [9].
The current advanced plethysmographic technique with oscillometric cuffs allows accurate pulse wave recording on the brachial and ankle arteries and the measurement of brachial-ankle PWV [10]. This new technique is easy to use, reproducible and less dependent on operators. It is closely correlated with carotid-femoral PWV and proposed as a measure of aortic stiffness [11]. There is accumulating evidence that brachial-ankle PWV also predicts cardiovascular events and mortality [12,13,14,15,16,17]. Brachial-ankle PWV is recommended by some [18], though not all [9], current hypertension guidelines and is increasingly used in clinical practice in China, Japan, and several other countries. Brachial-ankle PWV has been criticized for inclusion of muscular arteries and for the estimation of wave path from body height instead of real measurement. Nonetheless, it would be clinically relevant to quantitatively compare brachial-ankle with carotid-femoral PWV for the proper use of this new technique in the assessment of arterial stiffness.
In the present study, we quantified the interrelationship between brachial-ankle and carotid-femoral PWV and investigated their associations with demographic and clinical characteristics, such as age and systolic blood pressure, and with carotid intima-media thickness (IMT) in a Chinese workplace population.
Methods
Study Population
Our study was conducted in the framework of comprehensive cardiovascular health examinations for all employees (including those who were retired but still lived in the area of the factory at the time of the study) of a factory, located in an isolated coast area, 300 km south of Shanghai [19,20,21]. The family members of the employees being at least 15 years of age and living in the nearby region were also invited for participation. The Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine approved the study protocol. All subjects gave written informed consent.
Of the 1,052 study subjects (participation rate 80.7%), 98 were excluded from the present analysis, because carotid-femoral (n = 71, including 3 subjects with atrial fibrillation) or brachial-ankle PWV (n = 12) was not measured, and because data of physical examinations were missing (n = 15). Thus, the number of participants included in the present analysis was 954.
Filed Work
One experienced physician measured clinic blood pressure 5 times consecutively by standard mercury sphygmomanometry on the nondominant arm, after the subjects had rested for at least 5 min in the sitting position. These 5 blood pressure readings were averaged for analysis. The same observer also administered a standardized questionnaire to collect information on medical history, lifestyle, and use of medications. Hypertension was defined as a blood pressure of at least 140 mm Hg (systolic) or 90 mm Hg (diastolic) or as the use of antihypertensive drugs.
A trained technician measured body height and body weight. Body mass index was calculated as the body weight in kilograms divided by the height in meters squared. Venous blood samples were taken after overnight fasting for measurements of plasma glucose concentration and serum concentrations of total and high-density lipoprotein cholesterol, triglycerides, creatinine and uric acid. Diabetes mellitus was defined as a plasma fasting glucose level of at least 7.0 or 11.1 mmol/l at any time or as the use of antidiabetic medication.
Measurement of PWV
Carotid-femoral PWV was measured by applanation tonometry. A trained technician used a high-fidelity SPC-301 micromanometer (Millar Instruments, Houston, Tex., USA) interfaced with a laptop computer running the SphygmoCor software, version 7.1 (AtCor Medical, West Tyde, N.S.W., Australia) to record sequentially the waveforms of the carotid and femoral arteries. With the simultaneously recorded ECG (lead II) tracing, the time delay between the two arterial sites was calculated after both carotid and femoral waveforms were imported for at least 12 s. PWV (cm/s) was calculated by dividing the length between the two arterial sites by the time interval. The path length was the difference between the distance from the sternal notch to the femoral site and the distance from the sternal notch to the carotid site. A recent study showed that this calculating method underestimated the path length and hence also PWV [22]. Therefore, we had to use a lower threshold of the carotid-femoral PWV for the definition of arterial stiffness, i.e., 10 m/s [9,22] instead of 12 m/s [23].
Brachial-ankle PWV was measured by the Vascular Profiler-1000 device (Omron, Kyoto, Japan), with an oscillometric cuff technique. This measurement was performed on the same occasion as the carotid-femoral PWV measurement by another trained technician. There is no defined order between the two PWV measurements. After the subjects had rested in the supine position for 10 min, the cuffs were placed on both arms and ankles. The device automatically measures the pulse waves of the brachial and ankle arteries, and estimates the path length of the pulse wave according to the body height of the subjects [10]. Brachial-ankle PWV was then calculated as the ratio of the path length to the time interval between the ankle and brachial pulse waveforms for both sides. The brachial-ankle PWV of the right and left sides were averaged for analysis. The device also simultaneously measures blood pressure on the 4 limbs. The ankle-brachial index (ABI) was calculated as the ratio of the lower-side ankle to the higher-side brachial systolic blood pressure. An ABI of 0.90 or lower was considered abnormal [9,18,24].
Measurement of Carotid IMT
A trained sonographer performed IMT measurement of the right common carotid artery with an echo-tracking ultrasonic system (ArtLab, Esaote, Italy). The acquired data were analyzed offline with the automatic detection of the intima and media and the automatic measurement and reporting of the IMT. The segment of the common carotid artery 1 cm proximal to the bifurcation was chosen. The IMT readings on the far wall were averaged for statistical analysis.
Statistical Analysis
For database management and statistical analysis, we used SAS software (version 9.2, SAS Institute, Cary, N.C., USA). The categorical variables were described as number (percent) and the continuous variables as mean ± standard deviation. Means and proportions were compared with the Student t test and the χ2 test, respectively. The interrelationship was analyzed by correlation analysis with Pearson's method and by the Bland-Altman approach after normalization of the two PWV measurements. For the normalization of the two PWV measurements, we subtracted the mean from the original measurement and then divided the difference by the standard deviation. The receiver operating characteristic (ROC) curve was drawn for accuracy of brachial-ankle PWV in the evaluation of arterial stiffness diagnosed by carotid-femoral PWV. Single and multiple regression analyses were performed to study the associations of the two PWV measurements with cardiovascular risk factors and with carotid IMT.
Results
Characteristics of the Study Participants
The 954 participants [630 (66.0%) men] had a mean age (±standard deviation) of 42.6 ± 14.2 years, and included 203 (21.3%) hypertensive patients, 89 (9.4%) of whom took antihypertensive drugs. Table 1 shows the characteristics of the study participants by gender. Men and women differed significantly (p ≤ 0.03) in all characteristics except for age (42.6 ± 14.2 years, p = 0.19), pulse rate (73.5 ± 9.5 beats/min, p = 0.07), plasma fasting glucose (4.4 ± 1.0 mmol/l, p = 0.65), serum total cholesterol (4.8 ± 0.9 mmol/l, p = 0.13), the prevalence of diabetes mellitus (2.1%, p = 0.18) and the use of antihypertensive drugs (9.8%, p = 0.19).
Table 1.
Characteristics of the study participants by gender
| Characteristic | Men (n = 630) | Women (n = 324) | p value |
|---|---|---|---|
| Age, years | 43.0±14.8 | 41.8±12.8 | 0.19 |
| Body height, cm | 167.6±6.1 | 157.7±5.0 | <0.0001 |
| Body weight, kg | 66.9±9.9 | 56.1±8.1 | <0.0001 |
| Body mass index | 23.8±3.2 | 22.6±3.2 | <0.0001 |
| Pulse rate, beats/min | 73.1±9.9 | 74.2±8.6 | 0.07 |
| Current smoking, n (%) | 300 (48.4) | 2 (0.6) | <0.0001 |
| Alcohol intake, n (%) | 236 (38.0) | 5 (1.6) | <0.0001 |
| Systolic blood pressure, mm Hg | 123.8±18.9 | 117.7±21.3 | <0.0001 |
| Diastolic blood pressure, mm Hg | 76.3±11.3 | 71.9±10.9 | <0.0001 |
| Prevalence of hypertension, n (%) | 147 (23.7) | 56 (17.5) | 0.03 |
| On antihypertensive medication, n (%) | 64 (10.7) | 25 (8.0) | 0.19 |
| Fasting plasma glucose, mmol/l | 4.3±1.2 | 4.4±0.7 | 0.65 |
| Diabetes mellitus, n (%) | 16 (2.5) | 4 (1.2) | 0.18 |
| Serum total cholesterol, mmol/l | 4.8±0.9 | 4.7±0.9 | 0.13 |
| Serum HDL cholesterol, mmol/l | 1.3±0.3 | 1.5±0.4 | <0.0001 |
| Serum triglycerides, mmol/l | 2.1±1.9 | 1.6±1.1 | <0.0001 |
| Serum creatinine, µmol/l | 78.3±13.4 | 56.7±8.9 | <0.0001 |
| Serum uric acid, µmol/l | 364±76 | 273±64 | <0.0001 |
Values are means ± standard deviations unless indicated otherwise. For definitions of hypertension and diabetes mellitus, see Methods. HDL = High-density lipoprotein.
Men had significantly higher brachial-ankle (13.7 ± 2.5 vs. 12.7 ± 2.8 m/s, p < 0.001) and carotid-femoral PWV (7.4 ± 1.6 vs. 7.2 ± 1.7 m/s, p = 0.02) compared with women. In men as well as in women, both brachial-ankle and carotid-femoral PWV were significantly (p < 0.001) higher with age advancing (r ≥ 0.58) and with higher systolic blood pressure (r ≥ 0.58).
Interrelationship between Brachial-Ankle and Carotid-Femoral PWV
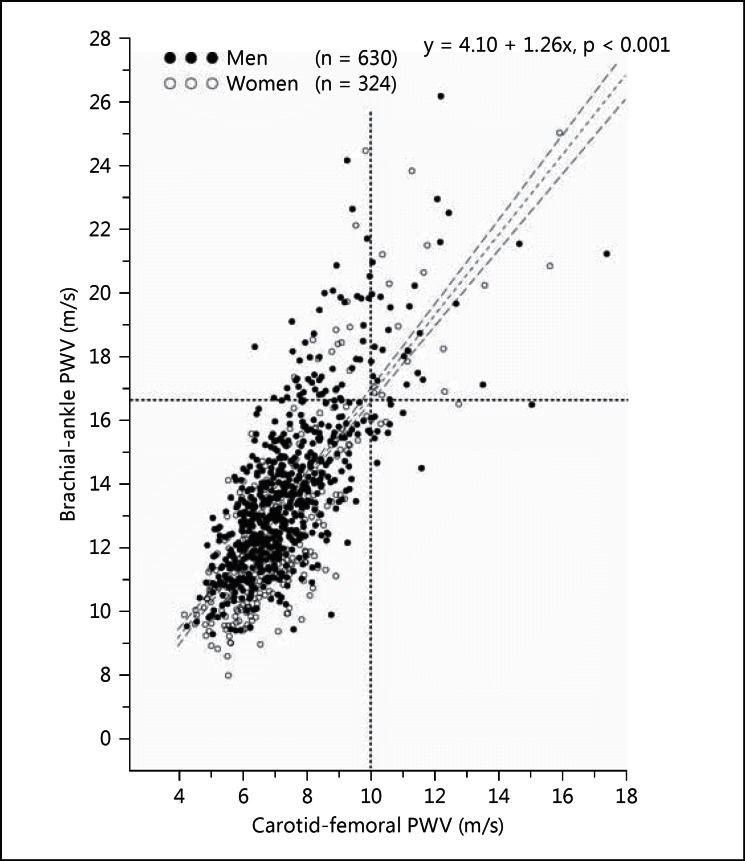
Brachial-ankle and carotid-femoral PWV were significantly (p < 0.001) and closely correlated with each other in all subjects (r = 0.75) and in men (r = 0.72) and women (r = 0.80) separately (fig. 1). In single regression with brachial-ankle and carotid-femoral PWV as dependent and independent variables, respectively, the slope and intercept were 1.26 and 4.10 m/s, respectively, in all subjects, 1.20 and 4.81 m/s, respectively, in men, and 1.32 and 3.22 m/s, respectively, in women.
Fig. 1.
Scatter plot for the interrelationship between brachial-ankle and carotid-femoral PWV. Dots and circles represent men and women, respectively. The dashed vertical lines indicate the diagnostic threshold for arterial stiffness for brachial-ankle (16.7 m/s) and carotid femoral PWV (10 m/s), respectively. The regression line was drawn with 95% confidence limits. The regression equation and the corresponding p value for the slope are given.
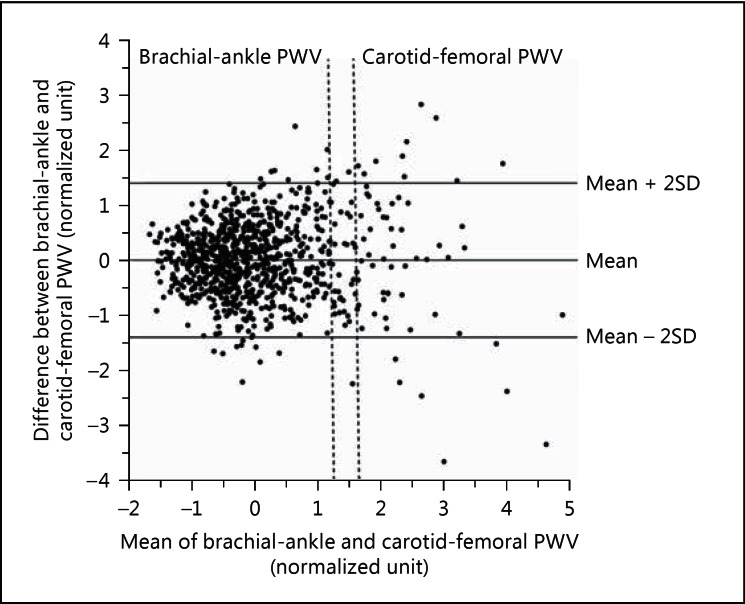
After normalization of both brachial-ankle and carotid-femoral PWV, we performed the Bland-Altman analysis to investigate the agreement between the two PWV measurements (fig. 2). The mean difference (±2 standard deviations) between the two measurements was 0.001 (±1.392), 0.075 (±1.39), and −0.144 (±1.347) normalized unit in all subjects, men and women, respectively. The correlation coefficients between the difference and the average of the two PWV measurements were 0.0004, −0.0248 and −0.0144, in all subjects, men and women, respectively (p ≥ 0.53 for all).
Fig. 2.
Bland-Altman plot for the agreement between brachial-ankle and carotid-femoral PWV after normalization. The continuous horizontal lines indicate the mean difference and ±2 standard deviations, respectively. The dashed vertical lines indicate the diagnostic threshold for arterial stiffness for brachial-ankle (16.7 m/s) and carotid femoral PWV (10 m/s), respectively.
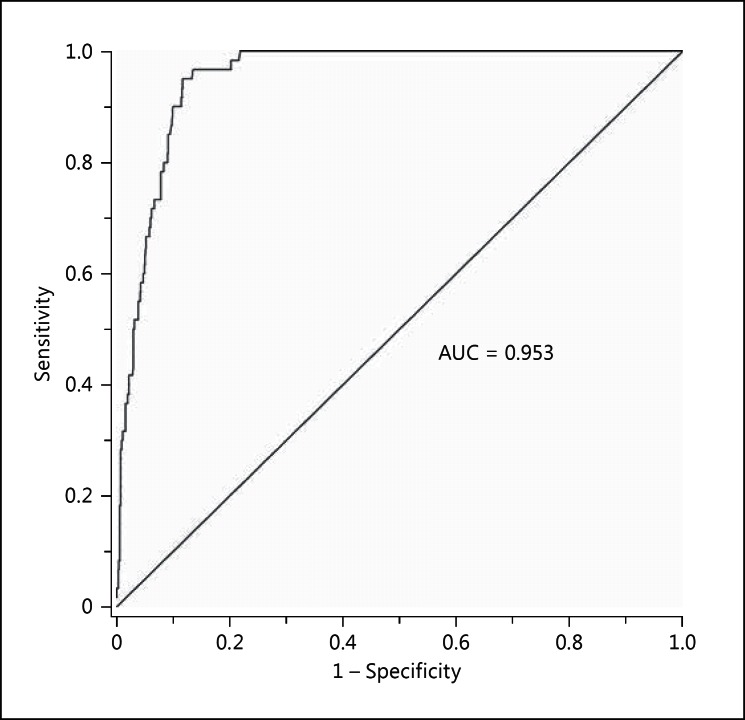
The prevalence of arterial stiffness according to carotid-femoral PWV (≥10 m/s) was 6.3%. According to the regression equation in all subjects, a brachial-ankle PWV of 16.7 m/s was an equivalence of the cutoff limit of carotid-femoral PWV of 10 m/s or higher. Brachial-ankle PWV of 16.7 m/s or higher had a sensitivity of 72% and a specificity of 94% in all subjects (table 2). The corresponding values were 73 and 73%, respectively, in hypertensive patients (n = 202) and 72 and 94%, respectively, in subjects without peripheral arterial disease as defined by an ABI of ≤0.90 (n = 937) and 82 and 72%, respectively, in subjects ≥60 years old (n = 146). Overall, the ROC curve showed an AUC of 0.953 for brachial-ankle PWV in the evaluation of arterial stiffness as diagnosed by carotid-femoral PWV (fig. 3).
Table 2.
Diagnostic accuracy of brachial-ankle PWV (>16.7 m/s) for arterial stiffness defined by carotid-femoral PWV (>10 m/s)
| Subject type | Sensitivity, % | Specificity, % |
|---|---|---|
| Overall (n = 954) | 72 | 94 |
| Hypertensive patients (n = 202) | 73 | 73 |
| Subjects with an ABI >0.90 (n = 937) | 72 | 94 |
| Subjects >60 years old (n = 146) | 82 | 72 |
Fig. 3.
ROC curve for arterial stiffness diagnosed by brachial-ankle PWV. A carotid-femoral PWV of ≥10 m/s was defined as arterial stiffness. The AUC is given.
In further analysis, we compared subjects with abnormal PWV on either carotid-femoral (≥10 m/s, n = 17) or brachial-ankle measurement alone (≥16.7 m/s, n = 57) to those with abnormal PWV on both measurements (n = 43). The former, compared with the latter, were younger (59.3 vs. 63.8 years, p = 0.004), and had a significantly (p = 0.02) lower systolic blood pressure (147.7 vs. 157.2 mm Hg).
Determinants of Brachial-Ankle and Carotid-Femoral PWV
In multiple stepwise regression analyses, we considered sex, age, body height, body weight, systolic and diastolic blood pressure, pulse rate, current smoking, alcohol intake, diabetes mellitus, the use of antihypertensive drugs, plasma fasting glucose, serum total/high-density lipoprotein cholesterol ratio, serum triglycerides, and serum creatinine and uric acid. We found that brachial-ankle and carotid-femoral PWV were positively and consistently associated with age (partial r = 0.33 and 0.34, p < 0.001), systolic blood pressure (partial r = 0.71 and 0.66, p < 0.001), pulse rate (partial r = 0.09 and 0.11, p < 0.001), the use of antihypertensive drugs (partial r = 0.08 and 0.05, p ≤ 0.03) and serum uric acid (partial r = 0.06 and 0.07, p ≤ 0.01, table 3). These major correlates altogether explained 62.7 and 57.0% of the variance of brachial-ankle and carotid-femoral PWV, respectively. Other significant (p ≤ 0.085) correlates included gender (r = 0.09), body height (r = −0.11), body weight (r = −0.05), serum triglycerides (r = 0.05) and diabetes mellitus (r = 0.05) for brachial-ankle PWV and gender (r = −0.05) and body weight (r = 0.04) for carotid-femoral PWV.
Table 3.
Determinants of brachial-ankle and carotid-femoral PWV in multiple stepwise regression analyses in all subjects
| Variable | Brachial-ankle PWV |
Carotid-femoral PWV |
||||
|---|---|---|---|---|---|---|
| partial r | β±SE | p | partial r | β±SE | p | |
| Systolic blood pressure, +10 mm Hg | 0.7129 | 0.58±0.04 | <0.001 | 0.6609 | 0.30±0.02 | <0.001 |
| Age, +10 years | 0.3314 | 0.68±0.05 | <0.001 | 0.3354 | 0.44±0.03 | <0.001 |
| Body height, +10 cm | −0.1091 | −0.33±0.11 | 0.003 | – | – | – |
| Pulse rate, +10 beats/min | 0.0949 | 0.23±0.06 | <0.001 | 0.1109 | 0.17±0.04 | <0.001 |
| Male gender | 0.0949 | 0.93±0.16 | <0.001 | −0.0469 | −0.23±0.09 | 0.010 |
| Use of antihypertensive medication | 0.0837 | 0.86±0.20 | <0.001 | 0.0520 | 0.28±0.13 | 0.033 |
| Serum uric acid, +100 µmol/l | 0.0574 | 0.20±0.08 | 0.012 | 0.0707 | 0.17±0.05 | 0.001 |
| Diabetes mellitus | 0.0500 | 1.02±0.40 | 0.011 | – | – | – |
| Serum triglycerides, mmol/l | 0.0480 | 0.08±0.03 | 0.015 | – | – | – |
| Body weight, +10 kg | −0.0469 | −0.25±0.07 | 0.001 | 0.0387 | 0.07±0.04 | 0.085 |
In multiple stepwise regression analyses, we considered gender, age, body height, body weight, systolic and diastolic blood pressure, pulse rate, current smoking, alcohol intake, diabetes mellitus, and the use of antihypertensive drugs, plasma fasting glucose, serum total/high-density lipoprotein cholesterol ratio, serum triglycerides, and serum creatinine and uric acid. We reported partial r, regression coefficient (β) ± standard error (SE) and p value for all variables entered and stayed in the model. Variables are listed according to the size of the absolute values of the correlation coefficients in the descending order for carotid-femoral and then brachial-ankle PWV.
In further analysis, we investigated associations of brachial-ankle and carotid-femoral PWV with carotid IMT. In single regression, both brachial-ankle and carotid-femoral PWV were significantly (p < 0.001) associated with carotid IMT (r = 0.57 and 0.55, respectively). However, after adjustment for the significant correlates identified in the above analysis, the associations became nonsignificant (p ≥ 0.08).
After exclusion of 89 subjects who took antihypertensive medication or 17 subjects with peripheral arterial disease as defined by an ABI of 0.90 or less separately, we performed sensitivity analyses. The results were confirmatory.
Discussion
Our study showed that brachial-ankle and carotid-femoral PWV were closely correlated. If a carotid-femoral PWV of 10 m/s or higher is considered abnormal, a brachial-ankle PWV of 16.7 m/s or higher could be considered as the threshold for the diagnosis of arterial stiffness. With this cutoff limit and carotid-femoral PWV as standard, the sensitivity and specificity of brachial-ankle PWV for the diagnosis of arterial stiffness were moderate or high. Furthermore, the two measurements of PWV had similar determinants, mainly age and systolic blood pressure.
Our finding on the close interrelationship between brachial-ankle and carotid-femoral PWV is in line with the results of several previous studies [11,25,26]. In a multicenter study of 2,287 subjects, Tanaka et al. [11] reported a correlation coefficient of 0.73 between brachial-ankle and carotid-femoral PWV. The corresponding correlation coefficients were 0.76 in healthy adults (n = 409) [25] and 0.99 in a study population of mixed normal healthy subjects (n = 24) and patients with coronary artery disease (n = 20) [26]. Taken together, the results of our and these previous studies suggest that brachial-ankle PWV and carotid-femoral PWV are closely correlated with correlation coefficients at a level similar to that for two repeated measurements of the same technique.
Our analysis on the determinants of the brachial-ankle and carotid-femoral PWV supports the consistency between the two measurements of PWV. Indeed, both measurements were closely correlated with age and systolic blood pressure. Age plus systolic blood pressure explained 61.3 and 55.1% of the variance of brachial-ankle and carotid-femoral PWV measurements, respectively. Other correlates, though different between the two PWV measurements, explained <5% of the variance. These observations are in agreement with the results of previous studies [10,25]. Multiple regression analysis was not performed in these previous studies [10,25]. In single regression, the correlation coefficients were at the size of approximately 0.5-0.6 for brachial-ankle and carotid-femoral PWV with age or systolic blood pressure [10,25].
Our analysis on the association between the two PWV measurements and carotid IMT, a structural measure of a large elastic artery, provided additional support for the consistency between the two PWV measurements. Both PWV measurements were significantly associated with carotid IMT in unadjusted analysis but not in analysis adjusted for age, systolic blood pressure, and other confounding factors. Our explanation is that the latter nonsignificant associations could be attributable to the high dependency of these arterial structural and functional measurements on age and systolic blood pressure [27].
Why the two distinct PWV measurements are so closely related and hence may be interchangeable remains to be investigated. Our explanation is that only or mainly the aortic artery becomes significantly stiffer with aging or with the presence of other cardiovascular risk factors, such as hypertension, diabetes mellitus, dyslipidemia, or cigarette smoking [28]. Although the brachial-ankle PWV measurement includes both elastic aortic and muscular arteries, the latter have much less influence on the measurement of PWV. Indeed, a previous study showed that carotid-femoral but not carotid-radial or femoral-pedis PWV significantly increased with aging [28]. We, therefore, believe that the close relationship between brachial-ankle and carotid-femoral PWV is probably physiological or pathophysiological instead of mathematical or statistical.
Our study should be interpreted within the context of its limitations. First, our study is cross-sectional and hence does not allow any causal interference for the associations. Second, our study included only a small proportion of subjects older than 60 years (n = 146), in whom the prevalence of peripheral arterial disease is high. Brachial-ankle PWV cannot be accurately measured in the presence of severe peripheral arterial disease. The device measures ABI and brachial-ankle PWV simultaneously and makes it possible to assess the validity of brachial-ankle PWV with ABI. Nonetheless, our study results have to be cautiously extrapolated to the elderly. Third, our study participants were enrolled in a workplace setting, and might be less representative than general population samples.
In conclusion, brachial-ankle and carotid-femoral PWV are closely related, and had similar major determinants. Brachial-ankle PWV can behave as an ease-of-use alternative measure of arterial stiffness for the assessment of cardiovascular risk and therapeutic effect.
Disclosure Statement
Dr. Wang reports receiving consulting and lecture fees from Astra-Zeneca, Boehringer Ingelheim, MSD, Novartis, Omron, Pfizer, Daiichi-Sankyo, Sanofi, and Servier. The other authors declare no conflicts of interest.
Acknowledgments
The authors gratefully acknowledge the voluntary participation of all study subjects and the technical assistance of the physicians and nurses of Haishen Hospital (Xiangshan County, Zhejiang Province). The authors also appreciate the expert assistance of Shou-Yu Mao, Gu-Liang Wang, Jie Wang, and Wei-Zhong Zhang (The Shanghai Institute of Hypertension, Shanghai, China).
The present study was financially supported by grants from the National Natural Science Foundation of China (grants 81170245, 81270373 and 81470533) and the Ministry of Science and Technology (grant 2013CB530700 and a grant for China-European Union collaborations [1012]), Beijing, China, the Shanghai Commission of Science and Technology (grants 11QH1402000 and 15XD1503200) and the Shanghai Bureau of Health (grant XBR2011004).
References
- 1.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. European Network for Non-invasive Investigation of Large Arteries: Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 2.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 6.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 7.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 8.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103:987–992. doi: 10.1161/01.cir.103.7.987. [DOI] [PubMed] [Google Scholar]
- 9.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. Task Force Members: 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 10.Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–364. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, Tomiyama H, Yamashina A, Yasuda H, Sawayama T, Ozawa T. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27:2022–2027. doi: 10.1097/HJH.0b013e32832e94e7. [DOI] [PubMed] [Google Scholar]
- 12.Ninomiya T, Kojima I, Doi Y, Fukuhara M, Hirakawa Y, Hata J, Kitazono T, Kiyohara Y. Brachial-ankle pulse wave velocity predicts the development of cardiovascular disease in a general Japanese population: the Hisayama Study. J Hypertens. 2013;31:477–483. doi: 10.1097/HJH.0b013e32835c5c23. [DOI] [PubMed] [Google Scholar]
- 13.Takashima N, Turin TC, Matsui K, Rumana N, Nakamura Y, Kadota A, Saito Y, Sugihara H, Morita Y, Ichikawa M, Hirose K, Kawakani K, Hamajima N, Miura K, Ueshima H, Kita Y. The relationship of brachial-ankle pulse wave velocity to future cardiovascular disease events in the general Japanese population: the Takashima Study. J Hum Hypertens. 2014;28:323–327. doi: 10.1038/jhh.2013.103. [DOI] [PubMed] [Google Scholar]
- 14.Sheng CS, Li Y, Li LH, Huang QF, Zeng WF, Kang YY, Zhang L, Liu M, Wei FF, Li GL, Song J, Wang S, Wang JG. Brachial-ankle pulse wave velocity as a predictor of mortality in elderly Chinese. Hypertension. 2014;64:1124–1130. doi: 10.1161/HYPERTENSIONAHA.114.04063. [DOI] [PubMed] [Google Scholar]
- 15.Munakata M, Konno S, Miura Y, Yoshinaga K. TOPP Study Group: Prognostic significance of the brachial-ankle pulse wave velocity in patients with essential hypertension: final results of the J-TOPP study. Hypertens Res. 2012;35:839–842. doi: 10.1038/hr.2012.53. [DOI] [PubMed] [Google Scholar]
- 16.Maeda Y, Inoguchi T, Etoh E, Kodama Y, Sasaki S, Sonoda N, Nawata H, Shimabukuro M, Takayanagi R. Brachial-ankle pulse wave velocity predicts all-cause mortality and cardiovascular events in patients with diabetes: the Kyushu Prevention Study of Atherosclerosis. Diabetes Care. 2014;37:2383–2390. doi: 10.2337/dc13-1886. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Song TJ, Song D, Lee KJ, Kim EH, Lee HS, Nam CM, Nam HS, Kim YD, Heo JH. Brachial-ankle pulse wave velocity is a strong predictor for mortality in patients with acute stroke. Hypertension. 2014;64:240–246. doi: 10.1161/HYPERTENSIONAHA.114.03304. [DOI] [PubMed] [Google Scholar]
- 18.Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S. Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension: The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37:253–390. doi: 10.1038/hr.2014.20. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Li Y, Sheng CS, Huang QF, Zheng Y, Wang JG. Association of serum uric acid with aortic stiffness and pressure in a Chinese workplace setting. Am J hypertens. 2010;23:387–392. doi: 10.1038/ajh.2009.277. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Li Y, Sheng CS, Huang QF, Fan HQ, Wang JG. Cardiac structure and function in relation to central blood pressure components in Chinese. J Hypertens. 2011;29:2462–2468. doi: 10.1097/HJH.0b013e32834c1e7d. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Li Y, Liu M, Sheng CS, Huang QF, Wang JG. Cardiac structure and function in relation to cardiovascular risk factors in Chinese. BMC Cardiovasc Disord. 2012;12:86. doi: 10.1186/1471-2261-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T. Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries: Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 23.Mancia G, De Backer G, Dominiczak A, et al. Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology. 2007 guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 24.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D. American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia: Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 25.Sugawara J, Hayashi K, Yokoi T, Cortez-Cooper MY, DeVan AE, Anton MA, Tanaka H. Brachial-ankle pulse wave velocity: an index of central arterial stiffness? J Hum Hypertens. 2005;19:401–406. doi: 10.1038/sj.jhh.1001838. [DOI] [PubMed] [Google Scholar]
- 26.Naidu MU, Reddy BM, Yashmaina S, Patnaik AN, Rani PU. Validity and reproducibility of arterial pulse wave velocity measurement using new device with oscillometric technique: a pilot study. Biomed Eng Online. 2005;4:49. doi: 10.1186/1475-925X-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelen L, Ferreira I, Stehouwer CD, Boutouyrie P, Laurent S. Reference Values for Arterial Measurements Collaboration: Reference intervals for common carotid intima-media thickness measured with echotracking: relation with risk factors. Eur Heart J. 2013;34:2368–2380. doi: 10.1093/eurheartj/ehs380. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Agnoletti D, Protogerou AD, Topouchian J, Wang JG, Xu Y, Blacher J, Safar ME. Characteristics of pulse wave velocity in elastic and muscular arteries: a mismatch beyond age. J Hypertens. 2013;31:554–559. doi: 10.1097/HJH.0b013e32835d4aec. [DOI] [PubMed] [Google Scholar]