Abstract
The intestinal epithelium is subjected to repetitive deformation during normal gut function by peristalsis and villous motility. In vitro, cyclic strain promotes intestinal epithelial proliferation and induces an absorptive phenotype characterized by increased dipeptidyl dipeptidase (DPPIV) expression. Schlafen 3 is a novel gene recently associated with cellular differentiation. We sought to evaluate whether Schlafen 3 mediates the effects of strain on the differentiation of intestinal epithelial cell (IEC)-6 in the absence or presence of cyclic strain. Strain increased Schlafen 3 mRNA and protein. In cells transfected with a control-nontargeting siRNA, strain increased DPPIV-specific activity. However, Schlafen 3 reduction by siRNA decreased basal DPPIV and prevented any stimulation of DPPIV activity by strain. Schlafen 3 reduction also prevented DPPIV induction by sodium butyrate (1 mM) or transforming growth factor (TGF)-β (0.1 ng/ml), two unrelated differentiating stimuli. However, Schlafen-3 reduction by siRNA did not prevent the mitogenic effect of strain or that of EGF. Blocking Src and phosphatidyl inositol (PI3)-kinase prevented strain induction of Schlafen 3, but Schlafen 3 induction required activation of p38 but not ERK. These results suggest that cyclic strain induces an absorptive phenotype characterized by increased DPPIV activity via Src-, p38-, and PI3-kinase-dependent induction of Schlafen 3 in rat IEC-6 cells on collagen, whereas Schlafen 3 may also be a key factor in the induction of intestinal epithelial differentiation by other stimuli such as sodium butyrate or TGF-β. The induction of Schlafen 3 or its human homologs may modulate intestinal epithelial differentiation and preserve the gut mucosa during normal gut function.
Keywords: deformation, enterocyte, signaling
the intestinal epithelium is subjected to repetitive deformation from diverse physical forces including peristalsis and villous motility during normal gut function (28, 72). Increasing in vitro and in vivo evidence suggests that such forces may substantially influence intestinal mucosal cell biology by flexing the matrix, altering integrin binding, and initiating matrix-dependent signals that regulate enterocyte proliferation (10, 20, 44). In vitro, such repetitive deformation stimulates intestinal epithelial proliferation and induces an absorptive phenotype characterized by increased dipeptidyl dipeptidase (DPPIV) expression in an amplitude-dependent fashion, changes opposite to those observed during prolonged fasting when peristaltic contractions and villous motility are diminished (5). In addition, different models of repetitive mechanical deformation also promote differentiation in other cell types including fetal type II epithelial cells (69), osteoblast-like cells (51), and embryonic stem cell-derived cardiomyocytes (23). However, the mechanisms of how strain modulates cell differentiation and particularly intestinal epithelial differentiation, a complex phenomenon during which the epithelial cells acquire the functional properties of mature enterocytes, are largely unknown.
Brush-border digestive enzymes such as dipeptidyl peptidase are canonical markers for enterocytic differentiation (4, 14, 55, 57, 73). DPPIV cleaves NH2-terminal dipeptides from polypeptides with either L-proline or L-alanine at the penultimate position, including chemokines and neuropeptides, leading to their inactivation and/or degradation (65). DPPIV-specific enzyme activity is also used in many other cell types as a marker of differentiation (2, 33, 67). Indeed, the loss or alteration of DPPIV expression is linked to the development of many cancers, including breast, prostate, lung, ovarian, hepatocellular cancer, and melanomas, and plays a key role in tumorigenesis and metastasis (2). In addition to brush-border digestive enzyme activity, intestinal differentiation is also frequently assessed by villin expression (24, 30, 36, 41, 49). Villin is a key Ca2+-regulated actin-binding protein in the microvillus core of the brush border (1, 24, 30, 36, 41, 46, 47, 49). Intestinal epithelial cells and kidney proximal tubule cells are notable examples of cells that have these highly specialized brush-border microvilli and villin accumulate at their apex. Villin content in differentiated HT29–18 cells, a clone derived from the HT-29 human colonic adenocarcinoma cell line, is 10 times higher than that in undifferentiated HT29–18 cells but close to that seen in normal human colonic cells (17).
We have previously observed that cyclic strain of a physiologically relevant frequency and magnitude modulates the differentiation of well-differentiated human intestinal epithelial Caco-2 cells, increasing in the specific activity of DPPIV (5). Schlafen 3 belongs to a family of growth regulatory genes that was first discovered in mice (61) and presently contains 10 intracellular protein members (53). A unique domain named “Schlafen box” along with the adjacent ATP/GTP binding AAA domain is common to all the members in the family (21). This family has been divided into three subgroups on the basis of their overall exon homology and size of the encoded proteins (21, 61). Schlafen 3, along with Schlafen 4, Schlafen 6, and Schlafen 7, belongs to intermediate subgroup. Schlafen 3 has recently been associated with cellular growth during aging and differentiation (52, 53). Other Schlafens, such as Slfn-1 or -8, plays an important role in modulation of T cell development (42, 61). Schlafen expression has been reported to increase during cellular differentiation in hematopoietic cell lines (7), and Slfn-2 induction has been shown to be critical during the process of differentiation of monocytes/macrophages to osteoclasts induced by receptor activator of NF-κB ligand (42). In this study, we sought to evaluate whether Schlafen 3 mediates the effects of strain on the differentiation of nontransformed rat small intestinal epithelial cells (IEC-6) cultured on collagen-coated membranes in the absence or presence of strain.
MATERIALS AND METHODS
Materials.
The nontransformed rat intestinal cell line, IEC-6, was obtained from American Type Culture Collection (Manassas, VA). DMEM, oligofectamine, and Plus Reagent were obtained from Invitrogen (Hercules, CA). Trypsin was obtained from Sigma (St. Louis, MO). Phosphospecific polyclonal antibodies to focal adhesion kinase (FAK) at Tyr576 were obtained from BIOSOURCE (Camarillo, CA). Phosphospecific rabbit polyclonal antibodies to p42/p44 at Tyr202/Thr204 (p-ERK1/2) and Akt at Thr473, rabbit polyclonal antibody to p42/44 (ERK1/2) and Akt, and horseradish peroxidase-conjugated anti-rabbit and anti-mouse IgG were obtained from Cell Signaling (Beverly, MA). Mouse monoclonal antibody to FAK was obtained from Upstate Cell Signaling Solutions (Charlottesville, VA). Western blot stripping reagent was obtained from Chemicon International (Temecula, CA). Goat polyclonal Schlafen 3, villin, Hsp 27 antibody, and horseradish peroxidase-conjugated anti-goat IgG were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibody to GAPDH was obtained from Biodesign (Saco, ME).
Cell culture.
Nontransformed rat IEC-6 cells (American Type Culture Collection) were maintained at 37°C 5% CO2 as previously described (21).
Strain application.
Cells were plated on Flexwell plates and maintained in a 37°C humidified incubator with 5% CO2. Once the cell monolayers were confluent, they were subjected to mechanical deformation using the Flexcell Strain Unit (FX-3000; Flexcell, McKeesport, PA) as described previously (19). Briefly, cells were subjected to cyclic deformation and relaxation at a magnitude of 10% strain and a frequency of 10 cycles/min by a computer-controlled 20-kPa vacuum. Control plates were not attached to the Flexcell Unit though placed in the same incubator. Placing a Plexiglas ring in the center addressed the nonuniformity of strain in the center of the flexible wells because cells were plated only around the periphery of the ring where strain is more uniform. Previous studies have demonstrated that the cells remain adherent during deformation and experience parallel elongation and relaxation with the repetitive deforming membrane (5).
Matrix and inhibitors.
Flexwell amino plates were precoated with collagen I. Cells were seeded at 300,000/well and grown to confluence. PD98059, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2), LY294002, and SB203580 (Calbiochem, La Jolla, CA) were each dissolved in DMSO, diluted immediately before use, and added to the cells 1 h before exposure to strain. Control cells in these studies were similarly treated with 0.1% DMSO as a vehicle control.
Western blot analysis.
Following strain, IEC-6 cells were lysed on ice in modified radioimmunoprecipitation buffer (50 mmol/l Tris, pH 7.4, 150 mmol/l NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mmol/l EDTA, 1 mmol/l phenylmethylsulfonyl fluoride, 1 mmol/l Na3VO4, 50 mmol/l NaF, 10 mmol/l sodium pyrophosphate, 2 μg/ml aprotinin, and 2 μg/ml leupeptin, pH 7.4), and lysates were centrifuged at 12,000 g for 10 min at 4°C. Supernatant protein concentrations were determined by bicinchoninic acid analysis (Pierce Chemical, Rockford, IL). Equal amounts of protein were resolved by SDS-PAGE and electrophoretically transferred to Hybond enhanced chemiluminescence nitrocellulose membrane (Amersham Pharmacia Biotech, Piscataway, NJ). Nonspecific binding sites were blocked with 5% bovine serum albumin in Tris-buffered saline (20 mM Tris·HCl, 137 mM NaCl, pH 7.6) with 0.1% Tween 20 for 1 h at room temperature. Membranes were probed with appropriate primary and secondary antibodies. Bands were visualized using enhanced chemiluminescence (Amersham Pharmacia Biotech) and analyzed with a Kodak Image Station 440CF. Membranes were then stripped and reprobed with appropriate primary and secondary antibodies specific for total protein. All exposures used for densitometric analysis were within the linear range.
Isolation of RNA from mucosal cells.
Total RNA was isolated from the IEC cells using RNA-STAT solution (Tel Test, Friendswood, TX) according to the manufacturer's instructions. The total RNA was treated with DNase I (Invitrogen) to remove contaminating genomic DNA. DNase I-treated RNA was purified using RNeasy Mini Kit (Qiagen, Valencia, CA). RNA concentration was measured spectrophotometrically at optical density (OD) 260.
RT-PCR.
The two-step RT-PCR was performed by using the GeneAmp Gold RNA PCR Kit (Applied Biosystems, Foster City, CA). Briefly, 1 μg of purified RNA was reverse transcribed in the presence of 2.5 mM MgCl2, 1× RT-PCR buffer, 1 mM dNTPs, 10 mM dithiothreitol, 10 U RNase inhibitor, 1.25 μM random hexamers, and 15 U Multiscribe Reverse Transcriptase in a final reaction volume of 20 μl. The components were mixed, briefly spun down, and incubated at 25°C, 10 min for hybridization; then reactions were carried out at 42°C for 15 min in a Gene Amp PCR system 9600 (Perkin-Elmer, Foster City, CA) and cooled to 4°C. The RT reactions were subjected to PCR amplification. Five microliters of cDNA products were amplified with 2.5 U of Ampli Taq Gold Polymerase (Applied Biosystems), 1× RT-PCR buffer, 1.75 mM MgCl2, 0.8 mM dNTPs, 0.15 μM upstream primers, and 0.15 μM downstream primers in final concentration. Reactions were carried out in the Gene Amp PCR system 9600. Reactions were performed for 10 min at 95°C for activated AmpliTaq Gold DNA Polymerase, then for 20 s at 94°C, and then for 60 s at 62°C for 40 cycles for amplification of the target gene. The rat Schlafen 3 primers used were 5′-ATTCTGCTGTGCAGTGTTCG-3′ (upstream) and 5′-TTGCTTGGAGAAACATGCTG-3 (downstream). The β-actin primers used were 5′-CCCAGCACAATGAAGATCAA-3′ (upstream) and 5′-ACATCTGCTGGAAGGTGGAC-3′ (downstream).
siRNA transfection.
Thirty-forty percent confluent IEC-6 cells were transfected with nontargeting siRNA (NT1), or siRNA to Schlafen 3 (Dharmacon, Lafayette, CO) as described previously (10). Protein reduction (routinely 70–90%) was confirmed by parallel Western blots.
Brush-border enzyme activity assay.
DPPIV activity was measured spectrophotometrically by substrate digestion of H-Gly-Pro-pNA·p-tosylate (Bachem, Torrance, CA), in protein-matched cellular lysates as quantitated by BCA (Pierce) as previously described (5). Standard DPPIV enzyme was purchased from Sigma.
Proliferation assay.
Proliferation assays were performed as previously described (10). Briefly, IEC-6 cells were plated on collagen-coated Flexwell plates at 10% confluence, cultured overnight, and then transfected with nontargeting siRNA (NT1), or siRNA to Schlafen 3. On the following day, cells were serum deprived for 6 h with 1 plate reserved for a time 0 measurement. The remaining serum-starved cells were switched back to normal growth medium with 10% FBS under static or repetitive strain conditions, or incubate without or with EGF for 24 h. Cells were then fixed and stained with crystal violet for dye elution assay. Using 10% acetic acid, dye was extracted, and absorbance at 550 nm was measured using a Thermomax microplate reader (Molecular Devices, Ramsey, MN). Each experiment contained six observations.
Statistics.
All data are expressed as means ± SE of at least three independent similar experiments. Statistical analysis was performed using paired or unpaired t-tests or analysis of variance as appropriate. P < 0.05 was considered statistically significant.
RESULTS
Strain increased both Schlafen 3 protein and mRNA.
To determine whether strain increases Schlafen 3 protein and mRNA in IEC-6 cells, we subjected confluent serum-deprived cells to cyclic strain for 24 h and assayed Schlafen 3 protein levels by Western blotting and gene expression by RT-PCR. Cyclic strain increased Schlafen 3 protein and mRNA levels to 1.73 ± 0.20 (n = 4, P < 0.05) and 1.92 ± 0.11 (n = 7, P < 0.05), respectively (Fig. 1, A and B). Furthermore, Schlafen 3 mRNA increased in a time-dependent fashion in response to strain. Cyclic strain for 0–48 h increased Schlafen 3 expression, with a maximal observed increase in expression of 2.46 ± 0.31 at the 12-h time point (Fig. 1C, n = 4, P < 0.05). We chose to study 24 h of an average 10% deformation at 10 cycles per minute as our primary stimulus because we have previously demonstrated that this stimulus modulates brush-border enzyme activity in human intestinal Caco-2 cell monolayers (5). These parameters are similar in magnitude and frequency to those observed during normal intestinal peristalsis (18) and villous motility (72).
Fig. 1.
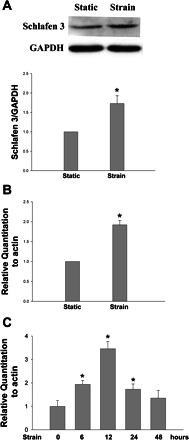
A: intestinal epithelial cell (IEC)-6 monolayers were subjected to cyclic strain for 24 h after confluence. Cells were lysed in buffer and equal protein aliquots resolved by SDS-PAGE, followed by Western analysis. Strain increased Schlafen 3 protein to 1.92 ± 0.11 (n = 7, *P < 0.05). Bars represent densitometric analysis; representative Western blots are shown above the graph. B: confluent IEC-6 monolayers were strained for 24 h. Total RNA was extracted followed by real-time RT-PCR analysis. Strain increased Schlafen 3 mRNA to 1.73 ± 0.20 (n = 4, *P < 0.05). C: Schlafen 3 mRNA increases in a time-dependent fashion in response to strain (n = 4, *P < 0.05).
Reducing Schlafen 3 by siRNA inhibited the stimulation of DPPIV activity and villin expression by strain.
We reduced Schlafen 3 protein by a specific siRNA before strain and examined DPPIV activity by DPPIV activity assay. Schlafen 3 levels were reduced by >70% 72 h after treatment before application of strain (Fig. 2A, n = 3). DPPIV enzymatic-specific activity was increased in lysate from IEC-6 cells exposed to cyclic strain for 24 h to 1.63 ± 0.09 compared with cells not exposed to strain for this period (Fig. 2B, n = 6, P < 0.05), similar in magnitude to previously reported effects of other differentiating stimuli in IEC-6 cells (37) as well as to the effects of cyclic strain in Caco-2 cells (5). Schlafen 3 reduction by >70% using a specific siRNA decreased basal DPPIV to 0.35 ± 0.05 (Fig. 2B, n = 6, P < 0.05) and prevented any stimulation of DPPIV activity by strain. Exposure to strain also increased villin protein levels to 1.34 ± 0.10, and this effect was also eliminated by Schlafen 3 reduction (Fig. 2C, n = 4, P < 0.05). Other differentiating stimuli in IEC-6 cells have similar magnitude effects on villin (64, 70).
Fig. 2.
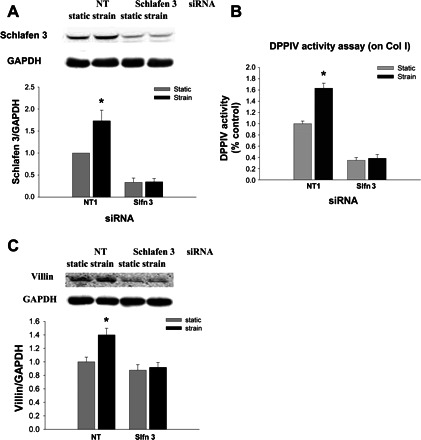
Reducing Schlafen 3 by siRNA inhibited the stimulation of dipeptidyl dipeptidase (DPPIV) activity and villin expression by strain. Schlafen 3 protein abundance was substantially reduced 72 h after transfection with siRNA to Schlafen 3. A: typical immunoblot showing Schlafen 3 protein level reduction in cells transiently transfected with siRNA targeted to Schlafen 3 compared with cells transfected with a nontargeting sequence (NT1) (A; 1 of 3 similar experiments). B: cells were plated on collagen I and transiently transfected with siRNA targeted to Schlafen 3 or with nontargeting NT1 sequences before lysis and DPPIV-specific activity assay. Strain increased DPPIV activity to 1.63 ± 0.09 (B; n = 6, *P < 0.05). Reducing Schlafen 3 prevented any stimulation of DPPIV activity by strain compared with unstretched cells, while basal activity lowered to 0.35 ± 0.05 (B; n = 6, *P < 0.05), strongly suggesting that Schlafen 3 mediates the strain effect on DPPIV stimulation. C: to determine the role of Schlafen 3 in villin expression following strain, we transiently transfected cells plated on collagen I with siRNA targeted to Schlafen 3 or with nontargeting NT1 sequences for 72 h before strain and assessed villin expression by Western blot. Schlafen 3 siRNA moderately reduced villin levels and eliminated the strain effect (n = 4, *P < 0.05).
Schlafen-3 reduction by siRNA also inhibited the stimulation of DPPIV activity by sodium butyrate or TGF-β.
Reducing Schlafen 3 by siRNA also prevented DPPIV induction by sodium butyrate (1 mM) or transforming growth factor (TGF)-β (0.1 ng/ml), two unrelated differentiating stimuli. Induction of DPPIV activity by sodium butyrate and TGF-β was significant in IEC-6 cells (n = 4, P < 0.01, Fig. 3, A and D). DPPIV activity induced by strain can be further increased by sodium butyrate, suggesting that sodium butyrate induces differentiation by a different mechanism from strain (n = 3, P < 0.05, Fig. 3B). These results further suggest the potential importance of Schlafen 3 in the regulation of intestinal epithelial differentiation because different differentiating stimuli each require Schlafen 3 for activity. We then treated IEC-6 cells with sodium butyrate (1 mM) or TGF-β (0.1 ng/ml) after transiently transfected cells with Schlafen 3-specific siRNA. DPPIV activity was increased by sodium butyrate (1 mM) in NT1-treated cells to 1.82 ± 0.09 (n = 5, P < 0.05), but this effect was completely blocked by transfection with Schlafen 3 siRNA, as shown in Fig. 3C. DPPIV activity was increased by TGF-β (0.1 ng/ml) in NT1-treated cells to 1.41 ± 0.04 (n = 3, P < 0.05); however, the effect of TGF-β on DPPIV activity was eliminated in cells transfected with siRNA to Schlafen 3 (n = 3, P < 0.05 Fig. 3E).
Fig. 3.
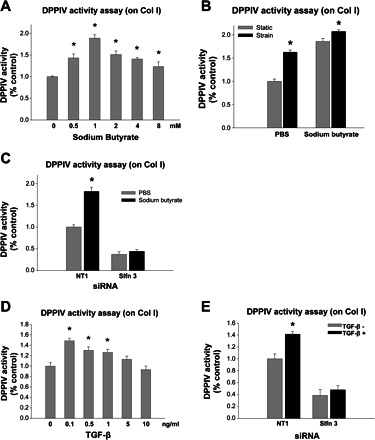
Schlafen-3 reduction by siRNA also inhibited the stimulation of DPPIV activity by 24-h treatment with sodium butyrate (1 mM) or transforming growth factor (TGF)-β (0.1 ng/ml). A: sodium butyrate, a differentiating agent, stimulated DPPIV activity in IEC-6 cells plated on collagen I (Col I) (n = 4, *P < 0.01). B: DPPIV activity was increased by strain and sodium butyrate (1 mM), indicating that sodium butyrate induces differentiation by a different mechanism from strain (n = 3; *P < 0.05). C: cells plated on collagen I and transiently transfected with siRNA targeted to Schlafen 3 (Slfn 3) or nontargeting NT1 sequences were treated with sodium butyrate (1 mM) before lysis and assay for DPPIV-specific activity. DPPIV activity was increased by sodium butyrate (1 mM) to 1.82 ± 0.09 in NT1-treated cells, but this effect was completely blocked by transfection with Schlafen 3 siRNA (n = 5, *P < 0.05). D: TGF-β, another well-characterized differentiating agent, stimulated DPPIV activity in IEC-6 cells plated on collagen I (n = 4, *P < 0.01). E: in IEC-6 cells plated on collagen I, addition of TGF-β stimulated DPPIV to 1.41 ± 0.04, and this effect was similarly prevented by reducing Schlafen 3 with siRNA (n = 3; *P < 0.05).
Schlafen-3 reduction by siRNA did not inhibit strain or EGF-induced proliferation in IEC-6 cells.
We have previously shown that cyclic strain also stimulates IEC-6 cell proliferation. However, Schlafen-3 reduction by siRNA did not prevent the mitogenic effect of strain or that of EGF (n = 3, P < 0.05 for each), as shown in Fig. 4.
Fig. 4.
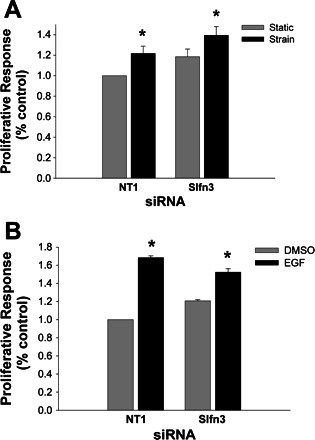
Schlafen 3 reduction did not modulate intestinal epithelial proliferation induced by strain or EGF. A: IEC-6 cells transiently transfected with siRNA targeted to Schlafen 3 or with a nontargeting NT1 sequence were maintained under static conditions or cyclic strain for 24 h before crystal violet staining. Strain stimulated proliferation in both NT1-treated cells and cells in which Schlafen 3 was reduced (n = 3, *P < 0.05). IEC-6 cell monolayers exposed to cyclic strain for 24 h display significantly increased cell numbers after strain initiation compared with static control cell monolayers at the same time point, as assessed by a colorimetric assay using crystal violet absorbance assay over the linear range of the assay, with interpolation against a standard curve. However, reducing Schlafen 3 by siRNA could not block this strain effect. B: incubation IEC-6 cells with 10 pM EGF for 24 h resulted in a 1.68 ± 0.02-fold (n = 3, *P < 0.05) increase in proliferation. However, reduction of Schlafen 3 by transient transfection with siRNA targeted to Schlafen 3 also did not block EGF-induced IEC-6 proliferation compared with static control.
The induction of Schlafen 3 protein by strain was prevented by blocking Src, p38, or PI3-K, but not ERK.
Although the upstream mediators by which strain influences differentiation are not known, we have previously described activation of Src, ERK, p38, and phosphatidyl inositol (PI3)-kinase by strain and shown that Src, ERK, and PI3-kinase mediate the mitogenic effect of strain, which is independent of p38 activation (10, 20). We therefore examined strain stimulation of Schlafen 3 protein levels in the absence or presence of PP2 (10 μM), PD98059 (20 μM), SB203580 (10 μM), and LY294002 (20 μM), which block Src, ERK, p38, and PI3-kinase, respectively. To confirm that the compounds were actually inhibiting Src, p38, and PI3-kinase activity, respectively, we took advantage of previous studies demonstrating that phosphorylation of FAK Y576, Hsp27 accumulation, and phosphorylation of Akt are downstream consequences of Src (12, 62), p38 (29, 34, 48), and PI3-kinase(27, 45) activation, respectively, in a variety of cell types. PD98059 is a specific MAPK/ERK kinase inhibitor that prevents downstream activation of ERK1 and ERK2 in response to various stimuli in diverse cell types (59, 74), so ERK phosphorylation served as a marker for efficacy for the PD98059 compound. We confirmed that all the agents used actually inhibited the activity of their target kinases (Fig. 5, A, B, C, and D, lower two blots for each, 1 of 3 similar experiments for each). Blocking Src or PI3-kinase prevented strain induction of Schlafen 3, similar to previous observations that Src and PI3-kinase are required for the mitogenic effects of strain in IEC lines (10, 20). However, Schlafen 3 induction required activation of p38 but not ERK in contrast to the mitogenic effects of strain, which requires ERK but not p38 (9, 10) (Fig. 5, A, B, C, and D; n = 4; P < 0.05 for each). Parallel RT-PCR studies confirmed that strain stimulation of Schlafen 3 gene expression was also prevented by Src blockade (Fig. 5E, n = 7, P < 0.01). These results suggest that cyclic strain modulates intestinal epithelial Schlafen 3 expression via Src, p38, and PI3-kinase activation and in particular identify for the first time a role for p38 induction by strain in IECs on a collagen substrate.
Fig. 5.
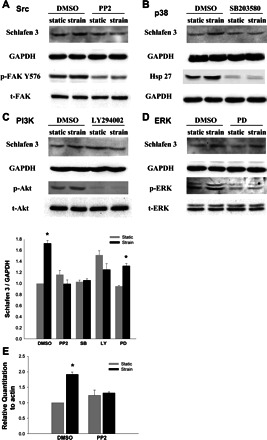
Cyclic strain (24 h) induction of Schlafen 3 protein was blocked by 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2; 10 μM) (A), SB203580 (SB) (10 μM) (B), or LY294002 (LY) (20 μM) (C), which block Src, p38, and phosphatidyl inositol (PI3)-K, but not by PD98059 (20 μM) (PD) (D), which blocks ERK (n = 4, *P < 0.05). p-FAK, phosphorylated FAK; t-FAK, total FAK. Each inhibitor significantly blocked basal as well as strain-stimulated phosphorylation or accumulation of the specific downstream target of each kinase (n = 3, lower two blots for each, *P < 0.05). E: Strain stimulation of Schlafen 3 expression was prevented by Src blockade with PP2 (10 μM) vs. actin mRNA (n = 7, *P < 0.01). FAK, focal adhesion kinase.
Strain did not induce Schlafen 3 expression or DPPIV activity in IEC-6 cells cultured on fibronectin.
We have previously reported that strain effect on cell biology is matrix dependent. Strain stimulates intestinal epithelial proliferation on collagen substrates but stimulates migration across fibronectin (10, 20, 76). The matrix dependence of the differentiating effects of strain on IECs has not previously been investigated. Therefore, we sought to determine whether strain effect on Schlafen 3 is similarly affected by different matrix substrates. We exposed normal small IEC-6 cells cultured on collagen or fibronectin to strain for 24 h and measured Schlafen 3 expression by Western blotting. Induction of Schlafen 3 expression across collagen by strain was statistically significant. In contrast, no stimulation of Schlafen 3 expression by strain was observed in cells cultured on fibronectin (n = 4, P < 0.05, Fig. 6A). In further studies, IEC-6 cells grown to confluence on either collagen or fibronectin were subjected to 24 h of strain, and DPPIV specific activity was measured. We observed that fibronectin slightly decreased basal DPPIV activity and prevented any stimulation of DPPIV activity by strain in contrast to collagen (n = 5, P < 0.01, Fig. 6B) and in parallel with our observations of the matrix dependence of the effects of strain on Schlafen 3.
Fig. 6.
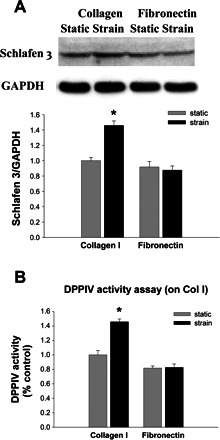
Strain increased Schlafen 3 protein and DPPIV activity on collagen but not on fibronectin. A: IEC-6 cells cultured on membranes coated with collagen or fibronectin were maintained under static conditions or repetitive deformation for 24 h before lysis and Western blot for Schlafen 3 protein. Schlafen 3 expression of IEC-6 cells was different in response to strain on collagen compared with cells on fibronectin. Induction of Schlafen 3 expression across collagen by strain was statistically significant. In contrast, little stimulation of Schlafen 3 expression by strain was observed in cells cultured on fibronectin (n = 4; *P < 0.05). B: DPPIV activity was determined by DPPIV activity assay in IEC-6 cells subjected to 24 h of strain after grown to confluence on either collagen or fibronectin. Fibronectin slightly decreased basal DPPIV activity and prevented any stimulation of DPPIV activity by strain in contrast to collagen (n = 5; *P < 0.01).
DISCUSSION
The gut mucosa repetitively experiences mechanical forces causing strain and pressure, including villus motility, contact with luminal contents, and peristalsis (28, 72). Although intestinal epithelial deformation in vivo is complex, we chose to study the effects on cultured nontransformed IEC-6 cells of a regular and rhythmic deformation pattern of physiologically relevant amplitude and frequency to facilitate reproducible analysis at acute time points. We found that repetitive strain induced an absorptive phenotype characterized by increased DPPIV activity and increased villin expression via induction of Schlafen 3 in rat IEC-6 cells on collagen. However, strain failed to induce Schlafen 3 or increase DPPIV activity on a fibronectin substrate, indicating that the strain effect on differentiation is somehow matrix dependent. Cyclic strain modulated intestinal epithelial Schlafen 3 expression via Src, p38, and PI3-kinase activation. Furthermore, Schlafen 3 might also be a key factor in the induction of intestinal epithelial differentiation by other stimuli such as sodium butyrate or TGF-β. However, these effects are independent of the mitogenic effect of strain because Schlafen-3 reduction by siRNA did not inhibit strain-induced proliferation in IEC-6 cells.
Considerable evidence indicates that cyclic mechanical deformation provides physical signals for differentiation in many cell types that experience strain normally in vivo (25, 31, 32, 43, 63, 69). Although cell differentiation is difficult to study in vivo, intestinal lines that reproducibly modulate brush-border enzyme expression permit the modeling of this process in culture. The expression of active brush-border enzymes and microvilli-specific structural protein has proven useful markers of differentiation of IECs. We have previously reported that brush-border enzyme DPPIV is amplitude dependently stimulated by strain in Caco-2 cells (5) and here confirm this observation in nonmalignant IEC-6 cells. Although we measured only DPPIV-specific activity in this study, we have previously reported that changes in DPPIV protein levels in response to cyclic strain correlate with DPPIV specific activity (5). In addition, we demonstrate here that strain affects the expression of another differentiation marker, the microvilli-specific protein villin, similarly.
Although the mechanism of strain-induced differentiation is poorly understood, our data suggest that Schlafen 3 expression stimulated by strain plays a critical role in regulating this strain effect, and Schlafen 3 might even be a common effector of differentiating agents such as butyrate (38, 40, 56) and TGF-β (26, 58, 71). Stimulation of Schlafen 3 expression was associated with increased activity of DPPIV and increased expression of villin, whereas transfection of IECs with siRNA to Schlafen 3 markedly attenuated those two, indicating a relationship between Schlafen 3 and intestinal epithelial differentiation. In contrast to the effects of strain on differentiation, Schlafen 3 was not required for the mitogenic effect of either strain or EGF despite many reports that Schlafen family members serve as negative regulators of growth (6, 52, 61). Indeed, Schlafen 1 and Schlafen 2 were also found to not regulate proliferative activities in vitro in murine fibroblasts or myeloid cell lines (77).
Although we have studied collagen I here, previous studies demonstrated that strain affects IECs similarly on type I and type IV collagen substrates (3). Interestingly, although the effects of strain on the differentiation of the intestinal epithelial cells cultured on collagen substrates had previously been studied (5), the effects of strain on the differentiation of intestinal epithelial cells cultured on fibronectin had not previously been investigated. Strain was unable to induce Schlafen 3 or stimulate DPPIV activity on fibronectin, consistent with the model that Schlafen 3 induction is required for strain to induce IEC-6 intestinal epithelial differentiation and suggesting that strain-induced differentiation is matrix dependent similarly to strain-induced proliferation (which also occurs on collagen or laminin but not fibronectin) but inversely to strain-induced migration (which occurs on fibronectin but not collagen) (8, 10, 11, 19, 20, 75, 76). This may be important because fibronectin deposition is characteristic of gut inflammatory states characterized by chronic mucosal injury (68) in which the sealing of breaks in the mucosal barrier by restitution may be more critical than enterocytic differentiation.
We have previously reported that PI3-kinase, Src, ERK, and P38 are activated in IECs subjected to repetitive mechanical strain on collagen substrates (9, 10, 20, 44). However, PI3-kinase, Src, and ERK had previously been implicated in the regulation of intestinal epithelial proliferation by strain (10, 20), whereas the role of p38 in mediating physical force effects in IECs is not yet well understood. Our present results suggest that strain-associated p38 activation plays an important role in mediating the effects of cyclic strain on the expression of differentiation genes in IEC-6 cells independently of mitogenic ERK signaling. Activation of specific MAPKs by mechanical strain has also been shown to be involved in phenotypic modulation in some other mechanosensitive cell types including vascular smooth muscle cells (54, 66) and osteoblasts (22, 35). However, the role of p38 in mediating strain effects seems likely to be cell-type and matrix specific. In human bone marrow stromal cells, ERK and p38 are each activated in response to strain, but neither seems required for strain-induced enhanced differentiation (15). In contrast, p38 activation in response to strain in embryonic stem cells is required for strain-stimulated differentiation (60). Moreover, p38 stimulates differentiation in intestinal cells subjected to some other stimuli as well, such as sodium butyrate (16) and oligosaccharides (39). Our observation that p38 inhibitor SB203580 blocks strain-induced Schlafen 3 expression suggests a possible role for p38 in intestinal differentiation via Schlafen 3, whereas the different roles of p38 in mediating strain effects in other cell types likely reflect the different regulatory machinery of these other cells.
We additionally demonstrated that blocking PI3-kinase by LY294002 or blocking Src by PP2 each prevented induction of Schlafen 3 by strain. This observation is consistent with previous suggestions that PI3-kinase activation is required for the stimulation of p38 MAPK during chondrogenesis of mesenchymal cells (50), and PP2 regulates human trophoblast cells differentiation by activating p38 (13). Thus it would appear then that repetitive deformation is an important trophic factor for IECs that promote a differentiated phenotype.
In conclusion, cyclic mechanical strain applied to cells plated on collagen activated cell signals necessary for cell differentiation in a matrix-dependent fashion. The induction of Schlafen 3 or its human homologs by strain may modulate intestinal epithelial differentiation and preserve the gut mucosa during normal gut function.
GRANTS
This work was supported in part by a VA Merit award (M. Basson).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Arpin M, Blair L, Coudrier E, Dudouet B, Finidori J, Carcia A, Huet C, Pringault E, Robine S, Sahuguillo-Merino C, Louvard D. Villin, a specific marker for some epithelia specialized in transport, to study the differentiation of intestinal and kidney cells in vivo and in a human colon adenocarcinoma line HT29 in culture. Mol Aspects Med 10: 257–272, 1988. [DOI] [PubMed] [Google Scholar]
- 2. Arscott WT, LaBauve AE, May V, Wesley UV. Suppression of neuroblastoma growth by dipeptidyl peptidase IV: relevance of chemokine regulation and caspase activation. Oncogene 28: 479–491, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Basson MD. Invited research review: cell-matrix interactions in the gut epithelium. Surgery 133: 263–267, 2003. [DOI] [PubMed] [Google Scholar]
- 4. Basson MD, Emenaker NJ, Rashid Z. Effects of modulation of tyrosine phosphorylation on brush border enzyme activity in human Caco-2 intestinal epithelial cells. Cell Tissue Res 292: 553–562, 1998. [DOI] [PubMed] [Google Scholar]
- 5. Basson MD, Li GD, Hong F, Han O, Sumpio BE. Amplitude-dependent modulation of brush border enzymes and proliferation by cyclic strain in human intestinal Caco-2 monolayers. J Cell Physiol 168: 476–488, 1996. [DOI] [PubMed] [Google Scholar]
- 6. Brady G, Boggan L, Bowie A, O'Neill LA. Schlafen-1 causes a cell cycle arrest by inhibiting induction of cyclin D1. J Biol Chem 280: 30723–30734, 2005. [DOI] [PubMed] [Google Scholar]
- 7. Bustos O, Naik S, Ayers G, Casola C, Perez-Lamigueiro MA, Chippindale PT, Pritham EJ, de la Casa-Esperon E. Evolution of the Schlafen genes, a gene family associated with embryonic lethality, meiotic drive, immune processes and orthopoxvirus virulence. Gene 447: 1–11, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chaturvedi LS, Gayer CP, Marsh HM, Basson MD. Repetitive deformation activates Src-independent FAK-dependent ERK mitogenic signals in human Caco-2 intestinal epithelial cells. Am J Physiol Cell Physiol 294: C1350–C1361, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaturvedi LS, Marsh HM, Basson MD. Src and focal adhesion kinase mediate mechanical strain-induced proliferation and ERK1/2 phosphorylation in human H441 pulmonary epithelial cells. Am J Physiol Cell Physiol 292: C1701–C1713, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Chaturvedi LS, Marsh HM, Shang X, Zheng Y, Basson MD. Repetitive deformation activates focal adhesion kinase and ERK mitogenic signals in human Caco-2 intestinal epithelial cells through Src and Rac1. J Biol Chem 282: 14–28, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Chaturvedi LS, Saad SA, Bakshi N, Marsh HM, Basson MD. Strain matrix-dependently dissociates gut epithelial spreading and motility. J Surg Res 156: 217–223, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ciccimaro E, Hanks SK, Blair IA. Quantification of focal adhesion kinase activation loop phosphorylation as a biomarker of Src activity. Mol Pharmacol 75: 658–666, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daoud G, Le Bellego F, Lafond J. PP2 regulates human trophoblast cells differentiation by activating p38 and ERK1/2 and inhibiting FAK activation. Placenta 29: 862–870, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Darmoul D, Lacasa M, Baricault L, Marguet D, Sapin C, Trotot P, Barbat A, Trugnan G. Dipeptidyl peptidase IV (CD 26) gene expression in enterocyte-like colon cancer cell lines HT-29 and Caco-2. Cloning of the complete human coding sequence and changes of dipeptidyl peptidase IV mRNA levels during cell differentiation. J Biol Chem 267: 4824–4833, 1992. [PubMed] [Google Scholar]
- 15. Diederichs S, Freiberger F, van Griensven M. Effects of repetitive and short time strain in human bone marrow stromal cells. J Biomed Mater Res A 88: 907–915, 2009. [DOI] [PubMed] [Google Scholar]
- 16. Ding Q, Wang Q, Evers BM. Alterations of MAPK activities associated with intestinal cell differentiation. Biochem Biophys Res Commun 284: 282–288, 2001. [DOI] [PubMed] [Google Scholar]
- 17. Dudouet B, Robine S, Huet C, Sahuquillo-Merino C, Blair L, Coudrier E, Louvard D. Changes in villin synthesis and subcellular distribution during intestinal differentiation of HT29–18 clones. J Cell Biol 105: 359–369, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Froehlich JM, Patak MA, von Weymarn C, Juli CF, Zollikofer CL, Wentz KU. Small bowel motility assessment with magnetic resonance imaging. J Magn Reson Imaging 21: 370–375, 2005. [DOI] [PubMed] [Google Scholar]
- 19. Gayer CP, Chaturvedi LS, Wang S, Alston B, Flanigan TL, Basson MD. Delineating the signals by which repetitive deformation stimulates intestinal epithelial migration across fibronectin. Am J Physiol Gastrointest Liver Physiol 296: G876–G885, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gayer CP, Chaturvedi LS, Wang S, Craig DH, Flanigan T, Basson MD. Strain-induced proliferation requires the phosphatidylinositol 3-kinase/AKT/glycogen synthase kinase pathway. J Biol Chem 284: 2001–2011, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Geserick P, Kaiser F, Klemm U, Kaufmann SH, Zerrahn J. Modulation of T cell development and activation by novel members of the Schlafen (slfn) gene family harbouring an RNA helicase-like motif. Int Immunol 16: 1535–1548, 2004. [DOI] [PubMed] [Google Scholar]
- 22. Granet C, Boutahar N, Vico L, Alexandre C, Lafage-Proust MH. MAPK and SRC-kinases control EGR-1 and NF-kappa B inductions by changes in mechanical environment in osteoblasts. Biochem Biophys Res Commun 284: 622–631, 2001. [DOI] [PubMed] [Google Scholar]
- 23. Gwak SJ, Bhang SH, Kim IK, Kim SS, Cho SW, Jeon O, Yoo KJ, Putnam AJ, Kim BS. The effect of cyclic strain on embryonic stem cell-derived cardiomyocytes. Biomaterials 29: 844–856, 2008. [DOI] [PubMed] [Google Scholar]
- 24. Hahn HP, Blount PL, Ayub K, Das KM, Souza R, Spechler S, Odze RD. Intestinal differentiation in metaplastic, nongoblet columnar epithelium in the esophagus. Am J Surg Pathol 33: 1006–1015, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hayward LN, Morgan EF. Assessment of a mechano-regulation theory of skeletal tissue differentiation in an in vivo model of mechanically induced cartilage formation. Biomech Model Mechanobiol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heldin CH, Landstrom M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol 21: 166–176, 2009. [DOI] [PubMed] [Google Scholar]
- 27. Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 4: 988–1004, 2005. [DOI] [PubMed] [Google Scholar]
- 28. Hennig GW, Costa M, Chen BN, Brookes SJ. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J Physiol 517: 575–590, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hirade K, Kozawa O, Tanabe K, Niwa M, Matsuno H, Oiso Y, Akamatsu S, Ito H, Kato K, Katagiri Y, Uematsu T. Thrombin stimulates dissociation and induction of HSP27 via p38 MAPK in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 283: H941–H948, 2002. [DOI] [PubMed] [Google Scholar]
- 30. Ho SB. Cytoskeleton and other differentiation markers in the colon. J Cell Biochem Suppl 16G: 119–128, 1992. [DOI] [PubMed] [Google Scholar]
- 31. Huang CH, Chen MH, Young TH, Jeng JH, Chen YJ. Interactive effects of mechanical stretching and extracellular matrix proteins on initiating osteogenic differentiation of human mesenchymal stem cells. J Cell Biochem 108: 1263–1273, 2009. [DOI] [PubMed] [Google Scholar]
- 32. Hwang IK, Kim IY, Kim DW, Yoo KY, Kim YN, Yi SS, Won MH, Lee IS, Yoon YS, Seong JK. Strain-specific differences in cell proliferation and differentiation in the dentate gyrus of C57BL/6N and C3H/HeN mice fed a high fat diet. Brain Res 1241: 1–6, 2008. [DOI] [PubMed] [Google Scholar]
- 33. Imai K, Maeda M, Fujiwara H, Kariya M, Takakura K, Kanzaki H, Mori T. Dipeptidyl peptidase IV as a differentiation marker of the human endometrial glandular cells. Hum Reprod 7: 1189–1194, 1992. [DOI] [PubMed] [Google Scholar]
- 34. Ito T, Kozawa O, Tanabe K, Niwa M, Matsuno H, Sakai N, Ito H, Kato K, Uematsu T. p38 MAP kinase is required for vasopressin-stimulated HSP27 induction in aortic smooth muscle cells. Hypertension 35: 673–678, 2000. [DOI] [PubMed] [Google Scholar]
- 35. Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T. Mechanical stress-mediated Runx2 activation is dependent on Ras/ERK1/2 MAPK signaling in osteoblasts. J Cell Biochem 101: 1266–1277, 2007. [DOI] [PubMed] [Google Scholar]
- 36. Kato M, Kusumi T, Tsuchida S, Tanaka M, Sasaki M, Kudo H. Induction of differentiation and peroxisome proliferator-activated receptor gamma expression in colon cancer cell lines by troglitazone. J Cancer Res Clin Oncol 130: 73–79, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kolinska J, Lisa V, Clark JA, Kozakova H, Zakostelecka M, Khailova L, Sinkora M, Kitanovicova A, Dvorak B. Constitutive expression of IL-18 and IL-18R in differentiated IEC-6 cells: effect of TNF-alpha and IFN-gamma treatment. J Interferon Cytokine Res 28: 287–296, 2008. [DOI] [PubMed] [Google Scholar]
- 38. Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem 42: 65–82, 1982. [DOI] [PubMed] [Google Scholar]
- 39. Kuntz S, Kunz C, Rudloff S. Oligosaccharides from human milk induce growth arrest via G2/M by influencing growth-related cell cycle genes in intestinal epithelial cells. Br J Nutr 101: 1306–1315, 2009. [DOI] [PubMed] [Google Scholar]
- 40. Langdon SP, Hawkes MM, Hay FG, Lawrie SS, Schol DJ, Hilgers J, Leonard RC, Smyth JF. Effect of sodium butyrate and other differentiation inducers on poorly differentiated human ovarian adenocarcinoma cell lines. Cancer Res 48: 6161–6165, 1988. [PubMed] [Google Scholar]
- 41. Lee M, Hadi M, Hallden G, Aponte GW. Peptide YY and neuropeptide Y induce villin expression, reduce adhesion, and enhance migration in small intestinal cells through the regulation of CD63, matrix metalloproteinase-3, and Cdc42 activity. J Biol Chem 280: 125–136, 2005. [DOI] [PubMed] [Google Scholar]
- 42. Lee NK, Choi HK, Yoo HJ, Shin J, Lee SY. RANKL-induced schlafen2 is a positive regulator of osteoclastogenesis. Cell Signal 20: 2302–2308, 2008. [DOI] [PubMed] [Google Scholar]
- 43. Lee SK, Lee CY, Kook YA, Kim EC. Mechanical stress promotes odontoblastic differentiation via the heme oxygenase-1 pathway in human dental pulp cell line. Life Sci 86: 107–114, 2009. [DOI] [PubMed] [Google Scholar]
- 44. Li W, Duzgun A, Sumpio BE, Basson MD. Integrin and FAK-mediated MAPK activation is required for cyclic strain mitogenic effects in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol 280: G75–G87, 2001. [DOI] [PubMed] [Google Scholar]
- 45. Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell 4: 257–262, 2003. [DOI] [PubMed] [Google Scholar]
- 46. Maroux S, Coudrier E, Feracci H, Gorvel JP, Louvard D. Molecular organization of the intestinal brush border. Biochimie 70: 1297–1306, 1988. [DOI] [PubMed] [Google Scholar]
- 47. Moll R, Robine S, Dudouet B, Louvard D. Villin: a cytoskeletal protein and a differentiation marker expressed in some human adenocarcinomas. Virchows Arch B Cell Pathol Incl Mol Pathol 54: 155–169, 1987. [DOI] [PubMed] [Google Scholar]
- 48. Niwa M, Hotta K, Kanamori Y, Hatakeyama D, Hirade K, Katayama M, Hara A, Mori H, Ito H, Kato K, Matsuno H, Uematsu T, Kozawa O. Involvement of p38 mitogen-activated protein kinase in heat shock protein 27 induction in human neutrophils. Eur J Pharmacol 466: 245–253, 2003. [DOI] [PubMed] [Google Scholar]
- 49. Niwa T, Ikehara Y, Nakanishi H, Tanaka H, Inada K, Tsukamoto T, Ichinose M, Tatematsu M. Mixed gastric- and intestinal-type metaplasia is formed by cells with dual intestinal and gastric differentiation. J Histochem Cytochem 53: 75–85, 2005. [DOI] [PubMed] [Google Scholar]
- 50. Oh CD, Chun JS. Signaling mechanisms leading to the regulation of differentiation and apoptosis of particular chondrocytes by insulin-like growth factor-1. J Biol Chem 278: 36563–36571, 2003. [DOI] [PubMed] [Google Scholar]
- 51. Ott CE, Bauer S, Manke T, Ahrens S, Rodelsperger C, Grunhagen J, Kornak U, Duda G, Mundlos S, Robinson PN. Promiscuous and depolarization-induced immediate-early response genes are induced by mechanical strain of osteoblasts. J Bone Miner Res 24: 1247–1262, 2009. [DOI] [PubMed] [Google Scholar]
- 52. Patel BB, Yu Y, Du J, Rishi AK, Sarkar FH, Tarca AL, Wali A, Majumdar AP. Schlafen 3, a novel gene, regulates colonic mucosal growth during aging. Am J Physiol Gastrointest Liver Physiol 296: G955–G962, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Patel VB, Yu Y, Das JK, Patel BB, Majumdar AP. Schlafen-3: a novel regulator of intestinal differentiation. Biochem Biophys Res Commun 388: 752–756, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Qu MJ, Liu B, Wang HQ, Yan ZQ, Shen BR, Jiang ZL. Frequency-dependent phenotype modulation of vascular smooth muscle cells under cyclic mechanical strain. J Vasc Res 44: 345–353, 2007. [DOI] [PubMed] [Google Scholar]
- 55. Quaroni A, Tian JQ, Seth P, Ap Rhys C. p27(Kip1) is an inducer of intestinal epithelial cell differentiation. Am J Physiol Cell Physiol 279: C1045–C1057, 2000. [DOI] [PubMed] [Google Scholar]
- 56. Ren M, Yan L, Shang CZ, Cao J, Lu LH, Min J, Cheng H. Effects of sodium butyrate on the differentiation of pancreatic and hepatic progenitor cells from mouse embryonic stem cells. J Cell Biochem 109: 236–244, 2009. [DOI] [PubMed] [Google Scholar]
- 57. Rimondi E, Secchiero P, Quaroni A, Zerbinati C, Capitani S, Zauli G. Involvement of TRAIL/TRAIL-receptors in human intestinal cell differentiation. J Cell Physiol 206: 647–654, 2006. [DOI] [PubMed] [Google Scholar]
- 58. Romano MF. Targeting TGFbeta-mediated processes in cancer. Curr Opin Drug Discov Devel 12: 253–263, 2009. [PubMed] [Google Scholar]
- 59. Romerio F, Zella D. MEK and ERK inhibitors enhance the anti-proliferative effect of interferon-α2b. FASEB J 16: 1680–1682, 2002. [DOI] [PubMed] [Google Scholar]
- 60. Schmelter M, Ateghang B, Helmig S, Wartenberg M, Sauer H. Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. FASEB J 20: 1182–1184, 2006. [DOI] [PubMed] [Google Scholar]
- 61. Schwarz DA, Katayama CD, Hedrick SM. Schlafen, a new family of growth regulatory genes that affect thymocyte development. Immunity 9: 657–668, 1998. [DOI] [PubMed] [Google Scholar]
- 62. Shikata Y, Birukov KG, Birukova AA, Verin A, Garcia JG. Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: role of Src and GIT. FASEB J 17: 2240–2249, 2003. [DOI] [PubMed] [Google Scholar]
- 63. Silbert O, Wang Y, Maciejewski BS, Lee HS, Shaw SK, Sanchez-Esteban J. Roles of RhoA and Rac1 on actin remodeling and cell alignment and differentiation in fetal type II epithelial cells exposed to cyclic mechanical stretch. Exp Lung Res 34: 663–680, 2008. [DOI] [PubMed] [Google Scholar]
- 64. Soubeyran P, Andre F, Lissitzky JC, Mallo GV, Moucadel V, Roccabianca M, Rechreche H, Marvaldi J, Dikic I, Dagorn JC, Iovanna JL. Cdx1 promotes differentiation in a rat intestinal epithelial cell line. Gastroenterology 117: 1326–1338, 1999. [DOI] [PubMed] [Google Scholar]
- 65. Tan EY, Richard CL, Zhang H, Hoskin DW, Blay J. Adenosine downregulates DPPIV on HT-29 colon cancer cells by stimulating protein tyrosine phosphatase(s) and reducing ERK1/2 activity via a novel pathway. Am J Physiol Cell Physiol 291: C433–C444, 2006. [DOI] [PubMed] [Google Scholar]
- 66. Tock J, Van Putten V, Stenmark KR, Nemenoff RA. Induction of SM-alpha-actin expression by mechanical strain in adult vascular smooth muscle cells is mediated through activation of JNK and p38 MAP kinase. Biochem Biophys Res Commun 301: 1116–1121, 2003. [DOI] [PubMed] [Google Scholar]
- 67. van Lingen RG, Poll MK, Seyger MM, de Jong EM, van de Kerkhof PC, van Erp PE. Distribution of dipeptidyl-peptidase IV on keratinocytes in the margin zone of a psoriatic lesion: a comparison with hyperproliferation and aberrant differentiation markers. Arch Dermatol Res 300: 561–567, 2008. [DOI] [PubMed] [Google Scholar]
- 68. Verspaget HW, Biemond I, Allaart CF, van Weede H, Weterman IT, Gooszen HG, Pena AS, Lamers CB. Assessment of plasma fibronectin in Crohn's disease. Hepatogastroenterology 38: 231–234, 1991. [PubMed] [Google Scholar]
- 69. Wang Y, Maciejewski BS, Soto-Reyes D, Lee HS, Warburton D, Sanchez-Esteban J. Mechanical stretch promotes fetal type II epithelial cell differentiation via shedding of HB-EGF and TGF-alpha. J Physiol 587: 1739–1753, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang Z, Chen WW, Li RL, Wen B, Sun JB. Effect of gastrin on differentiation of rat intestinal epithelial cells in vitro. World J Gastroenterol 9: 1786–1790, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Watabe T, Miyazono K. Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Res 19: 103–115, 2009. [DOI] [PubMed] [Google Scholar]
- 72. Womack WA, Barrowman JA, Graham WH, Benoit JN, Kvietys PR, Granger DN. Quantitative assessment of villous motility. Am J Physiol Gastrointest Liver Physiol 252: G250–G256, 1987. [DOI] [PubMed] [Google Scholar]
- 73. Yoshioka M, Erickson RH, Matsumoto H, Gum E, Kim YS. Expression of dipeptidyl aminopeptidase IV during enterocytic differentiation of human colon cancer (Caco-2) cells. Int J Cancer 47: 916–921, 1991. [DOI] [PubMed] [Google Scholar]
- 74. Zelivianski S, Spellman M, Kellerman M, Kakitelashvilli V, Zhou XW, Lugo E, Lee MS, Taylor R, Davis TL, Hauke R, Lin MF. ERK inhibitor PD98059 enhances docetaxel-induced apoptosis of androgen-independent human prostate cancer cells. Int J Cancer 107: 478–485, 2003. [DOI] [PubMed] [Google Scholar]
- 75. Zhang J, Li W, Sanders MA, Sumpio BE, Panja A, Basson MD. Regulation of the intestinal epithelial response to cyclic strain by extracellular matrix proteins. FASEB J 17: 926–928, 2003. [DOI] [PubMed] [Google Scholar]
- 76. Zhang J, Owen CR, Sanders MA, Turner JR, Basson MD. The mitogenic effects of cyclic mechanical strain on intestinal epithelial monolayer wound closure are matrix dependent. Gastroenterology 131: 1179–1189, 2006. [DOI] [PubMed] [Google Scholar]
- 77. Zhao L, Neumann B, Murphy K, Silke J, Gonda TJ. Lack of reproducible growth inhibition by Schlafen1 and Schlafen2 in vitro. Blood Cells Mol Dis 41: 188–193, 2008. [DOI] [PubMed] [Google Scholar]


