Abstract
During the first year of life, infants maintain their ability to discriminate faces from their own race but become less able to differentiate other-race faces. Though this is likely due to daily experience with own-race faces, the mechanisms linking repeated exposure to optimal face processing remain unclear. One possibility is that frequent experience with own-race faces generates a selective attention bias to these faces. Selective attention elicits enhancement of attended information and suppression of distraction to improve visual processing of attended objects. Thus attention biases to own-race faces may boost processing and discrimination of these faces relative to other-race faces. We used a spatial cueing task to bias attention to own- or other-race faces among Caucasian 9-month-old infants. Infants discriminated faces in the focus of the attention bias, regardless of race, indicating that infants remained sensitive to differences among other-race faces. Instead, efficacy of face discrimination reflected the extent of attention engagement.
Keywords: perceptual narrowing, infancy, selective attention, other-race effect, face perception
INTRODUCTION
Across the first year of life infants demonstrate increasingly optimal processing of frequently experienced faces and reduced sensitivity to face information that they do not routinely experience (Maurer & Werker, 2014; Pascalis, de Haan, & Nelson, 2002; Scott, Pascalis, & Nelson, 2007; Slater et al., 2010). Three month-old infants discriminate both own- and other-race faces, but by 9 months infants show increasing difficulty discriminating other-race faces while continuing to successfully discriminate own-race faces, a perceptual narrowing phenomenon termed the Other-Race Effect (ORE) (Kelly et al., 2007b, 2009).
The specific mechanisms underlying the ORE in infancy is a topic of active investigation, as it informs issues ranging from brain plasticity to developing social prejudice. The ORE has been described as a phenomenon in which frequent experience with own-race faces leads to optimal perceptual processing of own-race faces and reduced perceptual sensitivity to other-race faces (Pascalis et al., 2014; Scott et al., 2007). Infants develop a preference for own-race faces between birth and 3 months of age (Bar-Haim, Ziv, Lamy, & Hodes, 2006; Kelly et al., 2005, 2007a; Liu et al., 2015), suggesting that frequent exposure to own-race faces during this period supports the emergence of an attentional bias favoring own-race faces over other-race faces. At 4 months of age infants process both own-and other-race faces holistically but by 8 months of age infants process only own-race faces holistically (Ferguson, Kulkofsky, Cashon, & Casasola, 2009). Coupled with a decline in the ability to recognize other-race faces (Kelly et al., 2007b, 2009), these data suggest that frequent experience with own-race faces supports the development of visual representations of faces, and possible refinement of associated neural systems (Pascalis et al., 2014; Scott et al., 2007), that is optimal for differentiating own-race faces but results in less effective discrimination of other-race faces (Anzures et al., 2013).
Others have placed less emphasis on a decline in discrimination of infrequently experienced faces, instead suggesting that perceptual narrowing in the face domain is best characterized as a process of enhancement in which repeated experience promotes the development of perceptual sensitivity to experienced faces while sensitivity to other-race faces is never fully eliminated. (Fair, Flom, Jones, & Martin, 2012; Maurer & Werker, 2014). Fair et al. (2012) found that increased familiarization time counteracted the typical perceptual narrowing effect among 12-month-old infants, suggesting that infants do not lose the ability to discriminate less frequently experienced faces. Similar evidence that the ORE can be attenuated with increased exposure to other-race faces (Anzures et al., 2012; Heron-Delaney et al., 2011; Spangler et al., 2013) suggests that the frequency and timing of experience with faces is critical in determining the strength of the ORE in infancy. Recent research showing that older infants rely on an increased number of features for successful face discrimination also suggests that infants may learn to attend to the most salient features of experienced faces (Simpson, Jakobsen, Fragaszy, Okada, & Frick, 2014). Repeated experience with own-race faces supports the development of scanning strategies that are optimized for these faces, both in terms of the distribution of fixations across specific facial features and the frequency with which infants shift fixations from one feature to another within a face (Liu et al., 2015; Wheeler et al., 2011; Xiao, Xiao, Quinn, Anzures, & Lee, 2013). In particular, the emergence of the ORE tends to be associated with increased attention to the eyes of own-race faces (Wheeler et al., 2011; Xiao et al., 2013). Older infants also shift attention between paired own- and other-race faces more frequently, suggesting that increased exposure to own-race faces supports more efficient processing of these faces (Liu et al., 2015).
Cutting across all of these accounts is the idea that repeated experience with own-race faces during the first year of life leads to optimal perceptual processing of that face category. But what is it specifically about repeated experience that supports enhanced face processing? We suggest that one mechanism involves selective attention allocation—and the associated enhancement in visual processing—to own-race faces. The research described above demonstrates that infants’ orienting strategies—what is preferentially attended—are intricately linked to their experience with faces and the development of the ORE. However, selective attention comprises both orienting strategies as well as allocation of attention resources—how deeply information is processed. We investigate here the role of the latter form of attention biases in modulating visual processing and recognition of own- and other-race faces in infancy. Numerous studies with adults have established that selective attention acts to enhance processing of information at the attended location and to suppress distraction (Gandhi, Heeger, & Boynton, 1999; Kastner, Pinsk, De Weerd, Desimone, & Ungerleider, 1999; Markant, Worden, & Amso, 2015; Slotnick, Schwarzbach, & Yantis, 2003). This excitation/suppression mechanism improves the quality of early vision, enhancing contrast sensitivity, acuity, and visual processing of attended information (Carrasco, 2011, 2014; Zhang et al., 2011). This modulation of visual processing in turn supports improved encoding and recognition of attended information over unattended information among adults (Rutman, Clapp, Chadick, & Gazzaley, 2009; Uncapher & Rugg, 2009; Zanto & Gazzaley, 2009). If emerging selective attention biases support more effective processing of own-race faces during the first year of life, experimentally biasing selective attention to a face while suppressing distraction should improve online visual processing and subsequent discrimination/recognition of that face, regardless of race.
This hypothesis is consistent with, but extends, previous attention-based explanations of the ORE in infancy. Three-month-old infants showed differential attention orienting to own- and other-race face singletons surrounded by distracting faces (Hayden, Bhatt, Zieber, & Kangas, 2009; Hayden, Bhatt, Kangas, Zieber, & Joseph, 2012). Three- to 10-month-old infants additionally showed different fixation and scan patterns when looking at own- versus other-race faces (Liu et al., 2015; Wheeler et al., 2011; Xiao et al., 2013) and by 9 months of age infants preferentially oriented to other-race faces that were paired with an own-race face (Liu et al., 2015). We suggest that frequent experience with own-race faces may generate selective attention biases to these faces, which serve to bring visual details into focus, improving visual processing (Carrasco, 2011; Zhang et al., 2011) and in turn supporting enhanced memory encoding (Gazzaley & Nobre, 2012; Markant & Amso, 2013, 2014). We also suggest that observed differences in orienting/scanning strategies observed in previous studies may also derive from differences in the quality of visual processing of own- versus other-race faces resulting from these selective attention biases. Eyes and mouth hold contrast value and may be experienced more prominently when attention is differentially engaged by own-race faces (Herrmann, Montaser-Kouhsari, Carrasco, & Heeger, 2010), theoretically driving eye movements to these features. We thus hypothesize that infants’ superior learning of own-race faces is a function of selective attention biases to own-race faces that elicit greater attentional engagement and enhanced perceptual processing of these faces relative to other-race faces.
In previous work, Markant and Amso (2013) found that 9 month-old infants’ learning could be manipulated by biasing their attention to locations containing abstract objects for encoding. Specifically, infants successfully learned category exemplars that appeared in the location of the attention bias, but failed to learn category exemplars that appeared outside the location of the attention bias. Here, we capitalized on the same design to experimentally bias 9-month-old infants’ attention to locations containing own- versus other-race faces and examined their subsequent discrimination of those faces. Our primary question was whether experimentally inducing a selective attention bias—and the associated enhancement in visual cortex signal/perceptual processing—to other-race faces would boost recognition memory to the same level as frequently experienced own-race faces. The critical test of this question was whether infants showed similar recognition capabilities regardless of whether own- versus other-race faces appeared in the location of the attention bias.
We used a classic spatial cueing task (Posner, 1980) to manipulate infants’ attention. In this task a peripheral cue captures attention, followed by a delay and subsequent target presentation in the cued or noncued location. When the delay is sufficiently long, the cued location becomes suppressed and attention is biased to the noncued location, an effect called inhibition of return (IOR) (Posner, Rafal, & Choate, 1985). In a recent adult fMRI study we observed the expected selective attention dynamics in the context of an IOR spatial cueing task, with enhanced visual cortex signal associated with the location of the attention bias (i.e., the noncued location) and reduced/suppressed visual cortex signal associated with the previously cued location (Markant et al., 2015). Moreover, the extent of this attention modulation of visual cortex activity predicted adults’ subsequent memory for objects appearing in the location of the attention bias. Specifically, greater enhancement of signal at the attended target location and greater suppression at the previously cued location both predicted better recognition memory performance. These results suggest that inducing spatial selective attention (i.e., enhanced target/suppressed distractor cortical dynamics) in the context of an IOR spatial cueing task among 9-month-old infants should similarly result in enhanced perceptual processing and improved recognition memory for targets appearing in the location of the attention bias.
IOR is routinely observed by 9 months of age (Johnson & Tucker, 1996; Markant & Amso, 2013; Richards, 2000). However, previous work has also shown that using highly salient faces as stimuli in the spatial cueing task elicits arousal (Pérez-Dueñas, Acosta, & Lupiáñez, 2014; Weaver, Aronsen, & Lauwereyns, 2012) that could lead to individual variability in spatial cueing scores and add noise to the measurement of the IOR effect at the group level. Nonetheless, the IOR spatial cueing task is uniquely appropriate to our experimental question in that it mimics the covert orienting/cueing tasks that have been shown to support enhanced visual processing among adults (Carrasco, 2011; Markant et al., 2015). Moreover, although differences in orienting latencies provide an indication that attention has been biased to the noncued location, the attention bias itself is not a function of that difference in latency but rather of the cueing manipulation.
We specifically asked whether 9 month-old Caucasian infants’ discrimination of own- and other-race faces would be a function of those faces appearing in the selective attention-biased (noncued) versus unbiased (cued) locations. All infants saw the same number of own- and other-race faces; the only difference was which race category appeared in the attention bias location. If the ORE at 9 months reflects stable perceptual narrowing or learned differences in scanning strategies, infants will learn own-race faces better than other-race faces regardless of this manipulation. Alternatively, if biasing attention induces differences in online perceptual processing of faces, infants will successfully learn any face presented in the location of the attention bias, regardless of race.
METHOD
Participants
The final sample included forty 9-month-old infants (19M, 21F; MAge = 9 months, 14 days, SD = 12.32 days). Six additional infants were excluded due to fussiness (5) or technical error (1). All participants were reported by their parents to be Caucasian.
Eye Tracking Apparatus
We recorded eye movements using a remote eye tracker (SMI 60 Hz RED system, SensoMotoric Instruments, Inc., Boston, MA). Infants sat on their parent’s lap 70 cm from a 22” monitor. All parents were blind to the hypotheses of the study. A digital video camera (Canon ZR960) recorded infants’ head movements and allowed for online coding during the test phase. The video output was also recorded as a digital file.
Stimuli were presented using the SMI Experiment Center software (SensoMotoric Instruments Inc., Boston, MA). We used a 2-point calibration and 4-point calibration accuracy check as described in Markant and Amso (2013). Average deviation was 2.6° (SD = 2.4°). The digital eye recording was used for offline coding of left/right eye movements if an accurate calibration or stable position of gaze (POG) was not obtained. To confirm the accuracy of these data, reliability between coded and POG data was calculated for a subset of videos for infants who had successful eye movement recordings, r >.90, p <.05.
Procedure and Design
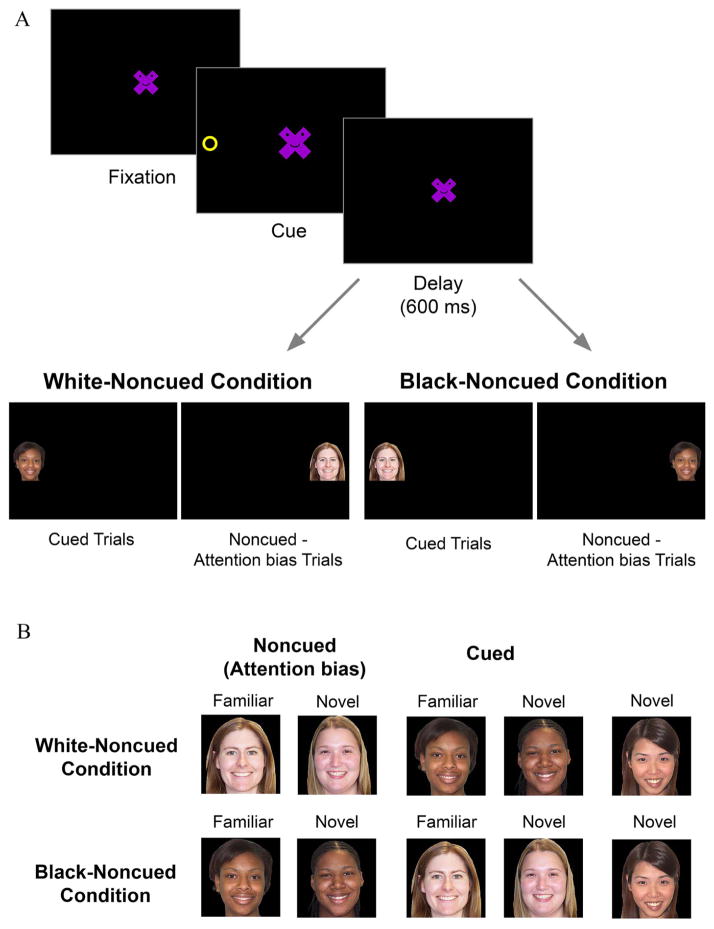
The task included a spatial cueing/encoding phase (Fig. 1) and a subsequent memory test. The first 56 trials comprised the spatial cueing phase of the session. Each trial began with a purple × with a cartoon face that loomed in and out (2.5–5.67 cm2) to engage infants’ fixation in the center of the display. The fixation was presented alone for 1,100 ms and then a cue (a yellow ring, 2.5 cm diameter; see Fig. 1) was presented 16° (19.4 cm) to the left/right of the central fixation, for 100 ms. This was followed by a 600 ms delay period in which only the fixation stimulus was visible. Then the target face was presented in either the cued or noncued location. A 600 ms delay between cue offset and target onset is sufficient to selectively bias attention to targets in the noncued location (i.e., IOR) (Hood, 1993; Johnson & Tucker, 1996; Markant & Amso, 2013; Richards, 2000).
FIGURE 1.
(A) Examples of spatial cueing trials presented to infants in the White-Noncued and Black-Noncued conditions. (B) Examples of images presented during the subsequent test phase.
After this delay the fixation disappeared and a target face appeared in the cued or noncued location for 1,500 ms. An equal number of cued and noncued faces were presented in random order. Infants were randomly assigned to one of two conditions (N = 20 per condition): in the “Black-noncued” condition, Black faces were presented in the noncued (biased) location and White faces were presented in the cued (unbiased) location, and in the “White-noncued” condition, White faces were presented in the noncued (biased) location and Black faces were presented in the cued (unbiased) location. Three Black and three White female faces (each 6.1 × 4.7 cm on the screen) were selected from the MacBrain Face Stimulus Set1 (Tottenham, 1998). For each infant, two Black and two White faces were selected as encoding stimuli. Thus, every infant received 14 trials with each White face and 14 trials with each Black face across the 56 spatial cueing trials.
After the spatial cueing phase, infants saw five test trials, one with each of five different faces: (1) a familiar face from the cued category, (2) a novel face from the cued category, (3) a familiar face from the noncued category, (4) a novel face from the noncued category, and (5) a completely novel Asian face (items two and four were the third exemplar from each category). Stimulus order was pseudo-randomized so that each of the five test items was presented first with equal frequency. Each test stimulus was presented individually in the center of the screen for up to 20 s. An experimenter (unaware of the stimulus) viewed the live video feed and advanced to the next trial if the infant looked away for more than 2 s. Look durations were validated offline, r >.90, p <.01.
Preliminary Data Processing
Eye Tracking Measures
Our primary variables for the spatial cueing phase were saccade latencies and duration of looking to the targets. Initial processing of the eye movement data utilized the SMI BeGaze analysis software (SensoMotoric Instruments Inc., Boston, MA). Three areas of interest (AOIs) were identified based on the central, left, and right stimulus locations. These AOIs were equivalent 14.2 cm2 regions over each of these locations. Usable looks were defined as segments of the data in which the POG remained within 7.1 cm2 (5.8°) for at least 100 ms. Saccade latencies were based on the time when a look first entered the AOI. Duration of looking was computed by summing the duration of all looks that occurred within the AOI following target onset.
On average, infants contributed 34 trials (SD = 8.9 trials) to their spatial cueing scores (out of 56 total trials), including an average of 16.9 cued trials (SD = 5.2 trials) and 16.9 noncued trials (SD = 5.2 trials). During these trials infants’ first looks were always directed to the target face (i.e., to the cued location during cued trials and to the noncued location during noncued trials). Individual trials were discarded for only the analyses of orienting latency during encoding if the infant looked at the cue prior to target onset (M = 4.3 trials, 7.6%; SD = 3.9 trials, 7.0%), looked away from the screen before looking at a target (M = 10.0 trials, 17.8%; SD = 5.7 trials, 10.1%), or if eye tracking data was unavailable (M = 8.0 trials, 14.2%; SD = 6.6 trials, 11.7%). There were no differences in the number of trials that were excluded across the cued and noncued trial types (all ps >.09) or across the Black-noncued and White-noncued conditions (all ps >.10). High rates of looking at the cued location or looking off-screen could reflect individual variability in infants’ attentional states at the time of testing. As such, these variables were included as covariates for analyses of spatial cueing effects and memory test performance.
Test
All looking time trials were used for analyses of test performance. No data were excluded. Individual infants’ overall mean looking times (averaging across the five test trials) ranged from 2.19–15.54 s. To correct for these individual differences in level of looking, we calculated z-scores based on each individual infant’s looking time during each test trial. Z-scores were computed as z = (trial look duration − overall mean look duration)/overall SD).
RESULTS
Spatial Cueing Effects
Orienting Latency
Our first set of analyses examined the effectiveness of our procedure in eliciting spatial cuing effects. We first evaluated infants’ orienting latencies to cued and noncued locations. To account for individual variability associated with using face stimuli we included the number of trials with Looks to the Cue as a covariate to index arousal/alerting and examined spatial cueing effects during the first half (Trials 1–28) and second half (Trials 29–56) of the task separately. We examined mean orienting latencies using an ANCOVA with Trial type (cued, noncued) as a within-subjects variable and Looks to the Cue treated as a covariate. Results showed that there was no effect of Trial type during the first half of the task (MCued = 520.85, SD = 126.31; MNoncued = 511.06, SD = 129.08; F(1,38) = .14, p = .709). However, there was a reliable main effect of Looks to the Cue (F(1,38) = 6.49, p = .015, ηp2 = .15), reflecting a negative relationship between infants’ overall mean reaction time and their rate of looking to the cue (r(40) = .38, p = .015). Thus, there was an alerting effect of the cue where infants who broke fixation and looked at the cue more frequently showed faster latencies overall, regardless of trial type. In contrast, in the second half of the task there was a trend-level significant main effect of trial type indicating IOR (F(1,35) = 3.23, p = .081, ηp2 = .08), with slower orienting latencies to the cued location (M = 528.31, SD = 148.18) relative to the noncued location (M = 512.32, SD = 139.26). The main effect of Looks to the Cue remained marginally significant (F(1,35) = 4.01, p = .053, ηp2 = .10), reflecting the same negative relationship between infants’ rates of looking to the cue and their overall mean latencies during the second half of the task (r(39) = −.30, p = .062). Importantly, Looks to the Cue did not interact with Trial type during the either portion of the task (F1stHalf(1,38) = .09, p = .770; F2ndHalf(1,38) = 2.34, p = .135) indicating that the alerting effect of the faces influenced orienting latencies equally during cued and noncued trial types. In other words, the increased alerting would make measurement of IOR difficult due to a floor effect in orienting latencies but did not alter the balance between enhancement at the noncued target location and suppression at the previously cued location. Overall these results suggest a progression over the course of the task in which the face stimuli led to initially higher levels of arousal/alerting (reflected in individual variability in sensitivity to the presence of the cue) and overall faster latencies that dampened the behavioral IOR effect. However, as this initial alerting effect attenuated the behavioral IOR effect began to emerge at the group level by the second half of the task.
Duration of Looking
We assume that enhanced processing at the noncued location associated with selective attention reflects deeper attentional engagement, not simply longer looking at the targets. Thus, it is important to show that infants are equally interested in targets presented in the cued and noncued locations. To confirm this we conducted an ANCOVA with duration of looking to the cued and noncued target locations as a within-subjects variable (Trial type), Condition (White-noncued, Black-noncued) as a between-subjects variable, and Looks to the Cue as a covariate. There were two infants with mean look durations that were >2SD above the group mean; these infants were excluded for this analysis only. Results indicated that there was no main effect of Trial type, indicating similar durations of looking to targets in the cued (M = 663.06 ms, SD = 197.82 ms) and noncued locations (M = 645.75 ms, SD = 181.65 ms; F(1,35) = 1.08, p = .305). There was also no main effect of Condition (F(1,35) = .01, p = .926), indicating similar looking times to the White (M = 657.08 ms, SD = 216.87 ms) and Black faces overall (M = 651.43 ms, SD = 145.71 ms). Importantly, the Trial type x Condition and Trial type x Looks to the Cue interactions were also not significant (F(1,35) = .24, p = .629; F(1,35) = .16, p = .692, respectively). These data confirm that infants in the Black-noncued condition and the White-noncued condition received similar durations of exposure to targets appearing in the cued and noncued locations during encoding. Any differences in learning of the faces appearing in the cued and noncued target locations or across the two conditions can thus be attributed to differential attentional engagement and associated improvement in perceptual processing resulting from the spatial cueing-induced attention bias rather than any difference in duration of exposure during encoding.
Test
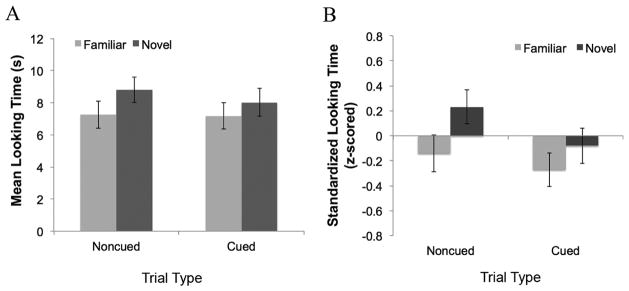
Our primary analyses were those that evaluated infants’ discrimination of the familiar and novel faces. If they did not sufficiently encode the familiar face, they will look equally at the two faces. We entered infants’ standardized looking times at test into a mixed ANCOVA with Trial type (cued, noncued) and Face Novelty (familiar, novel) as within-subjects factors, Condition (Black-noncued, White-noncued) as a between-subjects factor, and proportion of Looks to the Cue and Looks Away from the screen as covariates. Results indicated a Trial type x Face Novelty interaction, F(1, 36) = 7.35, p = .01, η2 = .17 and a Trial type x Face Novelty x Looks to Cue interaction, F(1, 36) = 5.96, p = .02, η2 = .14. Figure 2 shows that infants looked significantly longer at the novel than at the familiar face for items presented in the noncued (attention bias) location (MFamiliar = 7.25 s, SD = 5.34 s; MNovel= 8.8 s, SD = 4.94 s; F(1,37) = 10.23, p = .003, η2 = .22). However, their looking to the novel and familiar faces did not differ for items presented in the cued location, (MFamiliar = 7.18 s, SD = 5.29 s; MNovel= 8.02 s, SD = 5.53 s; F(1,37) = .75, p = .392). Thus, Caucasian infants successfully discriminated faces that were the focus of selective attention, regardless of race. These data indicate that, although spatial cueing measurements were variable, the cueing manipulation was successful at biasing attention to the noncued location and that this selective attention bias enhanced learning and subsequent memory for faces presented in the noncued relative to the cued locations during the spatial cueing/encoding phase of the task.
FIGURE 2.
Infants showed a significant novelty preference for faces presented in the noncued location but no significant difference in looking to faces presented in the cued location, regardless of race. (A) Raw looking time data, (B) Z-scored looking time data corrected for each infant’s mean look duration at test.
Importantly, the Trial type × Face Novelty × Condition interaction was not significant, F(1,36) = .17, p = .686. There were no differences in this general effect—a novelty preference for faces presented in the noncued location but not for faces presented in the cued location—as a function of own- versus other-race. Although statistically unnecessary, we wanted to further confirm this with planned comparisons, especially for the critical Black-noncued condition. We conducted separate Trial type (cued, noncued) x Face Novelty (familiar, novel) ANCOVAs with proportion of Looks to the Cue and Looks Away treated as covariates for the Black-noncued and White-noncued conditions. Caucasian infants who experienced the Black-noncued condition (i.e., were biased towards other-race faces) during the spatial cueing task looked significantly longer at the novel than the familiar other-race face at test(F(1,17) = 5.69, p = .029, η2 = .25). That is, they discriminated between the other-race faces at test. These same Caucasian infants showed no significant difference in looking times to the own-race White faces presented in the cued location (F(1,17) = .49, p = .493). These data constitute a reversal of the ORE. Caucasian infants in the White-noncued condition (i.e., who were biased towards own-race faces) during spatial cueing showed reliable preferential looking to the novel White face from the noncued location (F(1,17) = 4.26, p = .055, η2 = .20) but showed no significant difference in looking times to the Black other-race faces presented in the cued location (F(1,17) = .25, p = .627). Thus, these infants showed the expected ORE and this condition is perhaps most like the experience of a Caucasian infant, where selective attention is biased toward own-race faces. Thus, Caucasian infants discriminated either own- or other-race faces, provided that those faces were the focus of the attention bias. Conversely, they had difficulty discriminating both own- and other- race faces when they were presented outside the attention bias.
Recall that all infants saw the same number of Black and White faces during the spatial cueing phase of the experiment. Their response to the completely novel Asian face, therefore, provides additional insight into their processing of these faces. We next examined infants’ treatment of the completely novel Asian face as a function of condition. A Trial type (familiar noncued, novel Asian) x Condition (Black-noncued, White-noncued) ANCOVA with Looks to cue and Looks away as covariates revealed a main effect of Trial type, F(1,36) = 8.73, p = .005, η2 = .20, reflecting longer looking to the novel Asian face relative to the familiar noncued faces (MNoncued = 7.1 s, SD = 5.24 s, MNovel = 10.11 s, SD = 5.95 s). However, this main effect was moderated by the race of the faces appearing in the location of the attention bias, indicated by a significant Trial type x Condition interaction, F(1,36) = 4.63, p = .038, η2 = .11. This was driven by significantly longer looking times to the Asian face in the Black-noncued (M = 11.96 s, SD = 5.60 s) relative to the White-noncued condition (MNovel = 7.62 s, SD = 4.74 s; t(38) = 2.30, p = .012). There was no difference in looking times to the familiar face from the noncued location across the two conditions (MWhite = 8.02, SD = 5.81, MBlack = 6.49, SD = 4.85; t(38) = 1.59, p = .373). These data suggest that although Caucasian infants saw an equal number of White and Black faces in both conditions, the race of the face that is focus of selective attention bias influenced the extent to which Asian faces were treated as novel.
DISCUSSION
Although previous work has shown that 9 month-old infants have difficulty individuating other-race faces, we found that infants at this age discriminated both own- and other-race faces when they were the focus of an induced selective attention bias. This is particularly striking given that all infants saw the same number of own- and other-race faces; the only difference across conditions was whether these faces appeared in the attention bias location during the spatial cueing/encoding phase. To our knowledge, this is the first demonstration that infants show superior discrimination of other-race faces compared to own-race faces, and the first demonstration of infants’ differential sensitivity to own- versus other-race faces based on which category benefitted from selective attention engagement. We also found differences in infants’ treatment of Asian faces based on which race category appeared in the attention bias location. Caucasian infants biased to attend to Black faces treated the Asian faces as particularly novel relative to Caucasian infants biased to attend to White faces.
The present data suggest that inducing a selective attention bias, defined as enhancement at the attended location and suppression of the previously cued location, during online face processing benefits individuation of faces within race categories and shapes the boundaries of cross-race face categories. The selective attention bias resulted in successful face recognition regardless of race, suggesting that enhanced visual processing of other-race faces can boost recognition memory of these faces to the same level as frequently experienced own-race faces. The ORE may thus in part reflect the online cortical mechanisms of selectivity for frequently experienced own-race faces. Moreover, the same infants showed no discrimination of either own-or other-race faces when those faces appeared outside the selective attention bias. Adult fMRI studies have shown that allocating attention to a stimulus results in both enhanced visual cortex processing for that stimulus as well as reduced processing for those that appear outside the selected region (Gandhi et al., 1999; Kastner et al., 1999; Slotnick et al., 2003). Adults showed similar attention dynamics in the context of the IOR spatial cueing task design used in this study, with enhanced visual cortex signal in the location of the attention bias (i.e., the noncued location) and suppressed signal in the unattended (i.e., cued) location (Markant et al., 2015). Our data are consistent with these dynamics, as infants failed to discriminate the faces that had appeared in the cued location regardless of whether they were own- or other-race faces.
Importantly, infants’ performance at test cannot be explained by differences in overt orienting or duration of looking to the targets during encoding. There were no differences in infants’ frequency of orienting or duration of looking to the targets appearing in the cued versus noncued locations. Additionally, there were no differences in the rates of looking to the cue and looking away across the cued and noncued trial types. These points are critical for an attentional interpretation of the results, as they indicate that infants learned the faces in the noncued target location more effectively than those in the cued location despite receiving equal exposure to them during encoding. This differential learning can thus be attributed to differences in the extent of attention engagement rather than simple differences in overt looking patterns. Finally, given the attention dynamics inherent to IOR it is important to consider whether infants’ memory performance was driven by enhancement at the noncued location or suppression at the cued location. In previous work (Markant & Amso, 2013) 9-month-old infants encoded target objects in the context of IOR, facilitation, or no-cue baseline conditions in a between-subjects design. Results showed that infants successfully learned the target objects only in the context of IOR and performance was equivalent across the facilitation and baseline conditions. Similarly, adults showed superior memory performance for targets appearing in the noncued location of an IOR task but similar recognition memory for targets in the cued location and those appearing in a no-cue baseline condition (Markant et al., 2015). These data suggest that learning of targets in the noncued location during an IOR spatial cueing task reflects an enhancement above baseline rather than a suppression of learning at the cued location. The current findings are consistent with these previous studies. Nine-month-old infants typically do not discriminate other-race faces but were able to do so when those faces were encoded in the noncued location, reflecting an enhancement above the typical “baseline” for this age group. However, the lack of discrimination of own-race faces from the cued location suggests that in this study the suppression at the cued location may have also played a role in reducing learning at that location.
The IOR effect in the present study was weaker than would be typically expected by 9 months of age (Johnson & Tucker, 1996; Markant & Amso, 2013; Richards, 2000). We attribute this to the presence of face stimuli since previous adult studies have shown that the use of salient face stimuli in the spatial cueing task can elicit arousal that leads to generally fast orienting latencies and attenuates the typical IOR effect (Pérez-Dueñas et al., 2014; Weaver et al., 2012). Our data are consistent with this work and suggest that the face stimuli in the current task elicited a similar alerting effect that obscured our ability to measure a robust behavioral IOR effect at the group level. Specifically, infants who broke fixation and looked at the cue more frequently showed faster orienting latencies overall during encoding, suggesting that these infants may have experienced greater alerting in response to the face stimuli. Although this alerting should not interfere with the IOR effect elicited by the previous cue, these overall faster orienting latencies can lead to floor effects that make it more difficult to detect subtle differences in response times across cued and noncued trial types. Additionally, while there was no evidence of an IOR effect during the first half of the spatial cueing data, the IOR effect began to emerge at the group level by the second half of the task, when infants would be expected to begin to habituate to the initial alerting effect elicited by the faces. Given the complexities of using face stimuli in the spatial cueing task it will be important for future work to further examine the role of selective attention in the development of the ORE using a variety of attention manipulations.
These data suggest that emerging selective attention biases towards own-race faces during the first year of life (Hayden et al., 2012, 2009; Liu et al., 2015) may promote enhanced visual processing and recognition of these faces over other-race faces. Like all other lab-based studies, we cannot know for sure whether these results can directly extrapolate to infants’ daily experiences. Nonetheless, one possibility for further examination is that infants’ frequent experience with own-race faces generates a selective attention bias towards these faces, which in turn supports enhanced online perceptual processing and improved discrimination relative to other-race faces. That is, the ORE may in part reflect the value assigned to specific face categories, which in turn drives differential selective attention to the valued categories. Critically, our data do not address how repeated experience with own-race faces generates a selective attention bias to these faces. Related work sheds light on the issue. Previous ERP research highlights the role of attention networks at 5 and 9 months of age (Vogel, Monesson, & Scott, 2012). After viewing own- and other-race faces paired with laughing or crying sounds, 9-month-old infants showed the ORE and a race-specific ERP signal for the face/sound congruency manipulation. Five-month-old infants showed similar ERP signals for both own- and other-race faces, consistent with an absence of the ORE at this age. Furthermore, at this early age the activated ERP components were consistent with anterior attention networks whereas the ERP components activated at 9 months of age were consistent with posterior perceptual processing networks (Vogel et al., 2012). That is, emotional valence is associated with attentional strategies during face processing by 9 months.
One possibility is that an initial bias to selectively attend to own-race faces may promote visual processing of these faces, leading to greater reliance on perceptual systems as infants become more expert in encoding/discriminating these own-race faces. However, the system remains plastic and re-engagement of selective attention towards other-race faces can boost perceptual processing and recognition of these faces to the same level. One question for future research is whether there are time periods when re-engaging selective attention can more or less successfully boost perceptual processing and recognition of own- and other-race faces to the same level. This selective attention account is particularly compelling as it celebrates the plasticity inherent in the system. Although there is surely a benefit to increasing attention to own-race faces, it would be detrimental to lifelong flexible function if this benefit is obtained at the cost of a loss of ability to discriminate other-race faces. An attentional bias to own-race faces exploits the benefits of attentional enhancement of vision, allowing for a powerful yet efficient way to increase the gain to daily relevant faces while retaining flexibility if other-race faces become relevant.
Acknowledgments
Contract grant sponsor: National Institutes of Health
Contract grant numbers: NIGMS-NIH P20 GM103645, NIMH-NIH MH099078
Contract grant sponsor: James S. McDonnell Foundation
The authors gratefully acknowledge the National Institutes of Health (NIGMS-NIH P20 GM103645 and NIMH-NIH MH099078 to DA) and the James S. McDonnell Foundation (Scholar Award in Understanding Human Cognition to DA).
Footnotes
The faces depicted in Figure 1 were drawn from Center from Vital Longevity Face Database (Minear & Park, 2004) and are for illustration purposes only. The specific faces we used in our task could not be depicted due to copyright rules. The MacBrain stimulus set codes for these faces were 2F-HA-O, 6F-HA-O, 7F-HA-O, 11F-HA-O, 12F-HA-O, 14F-HA-O, 15F-HA-O.
References
- Anzures G, Quinn PC, Pascalis O, Slater AM, Tanaka JW, Lee K. Developmental origins of the other-rac effect. Current Directions in Psychological Science. 2013;22:173–178. doi: 10.1177/0963721412474459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzures G, Wheeler A, Quinn PC, Pascalis O, Slater AM, Heron-Delaney M, … Lee K. Brief daily exposures to Asian females reverses perceptual narrowing for Asian faces in Caucasian infants. Journal of Experimental Child Psychology. 2012;112:484–495. doi: 10.1016/j.jecp.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Ziv T, Lamy D, Hodes RM. Nature and nurture in own-race face processing. Psychological Science. 2006;17(2):159–163. doi: 10.1111/j.1467-9280.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- Carrasco M. Visual attention: The past 25 years. Vision Research. 2011;51:1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M. Spatial covert attention: Perceptual modulation. In: Nobre AC, Kastner S, editors. The Oxford Handbook of Attention. Oxford, UK: Oxford University Press; 2014. p. 183. [Google Scholar]
- Fair J, Flom R, Jones J, Martin J. Perceptual learning: 12-month-olds’ discrimination of monkey faces. Child Development. 2012;83(6):1996–2006. doi: 10.1111/j.1467-8624.2012.01814.x. [DOI] [PubMed] [Google Scholar]
- Ferguson KT, Kulkofsky S, Cashon CH, Casasola M. The development of specialized processing of own-race faces in infancy. Infancy. 2009;14(3):263–284. doi: 10.1080/15250000902839369. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Heeger DJ, Boynton GM. Spatial attention affects brain activity in human primary visual cortex. Proceedings of the National Academy of Sciences. 1999;96(6):3314–3319. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Nobre AC. Top-down modulation: Bridging selective attention and working memory. Trends in Cognitive Sciences. 2012;16(2):129–135. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden A, Bhatt RS, Kangas A, Zieber N, Joseph JE. Race-based perceptual asymmetry in face processing is evident early in life. Infancy. 2012;17(5):578–590. doi: 10.1111/j.1532-7078.2011.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden A, Bhatt RS, Zieber N, Kangas A. Race-based perceptual asymmetries underlying face processing in infancy. Psychonomic Bulletin & Review. 2009;16(2):270–275. doi: 10.3758/PBR.16.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron-Delaney M, Anzures G, Herbert JS, Quinn PC, Slater AM, Tanaka JW, … Pascalis O. Perceptual training prevents the emergence of the other race effect during infancy. PLoS ONE. 2011;6(5):e19858. doi: 10.1371/journal.pone.0019858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann K, Montaser-Kouhsari L, Carrasco M, Heeger DJ. When size matters: Attention affects performance by contrast or response gain. Nature Neuro-science. 2010;13(12):1554–1559. doi: 10.1038/nn.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood BM. Inhibition of return produced by covert shifts of visual attention in 6-month-old infants. Infant Behavior & Development. 1993;16:245–254. doi: 10.1016/0163-6383(93)80020-9. [DOI] [Google Scholar]
- Johnson MH, Tucker LA. The development and temporal dynamics of spatial orienting in infants. Journal of Experimental Child Psychology. 1996;63:171–188. doi: 10.1006/jecp.1996.0046. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Liu S, Lee K, Quinn PC, Pascalis O, Slater AM, Ge L. Development of the other-race effect during infancy: Evidence toward universality? Journal of Experimental Child Psychology. 2009;104(1):105–114. doi: 10.1016/j.jecp.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Liu S, Ge L, Quinn PC, Slater AM, Lee K, … Pascalis O. Cross-race preferences for same-race faces extend beyond the African versus Caucasian contrast in 3-month-old infants. Infancy. 2007a;11(1):87–95. doi: 10.1080/15250000709336871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Ge L, Pascalis O. The other-race effect develops during infancy: Evidence of perceptual narrowing. Psychological Science. 2007b;18:1084–1089. doi: 10.1111/j.1467-9280.2007.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Gibson A, Smith M, … Pascalis O. Three-month-olds, but not newborns, prefer own-race faces. Developmental Science. 2005;8(6):F31–F36. doi: 10.1111/j.1467-7687.2005.0434a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Xiao WS, Xiao NG, Quinn PC, Zhang Y, Chen H, … Lee K. Development of visual preference for own-versus other-race faces in infancy. Developmental Psychology. 2015;51(4):500–511. doi: 10.1037/a0038835. [DOI] [PubMed] [Google Scholar]
- Markant J, Amso D. Selective memories: Infants’ encoding is enhanced in selection via suppression. Developmental Science. 2013;16:926–940. doi: 10.1111/desc.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markant J, Amso D. Leveling the playing field: Attention mitigates the effects of intelligence on memory. Cognition. 2014;131:195–204. doi: 10.1016/j.cognition.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markant J, Worden MS, Amso D. Not all attention orienting is created equal: Recognition memory is enhanced when attention involves distractor suppression. Neurobiology of Learning and Memory. 2015 doi: 10.1016/j.nlm.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D, Werker JF. Perceptual narrowing during infancy: A comparison of language and faces. Developmental Psychobiology. 2014;56:154–178. doi: 10.1002/dev.21177. [DOI] [PubMed] [Google Scholar]
- Minear M, Park DC. A lifespan database of adult facial stimuli. Behavior Research Methods, Instruments, & Computers. 2004;36(4):630–633. doi: 10.3758/BF03206543. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Haan M, Nelson CA. Is face processing species-specific during the first year of life? Science. 2002;296(5571):1321–1323. doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Loevenbruck H, Quinn PC, Kandel S, Tanaka JW, Lee K. On the links among face processing, language processing, and narrowing during development. Child Development Perspectives. 2014;8(2):65–70. doi: 10.1111/cdep.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Dueñas C, Acosta A, Lupiáñez J. Reduced habituation to angry faces: Increased attentional capture as to override inhibition of return. Psychological Research. 2014;78(2):196–208. doi: 10.1007/s00426-013-0493-9. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rafal RD, Choate LS. Inhibition of return: Neural basis and function. Cognitive Neuropsycho logy. 1985;2:211–228. doi: 10.1080/02643298508252866. [DOI] [Google Scholar]
- Richards JE. Localizing the development of covert attention in infants with scalp event-related potentials. Developmental Psychology. 2000;36:91–108. doi: 10.1037/0012-1649.36.1.91. [DOI] [PubMed] [Google Scholar]
- Rutman AM, Clapp WC, Chadick JZ, Gazzaley A. Early top–down control of visual processing predicts working memory performance. Journal of Cognitive Neuroscience. 2009;22(6):1224–1234. doi: 10.1162/jocn.2009.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LS, Pascalis O, Nelson CA. A domain-general theory of the development of perceptual discrimination. Current Directions in Psychological Science. 2007;16:197–201. doi: 10.1111/j.1467-8721.2007.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EA, Jakobsen KV, Fragaszy DM, Okada K, Frick JE. The development of facial identity discrimination through learned attention. Developmental Psychobiology. 2014;56(5):1083–1101. doi: 10.1002/dev.21194. [DOI] [PubMed] [Google Scholar]
- Slater AM, Quinn PC, Kelly DJ, Lee K, Longmore CA, McDonald PR, Pascalis O. The shaping of the face space in early infancy: Becoming a native face processor. Child Development Perspectives. 2010;4(3):205–211. doi: 10.1111/j.1750-8606.2010.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Schwarzbach J, Yantis S. Attentional inhibition of visual processing in human striate and extrastriate cortex. NeuroImage. 2003;19(4):1602–1611. doi: 10.1016/s1053-8119(03)00187-3. [DOI] [PubMed] [Google Scholar]
- Spangler SM, Schwarzer G, Freitag C, Vierhaus M, Teubert M, Fassbender I, … Keller H. The other-race effect in a longitudinal sample of 3-, 6- and 9-month-old infants: Evidence of a training effect. Infancy. 2013;18(4):516–533. doi: 10.1111/j.1532-7078.2012.00137.x. [DOI] [Google Scholar]
- Tottenham N. MacBrain Face Stimulus Set. Chicago, IL: John D. & Catherine T; 1998. MacArthur foundation research network on early experience and brain development. [Google Scholar]
- Uncapher MR, Rugg MD. Selecting for memory? The influence of selective attention on the mnemonic binding of contextual information. The Journal of Neuroscience. 2009;29(25):8270–8279. doi: 10.1523/JNEUROSCI1043-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel M, Monesson A, Scott LS. Building biases in infancy: The influence of race on face and voice emotion matching. Developmental Science. 2012;15(3):359–372. doi: 10.1111/j.1467-7687.2012.01138.x. [DOI] [PubMed] [Google Scholar]
- Weaver MD, Aronsen D, Lauwereyns J. A short-lived face alert during inhibition of return. Attention, Perception & Psychophysics. 2012;74(3):510–520. doi: 10.3758/s13414-011-0258-8. [DOI] [PubMed] [Google Scholar]
- Wheeler A, Anzures G, Quinn PC, Pascalis O, Omrin DS, Lee K. Caucasian infants scan own- and other-race faces differently. PLOS ONE. 2011;6(4):e18621. doi: 10.1371/journal.pone.0018621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao WS, Xiao NG, Quinn PC, Anzures G, Lee K. Development of face scanning for own- and other-race faces in infancy. International Journal of Behavioral Development. 2013;37:100–105. doi: 10.1177/0165025412467584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. The Journal of Neuroscience. 2009;29(10):3059–3066. doi: 10.1523/JNEUROSCI4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Meyers EM, Bichot N, Serre T, Poggio TA, Desimone R. Object decoding with attention in inferior temporal cortex. Proceedings of the National Academy of Sciences. 2011;108(21):8850–8855. doi: 10.1073/pnas.1100999108. [DOI] [PMC free article] [PubMed] [Google Scholar]