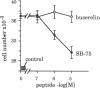
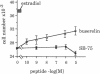
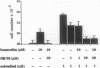
Abstract
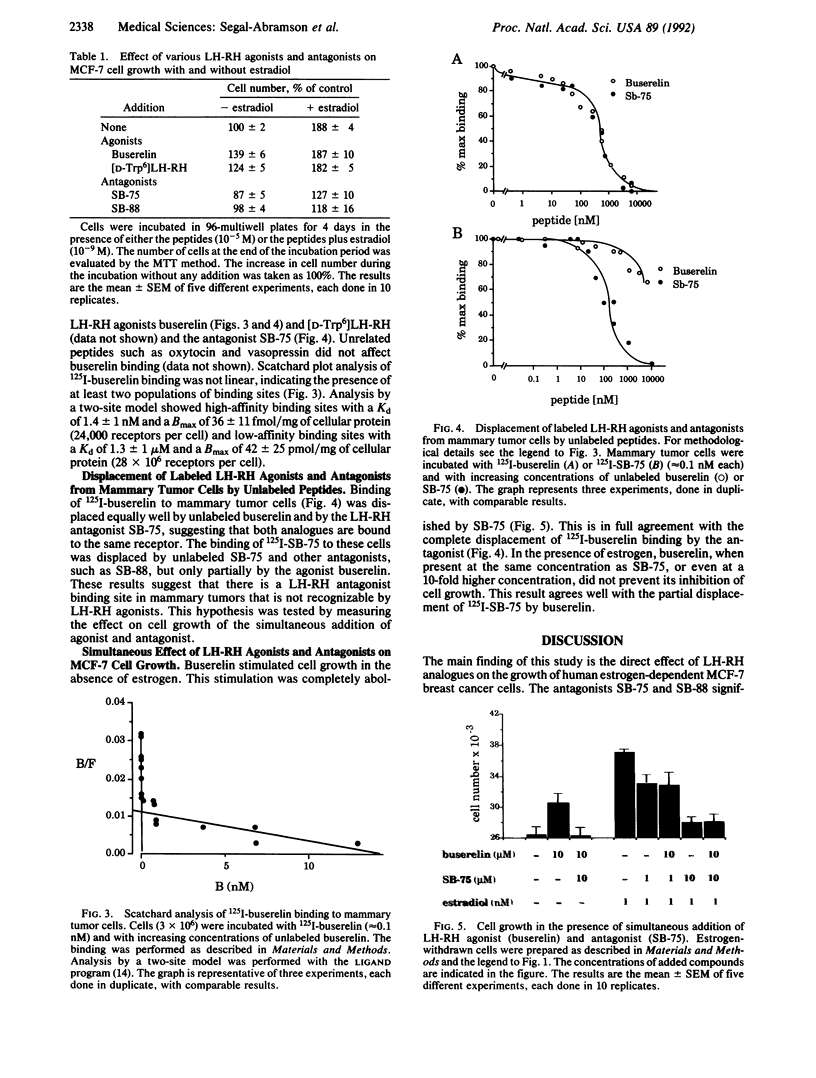
The binding of luteinizing hormone-releasing hormone (LH-RH) analogues to the human mammary tumor cell line MCF-7 and their effect on the cell proliferation was studied to elucidate their direct action on estrogen-dependent mammary tumors. The growth rate of these cells was doubled by the addition of 1 nM estradiol to cells maintained in an estrogen-deficient medium. Although the basal growth rate was only slightly inhibited by the LH-RH antagonist [Ac-D-Nal(2)1,D-Phe(pCl)2,D-Pal(3)3,D-Cit6,D-Ala10]LH-RH (SB-75), the estrogen-stimulated growth was completely abolished by the antagonist. In contrast, the LH-RH agonist buserelin stimulated cell growth in estrogen-deficient medium, whereas it had no effect in the presence of estrogen. 125I-labeled buserelin was used for the measurement of LH-RH receptors on MCF-7 cells. A Scatchard plot analysis of buserelin-specific binding revealed a nonlinear plot, which suggested the presence of one high-affinity binding site with a Kd of 1.4 +/- 1.0 nM and the remaining sites with low affinity (Kd = 1.3 +/- 1.0 microM). The binding of 125I-labeled buserelin was displaced equally well by unlabeled buserelin and by the LH-RH antagonist SB-75, suggesting that both analogues are bound to the same receptor. When parallel experiments were performed with 125I-labeled SB-75, the binding was displaced by unlabeled SB-75 and other antagonists, but only partially displaced by unlabeled buserelin. The results suggest that in these mammary tumor cells there is a LH-RH antagonist binding site that is not recognizable by LH-RH agonists. This hypothesis was tested by measuring cell growth in the presence of both agonists and antagonists. It was found that SB-75 inhibited the stimulation of growth by buserelin, but buserelin did not prevent the inhibition by the antagonist of the estrogen-dependent growth. These results suggest that antagonists directly inhibit mammary tumor growth, not only by competing with LH-RH high-affinity receptors, but also by other mechanisms mediated by low-affinity antagonist binding sites.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajusz S., Kovacs M., Gazdag M., Bokser L., Karashima T., Csernus V. J., Janaky T., Guoth J., Schally A. V. Highly potent antagonists of luteinizing hormone-releasing hormone free of edematogenic effects. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1637–1641. doi: 10.1073/pnas.85.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker G. H., Setyono-Han B., Henkelman M. S., de Jong F. H., Lamberts S. W., van der Schoot P., Klijn J. G. Comparison of the actions of the antiprogestin mifepristone (RU486), the progestin megestrol acetate, the LHRH analog buserelin, and ovariectomy in treatment of rat mammary tumors. Cancer Treat Rep. 1987 Nov;71(11):1021–1027. [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Dickson R. B., Lippman M. E. Estrogenic regulation of growth and polypeptide growth factor secretion in human breast carcinoma. Endocr Rev. 1987 Feb;8(1):29–43. doi: 10.1210/edrv-8-1-29. [DOI] [PubMed] [Google Scholar]
- Eidne K. A., Flanagan C. A., Harris N. S., Millar R. P. Gonadotropin-releasing hormone (GnRH)-binding sites in human breast cancer cell lines and inhibitory effects of GnRH antagonists. J Clin Endocrinol Metab. 1987 Mar;64(3):425–432. doi: 10.1210/jcem-64-3-425. [DOI] [PubMed] [Google Scholar]
- Fekete M., Wittliff J. L., Schally A. V. Characteristics and distribution of receptors for [D-TRP6]-luteinizing hormone-releasing hormone, somatostatin, epidermal growth factor, and sex steroids in 500 biopsy samples of human breast cancer. J Clin Lab Anal. 1989;3(3):137–147. doi: 10.1002/jcla.1860030302. [DOI] [PubMed] [Google Scholar]
- Hazum E. Some characteristics of GnRH receptors in rat-pituitary membranes: differences between an agonist and and antagonist. Mol Cell Endocrinol. 1981 Sep;23(3):275–281. doi: 10.1016/0303-7207(81)90125-8. [DOI] [PubMed] [Google Scholar]
- Klijn J. G. Long-term LHRH-agonist treatment in metastatic breast cancer as a single treatment and in combination with other additive endocrine treatments. Med Oncol Tumor Pharmacother. 1984;1(2):123–128. doi: 10.1007/BF02934984. [DOI] [PubMed] [Google Scholar]
- Manni A., Santen R., Harvey H., Lipton A., Max D. Treatment of breast cancer with gonadotropin-releasing hormone. Endocr Rev. 1986 Feb;7(1):89–94. doi: 10.1210/edrv-7-1-89. [DOI] [PubMed] [Google Scholar]
- Miller W. R., Scott W. N., Morris R., Fraser H. M., Sharpe R. M. Growth of human breast cancer cells inhibited by a luteinizing hormone-releasing hormone agonist. Nature. 1985 Jan 17;313(5999):231–233. doi: 10.1038/313231a0. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Santen R. J., Manni A., Harvey H., Redmond C. Endocrine treatment of breast cancer in women. Endocr Rev. 1990 May;11(2):221–265. doi: 10.1210/edrv-11-2-221. [DOI] [PubMed] [Google Scholar]
- Sharoni Y., Bosin E., Miinster A., Levy J., Schally A. V. Inhibition of growth of human mammary tumor cells by potent antagonists of luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1648–1651. doi: 10.1073/pnas.86.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolis G., Ackman D., Stellos A., Mehta A., Labrie F., Fazekas A. T., Comaru-Schally A. M., Schally A. V. Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1658–1662. doi: 10.1073/pnas.79.5.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignon F., Bouton M. M., Rochefort H. Antiestrogens inhibit the mitogenic effect of growth factors on breast cancer cells in the total absence of estrogens. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1502–1508. doi: 10.1016/0006-291x(87)90819-9. [DOI] [PubMed] [Google Scholar]