Abstract
Objectives
Interstitial lung disease (ILD) is an extramuscular manifestation that results in increased morbidity and mortality from polymyositis (PM) and dermatomyositis (DM). The aim of this study was to systematically evaluate risk factors associated with the development of ILD in PM/DM.
Methods
Observational studies were identified from searching PubMed, Medline, Embase, and the Cochrane Library. Pooled odds ratios (ORs) or standardized mean differences (SMDs) and corresponding 95% confidence intervals (CIs) were obtained for the relationships between risk factors and ILD in PM/DM using either fixed- or random-effects models, whichever were appropriate. Heterogeneity tests, sensitivity analyses, and publication bias assessments were also performed.
Results
Twenty-three studies were selected for a meta-analysis that included 834 patients and 1245 control subjects. Risk factors that may have increased the risk of developing ILD in PM/DM patients included older age at diagnosis (SMD, 0.35; 95% CI, 0.18–0.52; P < 0.0001), arthritis/arthralgia (OR, 3.17; 95% CI, 1.99–5.04; P < 0.00001), fever (OR, 2.31; 95% CI, 1.42–3.76; P = 0.0007), presence of anti-Jo-1 antibodies (OR, 3.34; 95% CI, 2.16–5.16; P < 0.00001), elevated erythrocyte sedimentation rate (ESR; SMD, 0.48; 95% CI, 0.32–0.64; P < 0.00001), presence of anti-MDA5 antibodies (OR, 18.26; 95% CI, 9.66–34.51; P < 0.00001), and elevated C-reactive protein level (CRP; OR, 3.50; 95% CI, 1.48–8.28; P = 0.004). Meanwhile, malignancy (OR, 0.36; 95% CI, 0.18–0.72; P = 0.004) reduced the risk of developing ILD in PM/DM patients.
Conclusion
Our meta-analysis results suggest that the association between PM/DM and ILD may be due to such risk factors as older age at diagnosis, arthritis/arthralgia, fever, presence of anti-Jo-1 antibodies, elevated ESR, presence of anti-MDA5 antibodies, and elevated CRP level, while malignancy was associated with a reduced risk of developing ILD. Thus, these variables may be used to guide screening processes for ILD in patients with PM/DM.
Introduction
Idiopathic inflammatory myopathies (IIMs) are a heterogeneous group of rare inflammatory systemic disorders with a complicated etiopathogenesis. Polymyositis (PM) and dermatomyositis (DM) are systemic inflammatory diseases with unknown etiologies and prognoses that are characterized by varying degrees of muscle inflammation. PM and DM share similar features, with the exception that DM involves a characteristic heliotrope skin rash and Gottron’s papules [1]. Interstitial lung disease (ILD) is an extramuscular manifestation that contributes to increased morbidity and mortality in PM/DM patients when it is present at admission [2]. ILD has been reported in 19.9% to 78% of PM/DM cases [3]. The most common patterns of myositis-associated ILD histology in lung biopsy include nonspecific interstitial pneumonia, general interstitial pneumonia, organizing pneumonia, diffuse alveolar damage, and lymphocytic interstitial pneumonia [4].
Although the incidence of ILD associated with PM/DM has increased, the underlying pathogenesis remains unknown. Many studies have focused on the components of the cellular immune system for inducing ILD in IIMs. In PM, CD8+ T cells, CD68+ cells, and TNF-α+ cells are closely associated with muscular inflammation [5]. In contrast to DM, PM involves a significant increase in the number of CD4+ T and B cells in the perivascular areas of muscle tissue [6]. Moreover, in our recent research, we found that CD8+ T cells and CD68+ cells predominate in lung tissues in both PM and DM, which further confirms that the pathogenesis in lung tissues is similar between PM and DM, and might play a role in ILD development in PM/DM [7]. In the presence of ILD, bronchoalveolar lavage has consistently revealed lymphocytosis with a marked predominance of CD8+ T cells, which is associated with anti-Jo-1 autoantibody expression [8].
The quality of life of PM/DM patients is poor; hence, those at high risk of developing ILD should be promptly identified. Of the eight known anti-isoleucyl-tRNA synthetase antibodies, anti-Jo-1 antibody has been shown to be significantly associated with a high prevalence of myositis-related ILD, whereas anti-OJ antibody, anti-PL-12 antibody, and anti-KS antibody have been shown to confer the greatest risk of developing ILD in PM/DM patients [9]. Amyopathic DM (ADM) and clinical ADM (CADM) are defined as disorders that show typical skin manifestations of DM without evidence of clinical myositis [10]. The presence of anti-CADM-140 antibodies is implicated in individual mortality risk in DM patients with ILD. CADM patients, especially those positive for anti-MDA5 (melanoma differentiation-associated gene 5) antibodies, are known to develop acute, life-threatening, and progressive ILD frequently [11]. Some studies have shown that stereotypical clinical features, including age, fever, Raynaud’s phenomenon, and mechanic’s hands, increase the risk of developing ILD in PM/DM [12–14]. However, previous studies that investigated such correlating factors of ILD in DM/PM patients were limited in size and had conflicting results [15]. In the present study, we identified risk factors for ILD in patients with PM/DM and performed a meta-analysis of published observational studies to assess these factors.
Materials and Methods
Data Sources
We identified all relevant studies on ILD associated with PM/DM published before January 1, 2016 that were listed in four international scientific databases: PubMed, Medline, Embase, and the Cochrane Library. Searches were restricted to articles written in English. The following keywords and text words were used: “myositis” OR “inflammatory myopathy” OR “polymyositis” OR “dermatomyositis” combined with “interstitial lung disease” OR “ILD”. Relevant references cited in the original articles were also reviewed.
Study Selection and Data Extraction
Studies had to meet the following eligibility criteria: (1) were retrospective studies with detailed information about the ILD status of PM and DM patients; (2) included cases in accordance with a probable or definitive diagnosis of PM or DM based on Bohan and Peter’s criteria [16,17]; (3) considered all types of ILD based on the American Thoracic Society and European Respiratory Society’s classification [18]; (4) included more than 20 subjects; (5) included sufficient information to calculate odds ratios (ORs) with 95% confidence intervals (CIs) and standardized mean differences (SMD) for the risk factors; and (5) included at least one potential risk factor.
Studies were excluded if (1) they were cadaveric or biomechanical studies, reviews, expert opinions, case reports, or letters that were not published in full; (2) they lacked a control group or provided data by comparing the difference in ILD between PM and DM (lacking a control group of PM/DM without ILD); or (3) it was impossible to extract relevant data from the outcomes. For studies that were conducted by the same research group with similar subjects, we prioritized the higher-quality study.
Two investigators (LZ and GQW) independently reviewed each retrieved article. Disagreement between the two reviewers was resolved by discussion and consensus. The senior investigator (QW) confirmed the final results. Information was extracted on the first author; publication year; geographical region of the population; study design; number of subjects enrolled; number of women; mean age at diagnosis, alanine aminotransferase (ALT) level, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) level; and the number of patients with Gottron’s sign, heliotrope rash, arthritis/arthralgia, Raynaud’s phenomenon, dysphagia, malignancy, fever, antinuclear antibodies (ANAs), anti-Jo-1 antibodies, anti-MDA5 antibodies, and ILD. In addition, the quality of nonrandomized studies was assessed with the Newcastle-Ottawa scale for subject groups, comparability, and outcome. The selected studies were assigned a high, moderate, or low methodological quality with scores >6, 4–6, and <4, respectively (http://www.ohri.ca/programs/clinical_epi-demiology/oxford.asp).
Data Analysis
We combined trial results for estimating risk factors using Review Manager 5.3 (RevMan 2012, http://tech.cochrane.org/revman/). We presented results as summary ORs or SMDs with 95% CIs.
Between-study heterogeneity was tested with the Cochrane Q test and I2 statistics. A P value of <0.05 for the Cochrane Q test was considered to indicate significant heterogeneity. An I2 value of >50% was considered to indicate significant heterogeneity. We used the random-effects model to calculate the ORs (or SMDs) and 95% CIs [19]. Publication bias was estimated with the Begg’s and Egger’s tests. A P value of <0.05 was considered statistically significant (Stata SE software, StataCorp, College Station, Texas).
Results
Database Search
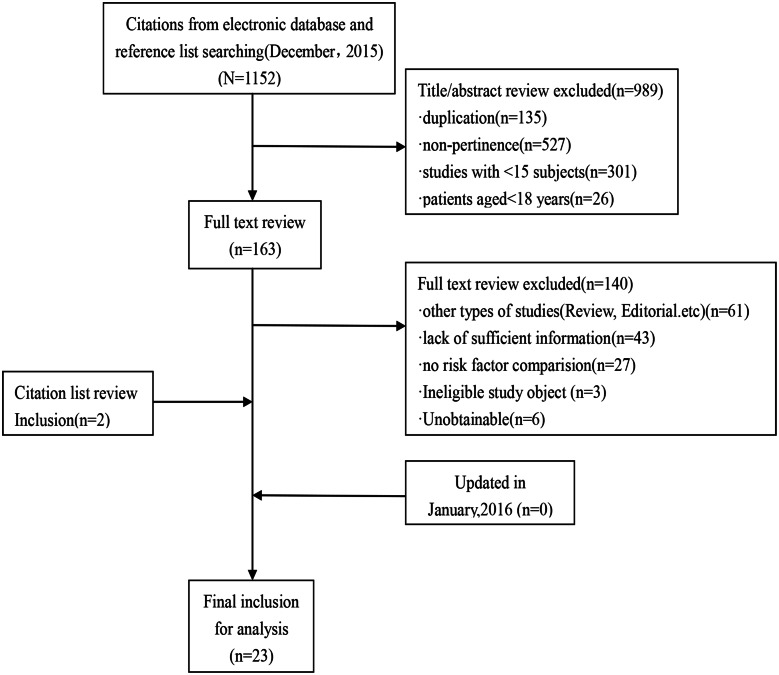
In the initial search, 1152 studies were identified. All titles and abstracts were screened, and 163 potentially relevant full-text papers were selected. After a detailed review, 15 variables associated with PM/DM-associated ILD from 23 studies met the selection criteria and were included in the final analysis (Fig 1).
Fig 1. A flow diagram of the studies.
Study Characteristics and Quality Assessment
The 23 selected studies [13–15,20–39] included 2079 patients who fulfilled the inclusion criteria. Of these patients, there were 834 with ILD and 1245 without ILD, who were considered control subjects. The studies analyzed the following characteristics (geographical region of the study, matched variables analyzed, study quality based on the Newcastle-Ottawa Scale, study size, and percentage of patients with ILD), which are listed in Table 1.
Table 1. Studies included in the meta-analysis.
| Study | Region | Matched or adjusted variables analysed | Quality | Study size (with ILD %) |
|---|---|---|---|---|
| E.H.Kang 2005 [20] | Korea | female sex, age, arthritis/arthralgia, dysphagia, malignancy, ANA, anti-Jo-1 antibody | 7 | 72 (40.3%) |
| Felix Chua 2012 [21] | England | female sex, age, anti-Jo-1 antibody, ANA, ESR | 7 | 107 (37.4%) |
| Hao Wu 2013 [22] | China | age, Gotrron's sign, heliotrope rash, arthritis/arthralgia, dysphagia, ANA, anti-Jo-1 antibody, ALT, ESR | 7 | 230 (49.6%) |
| I.MARIE 2002 [15] | France | female sex, arthritis/arthralgia, malignancy, ANA, anti-Jo-1 antibody | 7 | 156 (23.1%) |
| I-Jung chen 2009 [23] | China | female sex, heliotrope rash, Gotrron's sign, arthritis/arthralgia, raynaud's phenomenon, dysphagia, malignancy, ANA, anti-Jo-1 antibody, ALT | 7 | 151 (19.9%) |
| Jin Won Huh 2007 [24] | Korea | age, female sex, fever, ANA, anti-Jo-1 antibody, ESR | 7 | 99 (33.3%) |
| JI Su-yun 2010 [25] | China | female sex, heliotrope rash, Gotrron's sign, arthritis/arthralgia, raynaud's phenomenon, dysphagia, fever, anti-Jo-1 antibody, ALT | 7 | 197 (35.0%) |
| Kazuyoshi Ishigaski 2013 [26] | Japan | age, female sex, arthritis/arthralgia, fever, malignancy, ANA, anti-Jo-1 antibody | 7 | 39 (38.5%) |
| M.Fathi 2012 [27] | Sweden | female sex, arthritis/arthralgia, raynaud's phenomenon, ANA, anti-Jo-1 antibody | 6 | 26 (23.1%) |
| Takahisa Gono 2014 [28] | Japan | female sex | 7 | 38 (44.7%) |
| Thomas J.Richards 2009 [29] | America | arthritis/arthralgia, raynaud's phenomenon, fever | 6 | 90 (85.6%) |
| Xiaomin Cen 2013 [13] | China | age, female sex, heliotrope rash, Gotrron's sign, arthritis/arthralgia, raynaud's phenomenon, fever, ANA, anti-Jo-1 antibody | 8 | 134 (61.9%) |
| Yi Ju CHEN 2007 [14] | China | female sex, heliotrope rash, Gotrron's sign, arthritis/arthralgia, dysphagia, fever, ANA, anti-Jo-1 antibody | 6 | 56 (75%) |
| Yoshinao Muro 2013 [30] | Japan | age, female sex | 6 | 25 (68%) |
| Yuechi Sun 2013 [31] | China | female sex, heliotrope rash, Gotrron's sign, arthritis/arthralgia, fever, ANA, anti-Jo-1 antibody, ALT, | 7 | 41 (61.0%) |
| Zhiyong Chen 2013 [32] | China | MDA5 | 6 | 64 (75%) |
| Tomohiro Koga 2012 [33] | Japan | MDA5 | 7 | 79 (67.1%) |
| Ran Nakashima 2010 [34] | Japan | MDA5 | 7 | 37 (67.6%) |
| Kei Hoshino 2010 [35] | Japan | MDA5 | 7 | 61 (52.5%) |
| John C. Hall 2013 [36] | America | MDA5 | 7 | 160 (15.6%) |
| Moises Labrador-Horrillo 2014 [37] | Spain | MDA5 | 7 | 128 (8.6%) |
| Eun Ha Kang 2010 [38] | Korea | MDA5 | 7 | 49 (22.4%) |
| Yu. X 2015 [39] | China | female sex, arthritis/arthralgia, raynaud's phenomenon, ANA, anti-Jo-1 antibody, MDA5 | 7 | 40 (27.5%) |
Heterogeneity Test
No significant heterogeneity was observed for age at diagnosis (P = 0.12, I2 = 35%); proportion of women (P = 0.74, I2 = 0%); proportion of patients with Gottron’s sign (P = 0.23, I2 = 27%), heliotrope rash (P = 0.08, I2 = 50%), malignancy (P = 0.49, I2 = 0%), fever (P = 0.18, I2 = 33%), anti-Jo-1 antibodies (P = 0.54, I2 = 0%), or anti-MDA5 antibodies (P = 0.98, I2 = 0%); or levels of ALT (P = 0.17, I2 = 44%) or ESR (P = 0.62, I2 = 0%). Significant heterogeneity was observed for the proportion of patients with arthritis/arthralgia (P = 0.01, I2 = 53%), Raynaud’s phenomenon (P = 0.03, I2 = 59%), dysphagia (P = 0.003, I2 = 75%), and ANA (P = 0.001, I2 = 63%) (Table 2).
Table 2. Associations of PM/DM Associated ILD with Potential Factors In 23 Studies of 2079 Patients.
| Factors | Number of Studies | Number of Patients | OR/SMD[95%CI] | Heterogeneity | Begg’s test(P) | Egger’s test(P) | |
|---|---|---|---|---|---|---|---|
| P | I2(%) | ||||||
| Demographics | |||||||
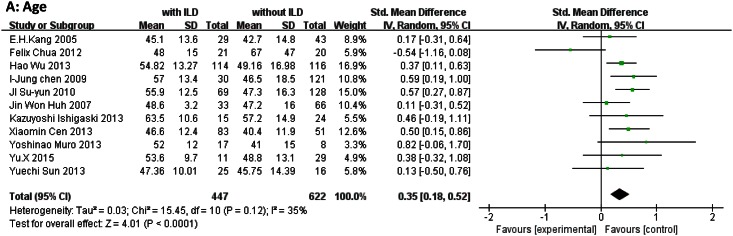
| Age | 11 | 1069 | SMD 0.35 [0.18, 0.52] | 0.12 | 35 | 0.876 | 0.398 |
| Female | 14 | 1181 | OR 0.94 [0.72, 1.23] | 0.74 | 0 | 1.000 | 0.458 |
| Clinical features | |||||||
| Gottron’s sign | 6 | 809 | OR 0.93 [0.63, 1.38] | 0.23 | 27 | 1.000 | 0.871 |
| Heliotrope rash | 6 | 809 | OR 1.42 [0.88, 2.28] | 0.08 | 50 | 1.000 | 0.942 |
| Arthritis/ Arthralgia | 12 | 1232 | OR 3.17 [1.99, 5.04] | 0.01 | 53 | 0.451 | 0.08 |
| Raynaud’s phenomenon | 6 | 638 | OR 1.62 [0.69, 3.84] | 0.03 | 59 | 0.452 | 0.277 |
| Dysphagia | 5 | 404 | OR 1.22 [0.50, 2.97] | 0.003 | 75 | 1.000 | 0.273 |
| Fever | 7 | 665 | OR 2.31 [1.42, 3.76] | 0.18 | 33 | 0.089 | 0.270 |
| Malignancy | 6 | 507 | OR 0.36 [0.18, 0.72] | 0.49 | 0 | 0.707 | 0.271 |
| Laboratory tests | |||||||
| ANA | 13 | 1288 | OR 0.89 [0.56, 1.40] | 0.001 | 63 | 0.059 | 0.022 |
| Anti-Jo-1 antibodies | 13 | 1128 | OR 3.34 [2.16, 5.16] | 0.54 | 0 | 0.300 | 0.018 |
| ALT | 3 | 389 | SMD 0.04 [-0.28, 0.37] | 0.17 | 44 | 1.000 | 0.919 |
| ESR | 5 | 674 | SMD 0.48 [0.32, 0.64] | 0.62 | 0 | 0.462 | 0.182 |
| Anti-MDA5 antibody | 8 | 618 | OR 18.26 [9.66, 34.51] | 0.98 | 0 | 0.108 | 0.108 |
| CRP | 2 | 174 | OR 3.50 [1.48, 8.28] | 0.26 | 23 | 1.000 | / |
Meta-analysis
The random-effects model was applied for the meta-analysis based on the results of heterogeneity testing. The potential risk factors that were evaluated for their association with the development of ILD were as follows: (Table 2)
Demographic characteristics: age at diagnosis (SMD, 0.35; 95% CI, 0.18–0.52; P < 0.0001) and female sex (OR, 0.94; 95% CI, 0.72–1.23; P = 0.65). (Fig 2)
Clinical features: Gottron’s sign (OR, 0.93; 95% CI, 0.63–1.38; P = 0.73), heliotrope rash (OR, 1.42; 95% CI, 0.88–2.28; P = 0.15), arthritis/arthralgia (OR, 3.17; 95% CI, 1.99–5.04; P < 0.00001), Raynaud’s phenomenon (OR, 1.62; 95% CI, 0.69–3.84; P = 0.27), dysphagia (OR, 1.22; 95% CI, 0.50–2.97; P = 0.65), malignancy (OR, 0.36; 95% CI, 0.18–0.72; P = 0.004), and fever (OR, 2.31; 95% CI, 1.42–3.76; P = 0.0007). (Fig 3)
Laboratory findings: Presence of ANA (OR, 0.89; 95% CI, 0.56–1.40; P = 0.60), anti-Jo-1 antibodies (OR, 3.34; 95% CI, 2.16–5.16; P < 0.00001), and anti-MDA5 antibodies (OR, 18.26; 95% CI, 9.66–34.51; P < 0.00001); and levels of ALT (OR, 0.04; 95% CI, −0.28 to 0.37; P = 0.79), ESR (SMD, 0.48, 95% CI, 0.32–0.64; P < 0.0001), and CRP (OR, 3.50; 95% CI, 1.48–8.28; P = 0.004). (Fig 4)
Fig 2. Forest plots generated by meta-analysis for the significant findings about demographics from the studies.
(A) Age at diagnosis.
Fig 3. Forest plots generated by meta-analysis for the significant findings about clinical features from the studies.
(A)heliotrope rash. (B) arthritis/arthralgia. (C) Malignancy. (D) fever.
Fig 4. Forest plots generated by meta-analysis for the significant findings about lab tests from the studies.
(A) anti-Jo-1 antibody. (B) ESR. (C) MDA5. (D) CRP.
Our findings demonstrate that age at diagnosis; the presence of arthralgia/arthritis, malignancy, fever, anti-Jo-1 antibodies, and anti-MDA5 antibodies; and ESR and CRP levels were associated with ILD in patients with PM/DM (Figs 1–3). No associations were observed between ILD and female sex, Gottron’s sign, heliotrope rash, Raynaud’s phenomenon, dysphagia, presence of ANA, or ALT levels (S1–S3 Figs).
Sensitivity Analysis
We conducted a sensitivity analysis to determine the relationships between arthritis/arthralgia, Raynaud’s phenomenon, dysphagia, ANA, and risks of ILD. In order to identify possible sources of heterogeneity, the analyses were repeated by removing one study per iteration by using Stata SE. The overall significance of the pooled ORs or SMDs remained the same when any single study was removed, except for Raynaud’s phenomenon. For Raynaud’s phenomenon, the OR derived from five studies was 1.10 (95% CI, 0.67–1.80), with the exception of the study by Xiaomin et al. [13]. For Raynaud’s phenomenon, we did not render the stable relationship between Raynaud’s phenomenon and ILD in PM/DM as conclusive (Fig 5).
Fig 5. Sensitivity Analysis of the studies.
(A) arthritis/arthralgia. (B) Raynaud's phenomenon. (C) dysphagia. (D) ANA.
Publication Bias
Publication bias of the included articles was examined. No significant publication bias was found by using Begg’s and Egger’s tests for age at diagnosis (P = 0.876, P = 0.398), female sex (P = 1.000, P = 0.458), Gottron’s sign (P = 1.000, P = 0.871), heliotrope rash (P = 1.000, P = 0.942), arthritis/arthralgia (P = 0.451, P = 0.08), Raynaud’s phenomenon (P = 0.452, P = 0.277), dysphagia (P = 1.000, P = 0.273), malignancy (P = 0.707, P = 0.271), fever (P = 1.000, P = 0.573), ALT level (P = 1.000, P = 0.919), ESR (P = 0.462, P = 0.182); or anti-MDA5 antibody (P = 0.108, P = 0.108; Table 2).
Discussion
To the best of our knowledge, this analysis is the first to demonstrate systematically the variables associated with the development of ILD in PM/DM patients. Disease progression is frequently aggressive and refractory for patients with PM or DM and is complicated when ILD is not recognized at an early stage [4]. However, little systematic evidence has yet shown a definitive relationship between the development of ILD and PM/DM.
In this meta-analysis and systematic review, we examined the clinical features and laboratory outcomes that influence the development of ILD associated with PM or DM. For the final analysis, we included 23 studies involving 2079 cases. Our results showed that nine factors (age at diagnosis, heliotrope rash, arthritis/arthralgia, malignancy, fever, presence of anti-Jo-1 antibody, elevated ESR, presence of anti-MDA5 antibody, and elevated CRP level) were associated with the development of ILD in patients with PM or DM. Among these characteristics, all except malignancy increased the risk of developing ILD. The presence of an underlying malignancy was associated with a reduced risk of ILD in PM/DM patients. Based on the results of this meta-analysis, female sex, Gottron’s sign, Raynaud’s phenomenon, dysphagia, ANA, and ALT did not show statistically significant relationships with ILD.
Statistical heterogeneity is a consequence of a greater variation among studies than would be expected by chance alone. A sensitivity analysis was performed to calculate these results (including for arthritis/arthralgia, dysphagia, and ANA) and demonstrate their stability and reliability.
ILD is frequently identified as an early manifestation of PM/DM on high-resolution computed tomography (HRCT). Up to 78% of patients with ILD have some degree of interstitial inflammation and fibrosis [40]. HRCT findings compatible with ILD show ground-glass attenuation, consolidation, or reticulation (i.e., intralobular reticular opacities, interlobular septal thickening, or nonseptal linear or plate-like opacity) [41]. Although many physicians are aware of the association between DM and ILD, screening practices are highly variable [42]. Pulmonary function tests (PFTs) are frequently used as a first-line screening modality, but physicians may be uncertain as to how to interpret results, when to repeat PFTs, when to obtain a chest CT, and when to refer patients to a pulmonary specialist for further care. Identifying patients in high-risk groups based on their risk factors at the time of diagnosis in order to provide better management is essential, and many clinical research studies have been conducted to elucidate the clinical features and prognostic factors of these patients [42–44].
Arthritis/arthralgia and anti-Jo-1 antibody have long been known as potential predictors of the development of ILD in patients with PM/DM [15,45]. Antisynthetase syndrome is characterized by PM/DM with the presence of antisynthetase antibodies, fever, arthritis, Raynaud’s phenomenon, mechanic’s hands, and ILD. Among the antisynthetase antibodies, anti-Jo-1 antibody is the most common (60%–80%) [46]. In anti-Jo-1 antibody-positive individuals, the most striking feature is the extraordinarily high incidence of ILD, which has been shown to approach 90%[29]. In PM/DM with ILD, serum CRP and the interferon (IFN)-γ-inducible chemokines CXC motif-ligand 9 (CXCL9) and CXCL10 seemed to be associated with anti-Jo-1 antibody expression, which is associated with ILD [29]. Immune complexes have been suggested to induce endogenous IFN in anti-Jo-1- or anti-Ro 52/anti-Ro 60-antibody-positive IIM patients [47]. ILD in myositis is an important extramuscular manifestation of the presence of anti-Jo-1 antibody (an RNA-binding protein) in patients. IFN induction could play a role in the pathogenesis of ILD, as its interference is confined to the IgG fraction and the RNA from necrotic cells [47]. Sy et al. [25] reported the results of a retrospective multivariate analysis that revealed older age at onset, fever, and arthritis/arthralgia as independent factors associated with ILD in PM/DM (after excluding anti-Jo-1 antibody). In that retrospective study, arthritis/arthralgia (OR, 2.274; 95% CI, 1.101–4.695; P = 0.026) was the predictor of ILD in PM/DM patients. Based on general data, fever was more apparent in patients with ILD-associated myositis than in those without ILD, in accordance with our results. Age at diagnosis (especially >45 years) was reported to be an important factor associated with poor prognosis [15,23,48]. Our analysis revealed that older age at diagnosis was associated with an increased risk of ILD. In addition, a higher ESR level was significantly more frequent in IIM patients with ILD, suggesting that patients with ILD have more severe systemic inflammation [13,15,24,31]. Thus, a high level of ESR was associated with ILD in PM/DM. Malignancy is another complication of IIM. The prevalence of malignancy has been shown to be lower in patients with ILD than in those without ILD [15], similar to the results of our analysis, in which malignancy was associated with a reduced risk of ILD in patients with PM/DM. High levels of serum ferritin, ALT, aspartate aminotransferase, creatine kinase, and lactate dehydrogenase have been reported as indicators of ILD in CADM patients [31]. However, our analysis revealed that ALT was not a predictor of PM/DM-ILD. Dermatological manifestations, such as heliotrope rash and Gottron’s sign, were common phenomena in DM patients [13]. However, in our analysis, these phenomena were not associated with the development of ILD in PM/DM.
Anti-MDA5 antibody expression has been reported to be found specifically in CADM patients and to predict acute progressive ILD with a poor prognosis [34]. Thus, anti-MDA5 antibody may act as a specific biomarker for a subset of DM and acute ILD patients [49]. MDA5 has been shown to have an analytical sensitivity of 85% and an analytical specificity of 100%, and was useful for identifying patients with CADM or rapidly progressive ILD [50]. In addition, a major histocompatibility complex has long been recognized as a major genetic region associated with DM [51]. An interaction between HLA-DRB1*03 and smoking was hypothesized for the formation of anti-Jo-1 antibody in IIM patients [52]. Furthermore, the HLA-DRB1*03-DQA1*05-DQB1*02 haplotype was associated with the expression of the ILD phenotype in both DM and PM when associated with a positive antisynthetase antibody [52,53]. This line of inquiry deserves further research to investigate the importance of MDA5 and genetic predispositions in predicting ILD in PM/DM. In our analysis, MDA5 expression was confirmed as a factor associated with ILD in PM/DM.
Recently, some promising biomarkers, such as Krebs von den Lungen-6 (KL-6) and serum surfactant protein D (SP-D) level, have been reported to be used in the diagnosis of ILD in PM/DM. Moreover, ethnicity was shown to be as a risk factor of IIM-ILD in a cohort study [21]. More clinical studies need to assess the potential value of these new biomarkers, as well as that of ethnicity.
Our study has limitations. The number of patients enrolled, PM/DM disease duration, population distribution, and extent of the relationship between ILD and PM/DM varied across studies. Some publication bias was observed in Begg’s and Egger’s test plots for anti-Jo-1 antibody, ANA, and CRP. Positive results that showed significant findings were more easily published than were negative or inconclusive results. Although the total number of studies included was not small, more studies, especially prospective studies with large sample sizes, are still needed to investigate the potential relationship between these factors and ILD in PM/DM. Other factors contributing to heterogeneity may have been unidentified in our review. The shortage of retrospective trials on this topic is a limitation, and more cohort or retrospective case-control studies are needed to better understand the variables associated with ILD in PM/DM.
In summary, this is the first comprehensive systematic review and meta-analysis that evaluated all factors presumed to be associated with ILD in PM/DM patients. The factors that were found to increase the risk of ILD associated with PM and DM significantly include age at diagnosis, presence of heliotrope rash, presence of arthritis/arthralgia, presence of fever, presence of anti-Jo-1 antibody, elevated ESR, presence of anti-MDA5 antibody, and elevated CRP level. Malignancy was associated with a reduced risk of ILD in PM/DM. Overall, our results are statistically robust, and the findings not only shed light on the clinical prognostic indicators of ILD in DM and PM but also demonstrate the potential pathogenesis associated with the disorders.
Supporting Information
(A) female sex.
(TIF)
(A) Gottron’s sign. (B) Raynaud's phenomenon. (C) dysphagia.
(TIF)
(A) ANA. (B) ALT.
(TIF)
(DOC)
Acknowledgments
The authors have declared no conflicts of interest.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1.Troyanov Y, Targoff IN, Payette MP, Raynauld JP, Chartier S, Goulet JR, et al. (2014) Redefining dermatomyositis: a description of new diagnostic criteria that differentiate pure dermatomyositis from overlap myositis with dermatomyositis features. Medicine (Baltimore) 93: 318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marie I, Hatron PY, Dominique S, Cherin P, Mouthon L, Menard JF (2011) Short-term and long-term outcomes of interstitial lung disease in polymyositis and dermatomyositis: a series of 107 patients. Arthritis Rheum 63: 3439–3447. 10.1002/art.30513 [DOI] [PubMed] [Google Scholar]
- 3.Hallowell RW, Ascherman DP, Danoff SK (2014) Pulmonary manifestations of polymyositis/dermatomyositis. Semin Respir Crit Care Med 35: 239–248. 10.1055/s-0034-1371528 [DOI] [PubMed] [Google Scholar]
- 4.Connors GR, Christopher-Stine L, Oddis CV, Danoff SK (2010) Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest 138: 1464–1474. 10.1378/chest.10-0180 [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, Ni L, Wang Q (2013) Administration of cathepsin B inhibitor CA-074Me reduces inflammation and apoptosis in polymyositis. J Dermatol Sci 72: 158–167. 10.1016/j.jdermsci.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 6.Reed AM, Ernste F (2009) The inflammatory milieu in idiopathic inflammatory myositis. Curr Rheumatol Rep 11: 295–301. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Fu XH, Yu Y, Shui RH, Li C, Zeng HY, et al. (2015) Treatment with CA-074Me, a Cathepsin B inhibitor, reduces lung interstitial inflammation and fibrosis in a rat model of polymyositis. Lab Invest 95: 65–77. 10.1038/labinvest.2014.135 [DOI] [PubMed] [Google Scholar]
- 8.Sauty A, Rochat T, Schoch OD, Hamacher J, Kurt AM, Dayer JM, et al. (1997) Pulmonary fibrosis with predominant CD8 lymphocytic alveolitis and anti-Jo-1 antibodies. Eur Respir J 10: 2907–2912. [DOI] [PubMed] [Google Scholar]
- 9.Mimori T, Nakashima R, Hosono Y (2012) Interstitial lung disease in myositis: clinical subsets, biomarkers, and treatment. Curr Rheumatol Rep 14: 264–274. 10.1007/s11926-012-0246-6 [DOI] [PubMed] [Google Scholar]
- 10.Gerami P, Schope JM, McDonald L, Walling HW, Sontheimer RD (2006) A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis sine myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol 54: 597–613. [DOI] [PubMed] [Google Scholar]
- 11.Mukae H, Ishimoto H, Sakamoto N, Hara S, Kakugawa T, Nakayama S, et al. (2009) Clinical differences between interstitial lung disease associated with clinically amyopathic dermatomyositis and classic dermatomyositis. Chest 136: 1341–1347. 10.1378/chest.08-2740 [DOI] [PubMed] [Google Scholar]
- 12.Targoff IN (1993) Humoral immunity in polymyositis/dermatomyositis. J Invest Dermatol 100: 116S–123S. [DOI] [PubMed] [Google Scholar]
- 13.Cen X, Zuo C, Yang M, Yin G, Xie Q (2013) A clinical analysis of risk factors for interstitial lung disease in patients with idiopathic inflammatory myopathy. Clin Dev Immunol 2013: 648570 10.1155/2013/648570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YJ, Wu CY, Shen JL (2007) Predicting factors of interstitial lung disease in dermatomyositis and polymyositis. Acta Derm Venereol 87: 33–38. [DOI] [PubMed] [Google Scholar]
- 15.Marie I, Hachulla E, Chérin P, Dominique S, Hatron PY, Hellot MF, et al. (2002) Interstitial lung disease in polymyositis and dermatomyositis. Arthritis Rheum 47: 614–622. [DOI] [PubMed] [Google Scholar]
- 16.Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (first of two parts). N Engl J Med 292: 344–347. [DOI] [PubMed] [Google Scholar]
- 17.Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (second of two parts). N Engl J Med 292: 403–407. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic S, European Respiratory S (2002) American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 165: 277–304. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 20.Kang EH, Lee EB, Shin KC, Im CH, Chung DH, Han SK, et al. (2005) Interstitial lung disease in patients with polymyositis, dermatomyositis and amyopathic dermatomyositis. Rheumatology (Oxford) 44: 1282–1286. [DOI] [PubMed] [Google Scholar]
- 21.Chua F, Higton AM, Colebatch AN, O'Reilly K, Grubnic S, Vlahos I, et al. (2012) Idiopathic inflammatory myositis-associated interstitial lung disease: ethnicity differences and lung function trends in a British cohort. Rheumatology (Oxford) 51: 1870–1876. [DOI] [PubMed] [Google Scholar]
- 22.Wu H, Geng D, Xu J (2013) An approach to the development of interstitial lung disease in dermatomyositis: a study of 230 cases in China. J Int Med Res 41: 493–501. 10.1177/0300060513476435 [DOI] [PubMed] [Google Scholar]
- 23.Chen IJ, Jan Wu YJ, Lin CW, Fan KW, Luo SF, Ho HH, et al. (2009) Interstitial lung disease in polymyositis and dermatomyositis. Clin Rheumatol 28: 639–646. 10.1007/s10067-009-1110-6 [DOI] [PubMed] [Google Scholar]
- 24.Won Huh J, Soon Kim D, Keun Lee C, Yoo B, Bum Seo J, Kitaichi M, et al. (2007) Two distinct clinical types of interstitial lung disease associated with polymyositis-dermatomyositis. Respir Med 101: 1761–1769. [DOI] [PubMed] [Google Scholar]
- 25.Ji SY, Zeng FQ, Guo Q, Tan GZ, Tang HF, Luo YJ, et al. (2010) Predictive factors and unfavourable prognostic factors of interstitial lung disease in patients with polymyositis or dermatomyositis: a retrospective study. Chin Med J (Engl) 123: 517–522. [PubMed] [Google Scholar]
- 26.Ishigaki K, Maruyama J, Hagino N, Murota A, Takizawa Y, Nakashima R, et al. (2013) Skin ulcer is a predictive and prognostic factor of acute or subacute interstitial lung disease in dermatomyositis. Rheumatol Int 33: 2381–2389. 10.1007/s00296-013-2735-y [DOI] [PubMed] [Google Scholar]
- 27.Fathi M, Barbasso Helmers S, Lundberg IE (2012) KL-6: a serological biomarker for interstitial lung disease in patients with polymyositis and dermatomyositis. J Intern Med 271: 589–597. 10.1111/j.1365-2796.2011.02459.x [DOI] [PubMed] [Google Scholar]
- 28.Gono T, Kaneko H, Kawaguchi Y, Hanaoka M, Kataoka S, Kuwana M, et al. (2014) Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology (Oxford) 53: 2196–2203. [DOI] [PubMed] [Google Scholar]
- 29.Richards TJ, Eggebeen A, Gibson K, Yousem S, Fuhrman C, Gochuico BR, et al. (2009) Characterization and peripheral blood biomarker assessment of anti-Jo-1 antibody-positive interstitial lung disease. Arthritis Rheum 60: 2183–2192. 10.1002/art.24631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muro Y, Sugiura K, Akiyama M (2013) Limitations of a single-point evaluation of anti-MDA5 antibody, ferritin, and IL-18 in predicting the prognosis of interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Clin Rheumatol 32: 395–398. 10.1007/s10067-012-2142-x [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Liu Y, Yan B, Shi G (2013) Interstitial lung disease in clinically amyopathic dermatomyositis (CADM) patients: a retrospective study of 41 Chinese Han patients. Rheumatol Int 33: 1295–1302. 10.1007/s00296-012-2545-7 [DOI] [PubMed] [Google Scholar]
- 32.Chen Z, Cao M, Plana MN, Liang J, Cai H, Kuwana M, et al. (2013) Utility of anti-melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res (Hoboken) 65: 1316–1324. [DOI] [PubMed] [Google Scholar]
- 33.Koga T, Fujikawa K, Horai Y, Okada A, Kawashiri SY, Iwamoto N, et al. (2012) The diagnostic utility of anti-melanoma differentiation-associated gene 5 antibody testing for predicting the prognosis of Japanese patients with DM. Rheumatology (Oxford) 51: 1278–1284. [DOI] [PubMed] [Google Scholar]
- 34.Nakashima R, Imura Y, Kobayashi S, Yukawa N, Yoshifuji H, Nojima T, et al. (2010) The RIG-I-like receptor IFIH1/MDA5 is a dermatomyositis-specific autoantigen identified by the anti-CADM-140 antibody. Rheumatology (Oxford) 49: 433–440. [DOI] [PubMed] [Google Scholar]
- 35.Hoshino K, Muro Y, Sugiura K, Tomita Y, Nakashima R, Mimori T (2010) Anti-MDA5 and anti-TIF1-gamma antibodies have clinical significance for patients with dermatomyositis. Rheumatology (Oxford) 49: 1726–1733. [DOI] [PubMed] [Google Scholar]
- 36.Hall JC, Casciola-Rosen L, Samedy LA, Werner J, Owoyemi K, Danoff SK, et al. (2013) Anti-melanoma differentiation-associated protein 5-associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res (Hoboken) 65: 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labrador-Horrillo M, Martinez MA, Selva-O'Callaghan A, Trallero-Araguas E, Balada E, Vilardell-Tarres M, et al. (2014) Anti-MDA5 antibodies in a large Mediterranean population of adults with dermatomyositis. J Immunol Res 2014: 290797 10.1155/2014/290797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang EH, Nakashima R, Mimori T, Kim J, Lee YJ, Lee EB, et al. (2010) Myositis autoantibodies in Korean patients with inflammatory myositis: anti-140-kDa polypeptide antibody is primarily associated with rapidly progressive interstitial lung disease independent of clinically amyopathic dermatomyositis. BMC Musculoskelet Disord 11: 223 10.1186/1471-2474-11-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, Yang CS, Li YJ, Liu XD, Wang JN, Zhao Q, et al. (2015) Predictive factors of rapidly progressive-interstitial lung disease in patients with clinically amyopathic dermatomyositis. Clin Rheumatol. [DOI] [PubMed] [Google Scholar]
- 40.Fathi M, Vikgren J, Boijsen M, Tylen U, Jorfeldt L, Tornling G, et al. (2008) Interstitial lung disease in polymyositis and dermatomyositis: longitudinal evaluation by pulmonary function and radiology. Arthritis Rheum 59: 677–685. 10.1002/art.23571 [DOI] [PubMed] [Google Scholar]
- 41.Tanizawa K, Handa T, Nakashima R, Kubo T, Hosono Y, Aihara K, et al. (2013) The prognostic value of HRCT in myositis-associated interstitial lung disease. Respir Med 107: 745–752. 10.1016/j.rmed.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 42.Fischer A, du Bois R (2012) Interstitial lung disease in connective tissue disorders. Lancet 380: 689–698. 10.1016/S0140-6736(12)61079-4 [DOI] [PubMed] [Google Scholar]
- 43.Fujisawa T, Hozumi H, Kono M, Enomoto N, Hashimoto D, Nakamura Y, et al. (2014) Prognostic factors for myositis-associated interstitial lung disease. PLoS One 9: e98824 10.1371/journal.pone.0098824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vij R, Strek ME (2013) Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest 143: 814–824. 10.1378/chest.12-0741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danko K, Ponyi A, Constantin T, Borgulya G, Szegedi G (2004) Long-term survival of patients with idiopathic inflammatory myopathies according to clinical features: a longitudinal study of 162 cases. Medicine (Baltimore) 83: 35–42. [DOI] [PubMed] [Google Scholar]
- 46.Marie I, Josse S, Decaux O, Dominique S, Diot E, Landron C, et al. (2012) Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmun Rev 11: 739–745. 10.1016/j.autrev.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 47.Eloranta ML, Barbasso Helmers S, Ulfgren AK, Ronnblom L, Alm GV, Lundberg IE. (2007) A possible mechanism for endogenous activation of the type I interferon system in myositis patients with anti-Jo-1 or anti-Ro 52/anti-Ro 60 autoantibodies. Arthritis Rheum 56: 3112–3124. [DOI] [PubMed] [Google Scholar]
- 48.Marie I, Hachulla E, Hatron PY, Hellot MF, Levesque H, Devulder B, et al. (2001) Polymyositis and dermatomyositis: short term and longterm outcome, and predictive factors of prognosis. J Rheumatol 28: 2230–2237. [PubMed] [Google Scholar]
- 49.Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T, et al. (2005) Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 52: 1571–1576. [DOI] [PubMed] [Google Scholar]
- 50.Sato S, Hoshino K, Satoh T, Fujita T, Kawakami Y, Fujita T, et al. (2009) RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: Association with rapidly progressive interstitial lung disease. Arthritis Rheum 60: 2193–2200. 10.1002/art.24621 [DOI] [PubMed] [Google Scholar]
- 51.Miller FW, Cooper RG, Vencovsky J, Rider LG, Danko K, Wedderburn LR, et al. (2013) Genome-wide association study of dermatomyositis reveals genetic overlap with other autoimmune disorders. Arthritis Rheum 65: 3239–3247. 10.1002/art.38137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chinoy H, Adimulam S, Marriage F, New P, Vincze M, Zilahi E, et al. (2012) Interaction of HLA-DRB1*03 and smoking for the development of anti-Jo-1 antibodies in adult idiopathic inflammatory myopathies: a European-wide case study. Ann Rheum Dis 71: 961–965. 10.1136/annrheumdis-2011-200182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chinoy H, Salway F, Fertig N, Shephard N, Tait BD, Wendy T, et al. (2006) In adult onset myositis, the presence of interstitial lung disease and myositis specific/associated antibodies are governed by HLA class II haplotype, rather than by myositis subtype. Arthritis Res Ther 8: R13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) female sex.
(TIF)
(A) Gottron’s sign. (B) Raynaud's phenomenon. (C) dysphagia.
(TIF)
(A) ANA. (B) ALT.
(TIF)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.