Abstract
The transient receptor potential melastatin 8 (TRPM8) ion channel is the primary cold sensor in humans. TRPM8 is gated by physiologically relevant cold temperatures and chemical ligands that induce cold sensations, such as the analgesic compound menthol. Characterization of TRPM8 ligand-gated channel activation will lead to a better understanding of the fundamental mechanisms that underlie TRPM8 function. Here, the direct binding of menthol to the isolated hTRPM8 sensing domain (transmembrane helices S1–S4) is investigated. These data are compared with two mutant sensing domain proteins, Y745H (S2 helix) and R842H (S4 helix), which have been previously identified in full length TRPM8 to be menthol insensitive. The data presented herein show that menthol specifically binds to the wild type, Y745H, and R842H TRPM8 sensing domain proteins. These results are the first to show that menthol directly binds to the TRPM8 sensing domain and indicates that Y745 and R842 residues, previously identified in functional studies as crucial to menthol sensitivity, do not affect menthol binding but instead alter coupling between the sensing domain and the pore domain.
Keywords: Transient receptor potential channel, TRPM8, Sensing domain, Voltage sensing domain, Menthol binding, WS-12 binding
Graphical abstract
The human transient receptor potential melastatin 8 (hTRPM8) ion channel is one of the 27 human TRP channels which have diverse roles in physiology.1–3 Specifically, hTRPM8 is activated at low temperatures (<25 °C) and functions as the primary cold sensor in humans.4, 5 This non-selective cation channel is also involved in pain sensation, regulation of thermogenesis, and is upregulated in various types of tumors such as prostate, breast, colon, lung, and skin cancers.6–10 As a result, hTRPM8 has been advocated as a target for therapeutic intervention as illustrated by ongoing preclinical trials of hTRPM8 modulators.11–13 Human TRPM8 is polymodally modulated by various stimuli including temperature, voltage, chemical ligands, lipids, and accessory proteins.14–22 Out of the chemical agonists, menthol is most well-known to significantly activate hTRPM8 (Figure 1). Currently the molecular mechanism of hTRPM8 activation by distinct stimuli including menthol is not fully understood.
Figure 1.
Representative whole cell patch clamp recording of a HEK-293 cell heterologously expressing hTRPM8 ion channel. Compared to the initial response in absence of menthol (empty squares), a significant increase in current is observed upon addition of 0.5 mM menthol to the bath solution (green circles). When menthol is depleted, the current level decreases to that of the initial response (red triangles). Menthol interacts with the sensing domain (SD, helices S1–S4), whereby the binding event is coupled to the pore domain (PD, S5–S6) which gates the channel.
TRP channels are composed of a six transmembrane (TM) α-helical architecture similar to voltage-gated potassium (Kv) channels, such that the first four TM helices (S1–S4) constitute the sensing domain (SD) and the fifth and sixth helices make up the pore domain (PD, S5–S6). Similar to the Kv Channels, TRP channels are tetramers with a centrally located pore and four flanking sensing domains.23–25 Previously reported electrophysiology and calcium imaging studies show that mutations in the SD affect ligand-dependent channel activation of TRPM8 and other TRP channels.18, 26–28 For example, high-throughput random mutagenesis studies of mouse TRPM8 showed that Y745 in S2 is crucial for menthol-dependent channel opening.26, 29 Similarly, various TRPM8 residues (R842, H845, R851, K856 and R862) in S4 and the S4–S5 linker affect both the voltage and temperature activation of the channel. A specific mutant among these residues, R842H, has been reported to significantly decrease menthol affinity resulting in attenuated TRPM8 menthol-dependent currents.30
To understand the role of the SD in menthol dependent TRPM8 gating, the hTRPM8-SD was heterologously produced from E. coli. After screening various expression, purification, and solubilization conditions, milligram quantities of hTRPM8-SD can be produced. Various biophysical techniques, including solution nuclear magnetic resonance spectroscopy (NMR), far-UV circular dichroism (CD), and microscale thermophoresis (MST), were used to directly detect hTRPM8-SD–menthol interactions. Furthermore, previously reported menthol insensitive mutations, Y745H and R842H, were subjected to binding studies, which provide additional insight into TRPM8 ligand-dependent gating.26, 30
EXPERIMENTAL PROCEDURES
Identification of the hTRPM8-SD
PSIPRED31, JPRED32, and JUFO 3D33 secondary structure algorithms were used in conjunction with TMHMM34, MEMSAT335, MEMSAT-SVM36, TMpred37, HMMTOP38, SOSIU39, TopPred40, and TMMOD41 transmembrane prediction algorithms to identify consensus transmembrane helices. These bioinformatics results were then compared with JUFO9D33. Separately, structural-based sequence alignments of the following S1–S4 protein domains from TRPV123, Kv1.242, Kv1.2/2.1 chimera24, KvAP43, Ci-VSP44, Hv145, MlotiK46, and NavAb47 were generated with MUSTANG48 to produce a multiple sequence alignment. TRPM8 was then aligned to the structural-based sequence alignment with Clustal Omega49 which was analyzed and adjusted manually in ALINE50 to enhance agreement with the independent transmembrane and secondary structure predictions. The consensus from these studies indicates that the S0 helix (an amphipathic helix at the N-terminus of the S1 transmembrane helix) begins near Val674 and the S4 helix terminates near Pro855. Given that proline residues often indicate a break in secondary structure, the TRPM8-SD construct used in these studies includes residues Pro672 through Pro855 (Figure 2A). The identity of the TRPM8 sensing domain arrived at by these bioinformatics tools is consistent with other published studies that identify specific regions of the TRPM8-SD.30, 51
Figure 2.
Transmembrane topology and NMR and CD spectra of the hTRPM8-SD. (A) The hTRPM8-sensing domain (TRPM8-SD) transmembrane topology depicting helices S1 to S4 composed of residues 672 to 855 from the full length human TRPM8 ion channel (Uniprot code : Q7Z2W7). Previously identified menthol sensitive sites, Y742 and R842, are highlighted in blue. The residues and topology that constitute the hTRPM8-SD were identified by bioinformatics as described in the experimental procedures section. (B) Optimized 1H-15N-TROSY HSQC NMR spectrum and, (C) CD spectrum of purified hTRPM8-SD in LPPG micelle at pH 6.0 and 45 °C are consistent with a folded helical protein.
Construction of pET16b-10×His-hTRPM8-SD
The pJ vector encoding the SD of hTRPM8 carrying an N-terminal 6×His tag purchased from DNA 2.0 and was modified to include a 10×His tag and thrombin cleavage site (TCS). The following primers which included NcoI / BamHI restriction sites (bold) were used to amplify the hTRPM8-SD gene: forward primer (5’- AGATATACCATGGGTCATCATCACCATCATCACCACCATCATCACGGCTTAG-3’) and reverse primer (5’-CAGCCGGATCCTCGAGTTACGGACCCAGGTTAC-3’). The NcoI and BamHI restriction sites were used to subclone the 10×His-TCS-hTRPM8-SD into the pET16b vector resulting in hTRPM8-SD with a thrombin cleavable N-terminal 10×His tag. Y745H- and R842H-hTRPM8-SD were prepared using site-directed mutagenesis. All genes were sequence verified.
Expression and Purification
To optimize the overexpression of hTRPM8-SD, the pET16b-10×His-TCS-hTRPM8-SD construct was transformed into the following E. coli expression cell lines: BL21 (DE3), BL21 (DE3) Star, BL21-Codon-Plus (DE3) RP, Rosetta 2 (DE3), C41 (DE3), C43 (DE3)-Rosetta 2 and BL21 (DE3) pLysS. Human TRPM8-SD expression was tested at three temperatures (18 °C, 25 °C and 37 °C) and three isopropyl β-D-1-thiogalactopyranoside (IPTG) concentrations (0.25, 0.5 and 1.0 mM) in M9 minimal media. The composition of M9 media per liter was as follows: 6 g/L Na2HPO4·7H2O (Fisher Scientific), 3 g/L KH2PO4 (Fisher Scientific), 0.5 g/L NaCl (Fisher Scientific), 1 g/L NH4Cl (Sigma Aldrich), 4 g/L Glucose (Sigma), 2 mM Mg(SO4)2, 0.1 mM CaCl2, 0.01 mM metals mix (FeCl3, CuSO4, MnSO4, and ZnSO4). A slot-blot apparatus (Bio-Rad) was used to detect hTRPM8-SD overexpression levels by applying an optical density (OD600nm) normalized quantity of whole cell lysate to a nitrocellulose membrane by vacuum, followed by detection with anti(His)5 antibody (Abgent). BL21 (DE3) produced the highest quantity of hTRPM8-SD and were used in subsequent studies. For liter scale expression, 10 mL of primary culture grown at 37 °C overnight was used to inoculate 1 L of M9 media, and the cultures were then grown at 18 °C. Induction was initiated at an OD600 nm of 0.6 to 0.7 with 0.25 mM IPTG for 32 h at 18 °C.
Cells expressing hTRPM8-SD were harvested by centrifugation at 5000 × g for 15 min at 4 °C and washed with buffer A (50 mM Tris-Cl, pH 7.8, 300 mM NaCl). The washed cell pellet was resuspended and homogenized in a volume of 15 mL per gram of cell pellet in buffer A. This was followed by addition of 0.2 mg/mL Lysozyme, 0.02 mg/mL DNase and 0.02 mg/mL RNase, 5 µL of 0.1 M PMSF and 10 µL of 0.5 M magnesium acetate per mL of buffer A. The resolubilized cell pellet solution was tumbled at room temperature for 30 min. Cell lysis was completed by ultrasonication (Misonix, S-4000 Ultrasonic Processor from Qsonica, LLC) for 7 min with a 50% duty cycle of 5 sec on / 5 sec off. Empigen (N,N-Dimethyl-N-dodecylglycine betaine, Sigma Aldrich) was then added to the cell lysate to a final concentration of 3% (v/v) and tumbled at room temperature for 1 h to extract the protein. This was followed by centrifugation at 18,000 × g for 30 min. The pH of the supernatant after centrifugation was adjusted to 7.8 with 1 M NH4OH. The supernatant was incubated with pre-equilibrated Ni2+-loaded NTA resin (Qiagen, 0.75 mL resin per 50 mL lysate) in buffer A containing 1% Empigen. The resin bound protein was washed with 15 column volumes of 40 mM imidazole in buffer A containing 1% (v/v) Empigen until the A280nm returned to baseline. Empigen was exchanged to a more suitable membrane mimic with at least 15 column volumes of buffer B (25 mM sodium phosphate, pH 7.8) containing an appropriate detergent (0.25% w/v for DPC and DHPC, or 0.2% w/v for TDPC, SDS, LMPC, LMPG, LPPC and LPPG, or 0.46% LDAO). Finally hTRPM8-SD was eluted with 500 mM imidazole in 8 mL of buffer B containing the desired detergent. Purification results were verified using 14% SDS-PAGE followed by Coomassie blue staining.
The eluted protein from Ni2+-NTA column purification was buffer exchanged by ultrafiltration using 10 kD cut-off Amicon Ultra-15 centrifugal filter (Millipore) with five iterations of 3 mL dilution to exchange the buffer to thrombin cleavage buffer (Buffer B containing 150 mM NaCl and 1 mM CaCl2). Restriction grade thrombin (Novagen) at 2 U/mg of hTRPM8-SD was used to cleave the N-terminal 10×His tag from hTRPM8-SD. The cleavage reaction was carried out at room temperature with continuous tumbling for 24 h and >90% cleavage efficiency was observed by SDS-PAGE analysis. The thrombin cleaved protein was incubated with 0.5 mL of Ni2+-NTA resin for 2 h and flowed over the column to eliminate the uncleaved 10×His-hTRPM8-SD. Cleaved hTRPM8-SD was obtained in the flow-through. This thrombin cleaved hTRPM8-SD was approximately 80% pure and was further purified by gel filtration using a 33 cm XK 16 Superdex S200 column (GE Healthcare) pre-equilibrated with 25 mM sodium phosphate, pH 6.0 containing 0.5 mM EDTA and 0.1% LPPG. The purity and homogeneity of the hTRPM8-SD was verified with 14% Tris-glycine SDS-PAGE followed by Coomassie blue staining. The purified protein was further analyzed by trypsin digestion and LC-MS/MS mass spectrometry (MS Bioworks), which produced 11 hTRPM8-SD exclusive unique peptides with sequence coverage of 40%. Peptides from both the N- and C-termini of the SD were detected, indicating that full SD was expressed and purified. The final sequence of the expressed protein includes residues Pro672 through Pro855 from human TRPM8 and an N-terminal Gly-Ser dipeptide that remains after thrombin cleavage. The resulting purified hTRPM8-SD was used for NMR, CD and MST studies.
Optimization of NMR Conditions
For preparation of 15N-labeled hTRPM8-SD, overexpression was carried out by supplementing the M9 media with 15NH4Cl (Cambridge Isotope Laboratories, Inc). A number of detergents were used to identify a suitable membrane mimic that produced high quality NMR spectra and specifically bound the TRPM8 agonist menthol. The impact of the membrane mimic on hTRPM8-SD was evaluated by 1H-15N TROSY-HSQC data as judged by the proton dimension dispersion, number of total resonances, and quantifying the glycine backbone and tryptophan side chain resonances. Various pH values from 5.0–7.8 and temperatures from 15–55 °C were also screened. In addition, a few less common membrane mimics were evaluated including, mixed micelles (equimolar ratio of LPPG/DHPC and LPPG/DPC) and Amphipol A 8–35. Unless otherwise mentioned all NMR spectra were recorded on a Bruker 850 MHz 1H Avance III HD spectrometer with a 5mm TCI cryoprobe. The data were processed in NMRPipe52 and analyzed in CcpNmr.53
NMR-detected Menthol Binding Studies
Menthol titrations of the hTRPM8-SD were followed by 15N TROSY-HSQC NMR experiments carried out in a 3 mm tube (180 µL volume), in 25 mM sodium phosphate buffer, pH 6.0. The protein concentration was 270 µM with LPPG concentrations of approximately 80 mM. Menthol and the TRPM8 SD are both hydrophobic, and are found to interact in vivo within the hydrophobic context of the membrane lipid bilayer. Therefore, the most accurate and appropriate units to represent the affinity of TRPM8 for menthol is as a mole percent of the constituents of the membrane mimic in which it is reconstituted, where mole percent is equal to:
Molarity in this case poorly describes the concentration of menthol because it depends on the volume of the solution. However, menthol is sparingly soluble in aqueous solutions (~0.49 mg/mL) as it is predominantly partitioned to the micelle mimic. A simulated menthol partition constant between octanol and water (log Pow = 3.2, Advanced Chemistry Development/ Labs Percepta Predictors, I., Ed.) is consistent with published values of log Pow = 3.3.54 These partition constants indicate that for every menthol molecule in the aqueous environment there are approximately 2000 in the membrane mimic. Given this, using units of molarity are not reflective of the molecular environment because the solubility of menthol primarily depends on the concentration of membrane mimic and not the volume of solution. As a result, we prefer the more accurate and commonly used units for membrane proteins of mole percent when expressing binding affinities for lipophilic ligands. We note that this has been done in other hydrophobic ligand/membrane protein interaction studies.55, 56 Hence, to observe the interaction between menthol and Y745H-, R842H- and hTRPM8-SD, menthol titration experiments were carried out by acquiring 1H-15N TROSY NMR spectra at 45 °C with various menthol concentrations ranging from zero to ten mole percent.
The observed resonance chemical shift perturbation (Δδ) upon titration with menthol was monitored according to the following equation:
where ΔδH and ΔδN are the proton and nitrogen chemical shift position differences between the initial titration point (0 mol% menthol) and a given menthol concentration for a given resonance. For a number of resonances, when Δδ values were plotted as a function of menthol concentration, saturable binding isotherms were detected, indicating that the hTRPM8-SD specificly binds menthol. The dissociation constant, Kd was calculated by fitting (SigmaPlot) the curve with the following single site binding equation:
Where, (Δδ)max is maximum chemical shift perturbation, x is the mol% of menthol, and f(x) is correlated to the absolute value of the change in chemical shift.
Far-UV Circular Dichroism (CD) Spectroscopy
CD spectra were recorded using a temperature controlled JASCO J710 spectropolarimeter. 10 µM hTRPM8-SD in 25 mM sodium phosphate, 0.5 mM EDTA and 0.1% LPPG at pH 6.0 were used for the CD measurements. CD spectra were acquired at 45 °C in the far-UV (190 −250 nm) region with standard 100 mDeg sensitivity with a data pitch of 0.5 nm and scanning speed of 50 nm per minute. Additional CD spectra were also recorded at pH 7.36 to verify that the protonation state of the histidine imidazole side chains in the Y745H and R842H SD mutations did not affect menthol binding. All spectra were signal averaged over 5 scans and represented as mean residue ellipticity (MRE) in deg·cm2·dmol−1.
To study the effect of menthol on the qualitative change in secondary structure of Y745H-, R842H- and hTRPM8-SD, CD spectra were recorded in the absence and presence of 10 mole % menthol (stock menthol was prepared in 50% ethanol). Blank measurements were carried out by either buffer alone or buffer containing 10 mole % menthol, which does not produce significant absorbance in the far-UV region.
Microscale Thermophoresis (MST) Menthol Binding Assay
MST was carried out on a monolith NT.115 series instrument (NanoTemper Technologies GmbH). NT-647 based maleimide dye was prepared as a stock solution in 25% DMSO (v/v) and standard treated capillaries were purchased from NanoTemper Technologies GmbH. For protein labeling, hTRPM8-SD carrying the 10×His tag was reacted with the fluorescent label in 1:1 mole ratio and incubated overnight at room temperature. To eliminate the free non-reacted NT-647 label, the protein-dye reaction mixture was incubated with 200 µL of Ni2+-NTA resin for 2 h at room temperature and was further washed with 20 mL (100 column volumes) of 25 mM sodium phosphate, pH 7.8 and 50 mM NaCl. The fluorescently labeled hTRPM8-SD was eluted with 500 mM imidazole and the 10×His tag was then cleaved with thrombin as described above. To validate the interaction between menthol and hTRPM8-SD, various concentrations of menthol or WS-12 (a potent TRPM8 agonist menthol derivative) with hTRPM8-SD were prepared by serial dilution. The protein-agonist mixtures were transferred to the capillaries and thermophoresis was measured at 45 °C with the LED and MST power both set to 75%, 30 s MST on, with 5 s fluorescence recording before and after applying MST and with a delay of 25 s between each measurement. MST experiments were performed in triplicate and the average data were plotted with the error calculated as the standard error of mean (SEM).
Cell Culture and Electrophysiology Measurements
Human embryonic kidney (HEK) 293 cells (ATCC CRL-1573) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/mL penicillin-streptomycin, and 2 mM L-glutamine (Gibco). Cells were cultured in 35 mm polystyrene dishes (Falcon) at 37 °C in the presence of 5% CO2. Cells were transiently co-transfected with hTRPM8 in a pIRES2-EGFP vector. This construct expresses bicistronic mRNA with an internal ribosome entry site positioned between hTRPM8 and the EGFP (Enhanced Green Fluorescent Protein) reporter gene such that the reporter is not covalently fused to the protein of interest. Transfection was achieved using Fugene 6 transfection reagent (Promega) and 0.5 µg of plasmid at a ratio of 1 µL transfection reagent per µg DNA. Cells were plated on glass coverslips for 36–48 h after transfection, and whole-cell voltage-clamp electrophysiology recordings were performed 2–5 h later.
Transfected cells were released from culture dishes by brief exposure to 0.25% trypsin/EDTA and resuspension in supplemented DMEM; cells were then plated on glass coverslips and allowed to recover for 1–2 h at 37 °C in 5% CO2. Cells that exhibited green fluorescence indicating successful transfection were selected for electrophysiology measurements. Whole-cell voltage-clamp current measurements were performed using an Axopatch 200B amplifier (Axon Instruments) and pClamp 10.3 software (Axon Instruments). Data was acquired at 2 kHz and filtered at 1 kHz. Patch pipettes were pulled using a P-2000 laser puller (Sutter Instruments) from borosilicate glass capillaries (World Precision Instruments) and heat-polished using a MF-830 microforge (Narishige). Pipettes had resistances of 2–5 MΩ in the extracellular solution. A reference electrode was placed in a 2% agar bridge made with a composition similar to the extracellular solution. Experiments were performed at 22 ± 1 °C. Cells were placed in a chamber with extracellular solution containing 132 mM NaCl, 4.8 mM KCl, 1.2 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and 5 mM glucose, with pH adjusted to 7.4 using NaOH and osmolality adjusted to 310 mOsm using sucrose. Pipettes were filled with a solution containing 135 mM K+ gluconate, 5 mM KCl, 1 mM MgCl2, 5 mM EGTA, and 10 mM HEPES, with pH adjusted to 7.2 with KOH, and osmolality adjusted to 300 mOsm using sucrose. Osmolality was measured using a Vapro 5600 vapor pressure osmometer (Wescor). For menthol perfusion, a stock solution was prepared by dissolving menthol in ethanol at a concentration of 100 mg/mL and diluted to specified concentrations with extracellular solution. Solutions were perfused across cells at a flow rate of ~0.5 mL/min.
RESULTS
Optimization of hTRPM8-SD Expression, Purification, and NMR Conditions
hTRPM8-SD expression was optimized from different E. coli strains with the highest expression level observed from the BL21 (DE3) cell line. hTRPM8-SD was primarily localized to inclusion bodies and after subsequent whole cell lysate extraction using 3% Empigen, purification using Ni-NTA affinity chromatography, thrombin cleavage, and size exclusion chromatography in various detergent micelles resulted in an average yield of 0.75 mg of purified protein per liter of M9 culture (Figure S1). After screening various NMR suitable detergents, poor quality 1H-15N TROSY-HSQC spectra were obtained in DPC, TDPC, LPPC, LMPG, LMPC and LDAO as assessed by the fact that <50% of the expected backbone resonances were detected (Figure S2). However, a comparatively better 1H-15N TROSY-HSQC spectrum was obtained in LPPG micelles, which contained the expected number of back bone resonances, the expected six tryptophan indole side chain resonances, and good resolution and dispersion.
The use of mixed micelles has been shown in some cases to enhance the quality of NMR spectra of membrane proteins.57–59 Applied to hTRPM8-SD using LPPG/DPC or LPPG/DHPC mixed micelles, the data suggest that the mixed micelles potentially stabilize some parts of hTRPM8-SD as evidenced by higher resolution and better resonance dispersion in the central part of the 1H-15N TROSY-HSQC spectrum; however, four out of six tryptophan side chain indole amine resonances disappeared, indicative of increased flexibility in other parts of the protein (Figure S2). In addition to optimization of the membrane mimic, the effect of pH and temperature on the quality of 1H-15N TROSY-HSQC spectrum was probed. On the basis of the expected number of well dispersed resonances, the quality of the NMR spectrum was best at pH 6.0 and at 45 °C and subsequently used for further NMR studies (Figure 2B). The narrow dispersion of hTRPM8-SD resonances over 1H chemical shift of 7 to 9 ppm is consistent with folded helical membrane protein. The far-UV CD spectrum under the same conditions had negative minima at 221 and 208 nm and a positive maximum at 195 nm consistent with a helical protein (Figure 2C).
Menthol Binding Studies of hTRPM8-SD
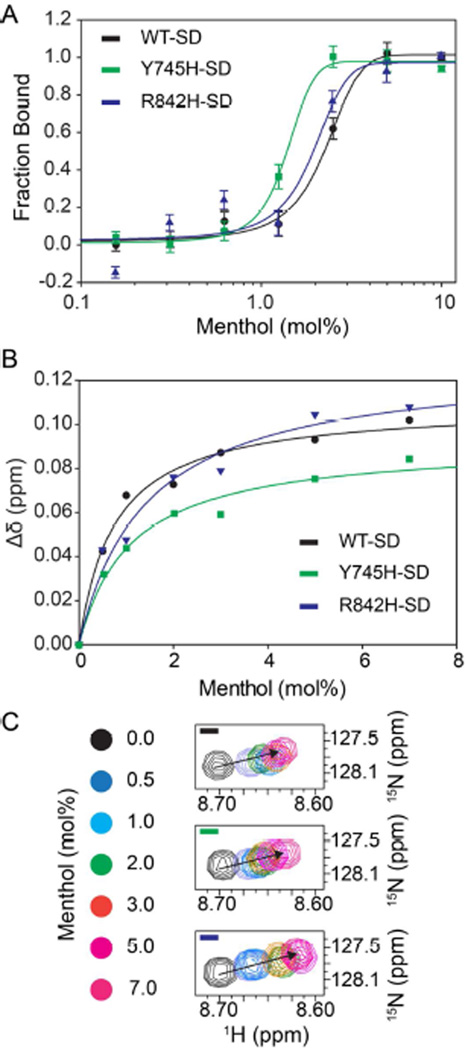
Functional whole-cell patch-clamp electrophysiology measurements show that hTRPM8 mediated currents are dramatically increased when cells are exposed to menthol (Figure 1). Binding studies of the purified hTRPM8-SD were carried out as a function of added menthol using NMR, CD, and MST. First, 1H-15N TROSY-NMR experiments were recorded at different menthol concentrations and menthol-dependent chemical shift perturbation was measured. Mole percent Kd values were extracted as described above from resonances that displayed specific binding with an average value of 1.1 ± 0.2 mole% (Figure 3). The specific binding of menthol indicates that the purified hTRPM8-SD in LPPG micelles is properly folded. Second, far-UV CD experiments were carried out in the absence and presence of 10 mole% menthol. The CD data are consistent with a reduction in helical content at increased menthol concentrations as indicated by reduced MRE values at 208 and 222 nm (Figure 4A). The CD data for hTRPM8-SD in LPPG micelles are consistent with the NMR binding data that hTRPM8-SD binds to menthol, and suggests that menthol induces a change in secondary structural content. Finally, menthol binding to the hTRPM8-SD was also shown by using MST, which has been used to detect ligand binding in other membrane proteins including GPCRs and transporters.60–63 The MST-detected Kd for menthol binding to hTRPM8-SD is 1.3 ± 0.2 mol% (Figure 5A). As a positive control, the menthol derived TRPM8 agonist, WS-12, was also subjected to binding studies by MST (Figure S3). WS-12 is reported to have an EC50 value that is ~10× higher and an efficacy that is double that of menthol.64 As such one would expect an increase in binding affinity for WS-12 relative to menthol. Figure S3 shows the expected leftward shift in the binding curve and fitting the data indicates that the WS-12 affinity is double that of menthol under identical conditions. In summary, menthol binding studies with three biophysical techniques clearly demonstrate that the heterologously expressed and purified hTRPM8-SD in LPPG micelles is properly folded and is suitable for a variety of additional studies.
Figure 3.
NMR detection of specific menthol binding to the hTRPM8-SD. 1H-15N-TROSY NMR spectra of the hTRPM8-SD in LPPG micelles at pH 6.0 and 45 °C as a function of menthol concentration show chemical shift perturbation. A number of resonance positions shift with increased menthol concentrations (upper right), four representative resonances are highlighted in the overlaid spectra (left panel) which show saturable and menthol binding (bottom right). Fitting the chemical shift perturbations (Δδ) to a single site binding equation identify the Kd of menthol binding to the hTRPM8 SD as 1.1 ± 0.2 mole percent.
Figure 4.
CD and NMR detected menthol dependent hTRPM8-SD structural changes. CD spectra (left panels) and NMR spectra (right panels) of hTRPM8-SD (WT-SD), Y745H hTRPM8-SD, and R842H hTRPM8-SD in LPPG micelles at pH 6.0 and 45 °C with zero and 10 mole % menthol concentrations are consistent with menthol induced structural perturbation. CD spectra of the WT-SD suggest secondary structure changes of the protein upon binding to menthol. This loss of helicity is detected as a decrease in magnitude of mean residue ellipticity at 208 and 221 nm. NMR data (right panels) highlight changes in chemical shift position upon addition of menthol.
Figure 5.
The menthol insensitive hTRPM8-SD mutants, Y745H and R842H, bind menthol specifically. (A) Microscale thermophoresis binding curves of hTRPM8-SD (WT-SD), Y745H-hTRPM8-SD and R842H-hTRPM8-SD illustrate that the menthol binds to the SD and SD mutants with similar affinity as indicated by Kd values of 1.3 ± 0.2, 1.4 ± 0.1 and 1.7 ± 0.3 mole percent respectively. (B) NMR-detected binding isotherms of hTRPM8-SD (WT-SD), Y745H-hTRPM8-SD and R842H-hTRPM8-SD validate the MST affinity measurements with Kd values of 1.1 ± 0.1, 1.1 ± 0.2 and 1.0 ± 0.1 mole percentage respectively. The NMR determined Kd values between the WT and mutant SD proteins are derived from a representative resonance (C). This resonance undergoes similar chemical shift perturbation between the three SD proteins. Taken together the Y745H and R842H mutations do not significantly alter the menthol binding affinity.
Effect of Menthol Binding on Y745H- and R842H-hTRPM8-SD Mutants
Previous TRPM8–menthol studies have identified a handful of residues critical for menthol-dependent activation of TRPM8. These studies have identified mutants by random mutagenesis and detected the functional outcomes by electrophysiology and/or calcium imaging experiments (i.e. structure-function studies). Two prominently identified menthol binding residues are Tyr745 and Arg842 from the mouse and human orthologous proteins respectively. Mutations of these residues have been reported to decrease menthol affinity and simultaneously play a significant role in TRPM8 voltage dependence and temperature sensitivity. Mutations in the hTRPM8-SD were generated in an effort to probe the direct menthol binding of these functionally identified residues. As shown in Figure 5, in the context of an isolated hTRPM8-SD, these mutations do not significantly affect menthol binding as detected by MST and NMR. Indeed, the observed binding curves are similar to the wild-type (WT) protein domain with only minor changes in Kd values. An overlay of the WT and mutant protein spectra (Figure S4) show only minor resonance changes, suggesting that these mutations at Y745 and R842 to histidine do not likely alter the overall structure of the protein domain. This interpretation is congruent with the far-CD data, in which the Y745H and R842H spectra are comparable to the wild-type (WT) domain spectrum (Figure 4). The CD spectral changes resulting from menthol binding to the TRPM8-SD are consistent at pH 6.0 and physiological pH (7.36) (Figures 4 and S5). This indicates that protonation state of the Y745H and R842H histidine imidazole side chain is not important in binding. We note that these results are congruent with previous full length TRPM8 studies which showed that menthol induced TRPM8 currents are relatively stable as a function of pH between pH values of 6.1 and 7.4.14, 65 Taken together, these data show that mutating the TRPM8 residues, Y745 and R842 to histidine does not significantly affect menthol binding. Moreover, the NMR, CD, and MST data show direct and specific binding to the TRPM8-SD which has implications for the menthol dependent-gating mechanism.
DISCUSSION
Dissecting the mechanism(s) of polymodal TRPM8 channel gating via diverse stimuli such as temperature, voltage, ligands, lipids and accessory proteins, is challenging. One current limitation is the ability to obtain sufficient quantities of properly folded TRPM8 for structural biology and biophysical pursuits. After screening more than 100 expression conditions including cell lines, plasmids, growth and induction temperatures, we isolated conditions that allow for production of milligram quantities of pure and stable hTRPM8-SD. The menthol binding data indicate that the E. coli produced hTRPM8-SD is in a biologically relevant state.
In the present study, we used NMR as a tool to screen a number of micelles and mixed-micelle compositions and observed the highest quality spectrum in LPPG lysolipid micelles. In LPPG micelles, hTRPM8-SD binds specifically to menthol as demonstrated using NMR, CD, and MST (Figures 3 and 5). The reconstituted protein not only binds to menthol, but it also interacts with WS-12, a potent TRPM8 agonist derived from menthol (Figure S3). It is interesting to note that lysolipid based PG detergents have also been successfully used in previous voltage-sensing domain studies of human KCNQ1 and hERG ion channels.66, 67 TRPM8, KCNQ1, and hERG, have all been shown to reside in lipid micro-domains, suggesting that lysolipids could be a generally suitable detergent environment for other micro-domain trafficked human ion channels.68–70
The general topology of TRPM8 is homologous to other TRP and voltage-gated ion channels (VGICs), but unlike VGICs, the functional role of the sensing domain in TRP channels is currently not well understood. For example, in VGICs it is clear that the VSD senses and transduces a change in electrical potential into mechanical force, which gates the channel.71 Other voltage-sensing proteins, including phosphatases, share the sensing, conformational changes, and information coupling mechanisms of VSDs from VGICs. Recently, X-ray structures of the VSD from Ci-VSP highlight this mechanistic conservation with significant conformational rearrangements of the VSD, particularly with regards to the translocation of the S4 helix upon activation.44 In contrast, cryo-EM structures of a truncated rat TRPV1 construct in the presence and absence of agonists suggest that the TRPV1-SD does not undergo appreciable conformational change, even though the primary functional determinant of capsaicin agonism has been pinpointed to residue Y511 in the S3 helix of the TRPV1 SD.72–74
The CD and NMR data of the hTRPM8-SD are consistent with menthol induced conformational change (Figures 3 and 4) and suggest a mechanism in which hTRPM8 menthol-dependent gating is initiated in the SD. This conformational change is then coupled to the pore domain leading to menthol induced activation (Figure 6). This indicates that the mechanism of menthol activation of TRPM8 may be distinct from capsaicin activation of TRPV1. Further biophysical and structural studies will be required to distinguish the differences and similarities of ligand-based TRPM8 and TRPV1 gating.
Figure 6.
Mechanistic model of menthol activated hTRPM8 channel gating. TRPM8 ligand activation is at least a two-step mechanism, where menthol dependent gating is initiated in the sensing domain (blue, middle panel) where via conformational changes, the binding information is subsequently coupled to the pore domain resulting in channel opening (bottom panel). The Y745H and R842H SD mutations are menthol binding competent, but affect the ability of TRPM8 to couple menthol binding to the resulting conformational change in the pore domain (green) that in turn activates the channel.
Previous studies have suggested that menthol binding of the channel is dependent on the residues Y745 and R842.26, 30 In this study, we have shown that individually mutating these residues in the sensing domain does not appreciably alter the TRPM8-SD affinity for menthol (Figures 4 and 5). Previous TRPM8 menthol binding studies have been indirect, where ion conductance is monitored as a function of menthol concentration. To our knowledge, the data presented here are the first to show directly measured TRPM8 affinities, whereas previous studies measure an apparent menthol affinity (EC50). EC50 is an indirect measure of agonist affinity that depends on multiple mechanistic steps. For ligand activated processes, like menthol induced TRPM8 currents, EC50 values measure information that depends on at least two equilibrium constants. One equilibrium constant that reports on ligand binding and a second that depends on a conformational change (gating) that leads to channel activation (coupling).75 For a simple two-step mechanism we expect the following:
Where menthol (A) and TRPM8 (R) bind to form the inactive complex (AR); this binding event is coupled from the TRPM8-SD to the PD which undergoes a conformational change resulting in the active state (AR*). In structure-function studies, the current is measured relative to the agonist concentration and determines the apparent menthol affinity (EC50). The mechanistic steps can be written in terms of equilibrium constants, Kd, the dissociation constant, which describes menthol binding and E, which is a measure of the coupling to conformational change. These equilibrium constants are defined as: from the equation above. For a simple two-step menthol activation mechanism, the apparent affinity from structure-function measurements is:
In the current work, the Kd is directly measured with various biophysical techniques, and as is noted in the preceding equation, affinity and apparent affinity are proportional but not equal to each other. This makes direct comparisons between the current and previous menthol binding studies challenging.75 It is noted that in reality the menthol agonism of TRPM8 is likely more complex mechanistically than the simple mechanism shown above, making the relationship between affinity and apparent affinity more opaque. Additional issues that complicate comparisons between the direct and apparent menthol affinities include the tetrameric nature and presumed cooperativity in TRPM8 menthol activation and the fact that the hTRPM8-SD lacks the TRPM8 S4–S5 linker and pore domain which may impact the absolute menthol affinity. Nonetheless, the simple two-step mechanism is useful in illustrating the relationship between the data presented here and existing data in the literature.
Heterologously produced hTRPM8-SD specifically binds menthol. These data show that ligand binding and channel gating are likely coupled mechanistically via conformational change in the hTRPM8-SD, and that R842 and Y745 are not integral to the affinity for menthol, but instead must play a role in coupling. The resulting mechanistic picture that emerges is that TRPM8-SD binds to menthol and the structural coupling that results in gating are independent processes. Elucidation of this mechanism will be benefited with high-resolution structural data in the absence and presence of menthol, which should be an achievable goal.
Supplementary Material
Acknowledgments
The authors acknowledge other Van Horn lab members for discussion and the laboratory of Prof. John Chaput (Department of Pharmaceutical Sciences, University of California, Irvine) for assistance with and access to microscale thermophoresis instrumentation.
Funding Sources
WVH acknowledges support from a Bisgrove Early Career Award from Science Foundation Arizona and the National Institutes of Health (NIH R01GM112077).
ABBREVIATIONS
- CD
circular dichroism
- DPC
n-dodecylphosphocholine
- DHPC
1,2-dihexanoyl-sn-glycero-3-phosphocholine
- hTRPM8
human transient receptor potential melastatin 8
- LMPC
1-myristoyl-2-hydroxy-sn-glycero-3-phosphocholine
- LMPG
1-myristoyl-2-hydroxy-sn-glycero-3-phosphoglycerol
- LPPC
1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine
- LPPG
1-palmitoyl-2-hydroxy-sn-glycero-3-phosphoglycerol
- MST
microscale thermophoresis
- MRE
mean residue ellipticity
- NMR
nuclear magnetic resonance
- SD
sensing domain
- SDS
sodium dodecyl sulfate
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- TDPC
n-tetradecylphosphocholine
- Tris
tris(hydroxymethyl)aminomethane
- WS-12
(2S,5R)-2-Isopropyl-N-(4-methoxyphenyl)-5-methylcyclohexanecarboximide
- WT
wild-type
Footnotes
Author Contributions
WVH and PR designed the experiments. PR and NJS acquired and processed NMR data. PR acquired and processed CD and MST data. JKH performed electrophysiology experiments. All authors contributed in analysis and manuscript writing. All authors have given approval to the final version of the manuscript.
REFERENCES
- 1.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol. Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 3.Venkatachalam K, Montell C. TRP Channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 5.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 6.Bai VU, Murthy S, Chinnakannu K, Muhletaler F, Tejwani S, Barrack ER, Kim SH, Menon M, Reddy GPV. Androgen regulated TRPM8 expression: A potential mRNA marker for metastatic prostate cancer detection in body fluids. Int. J. Onc. 2010;36:443–450. [PubMed] [Google Scholar]
- 7.Bidaux G, Flourakis M, Thebault S, Zholos A, Beck B, Gkika D, Roudbaraki M, Bonnal JL, Mauroy B, Shuba Y, Skryma R, Prevarskaya N. Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function. J. Clin. Invest. 2007;117:1647–1657. doi: 10.1172/JCI30168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Wang XH, Yang ZH, Wang B, Li SL. Menthol Induces Cell Death via the TRPM8 Channel in the Human Bladder Cancer Cell Line T24. Oncology. 2009;77:335–341. doi: 10.1159/000264627. [DOI] [PubMed] [Google Scholar]
- 9.Yamamura H, Ugawa S, Ueda T, Morita A, Shimada S. TRPM8 activation suppresses cellular viability in human melanoma. Am. J. Physiol.Cell Physiol. 2008;295:C296–C301. doi: 10.1152/ajpcell.00499.2007. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Barritt GJ. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004;64:8365–8373. doi: 10.1158/0008-5472.CAN-04-2146. [DOI] [PubMed] [Google Scholar]
- 11.Duncan D, Stewart F, Frohlich M, Urdal D. Preclinical Evaluation of the Trpm8 Ion Channel Agonist D-3263 for Benign Prostatic Hyperplasia. J. Urol. 2009;181:503–503. [Google Scholar]
- 12.Fallon MT, Storey DJ, Krishan A, Weir CJ, Mitchell R, Fleetwood-Walker SM, Scott AC, Colvin LA. Cancer treatment-related neuropathic pain: proof of concept study with menthol-a TRPM8 agonist. Support Care Cancer. 2015;23:2769–2777. doi: 10.1007/s00520-015-2642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grace MS, Dubuis E, Birrell MA, Belvisi MG. Pre-clinical studies in cough research: role of Transient Receptor Potential (TRP) channels. Pulm. Pharmacol. Ther. 2013;26:498–507. doi: 10.1016/j.pupt.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersson DA, Chase HW, Bevan S. TRPM8 activation by menthol, icilin, and cold is differentially modulated by intracellular pH. J. Neurosci. 2004;24:5364–5369. doi: 10.1523/JNEUROSCI.0890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao C, Yudin Y, Bikard Y, Chen W, Liu T, Li H, Jendrossek D, Cohen A, Pavlov E, Rohacs T, Zakharian E. Polyester modification of the mammalian TRPM8 channel protein: implications for structure and function. Cell Rep. 2013;4:302–315. doi: 10.1016/j.celrep.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gkika D, Lemonnier L, Shapovalov G, Gordienko D, Poux C, Bernardini M, Bokhobza A, Bidaux G, Degerny C, Verreman K, Guarmit B, Benahmed M, de Launoit Y, Bindels RJ, Fiorio Pla A, Prevarskaya N. TRP channel-associated factors are a novel protein family that regulates TRPM8 trafficking and activity. J. Cell Biol. 2015;208:89–107. doi: 10.1083/jcb.201402076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilton JK, Rath P, Helsell CV, Beckstein O, Van Horn WD. Understanding thermosensitive transient receptor potential channels as versatile polymodal cellular sensors. Biochemistry. 2015;54:2401–2413. doi: 10.1021/acs.biochem.5b00071. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn FJ, Kuhn C, Luckhoff A. Inhibition of TRPM8 by icilin distinct from desensitization induced by menthol and menthol derivatives. J. Biol. Chem. 2009;284:4102–4111. doi: 10.1074/jbc.M806651200. [DOI] [PubMed] [Google Scholar]
- 19.Rohacs T. Phosphoinositide Regulation of TRP Channels. Handb. Exp. Pharmacol. 2014;223:1143–1176. doi: 10.1007/978-3-319-05161-1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Z, Kim A, Masuch T, Park K, Weng H, Wetzel C, Dong X. Pirt functions as an endogenous regulator of TRPM8. Nat. Commun. 2013;4:2179. doi: 10.1038/ncomms3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zakharian E, Cao C, Rohacs T. Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J. Neurosci. 2010;30:12526–12534. doi: 10.1523/JNEUROSCI.3189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zakharian E, Thyagarajan B, French RJ, Pavlov E, Rohacs T. Inorganic polyphosphate modulates TRPM8 channels. PLoS One. 2009;4:e5404. doi: 10.1371/journal.pone.0005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 25.Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015;520:511–517. doi: 10.1038/nature14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW, Patapoutian A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat. Neurosci. 2006;9:493–500. doi: 10.1038/nn1665. [DOI] [PubMed] [Google Scholar]
- 27.Chuang HH, Neuhausser WM, Julius D. The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron. 2004;43:859–869. doi: 10.1016/j.neuron.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 28.Gavva NR, Klionsky L, Qu Y, Shi L, Tamir R, Edenson S, Zhang TJ, Viswanadhan VN, Toth A, Pearce LV, Vanderah TW, Porreca F, Blumberg PM, Lile J, Sun Y, Wild K, Louis JC, Treanor JJ. Molecular determinants of vanilloid sensitivity in TRPV1. J. Biol. Chem. 2004;279:20283–20295. doi: 10.1074/jbc.M312577200. [DOI] [PubMed] [Google Scholar]
- 29.Malkia A, Pertusa M, Fernandez-Ballester G, Ferrer-Montiel A, Viana F. Differential role of the menthol-binding residue Y745 in the antagonism of thermally gated TRPM8 channels. Mol. Pain. 2009;5:62. doi: 10.1186/1744-8069-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voets T, Owsianik G, Janssens A, Talavera K, Nilius B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat. Chem. Biol. 2007;3:174–182. doi: 10.1038/nchembio862. [DOI] [PubMed] [Google Scholar]
- 31.Buchan DW, Minneci F, Nugent TC, Bryson K, Jones DT. Scalable web services for the PSIPRED Protein Analysis Workbench. Nucleic. Acids. Res. 2013;41:W349–W357. doi: 10.1093/nar/gkt381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole C, Barber JD, Barton GJ. The Jpred 3 secondary structure prediction server. Nucleic. Acids. Res. 2008;36:W197–W201. doi: 10.1093/nar/gkn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leman JK, Mueller R, Karakas M, Woetzel N, Meiler J. Simultaneous prediction of protein secondary structure and transmembrane spans. Proteins. 2013;81:1127–1140. doi: 10.1002/prot.24258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 35.Jones DT, Taylor WR, Thornton JM. A model recognition approach to the prediction of all-helical membrane protein structure and topology. Biochemistry. 1994;33:3038–3049. doi: 10.1021/bi00176a037. [DOI] [PubMed] [Google Scholar]
- 36.Nugent T, Jones DT. Transmembrane protein topology prediction using support vector machines. BMC Bioinformatics. 2009;10:159. doi: 10.1186/1471-2105-10-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofman KaSW. TMbase - A database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler. 1993;374:166. [Google Scholar]
- 38.Tusnady GE, Simon I. The HMMTOP transmembrane topology prediction server. Bioinformatics. 2001;17:849–850. doi: 10.1093/bioinformatics/17.9.849. [DOI] [PubMed] [Google Scholar]
- 39.Hirokawa T, Boon-Chieng S, Mitaku S. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics. 1998;14:378–379. doi: 10.1093/bioinformatics/14.4.378. [DOI] [PubMed] [Google Scholar]
- 40.von Heijne G. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J. Mol. Biol. 1992;225:487–494. doi: 10.1016/0022-2836(92)90934-c. [DOI] [PubMed] [Google Scholar]
- 41.Kahsay RY, Gao G, Liao L. An improved hidden Markov model for transmembrane protein detection and topology prediction and its applications to complete genomes. Bioinformatics. 2005;21:1853–1858. doi: 10.1093/bioinformatics/bti303. [DOI] [PubMed] [Google Scholar]
- 42.Chen XR, Wang QH, Ni FY, Ma JP. Structure of the full-length Shaker potassium channel Kv1.2 by normal-mode-based X-ray crystallographic refinement. Proc. Natl. Acad. Sci.U.S.A. 2010;107:11352–11357. doi: 10.1073/pnas.1000142107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang YX, Lee A, Chen JY, Ruta V, Cadene M, Chait BT, MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 44.Li Q, Wanderling S, Paduch M, Medovoy D, Singharoy A, McGreevy R, Villalba-Galea CA, Hulse RE, Roux B, Schulten K, Kossiakoff A, Perozo E. Structural mechanism of voltage-dependent gating in an isolated voltage-sensing domain. Nat. Struct. Mol. Biol. 2014;21:244–252. doi: 10.1038/nsmb.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeshita K, Sakata S, Yamashita E, Fujiwara Y, Kawanabe A, Kurokawa T, Okochi Y, Matsuda M, Narita H, Okamura Y, Nakagawa A. X-ray crystal structure of voltage-gated proton channel. Nat. Struct. Mol. Biol. 2014;21:352–U170. doi: 10.1038/nsmb.2783. [DOI] [PubMed] [Google Scholar]
- 46.Clayton GM, Altieri S, Heginbotham L, Unger VM, Morais-Cabral JH. Structure of the transmembrane regions of a bacterial cyclic nucleotide-regulated channel. Proc. Natl. Acad. Sci.U.S.A. 2008;105:1511–1515. doi: 10.1073/pnas.0711533105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konagurthu AS, Reboul CF, Schmidberger JW, Irving JA, Lesk AM, Stuckey PJ, Whisstock JC, Buckle AM. MUSTANG-MR structural sieving server: applications in protein structural analysis and crystallography. PLoS One. 2010;5:e10048. doi: 10.1371/journal.pone.0010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bond CS, Schuttelkopf AW. ALINE: a WYSIWYG protein-sequence alignment editor for publication-quality alignments. Acta. Crystallogr. D. Biol. Crystallogr. 2009;65:510–512. doi: 10.1107/S0907444909007835. [DOI] [PubMed] [Google Scholar]
- 51.Kalia J, Swartz KJ. Exploring structure-function relationships between TRP and Kv channels. Scientific Reports. 2013:3. doi: 10.1038/srep01523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. Nmrpipe - a Multidimensional Spectral Processing System Based on Unix Pipes. J. Biomol. Nmr. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 53.Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas M, Ulrich EL, Markley JL, Ionides J, Laue ED. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins. 2005;59:687–696. doi: 10.1002/prot.20449. [DOI] [PubMed] [Google Scholar]
- 54.Chemicals Inspection and Testing Institute, Biodegradation and Bioaccumulation data of existing chemicals based on the CSCL Japan. Japan: Chemical Industry Ecology-Toxicology & Information Center; 1992. ISBN 4-98074-98101-98071. [Google Scholar]
- 55.Barrett PJ, Song Y, Van Horn WD, Hustedt EJ, Schafer JM, Hadziselimovic A, Beel AJ, Sanders CR. The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science. 2012;336:1168–1171. doi: 10.1126/science.1219988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beel AJ, Mobley CK, Kim HJ, Tian F, Hadziselimovic A, Jap B, Prestegard JH, Sanders CR. Structural studies of the transmembrane C-terminal domain of the amyloid precursor protein (APP): does APP function as a cholesterol sensor? Biochemistry. 2008;47:9428–9446. doi: 10.1021/bi800993c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Columbus L, Lipfert J, Jambunathan K, Fox DA, Sim AYL, Doniach S, Lesley SA. Mixing and Matching Detergents for Membrane Protein NMR Structure Determination. J. Am. Chem. Soc. 2009;131:7320–7326. doi: 10.1021/ja808776j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poget SF, Girvin ME. Solution NMR of membrane proteins in bilayer mimics: Small is beautiful, but sometimes bigger is better. Biochim. Biophys. Acta-Biomembr. 2007;1768:3098–3106. doi: 10.1016/j.bbamem.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warschawski DE, Arnold AA, Beaugrand M, Gravel A, Chartrand E, Marcotte I. Choosing membrane mimetics for NMR structural studies of transmembrane proteins. Biochim. Biophys. Acta-Biomembr. 2011;1808:1957–1974. doi: 10.1016/j.bbamem.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 60.Jerabek-Willemsen M, Wienken CJ, Braun D, Baaske P, Duhr S. Molecular interaction studies using microscale thermophoresis. Assay Drug Dev. Technol. 2011;9:342–353. doi: 10.1089/adt.2011.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parker JL, Newstead S. Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature. 2014;507:68–72. doi: 10.1038/nature13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seidel SA, Dijkman PM, Lea WA, van den Bogaart G, Jerabek-Willemsen M, Lazic A, Joseph JS, Srinivasan P, Baaske P, Simeonov A, Katritch I, Melo FA, Ladbury JE, Schreiber G, Watts A, Braun D, Duhr S. Microscale thermophoresis quantifies biomolecular interactions under previously challenging conditions. Methods. 2013;59:301–315. doi: 10.1016/j.ymeth.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh SK, Gluck JM, Mockel L, Hung YF, Willbold D, Koenig BW. Membrane Protein Interaction Studies using Microscale Thermophoresis. Biophysical J. 2013;104:557a–558a. [Google Scholar]
- 64.Ma S, G G, Ak VE, Jf D, H H. Menthol derivative WS-12 selectively activates transient receptor potential melastatin-8 (TRPM8) ion channels. Pak. J. Pharm. Sci. 2008;21:370–378. [PubMed] [Google Scholar]
- 65.Sherkheli MA, Vogt-Eisele AK, Bura D, Beltran Marques LR, Gisselmann G, Hatt H. Characterization of selective TRPM8 ligands and their structure activity response (S.A.R) relationship. J. Pharm. Pharm. Sci. 2010;13:242–253. doi: 10.18433/j3n88n. [DOI] [PubMed] [Google Scholar]
- 66.Ng HQ, Kim YM, Huang QW, Gayen S, Yildiz AA, Yoon HS, Sinner EK, Kang C. Purification and structural characterization of the voltage-sensor domain of the hERG potassium channel. Protein Expression and Purif. 2012;86:98–104. doi: 10.1016/j.pep.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Peng D, Kim JH, Kroncke BM, Law CL, Xia Y, Droege KD, Van Horn WD, Vanoye CG, Sanders CR. Purification and structural study of the voltage-sensor domain of the human KCNQ1 potassium ion channel. Biochemistry. 2014;53:2032–2042. doi: 10.1021/bi500102w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ganapathi SB, Fox TE, Kester M, Elmslie KS. Ceramide modulates HERG potassium channel gating by translocation into lipid rafts. Am. J. Physiol. Cell Physiol. 2010;299:C74–C86. doi: 10.1152/ajpcell.00462.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martens JR, O’Connell K, Tamkun M. Targeting of ion channels to membrane microdomains: localization of KV channels to lipid rafts. Trends Pharmacol. Sci. 2004;25:16–21. doi: 10.1016/j.tips.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 70.Roura-Ferrer M, Sole L, Oliveras A, Dahan R, Bielanska J, Villarroel A, Comes N, Felipe A. Impact of KCNE subunits on KCNQ1 (Kv7.1) channel membrane surface targeting. J. Cell Physiol. 2010;225:692–700. doi: 10.1002/jcp.22265. [DOI] [PubMed] [Google Scholar]
- 71.Long SB, Campbell EB, Mackinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–908. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- 72.Cao E, Liao M, Cheng Y, Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504:113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- 74.Yang F, Xiao X, Cheng W, Yang W, Yu P, Song Z, Yarov-Yarovoy V, Zheng J. Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat. Chem. Biol. 2015;11:518–524. doi: 10.1038/nchembio.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br. J. Pharmacol. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.