Abstract
Idiopathic Parkinson's disease (PD) is a late-onset, chronic, and progressive motor dysfunction attributable to loss of nigrostriatal dopamine neurons. Patients with PD experience significant gastrointestinal (GI) issues, including gastroparesis. We aimed to evaluate whether 6-hydroxy-dopamine (6-OHDA)-induced degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) induces gastric dysmotility via dysfunctions of the brain-gut axis. 6-OHDA microinjection into the SNpc induced a >90% decrease in tyrosine hydroxylase-immunoreactivity (IR) on the injection site. The [13C]-octanoic acid breath test showed a delayed gastric emptying 4 wk after the 6-OHDA treatment. In control rats, microinjection of the indirect sympathomimetic, tyramine, in the dorsal vagal complex (DVC) decreased gastric tone and motility; this inhibition was prevented by the fourth ventricular application of either a combination of α1- and α2- or a combination of D1 and D2 receptor antagonists. Conversely, in 6-OHDA-treated rats, whereas DVC microinjection of tyramine had reduced effects on gastric tone or motility, DVC microinjection of thyrotropin-releasing hormone induced a similar increase in motility as in control rats. In 6-OHDA-treated rats, there was a decreased expression of choline acetyl transferase (ChAT)-IR and neuronal nitric oxide synthase (NOS)-IR in DVC neurons but an increase in dopamine-β-hydroxylase-IR in the A2 area. Within the myenteric plexus of the esophagus, stomach, and duodenum, there were no changes in the total number of neurons; however, the percentage of NOS-IR neurons increased, whereas that of ChAT-IR decreased. Our data suggest that the delayed gastric emptying in a 6-OHDA rat model of PD may be caused by neurochemical and neurophysiological alterations in the brain-gut axis.
Keywords: brainstem, gastric motility, immunohistochemistry, Parkinson’s disease
idiopathic parkinson's disease (PD) is generally considered a movement disorder characterized by bradykinesia, rigidity, tremor, and gait/postural disorders related to the severe degeneration of the dopaminergic neurons in the substantia nigra pars compacta (SNpc) of the basal ganglia. Although the movement disorder has dominated the attention of clinicians and researchers, it is becoming increasingly recognized that PD also involves a prominent nonmotor pathology. In fact, patients with PD experience a remarkably broad spectrum of prodromic nonmotor symptoms that include sleep disorders, orthostatic hypotension, and gastrointestinal (GI) dysfunctions, all of which add significantly to the overall disability caused by PD (9, 22, 26, 33, 38, 42).
GI symptoms, such as dysphagia, nausea, delayed gastric emptying and dysmotility, and constipation, often precede the onset of motor symptoms; indeed, their occurrence in otherwise healthy people has been associated with an increased PD risk (9, 14, 27, 40). Furthermore, the GI-related dysfunctions experienced by patients with PD often adversely impact the quality of life, and, perhaps worse, their management is limited and often restricted to supportive measures (38, 47). Gastric dysmotility is particularly troublesome, as it contributes directly to the morbidity of PD and complicates clinical management of the disease. Delayed gastric emptying, for example, leads to nausea, contributes to weight loss, and adds to fluctuations in motor impairment attributable to unpredictable absorption of medications such as levodopa (10).
The multifactorial pathophysiological mechanisms responsible for the GI-related manifestations are, however, still unclear, limiting the success of developing treatments for PD-related GI symptoms. It has been recognized recently that dysmotilities reflect an early involvement of both extrinsic and intrinsic innervation of the gut, i.e., vagal and enteric nervous system (ENS), particularly the myenteric plexus (9, 27). Indeed Lewy bodies and neurites, i.e., the misfolded α-synuclein that is a histological hallmark of PD, are found in vagal motor nuclei as well as in the ENS of patients with PD (6, 35, 55). Furthermore, the delayed emptying of solids observed in patients with PD (19) may indicate vagal degeneration, and α-synuclein-mutant mice show that the presence of α-synuclein in vagal efferent fibers decreases with age in a manner that is similar to the deterioration of GI motility observed in PD (39).
The coordinated control of digestive and reflexive processes in the upper GI tract is under vagal control (54). Visceral sensory information is transmitted through the afferent vagus into the brainstem at the level of the second-order neurons of the nucleus tractus solitarius (NTS), which integrate visceral information with converging inputs from higher centers to shape the resulting output response (2, 25, 54). NTS neurons project to, among other areas, the adjacent preganglionic parasympathetic neurons of the dorsal motor nucleus of the vagus (DMV). Activation of DMV neurons excites postganglionic myenteric neurons located in the ENS, primarily by activation of nicotinic receptors (48). In the stomach, postganglionic parasympathetic neurons form two distinct pathways: 1) an excitatory cholinergic pathway that increases gastric tone, motility, and secretion via activation of muscarinic cholinergic receptors, and 2) an inhibitory nonadrenergic, noncholinergic (NANC) pathway that inhibits gastric functions via release of predominantly nitric oxide (NO) or vasoactive intestinal polypeptide (VIP) (54). Gastric functions may be inhibited, therefore, either by activation of the NANC pathway or by inhibition of the tonically active cholinergic pathway.
Considering the involvement of the DMV very early in PD (18), it is tempting to speculate that many of the very common GI symptoms observed in patients with PD, some of them occurring many years before the onset of the motor symptoms, may be linked to damage of this nucleus. Brainstem vagal and myenteric ganglionic neurons of the ENS thus represent a prime target of interest for the understanding of the neural mechanisms underlying parkinsonian-related GI dysfunctions.
There are several animal models of extrapyramidal motor dysfunctions of PD; many of these models utilize neurotoxins such as 6-hydroxy-dopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, or pesticides such as rotenone to more or less selectively destroy the dopaminergic neurons of the SNpc (33). Very little attention, however, has been focused on the GI dysfunctions in models of toxin-mediated parkinsonian-like neurodegeneration in rats. Most of the studies have focused on the effects of neurotoxins on the ENS (4, 12, 15, 33, 38, 41, 51, 56, 57, 61), and, essentially, limited information has been generated linking CNS neurodegeneration with parkinsonian gastric dysmotility. Relatively recent studies by Zheng and colleagues (58, 59) show that degeneration of nigral neurons, as obtained either with administration of 6-OHDA or lipopolysaccharide in the SNpc, decreases antral motility, possibly via alterations of the neurochemical phenotype of DMV neurons; the model, however, used bilateral microinjections in the SNpc, which may limit severely the relevance of the observation because of the large array of behavioral side effects induced by the bilateral damage of SNpc (33).
The aim of this study was to investigate whether the 6-OHDA-induced neuronal degeneration of the SNpc in rats is associated with neurofunctional and neuroanatomical modifications of the brain-gut axis, leading to gastric dysfunction.
MATERIALS AND METHODS
Male Sprague-Dawley rats (180–260 g) were housed, four per cage, in an AAALAC accredited Animal Care Facility maintained at 24°C with a 12-h:12-h light/dark cycle with food and water provided ad libitum.
All procedures were performed using aseptic techniques and were conducted in accordance with the National Institutes for Health guidelines, with the approval of the Penn State University College of Medicine Institutional Animal Care and Use Committee and according to the policies and regulations of journal policy on animal experimentation.
SNpc Degeneration Model
Animals were anaesthetized with a mixture of ketamine/xyalzine/acepromazine (80/1.6/5 mg·ml−1·kg−1 ip) and placed in a stereotaxic frame. The scalp was retracted to expose the skull, and two holes of ∼2-mm diameter were made over the SNpc to allow insertion of a glass pipette. In a group of rats, 1 μl of a solution containing 6-OHDA (4 μg/μl) in saline containing 0.02% ascorbic acid was unilaterally microinjected in two sides of the SNpc at a rate of 0.2 μl/min at the following coordinates, in mm: 1) rostro-caudal (RC): −5.0 from Bregma, medio-lateral (ML): 2.4 from midline, dorso-ventral (DV): −7.6 from the surface of the dura mater; and 2) RC: −5.6, ML: 1.6, DV: −7.8. After each injection, the pipette was kept in place for 5–10 min before being withdrawn. Control rats were injected with saline containing 0.02% ascorbic acid at the same coordinates. The incision was sutured and swabbed with a solution of 2% lidocaine in Neosporin before the rats were returned to their home cage.
Measurement of Gastric Emptying Using [13C]-Octanoic Acid Breath Test
The [13C]-octanoic acid breath test was performed 1 wk before the 6-OHDA microinjection (baseline) and weekly for 4 wk after (SNpc degeneration) using procedures described previously (44). Briefly, rats were fasted overnight with ad libitum access to water before being placed in metabolic chambers (∼7-l capacity) under a continuous flow of fresh air (0.65–0.70 l/min) to maintain the rate of CO2 at 0.5%. After ambient baseline [13C]- to [12C]-carbon dioxide measurement, they were fed with 1 g of a pancake containing 5 μl of [13C]-octanoic acid. Samples of air containing the exhaled breath were collected at 5-min intervals for the first 70 min; subsequent air samples were analyzed every 15 min for a total testing time of 370 min and analyzed to determine the ratio [13C]- to [12C]-carbon dioxide by the use of the nondispersive Infra-Red Isotope Analyzer (IRIS; Wagner Analysen Technik, Bremen, Germany). The data collected for each air sample were analyzed using IRIS software (Wagner Analysen Technik); specifically the following parameters were considered: change in 13CO2 level over baseline (DOB), the cumulative percent recovery, the time to reach the maximum concentration in fractional dose per hour (tmax), and the gastric half emptying time (t1/2).
Gastric Studies
Gastric tone and motility recordings were conducted, 1 wk after the last [13C]-octanoic acid breath test, as described previously (8). Briefly, rats were fasted overnight (water ad libitum) before being anaesthetized deeply with thiobutabarbitol (Inactin; Sigma, St. Louis, MO; 100–150 mg/kg ip). Once an adequate depth of anesthesia was assessed, rats were intubated with a tracheal catheter, and, following a laparotomy, a 6 × 8 mm encapsulated miniature strain gauge (RB Products, Minneapolis, MN) was sutured to the anterior gastric corpus in alignment with the circular smooth muscle. The abdominal incision and the wound margins were sutured; the strain gauge leads were exteriorized, the signal was low-pass filtered (0.5-Hz frequency cut off), amplified (EXP CLSG-2; QuantaMetrics, Newton, PA), and recorded on a computer using Axotape software (Molecular Devices, Sunnydale, CA). Following surgical instrumentation, animals were placed in a stereotaxic frame, and rectal temperature was monitored and maintained at 37 ± 1°C with the use of a heating pad (TCAT 2LV; Physitemp Instruments, Clifton, NJ). The head of the animal was oriented to expose the fourth ventricle via a midline incision and removal of the overlying neck musculature. The pial membrane above the vagal trigone was dissected, and the exposed tissues were covered with a warm, saline-infused cotton patch. Following about 1 h of stabilization, baseline values of corpus tone and motility were determined as the mean value of the 2-min period immediately preceding drug application.
The strain gauges used for measurements of corpus motility and tone were calibrated before the experiment. The effects on gastric tone obtained upon microinjection of neuroactive substances were extrapolated from the average value of the calibration measures. Although the basal gastric tone was not preset to a fixed value, the strain gauge transducer was sewn to provide a baseline tension of ∼0.5 g; the data reported are thus absolute values of variations in corpus tone over baseline. Note that the recorded magnitude of the drug-induced corpus relaxation can be influenced by such factors as the size of the animal and variations in the strain gauge placement. Because these variations may lead to slight differences in responses between individual animals, each animal served as its own control, and data were measured as absolute changes over baseline. Some animals (n = 5) received a microinjection of PBS in the DVC, and these microinjections per se did not vary either gastric tone or motility index (see below).
The catecholaminergic A2 area plays a relevant role in the modulation of vago-vagal reflexes (46, 54). To assess the effects of endogenous catecholamines, the indirect sympatomimetic tyramine (4.5 nmol/60 nl) was microinjected in the DVC at (in mm): 0.2–0.3 RC from calamus scriptorius, 0.1–0.3 ML from midline, and −0.5 DV from the brainstem surface. To assess the effects of direct activation of vagal efferent motoneurons, thyrotropin-releasing hormone (TRH, 1–100 pmol/60 nl) was injected in the DVC because it is well recognized that TRH effects are mediated by vagal efferent fibers (34, 50, 53). Thirty minutes after the first tyramine microinjection, either a combination of α1- and the α2-adrenergic receptor antagonists, prazosin (100 pmol/2 μl) and yohimbine (500 pmol/2 μl), or a combination of D1 and D2 dopamine receptor antagonists, SCH 23390 (45 nmol/2 μl) and L-741,626 (45 nmol/2 μl), were applied to the surface of the fourth ventricle at the level of obex, followed two 5 min later by a second tyramine microinjection; all drugs were dissolved in PBS. The strain gauge output was monitored for any changes for at least 10 min following drug infusion.
Gastric motility was calculated using the following formula, as described previously (8): Motility index percent = [(N1 × 1) + (N2 × 2) + (N3 × 4) + (N4 × 8)/t] × 100, where N equals the number of peaks in a particular force range, and t equals the interval time in which the gastric motility was measured. Presuming that a 0-mV signal is indicative of no gastric motility, the grouping of peak-to-peak sinusoidal signals reflected 25–50 mg for N1, 60–100 mg for N2, 110–200 mg for N3, and signals >210 mg for N4.
The drug-induced effects on gastric motility were measured in percent against the averaged value of gastric motility before microinjection (baseline = 100%). Some animals (n = 5) received a microinjection of PBS in the DVC; these microinjections per se did not vary either gastric tone or motility index.
The results are represented as the means ± SE. Data were evaluated within each group by ANOVA and the appropriate t-test (SPSS, Chicago, IL). In all instances, significance was set at P < 0.05.
Immunohistochemistry Processing
Tissue preparation.
Immunohistochemical analyses were conducted on rats 5 wk after 6-OHDA or vehicle microinjections into SNpc. At the conclusion of measurements of corpus tone and motility experiments, rats were perfused transcardially with heparinized saline followed by paraformaldehyde fixative (4% PFA in PBS). Brains were removed and postfixed for 4 days at room temperature with 4% PFA containing 20% sucrose before being transferred to a solution containing PBS and 20% sucrose at 4°C for at least 1 day. The entire rostrocaudal extension of SNpc at the level of the midbrain and of nucleus ambiguus (NAmb) and DVC at the level of brainstem were sliced, using a microtome, into four series of 50-μm transverse sections and preserved in long-term storage buffer (Phosphate buffer 0.1 M, sucrose 30%, ethylene glycol 30%).
Segments of the GI tract comprising the esophagus, stomach, and duodenum were extracted before PFA perfusion and immersed in PBS. Tissues were opened along the mesenteric border, washed, and pinned under tension to the bottom of silicon-coated dishes. Specimens were fixed 1–2 days in 4% PFA at 4°C, washed in PBS, and stored in PBS + 0.05% sodium azide until used, generally within 2–5 days. Specimens of esophagus, fundus, and corpus of the stomach and duodenum were then processed as longitudinal muscle-myenteric plexus whole-mount preparation by peeling away the mucosa, submucosa, and circular muscle.
All steps were performed at room temperature on a shaker. The primary antibodies used [mouse anti-tyrosine hydroxylase (TH), 1:10,000 dilution for brainstem and SNpc, 1:500 for myenteric plexus (Immunostar, Hudson, WI); mouse anti-dopamine-β-hydroxylase (DβH), 1:30,000 dilution (Millipore, Bedford, MA); mouse anti-neuronal nitric oxide synthase (nNOS), 1:3,000 dilution for brainstem, 1:500 for myenteric plexus (Sigma); goat anti-choline acetyl transferase (ChAT), 1:5,000 dilution for brainstem, 1:250 for myenteric plexus (Millipore); and rabbit anti-protein gene product (PGP9.5), 1:5,000 dilution (Millipore)] and their dilutions were determined by titration in tissue fixed and processed in the same ways as the experimental tissue. At the antibody dilutions used, immunoperoxidase labeling produced the maximum number of immunoreactive structures with a minimal amount of nonspecific staining. For permanent staining, secondary antibodies were biotinylated donkey immunoglobulins (IgG, whole molecules, not fragments) optimized for multiple labeling (Jackson ImmunoResearch Laboratories, West Grove, PA) and were used at a dilution of 1:500 for brain section or 1:100 for myenteric plexus; the detection complex was ExtrAvidin-horseradish peroxidase (catalogue no. E-2886; Sigma) diluted 1:1,500. For immunofluorescence, the secondary antibodies used were goat anti-mouse-Alexa Fluor 488, donkey anti-goat Alexa Fluor 488, or donkey anti-rabbit Alexa Fluor 568; all fluorescent secondary antibodies were purchased from Life Technologies (Grand Island, NY) and were used at 1:100 dilution.
Immunoperoxidase labeling.
One of every four brainstem sections was permeabilized by three 10-min washes in 10 mM Tris-buffered saline containing 0.3% Triton X-100 and 0.05% thimerosal, pH 7.4 (TPBS). The sections were then exposed to 10% normal horse serum (NHS) in TPBS-Triton for at least 30 min. TPBS-Triton containing 10% NHS was also used for diluting primary antibodies; TPBS-Triton containing 1% NHS was used for diluting secondary antibodies; and TPBS-Triton was used for diluting the avidin-horseradish peroxidase complex. Incubations in primary antisera were conducted on a shaker at room temperature for 2–4 days (for brain slices) or 4–7 days (for myenteric plexus); incubation in biotinylated secondary antibodies occurred overnight, and incubations in ExtrAvidin-horseradish peroxidase were for 4–6 h. After each exposure to immunoreagents, sections were washed for three 10-min sessions in TPBS. Immunostained sections were mounted on subbed slides, dehydrated, and coverslipped with Cytoseal (Thermo Fisher Scientific, Waltham, MA).
One-color immunoperoxidase labeling was used to localize TH-immunoreactivity (IR) neurons with a dark blue reaction product at the level SNpc or ChAT-IR neurons with a brown reaction product at the level of DMV and NAmb. Details of the procedure are described previously (31).
Two-color immunoperoxidase labeling was used to localize 1) NOS-IR with a dark blue reaction product and ChAT-IR with a brown reaction product, 2) DβH-IR with a dark blue reaction product and TH-IR with a brown reaction product at the level of brainstem, and 3) TH-IR with a dark blue reaction product and PGP9.5-IR with a brown react product for myenteric plexus. After being washed in TPBS-Triton and incubation in 10% NHS, sections from control rats or 6-OHDA rats were exposed to either of the following: mouse anti-TH, NOS, or DβH antiserum. After treatment with the first primary antibody, the sections were incubated overnight in biotinylated anti-mouse IgG and then in ExtrAvidin-horseradish peroxidase. A Vector SG (Burlingame, CA) reaction with peroxide generated by glucose oxidase produced a dark blue reaction. After a second blocking step in TPBS-Triton containing 10% NHS, sections from untreated rats were exposed to either goat anti-ChAT, mouse anti-TH, or rabbit anti-PGP9.5. Secondary antibodies were biotinylated donkey anti-goat for the sections incubated in anti-ChAT or anti-mouse IgG for the sections incubated in anti-TH. After a final incubation in ExtrAvidin-horseradish peroxidase, immunoreactive neurons were visualized with an imidazole-intensified diaminobenzidine reaction with peroxide generated by glucose oxidase (31).
Immunofluorescence.
Two-color immunofluorescence in myenteric plexus tissues was used to localize NOS- or ChAT-IR with a green staining product and PGP9.5-IR with a red staining. After being washed in TPBS-Triton and incubation in 10% NHS, specimens from control rats or 6-OHDA rats were incubated in primary antibodies diluted in TPBS-Triton containing 10% NHS for 5–7 days. After treatment with primary antibodies, the sections were exposed overnight in secondary antibodies 1) goat anti-mouse-Alexa Fluor 488 for NOS-IR, 2) donkey anti-goat Alexa Fluor 488 for ChAT-IR, and 3) donkey anti-rabbit Alexa Fluor 568 (all used at 1:100, Life Technologies) for PGP9.5-IR diluted in TPBS containing 1% NHS. After several washes, tissues were mounted on gelatin-subbed slides and coverslipped using Fluoromount-G (Southern Biotechnology Associated, Birmingham, AL).
Data collection, analysis, and preparation of figures.
For permanent staining immunohistochemistry, images were captured with a SPOT RT color camera mounted on a Nikon E400 microscope and digitally enhanced (adjusting brightness, contrast, and sharpness only) with Adobe Photoshop, Picasa, or Image J software. TH- and DβH-IR neurones at the level of A2 and C2 areas, ChAT-IR neurons at the level of DMV and NAmb. and NOS-IR neurons at the level of caudal NTS were counted every fourth slice. Cell count values are given as means ± SE neurones/section.
Images for immunofluorescence myenteric plexuses were captured with a Olympus Fluoview FV1000 confocal laser scanning microscope and digitally enhanced (adjusting brightness, contrast, and sharpness only) with FV10-ASV 2.0 viewer software. ChAT, NOS, and PGP9.5-IR neurons in the myenteric plexuses of any segment and animal were counted per microscopic field (0.1 mm2). The percentage of ChAT and NOS-IR neurons were calculated and expressed as a percentage of PGP9.5-IR-positive neurons, representing 100% of neural neurons.
Data were analyzed with ANOVA followed by Student's t-test (either grouped or paired) with significance set at P < 0.05.
RESULTS
6-OHDA Microinjection Induces Degeneration of SNpc Neurons
To assess the extent of the 6-OHDA-induced degeneration of the dopaminergic neurons of SNpc, TH-IR was examined in midbrain slices at the level of the SNpc 4 wk after unilateral 6-OHDA microinjection in the SNpc. The treated side showed a 96 ± 0.8% loss of dopaminergic neurons compared with the untreated side (n = 23, P < 0.05); conversely, microinjection of vehicle did not induce degeneration of dopaminergic neurons (100 ± 3.8%; n = 9, P > 0.05) compared with the untreated side (Fig. 1). These data indicate that 6-OHDA induced an almost complete degeneration of dopaminergic neurons of the SNpc.
Fig. 1.
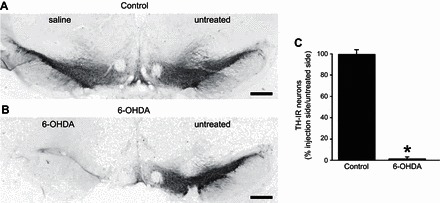
6-Hydroxy-dopamine (6-OHDA) microinjection in substantia nigra pars compacta (SNpc) induces degeneration of midbrain dopaminergic neurons. A: representative micrographs showing tyrosine hydroxylase immunoreactivity (TH-IR; blue stain) in the SNpc of control rats 4 wk after either no treatment (right) or saline was microinjected (left). B: age-matched rats 4 wk after either no treatment (right) or 6-OHDA was microinjected (left). Note the almost complete degeneration of TH-IR neurons in the 6-OHDA-treated side. C: graphic representation of the proportion of untreated/treated (i.e., saline-control, or 6-OHDA) TH-IR neurons. Note that, in control rats (n = 9), an equal number of neurons was present in both sides; conversely, in 6-OHDA-treated rats (n = 23), there was an almost completed degeneration of TH-IR neurons (i.e., 4 ± 0.8% of neurons were still present in the 6-OHDA-treated side). *P < 0.05 vs. control. Scale bar = 600 μm.
6-OHDA in SNpc Delays Gastric Emptying
The noninvasive [13C]-octanoic acid breath test was used to investigate whether unilateral degeneration of SNpc induced delayed gastric emptying. Sprague-Dawley rats (n = 8 for each group) did not differ in gastric emptying parameters either before or up to 3 wk after the injection of saline or 6-OHDA (P > 0.05 for all parameters). Four weeks after microinjection in SNpc, however, rats that received 6-OHDA showed a delayed gastric emptying as indicated by the altered values of DOB, cumulative percent recovery curves, tmax, and t1/2 (P < 0.05 for all parameters; Fig. 2).
Fig. 2.
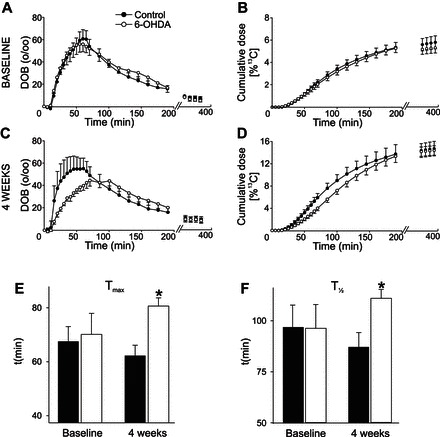
Pretreatment of SNpc with 6-OHDA induces delayed gastric emptying. Summary curve showing the results of the [13C]-octanoic acid breath test obtained before (A and B) or 4 wk after either saline (control) or 6-OHDA microinjections in the SNpc (C and D; n = 8 in each group). In C and D, note the significant reduction and rightward shift of the change over baseline (DOB) curve as well as the rightward shift of the cumulative dose curve. Note also the significant delay in both tmax (62 ± 3.8 and 81 ± 3.0 min in control and 6-OHDA-treated rats; E) and t1/2 (87 ± 7.1 and 111 ± 4.3 min in control and 6-OHDA-treated rats; F). *P < 0.05 vs. control.
Response of Corpus Tone and Motility to Microinjection of Neuroactive Drugs in DVC is Altered in 6-OHDA-Treated Rats
To investigate whether SNpc degeneration induced alterations in corpus tone and motility, experiments were conducted 5 wk after microinjection of either 6-OHDA or vehicle in the SNpc. In control rats, microinjection of tyramine (4.5 nmol/60 nl) in the left DVC decreased gastric tone and motility by −164 ± 19.4 mg and −61 ± 11.8% vs. baseline, respectively (n = 9, 4; P < 0.05); tone and motility returned to baseline values within 10 min of the microinjection; the decreased gastric tone and motility in response to tyramine microinjections in the DVC were reproducible when tyramine microinjections were repeated at 30–45-min intervals; microinjections of PBS did not alter gastric tone or motility (n = 5; not shown). The inhibitory effects of tyramine microinjection were antagonized by pretreatment with a combination of the α1- and α2-adrenoceptor antagonists, prazosin and yohimbine, which per se did not alter either gastric tone or motility. In detail, microinjection of tyramine decreased corpus tone and motility by −194 ± 22.1 mg and −61 ± 11.8% vs, baseline (n = 5, 4). Following 30–45-min recovery, the α1- and α2-adrenergic receptor antagonists, prazosin (100 pmol/2 μl) and yohimbine (500 pmol/2 μl), were applied to the floor of the fourth ventricle. Three minutes later, a second microinjection of tyramine was administered to the DVC. In the presence of prazosin and yohimbine, the effects of tyramine on both corpus tone (−28 ± 28.4 mg) and motility (+5 ± 23.2% of baseline) were attenuated significantly (P < 0.05 vs. tyramine alone).
In four other rats, the effects of tyramine microinjection in the DVC were tested in the presence of a combination of D1 and D2 receptor antagonists, SCH-23390 (45 nmol/2 μl) and L-741,626 (45 nmol/2 μl), which per se did not alter tone or motility. Upon application of SCH-23390 and L-741,626 to the floor of the fourth ventricle, the decrease in corpus tone induced by the second microinjection of tyramine was attenuated significantly (−31 ± 18.8 mg; P < 0.05 vs. tyramine alone); however, pretreatment with SCH-23390 and L-741,626 on the floor of the fourth ventricle did not attenuate the effects of tyramine on corpus motility (−53 ± 5.0% of baseline; P > 0.05 vs. tyramine alone).
In rats that received 6-OHDA 5 wk before the recordings, baseline motility index values were not significantly different from controls. In these animals, microinjection in the DVC of tyramine induced a decrease in corpus tone and motility that was attenuated significantly compared with control rats (−54 ± 22.3 mg and −18 ± 10.5% of baseline; P < 0.05 vs. control; Fig. 3). These data indicate that 6-OHDA-induced degeneration of SNpc altered the corpus tone and motility response to sympathomimetic activation in the DVC.
Fig. 3.
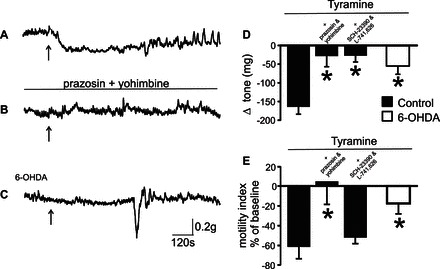
The tyramine-induced decrease in corpus tone and motility is altered in 6-OHDA-treated rats. Representative recording of gastric motility tone from the anterior corpus of control rats showing that microinjection of tyramine in the left dorsal vagal complex (DVC) decreased tone and motility (A). 30–45 min after the tyramine microinjection, i.e., upon return of tone and motility to baseline values, a combination of α1- and the α2-adrenergic receptor antagonists, prazosin (100 pmol/2 μl) and yohimbine (500 pmol/2 μl), which per se did not alter tone or motility, were applied to the surface of the 4th ventricle at the level of obex, followed 2–5 min later by another tyramine microinjection (B). Conversely, in rats that received 6-OHDA 5 wk before the recordings, tyramine microinjection induced a significantly attenuated decrease in corpus tone and motility (C). Arrows in panels A–C indicate the time of tyramine microinjection. Graphic summary of the effects of DVC tyramine microinjections on corpus tone (D) and motility (E). Note that, in control animals, the tyramine-induced decrease in corpus tone and motility were attenuated significantly by pretreatment with a combination of the α1- and α2-adrenoceptor antagonists, prazosin and yohimbine, or with a combination of the D1 and D2 dopamine antagonists, SCH-23390 and L-741,626, which per se did not alter tone or motility. Conversely, the corpus motility response to tyramine microinjection in the DVC was decreased significantly by pretreatment with prazosin and yohimbine but not by pretreatment with SCH-23390 and L-741,626. Note also that the effects of tyramine microinjections were attenuated significantly in 6-OHDA-treated rats. *P < 0.05 vs. control.
To test whether the decreased response to tyramine in 6-OHDA-treated rats is due to reduced vagal responsiveness, the effects of TRH microinjection in the DVC were assessed in naïve rats. TRH is well known to increase gastric motility via direct activation of vagal efferent motoneurons involved in the cholinergic excitatory pathway only (34, 50, 53). If microinjection of TRH in the DVC of 6-OHDA-treated rats results in an altered gastric motor response, it is reasonable to conclude that the efferent vagal pathway controlling cholinergic postganglionic neurons has been disrupted by SNpc degeneration. Conversely, if the response to TRH is still unaltered, we can conclude that 6-OHDA has induced alterations to gastric motility that involve other pathways or areas.
Microinjections of TRH (1–100 pmol/60 nl) were conducted in 22 rats (13 controls and 9 treated with 6-OHDA). The dose response to TRH effects on gastric motility was not different at any of the doses tested (Fig. 4). For example, in control rats (n = 13), microinjections of TRH (100 pmol/60 nl) in the left DVC increased corpus motility index values from 68 ± 9.8 to 260 ± 27.9 (i.e., +461 ± 66.3% compared with baseline, P < 0.05 vs. baseline). Conversely, in 6-OHDA-treated rats (n = 7), microinjections of TRH increased the corpus motility index values from 85 ± 12 to 437 ± 25.1 (i.e., +589 ± 106.3% compared with baseline; P > 0.05 vs. control rats) (Fig. 4).
Fig. 4.
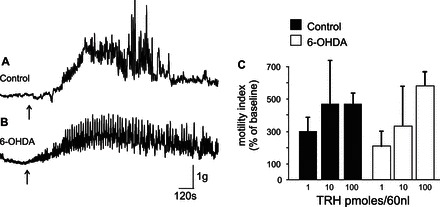
The response of corpus motility to microinjection of thyrotropin-releasing hormone (TRH) in DVC is similar in 6-OHDA-treated and control rats. Representative recording from the anterior corpus of control rats showing that microinjection of TRH (100 pmol/60 nl) in the left DVC increased corpus motility index in both control (A) and 6-OHDA-treated (B) rats. C: graphic summary of the DVC TRH microinjections (1–100 pmol/60 nl) on corpus motility. Note that, in 6-OHDA-treated rats, the increase in corpus motility was similar (P > 0.05) to that obtained in control animals at any of the TRH doses tested.
These data suggest that the vagal efferent cholinergic pathway is functional in 6-OHDA-treated rats and that the decreased gastric emptying observed in these animals may be due alterations in the vagal efferent pathways impinging on NANC postsynaptic myenteric neurons and, possibly, the neurochemical phenotype of either vagal or myenteric plexus neurons.
Alterations in the Neurochemical Phenotype of Caudal Brainstem Neurons
To assess whether 6-OHDA-induced degeneration of SNpc dopaminergic neurons induces alterations in the neurochemical phenotype of neurons in the DVC, brainstems were extracted 4–5 wk after microinjections of 6-OHDA in the SNpc and compared with age-matched control rats to quantify the presence of ChAT, NOS, TH, and DβH, as markers of cholinergic, nitrergic, dopaminergic, and noradrenergic neurons, respectively.
In 6-OHDA-treated rats there was a significant decrease in ChAT-IR in both the DMV (n = 6 and 7 in control and 6-OHDA-treated rats, respectively) and the NAmb (n = 6, 7), i.e., the nuclei controlling GI tone/motility and esophageal motility and heart rate, respectively (Fig. 5, A–F). Similarly, NOS-IR was reduced significantly in neurons of the NTS centralis (cNTS; n = 4, 6), i.e., the nucleus that comprises second-order neurons related to esophageal sensation (Fig. 5, J–L).
Fig. 5.
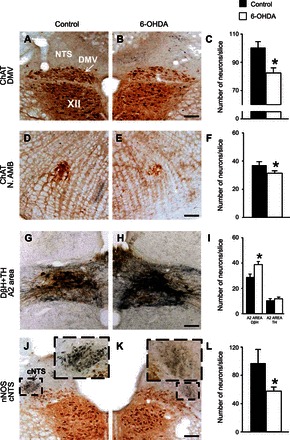
6-OHDA-treated rats have altered neurochemical phenotypes in vagal brainstem neurons. Representative micrographs of brainstem from control (A, D, G, and J) and 6-OHDA (B, E, H, and K) rats showing choline acetyltransferase (ChAT, A–D, J, and K, brown stain), tyrosine hydroxylase (TH, E and F, brown stain), dopamine-β-hydroxylase (DβH, E and F, black stain), and neuronal nitric oxide synthase (nNOS, J and K, blue stain) IR in the dorsal motor nucleus of the vagus (DMV), the nucleus ambiguus (N.AMB), the A2 area, and the subnucleus centralis of the nucleus tractus solitarius (cNTS). Summary graphics show that, in 6-OHDA-treated rats, the number of ChAT-IR-positive neurons was reduced in both DMV (C) and N.AMB (F). Similarly, the number of nNOS-IR-positive neurons was reduced in cNTS of 6-OH-treated rats (L). Conversely, whereas the number of TH-IR-positive neurons was similar in the A2 area of control and 6-OHDA-treated rats, the number of DβH-IR-positive neurons was increased in 6-OHDA-treated rats (I). *P < 0.05 vs. control. Scale bar = 70 μm.
In contrast, the total number of catecholaminergic-positive neurons of the A2 area was increased significantly in 6-OHDA-treated rats vs. control rats (n = 11, 7). In particular, the increase was observed in noradrenergic (i.e., expressing both TH and DβH) but not in dopaminergic neurons (i.e., expressing TH only; Fig. 5, G–I). Catecholaminergic neurons of the C2 area did not show significant differences between control and 6-OHDA-treated rats, i.e., 17 ± 4.7 and 16 ± 1.7 neurons/slice, respectively (n = 6, 11; data not shown).
Alterations in Neurochemical Phenotype of Myenteric Plexus Neurons
To investigate whether the alterations at the level of the brainstem nuclei controlling GI motility were associated also with changes at the level of myenteric plexus of the enteric nervous system, the GI tract from the lower third of the esophagus to the proximal duodenum was extracted from rats that received 6-OHDA 4–5 wk before experimentation and from age-matched control rats to quantify the total number of myenteric neurons (using PGP9.5 as a neuronal marker) and the relative percentage of ChAT- or NOS-positive neurons.
The total count of PGP9.5 neurons within the myenteric plexus of every GI area analyzed did not differ between control and 6-OHDA-microinjected rats (n = 8 for both groups). In contrast, the percentage of cholinergic neurons (ChAT-IR-positive; n = 4 for both groups) was reduced significantly, whereas the percentage of nitrergic neurons (NOS-IR-positive; n = 4 for both groups) was increased significantly in 6-OHDA-treated vs. control rats. Representative images are shown in Fig. 6, and data are summarized in Table 1.
Fig. 6.
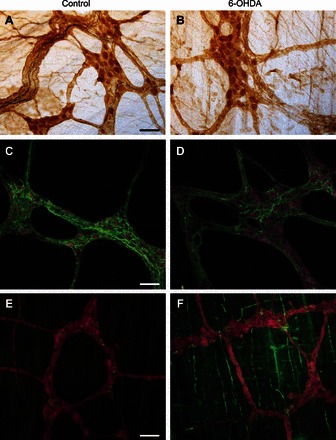
6-OHDA-treated rats have altered neurochemical phenotypes in myenteric neurons. Representative micrographs of myenteric neurons of fundus (A and B), corpus (C and D) and duodenum (E and F) from control (A, C, and E) and 6-OHDA-treated rats (B, D, and F) showing protein gene product (PGP9.5) (A and B, brown stain; C–F red stain), ChAT (C and D, green stain), TH (A and B, black stain), and nNOS (E and F, green stain) IR. Note that the total number of PGP9.5-labeled (brown stain in A and B, or red stain in C–F) neurons did not vary between control and 6-OHDA-treated rats. Whereas TH-IR-positive neurons were not found in any animal groups, A and B show a qualitative decrease in the density of TH-IR-positive fibers (dark blue stain). Note that the density and percentage of ChAT-IR-positive fibers or neurons (green stain in C and D) were decreased, whereas the percentage of nNOS-IR neurons (green stain in E and F) was increased significantly in 6-OHDA-treated rats vs. control rats. Scale bar = 70 μm. Data are summarized in Table 1.
Table 1.
Number of myenteric neurons/field of view in the proximal gastrointestinal tract
| Control | 6-OHDA | ||
|---|---|---|---|
| Esophagus | PGP9.5 neurons | 23 ± 2.9 | 28 ± 4.8 |
| % ChAT-IR neurons | 44 ± 2.2 | 35 ± 3.1* | |
| % NOS-IR neurons | 57 ± 2.5 | 69 ± 5.8* | |
| Fundus | PGP9.5 neurons | 88 ± 12.4 | 86 ± 10.9 |
| % ChAT-IR neurons | 58 ± 1.5 | 49 ± 1.2* | |
| % NOS-IR neurons | 27 ± 2.2 | 33 ± 2.1* | |
| Corpus | PGP9.5 neurons | 114 ± 7.2 | 136 ± 12.2 |
| % ChAT-IR neurons | 56 ± 3.8 | 45 ± 3.1* | |
| % NOS-IR neurons | 27 ± 0.3 | 33 ± 1.4* | |
| Duodenum | PGP9.5 neurons | 150 ± 9.3 | 134 ± 8.4 |
| % ChAT-IR neurons | 56 ± 3.9 | 46 ± 0.9* | |
| % NOS-IR neurons | 26 ± 2.3 | 32 ± 1.7* |
Data are means ± SE.
P < 0.05 vs. control. 6-OHDA, 6-hydroxy-dopamine; PGP9.5, protein gene product; ChAT, choline acetyl transferase; IR, immunoreactive; NOS, nitric oxide synthase.
As described previously (13, 17), TH-IR-positive neurons were rarely found either in 6-OHDA-treated or control animals in the GI segments analyzed; we observed, however, a qualitative decrease of extrinsic TH-IR fibers in 6-OHDA animals compared with control rats along the GI tracts (Fig. 6, A and B, images from the gastric fundus). These data suggest that microinjection of 6-OHDA into the SNpc impairs gastric motility possibly via alterations of the neurochemical phenotype of both brainstem vagal as well as myenteric neurons.
DISCUSSION
In the present study, we have shown that rats that received 6-OHDA microinjections in the SNpc, develop 1) delayed gastric emptying, 2) decreased response of corpus tone and motility to DVC injection of tyramine but unaltered gastric motility in response to DVC injection of TRH, 3) decreased number of cholinergic neurons in DMV and NAmb, 4) decreased number of NO-containing neurons in the NTS pars centralis, 5) increased number of DβH-positive but not TH-positive neurons in the A2 area, 6) decreased cholinergic and catecholaminergic innervation of myenteric plexus neurons, and 7) increased number of nitrergic myenteric neurons.
Taken together, these data indicate that the neuronal dopaminergic degeneration of the SNpc induced by local unilateral microinjection of 6-OHDA is associated with neurofunctional and neuroanatomical alterations of the brain-gut axis neurocircuitry controlling the stomach. The delayed gastric emptying associated with these alterations is reminiscent of the gastric dysfunctions observed in many patients with PD.
An essential question arising from this study is how the nigrostriatal dopaminergic denervation may affect both vagal structures in the brainstem as well as myenteric neurons in the ENS. It is unlikely that the centrally administered 6-OHDA has systemic effects attributable to diffusion from the injection site into the blood stream. Similarly, it is unlikely that 6-OHDA, leaking from SNpc and diffusing into the cerebrospinal fluid, exerts a cytotoxic action on the brainstem at the level of the dorsal vagal complex because the number of TH-IR-positive neurons in the A2 area increases rather than decreases, as expected by these potential, though unlikely, contaminations. It is reasonable to assume, therefore, that the neuronal degeneration of dopaminergic neurons in the SNpc influences neuronal function in DMV, resulting in variations in the neurochemical phenotype of vagal and myenteric neurons.
As of now, the mechanisms responsible for this puzzling observation remain unknown. Recently, however, we presented evidence for the existence of a previously undescribed, monosynaptic, reciprocal nigro-solitary pathway that controls gastric tone and motility via both dopaminergic and noradrenergic pathways (52); this nigro-vagal pathway could theoretically function as a conveyor of the alterations occurring at the level of the SNpc.
As described previously with regard to GI and especially intestinal motility (4, 12, 51, 56–58, 61), the 6-OHDA model of unilateral degeneration of dopaminergic nigral neurons induces a clear gastric/GI dysfunction. In fact, following 6-OHDA, we report a significant delay in gastric emptying as well as a reduced gastric motility response to DVC microinjection of the sympathomimetic tyramine. In control rats, the effects of tyramine are mediated by release of both dopamine and norepinephrine, and a series of manuscripts from our group have reported the multifaceted responses obtained upon administration of catecholamines in the DVC. In particular, we distinguished three separate populations of gastric-projecting DMV neurons in response to brainstem perfusion with dopamine. The majority of responsive neurons were inhibited via activation of D2-like receptors, whereas smaller subpopulations of DMV neurons were either excited via activation of D1-like receptors or unresponsive to dopamine administration (60). Similar responses of the DMV membrane were obtained upon perfusion of norepinephrine, which excited the vast majority and inhibited a small minority of responsive neurons (32), as described previously in unidentified brainstem vagal neurons (16). The effects of microinjections of norepinephrine or its antagonists in the DVC have been reported by several groups, including ours, and include a norepinephrine-mediated decrease in gastric tone as well as a reduced esophago-gastric reflex (24, 36, 46). These inhibitory effects were antagonized by different combinations of α1- and α2-adrenoreceptors antagonists; because α1-receptors induce an excitation of the DMV membranes (32), this would indicate that at least a portion of the gastroinhibitory effects of norepinephrine are attributable to activation of a vagally controlled postganglionic NANC pathway.
The gastric response to microinjection of dopamine or its antagonists in the DVC, however, has received less attention. Indeed, we are aware of just one report by Kessler and Jean (29), which showed that microinjection of DA or the agonist apomorphine into the NTS induced an immediate decrease in the number and amplitude of swallows initiated by stimulation of the superior laryngeal nerve, indicating a possible physiological role of dopaminergic neurotransmission in vagal brainstem circuits particularly in relation to swallowing and the A2 area (5, 11, 25). Interestingly, swallowing dysfunctions are a common outcome in patients with PD (9, 10, 22, 27, 38, 43). Thus the data reported herein would suggest that, in 6-OHDA-treated rats, the decreased response to DVC microinjection of tyramine may be due to some degree of impairment of the NANC pathway. Conversely, the similar response of control and 6-OHDA-treated rats to microinjection of TRH would suggest that nigral degeneration does not affect the TRH-sensitive vagal pathways that project cholinergic postganglionic neurons.
Previous studies that utilized the chemical degeneration of the SNpc by uni- or bilateral microinjection of 6-OHDA have reported variations in the neurochemical phenotype at the level of the myenteric neurons of the stomach or distal intestine. In fact, although ChAT-IR or HuC/D-IR appear to remain unchanged throughout the length of the GI tract (4, 12, 61), there was an increase in TH-IR or VIP-IR and a decrease in NOS-IR in the distal intestine (12, 51, 61) and an increase in dopamine-IR and dopamine transporter-IR in the stomach (51, 57). Focusing on the proximal portion of the GI tract, we confirm that, in agreement with previous studies as well as a report from patients with PD (3), in the 6-OHDA model, there appear to be no overall changes in the total number of myenteric neurons. We observe, however, a small but significant decrease of ChAT-immunopositive neurons, which was accompanied by a small, although significant, increase in nNOS-IR. Decreased ChAT-IR and increased TH-IR in the DVC, when combined with a decreased ChAT-IR and increased nNOS-IR in esophageal and gastric myenteric neurons, may contribute effectively to the gastric dysmotility observed in our experiments. Furthermore, we have also observed a significant decrease in nNOS in NTS pars centralis and in ChAT-IR in NAmb, i.e., the vagal nuclei receiving esophageal information and controlling esophageal motility, respectively (1, 21, 25, 45). Indeed, we have provided evidence that the NTS pars centralis is the main NTS nucleus involved in the esophago- gastric reflex (i.e., the receptive relaxation reflex) (46); however, as of now, it is not clear whether these alterations relate exclusively to esophageal functions or are reflective of a more compromised picture also involving esophago-gastric functions.
The overall impairment of the brain-gut axis with the decreased vagal responsiveness reported herein fits with previous reports showing that electrogastroenterography in patients with PD is reminiscent of the changes observed in the acute stage of vagotomy (28, 49). The mechanisms, however, may differ between these conditions; in fact, we observed an increase in nNOS-IR following 6-OHDA administration in SNpc, whereas a previous study indicated that vagotomy decreases the overall NO influence in the gastric myenteric plexus (37). Some of the observations in the present manuscript are difficult to reconcile with the decreased gastric emptying we reported, and more mechanistic studies of this model are necessary.
The appropriate management of gastric symptoms is essential for improving the patient's quality of life, and the clinical and therapeutic approach depends on the recognition of the associated pathology. Because there are several indications that the vagus nerve is involved in the initial stages of PD (7, 18, 23) and many nonmotor symptoms progress in concurrence with motor disorder (20, 30), we suggest that more attention should be paid to the brainstem and the vagus nerve in the search for treatment options for the gastric disturbances in PD.
GRANTS
This work was supported in part by a RRIA grant from the Michael J. Fox Foundation for Parkinson's Disease.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.T. and R.A.T. conception and design of research; L.T. and R.A.T. performed experiments; L.T. and R.A.T. analyzed data; L.T. and R.A.T. interpreted results of experiments; L.T. and R.A.T. prepared figures; L.T. and R.A.T. drafted manuscript; L.T. and R.A.T. edited and revised manuscript; L.T. and R.A.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Cesare M. and Zoraide Travagli for support and encouragements, Dr. K. N. Browning for critical comments to earlier versions of the manuscript, and G. M. Holmes for providing the [13C]-octanoic acid breath test equipment.
REFERENCES
- 1.Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol 283: 248–268, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Andresen MC, Kunze DL. Nucleus tractus solitarius–gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Annerino DM, Arshad S, Taylor GM, Adler CH, Beach TG, Greene JG. Parkinson's disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol (Berl) 124: 665–680, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blandini F, Balestra B, Levandis G, Cervio M, Greco R, Tassorelli C, Colucci M, Faniglione M, Bazzini E, Nappi G, Clavenzani P, Vigneri S, De Giorgio R, Tonini M. Functional and neurochemical changes of the gastrointestinal tract in a rodent model of Parkinson's disease. Neurosci Lett 467: 203–207, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Blessing WW. The Lower Brainstem and Bodily Homeostasis. Oxford, UK: Oxford University Press, 1997. [Google Scholar]
- 6.Braak H, Braak E. Pathoanatomy of Parkinson's disease. J Neurol 247, Suppl 2: II3–II10, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 110: 517–536, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Browning KN, Babic T, Holmes GM, Swartz EM, Travagli RA. A critical re-evaluation of the specificity of action of perivagal capsaicin. J Physiol 591: 1563–1580, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cersosimo MG, Benarroch EE. Neural control of the gastrointestinal tract: Implications for Parkinson disease. Mov Disord 23: 1065–1075, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Cersosimo MG, Benarroch EE. Autonomic involvement in Parkinson's disease: pathology, pathophysiology, clinical features and possible peripheral biomarkers. J Neurol Sci 313: 57–63, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Chang HY, Mashimo H, Goyal RK. Musings on the wanderer: What's new in our understanding of vago-vagal reflex?: IV. Current concepts of vagal efferent projections to the gut. Am J Physiol Gastrointest Liver Physiol 284: G357–G366, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Colucci M, Cervio M, Faniglione M, De AS, Pajoro M, Levandis G, Tassorelli C, Blandini F, Feletti F, De Giorgio R, Dellabianca A, Tonini S, Tonini M. Intestinal dysmotility and enteric neurochemical changes in a Parkinson's disease rat model. Auton Neurosci 169: 77–86, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Costa M, Furness JB, Gibbins IL. Chemical coding of enteric neurons. Prog Brain Res 68: 217–239, 1986. [DOI] [PubMed] [Google Scholar]
- 14.Derkinderen P, Rouaud T, Lebouvier T, Bruley D, V, Neunlist M, De GR. Parkinson disease: the enteric nervous system spills its guts. Neurology 77: 1761–1767, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Drolet RE, Cannon JR, Montero L, Greenamyre JT. Chronic rotenone exposure reproduces Parkinson's disease gastrointestinal neuropathology. Neurobiol Dis 36: 96–102, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda A, Minami T, Nabekura J, Oomura Y. The effects of noradrenaline on neurones in the rat dorsal motor nucleus of the vagus, in vitro. J Physiol 393: 213–231, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst 81: 87–96, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Goedert M, Spillantini MG, Del TK, Braak H. 100 years of Lewy pathology. Nat Rev Neurol 9: 13–24, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Goetze O, Nikodem AB, Wiezcorek J, Banasch M, Przuntek H, Mueller T, Schmidt WE, Woitalla D. Predictors of gastric emptying in Parkinson's disease. Neurogastroenterol Motil 18: 369–375, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein DS, Sewell L, Sharabi Y. Autonomic dysfunction in PD: a window to early detection? J Neurol Sci 310: 118–122, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Goyal RK, Paterson WG. Esophageal motility. In: Handbook of Physiology. The Gastrointestinal System. The Alimentary Canal. Bethesda, MD: Am. Physiol. Soc., 1989, sect. 6, vol. II, p. 865–908. [Google Scholar]
- 22.Grinberg LT, Rueb U, Alho AT, Heinsen H. Brainstem pathology and non-motor symptoms in PD. J Neurol Sci 289: 81–88, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Hawkes CH, Del TK, Braak H. A timeline for Parkinson's disease. Parkinsonism Relat Disord 16: 79–84, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Herman MA, Niedringhaus M, Alayan A, Verbalis JG, Sahibzada N, Gillis RA. Characterization of noradrenergic transmission at the Dorsal Motor Nucleus of the Vagus involved in reflex control of fundus tone. Am J Physiol Regul Integr Comp Physiol 294: R720–R729, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Jean A. Brainstem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 81: 929–969, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Jellinger KA. Synuclein deposition and non-motor symptoms in Parkinson disease. J Neurol Sci 310: 107–111, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Jost WH. Gastrointestinal dysfunction in Parkinson's Disease. J Neurol Sci 289: 69–73, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Kaneoke Y, Koike Y, Sakurai N, Washimi Y, Hirayama M, Hoshiyama M, Takahashi A. Gastrointestinal dysfunction in Parkinson's disease detected by electrogastroenterography. J Auton Nerv Syst 50: 275–281, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Kessler JP, Jean A. Effect of catecholamines on the swallowing reflex after pressure microinjections into the lateral solitary complex of the medulla oblongata. Brain Res 386: 69–77, 1986. [DOI] [PubMed] [Google Scholar]
- 30.Kim JB, Kim BJ, Koh SB, Park KW. Autonomic dysfunction according to disease progression in Parkinson's disease. Parkinsonism Relat Disord 20: 303–307, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Llewellyn-Smith IJ, Kellett DO, Jordan D, Browning KN, Travagli RA. Oxytocin-immunoreactive innervation of identified neurons in the rat dorsal vagal complex. Neurogastroenterol Motil 24: e136–e146, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez-Pena y, Valenzuela IM, Rogers RC, Hermann GE, Travagli RA. Norepinephrine effects on identified neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol 286: G333–G339, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDowell K, Chesselet MF. Animal models of the non-motor features of Parkinson's disease. Neurobiol Dis 46: 597–606, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miampamba M, Yang H, Sharkey KA, Tache Y. Intracisternal TRH analog induces Fos expression in gastric myenteric neurons and glia in conscious rats. Am J Physiol Gastrointest Liver Physiol 280: G979–G991, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's Disease. Annu Rev Neurosci 28: 57–87, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Nagata M, Osumi Y. Central noradrenergic inhibition of gastric motility in rats. Eur J Pharmacol 223: 153–156, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura K, Takahashi T, Taniuchi M, Hsu CX, Owyang C. Nicotinic receptor mediates nitric oxide synthase expression in the rat gastric myenteric plexus. J Clin Invest 101: 1479–1489, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natale G, Pasquali L, Ruggieri S, Paparelli A, Fornai F. Parkinson's disease and the gut: a well known clinical association in need of an effective cure and explanation. Neurogastroenterol Motil 20: 741–749, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Noorian AR, Rha J, Annerino DM, Bernhard D, Taylor GM, Greene JG. Alpha-synuclein transgenic mice display age-related slowing of gastrointestinal motility associated with transgene expression in the vagal system. Neurobiol Dis 48: 9–19, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Palma JA, Kaufmann H. Autonomic disorders predicting Parkinson's disease. Parkinsonism Relat Disord 20, Suppl 1: S94–S98, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, Jung R, Jackson S, Gille G, Spillantini MG, Reichmann H, Funk RH. Progression of Parkinson's disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One 5: e8762, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol 2: 107–116, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Poewe W. Non-motor symptoms in Parkinson's disease. Eur J Neurol 15, Suppl 1: 14–20, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Qualls-Creekmore E, Tong M, Holmes GM. Gastric emptying of enterally administered liquid meal in conscious rats and during sustained anaesthesia. Neurogastroenterol Motil 22: 181–185, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogers RC, Hermann GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol 514: 369–383, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol Regul Integr Comp Physiol 285: R479–R489, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schapira AH. Neurobiology and treatment of Parkinson's disease. Trends Pharmacol Sci 30: 41–47, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Schemann M, Grundy D. Electrophysiological identification of vagally innervated enteric neurons in guinea pig stomach. Am J Physiol Gastrointest Liver Physiol 263: G709–G718, 1992. [DOI] [PubMed] [Google Scholar]
- 49.Soykan I, Lin Z, Bennett JP, McCallum RW. Gastric myoelectrical activity in patients with Parkinson's disease: evidence of a primary gastric abnormality. Dig Dis Sci 44: 927–931, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Tache Y, Stephens RL, Jr, Ishikawa T. Central nervous system action of TRH to influence gastrointestinal function and ulceration. Ann NY Acad Sci 553: 269–285, 1989. [DOI] [PubMed] [Google Scholar]
- 51.Tian YM, Chen X, Luo DZ, Zhang XH, Xue H, Zheng LF, Yang N, Wang XM, Zhu JX. Alteration of dopaminergic markers in gastrointestinal tract of different rodent models of Parkinson's disease. Neuroscience 153: 634–644, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Toti L, Travagli RA. A nigro-vagal pathway controls gastric motility. Digestive Disease Week. Chicago, IL: May 3–6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Travagli RA, Gillis RA, Vicini S. Effects of thyrotropin-releasing hormone on neurons in rat dorsal motor nucleus of the vagus, in vitro. Am J Physiol Gastrointest Liver Physiol 263: G508–G517, 1992. [DOI] [PubMed] [Google Scholar]
- 54.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 68: 279–305, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson's disease: the presence of Lewy bodies in Auerbach's and Meissner's plexuses. Acta Neuropathol (Berl) 76: 217–221, 1988. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Mogami S, Karasawa H, Yamada C, Yakabi S, Yakabi K, Hattori T, Tache Y. Preventive effect of rikkunshito on gastric motor function inhibited by L-dopa in rats. Peptides 55: 136–144, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng LF, Song J, Fan RF, Chen CL, Ren QZ, Zhang XL, Feng XY, Zhang Y, Li LS, Zhu JX. The role of the vagal pathway and gastric dopamine in the gastroparesis of rats after a 6-hydroxydopamine microinjection in the substantia nigra. Acta Physiol (Oxf) 211: 434–446, 2014. [DOI] [PubMed] [Google Scholar]
- 58.Zheng LF, Wang ZY, Li XF, Song J, Hong F, Lian H, Wang Q, Feng XY, Tang YY, Zhang Y, Zhu JX. Reduced expression of choline acetyltransferase in vagal motoneurons and gastric motor dysfunction in a 6-OHDA rat model of Parkinson's disease. Brain Res 1420: 59–67, 2011. [DOI] [PubMed] [Google Scholar]
- 59.Zheng LF, Zhang Y, Chen CL, Song J, Fan RF, Cai QQ, Wang ZY, Zhu JX. Alterations in TH- and ChAT-immunoreactive neurons in the DMV and gastric dysmotility in an LPS-induced PD rat model. Auton Neurosci 177: 194–198, 2013. [DOI] [PubMed] [Google Scholar]
- 60.Zheng Z, Travagli RA. Dopamine effects on identified rat vagal motoneurons. Am J Physiol Gastrointest Liver Physiol 292: G1002–G1008, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu HC, Zhao J, Luo CY, Li QQ. Gastrointestinal dysfunction in a Parkinson's disease rat model and the changes of dopaminergic, nitric oxidergic, and cholinergic neurotransmitters in myenteric plexus. J Mol Neurosci 47: 15–25, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


