Abstract
Objectives
Rapidly escalating rates of heroin and prescription opioid use have been widely observed in rural areas across the United States. Although US Food and Drug Administration-approved medications for opioid use disorders exist, they are not routinely accessible to patients. One medication, buprenorphine, can be prescribed by waivered physicians in office-based practice settings, but practice patterns vary widely. This study explored the use of a learning collaborative method to improve the provision of buprenorphine in the state of Vermont.
Methods
We initiated a learning collaborative with 4 cohorts of physician practices (28 total practices). The learning collaborative consisted of a series of 4 face-to-face and 5 teleconference sessions over 9 months. Practices collected and reported on 8 quality-improvement data measures, which included the number of patients prescribed buprenorphine, and the percent of unstable patients seen weekly. Changes from baseline to 8 months were examined using a p-chart and logistic regression methodology.
Results
Physician engagement in the learning collaborative was favorable across all 4 cohorts (85.7%). On 6 of the 7 quality-improvement measures, there were improvements from baseline to 8 months. On 4 measures, these improvements were statistically significant (P < 0.001). Importantly, practice variation decreased over time on all measures. The number of patients receiving medication increased only slightly (3.4%).
Conclusions
Results support the effectiveness of a learning collaborative approach to engage physicians, modestly improve patient access, and significantly reduce practice variation. The strategy is potentially generalizable to other systems and regions struggling with this important public health problem.
Keywords: buprenorphine, learning collaborative, opioid use disorders, quality improvement
By current estimates, 1.9% of the US population engaged in nonmedical use of prescription opioids in the past year, whereas 335,000 people used heroin (Substance Abuse and Mental Health Services Administration, 2013). The chronic misuse of prescription opioids has increased by nearly 75% over the past decade, resulting in escalating rates of fatal overdoses and increased rates of admission to addiction treatment for opioid use disorders (Jones, 2012; Paulozzi et al., 2011). The current rise in opioid use problems includes a shift from more urban to rural areas (Cicero et al., 2014).In addition, the pathway from prescription opioids to heroin has become typical, and often results in intravenous (IV) routes of administration. Government officials have referred to the increase in prescription drug abuse as an “epidemic,” and identified increasing treatment access as one important strategy to meet this challenge (Office of National Drug Control Policy, 2011).
There are, at present, three US Food and Drug Administration (FDA)-approved medications for the management of opioid use disorders: methadone, buprenorphine, and naltrexone. Buprenorphine is a partial agonist with a very high binding affinity at the mu opioid receptor (Cowan, 2007). This allows buprenorphine to both prevent opioid withdrawal and block the euphoria associated with opioid use. In a recent meta-analysis, buprenorphine was shown to be effective for reducing opioid use and increasing treatment retention (Mattick et al., 2008).
Because buprenorphine can be prescribed by physicians in office-based prescribing programs and is not subject to the regulatory constraints of methadone, the availability of buprenorphine improves access to care for opioid-dependent patients (Arfken et al., 2010). Yet, despite its potential, buprenorphine is an underutilized treatment in primary care and in specialty addiction treatment settings, especially in rural areas (Knudsen et al., 2006, 2007). Physicians cite a number of barriers to the adoption of buprenorphine treatment such as a lack of expertise in treating addiction and concerns about the logistics of treating opioid-dependent patients (Walley et al., 2008; Barry et al., 2009). Increased familiarity with the provision of buprenorphine has been found to reduce perceived barriers and increase its prescription (Netherland et al., 2009).
State initiatives to fund buprenorphine and support providers have also been shown to reduce barriers and to increase physicians' utilization of buprenorphine (Ducharme and Abraham, 2008; Stein et al., 2012). Learning collaboratives are an established strategy for reducing practice variation, caring for complex patients, and implementing guidelines (Institute for Healthcare Improvement, 2003). The methodology of learning collaboratives consist of 3 main components: didactic or expert presentation on aspects of the topic including research-based evidence and practice guidelines; practice-based learning via case discussion; and the collection of common data measures across providers and/or practices. Participants frequently include individual physicians, multidisciplinary teams and/or other healthcare professionals, and typically involve some mix of face-to-face and video or teleconference encounters over an 18-month time-frame (Institute for Healthcare Improvement, 2003). Learning collaboratives blend research and practice-based mechanisms for producing quality improvement (QI) in healthcare (Mold and Peterson, 2005; Becker et al., 2011; Vannoy et al., 2011). As an implementation strategy, learning collaboratives are well suited to implement not only simple procedures (eg, a new medication) but complex interventions (Clarke, 2013). Complex interventions are common in health care, and often involve systems and multidisciplinary teams including the physician and other practice staff, such as nurses and behavioral health clinicians. A systematic review of learning collaboratives found that the evidence supporting their effectiveness was positive (Schouten et al., 2008; Hulscher et al., 2013). The value of learning collaboratives includes the benefits of gaining systematic knowledge, integrated with sharing practice-based experience, and having common data to guide and structure the conversation among participants (Clarke, 2013). Although learning collaboratives are being deployed in mental health contexts to implement psychiatric services (Becker et al., 2014), they have not been systematically used to enhance the implementation and dissemination of evidence-based addiction treatments or medications (McGovern et al., 2007). One well known approach to process improvement and implementation in addiction treatment, the Network for Improvement in Addiction Treatment (NIATx), utilizes some aspects of learning collaboratives. However, NIATx has additional components such as “walk-thru” to obtain insight into the patient perspective, clinic-level coaching in a content area and in-process improvement tactics (eg, developing Plan-Do-Study-Act [PDSA] cycles), and “interest circle” phone calls among subgroups of the project participants (Gustafson et al., 2013). Most notably, NIATx has been used to implement relatively simple practice changes, such as reduce wait time and improve show rates, rather than complex addiction treatment practices. Therefore, the effectiveness of learning collaboratives on complex practice changes in addiction treatment has still been relatively unexamined.
The program described in this article innovatively uses the learning collaborative strategy to improve the provision of buprenorphine throughout the state of Vermont. The rise of opioid use problems in Vermont received national attention in 2014 as the major focus of the Governor's annual state-of-the-state address. To address this issue, Vermont sought to expand and improve buprenorphine treatment for individuals with opioid use disorders, in part, by using a learning collaborative approach. In this learning collaborative, we examined physician engagement and change in buprenorphine practice. We hypothesized that the learning collaborative strategy would increase the number of patients prescribed buprenorphine and improve the quality of buprenorphine practice.
Methods
Participants
We recruited office-based opioid treatment (OBOT) practices serving medication-assisted treatment (MAT) patients in Vermont and formed 4 regional cohorts of 5 to 10 practices each. The total sample consisted of 28 physician practices. There were 7 practices in the Northwest region, 10 in the Northeast/Central region, 6 in the Southeast region, and 5 in the Southwestern region of the state. Practice specialty also varied. Whereas 11 practices were in family practice, 9 were specialists (psychiatry: 6; pain management: 2; obstetrics and gynecology (OB/GYN): 1), 3 were based in addiction-treatment agencies, and 5 in federally-qualified health centers (FQHCs). Though not required, all members of a practice team were asked to attend sessions because buprenorphine patients engage not only the physician but also his/her nurses, support staff, and behavioral health clinicians. In some cases, the physicians wanted to send staff members, but not attend themselves, whereas in other instances, physicians wanted to attend without including staff. We maintained that a full clinical care team was necessary to achieve the learning collaborative goals and encouraged practices to send a representative from each discipline. In total, 34 physicians attended the learning collaborative sessions, along with 100 nurses, medical assistants, office support staff, and behavioral health clinicians.
Developing the OBOT Learning Collaborative Approach
A scientific leadership group composed of 3 physician providers, 2 state regulatory authorities, and 2 addiction treatment and research experts who drafted a set of recommended practice guidelines and QI measures for OBOT in Vermont. Specific, pragmatic, and measurable guidelines were drawn from the research literature, existing documents from the Substance Abuse and Mental Health Services Administration (SAMHSA), and the pharmaceutical company recommendations (SAMHSA, 2004; Reckitt Benkiser Pharmaceuticals, 2014). In collaboration with Vermont state agency officials from the Department of Vermont Health Access and the Alcohol and Drug Abuse Programs, 2 of the study authors organized the curriculum and recruited physician practices. The learning collaboratives were led and facilitated by a practicing addiction psychiatrist with expertise in managing a buprenorphine clinic, along with a clinical research psychologist with expertise in integrated care models and learning collaboratives. Participants obtained level 1 continuing medical education credits (CMEs) for every hour of involvement in the learning collaborative, including in-person and teleconference sessions. Because attendance at the learning collaborative sessions and data collection were completely voluntary and focused on QI at the practice level, informed consent of the practice physicians and staff was not required.
OBOT Learning Collaborative Structure
In September 2012, 2 cohorts of learning collaboratives were launched, with 5 practices recruited from Southwestern Vermont, and 7 practices from Northwestern Vermont. In September 2013, a second cohort was launched with 6 practices in Southeastern Vermont and 10 in Central Vermont. At baseline, the recruited practices were treating more than 1400 buprenorphine patients in the state. Over 9 months, a total of 9 sessions were held for each of the 4 cohorts. Sessions alternated between 3-hour face-to-face sessions (5) and 1-hour teleconference webinars (4). Each cohort attended didactic lectures, engaged in case presentations, collected common QI data, and shared these data and practice-improvement strategies at the sessions.
An experienced panel of local prescribers and MAT providers recommended specific CME topics for the didactic component. These topics were: assessing patients for appropriateness for buprenorphine; treatment planning (induction, maintenance, tapering); treatment response monitoring (toxicology and pharmacy database monitoring); challenging behavioral issues; and coordination of care with other healthcare providers and the criminal justice system.
Procedure
This study used a single group, repeated-measures design. Data were collected bimonthly (mo 2, 4, 6, and 8) over 8 months. Cohort A collected and reported data from October 2012 through June 2013. Cohort B collected and reported data from October 2013 through June 2014. Practices collected baseline data on all active buprenorphine patients as of October 1 of 2012 and 2013, respectively. During months 4, 6, and 8, data were gathered on the baseline patient panel and any newly inducted buprenorphine patients, minus any patients who were discharged or left the practice. Therefore, data were collected at the practice level to assess change within the practices over time. Since change was measured at the practice level, individual patient data were not identifiable and contained no protected health information. As practices reported data that were already collected as part of patients' practice records, the QI learning collaborative was exempt from internal review board (IRB) monitoring as per 45 CFR 46.
OBOT QI Measures
The research team provided each practice with a 1-page chart audit tool to record the results of each QI measure. All QI measures were dichotomous. Practices examined all active buprenorphine patients for each QI measure during the collection period. Then, practices reported the percent of patients to meet criteria for each QI measure during the current data collection time interval. For every measure, the denominator of the aggregate data was the number of all active buprenorphine patients enrolled in all practices involved in the learning collaborative, which varied during each data collection period. The QI measures are described below.
The percent of patients for whom a diagnosis of an opioid use disorder is documented. The purpose of this measure was to ensure only patients meeting diagnostic criteria were prescribed buprenorphine, and to minimize prescribing to those seeking the medication for diversion or misuse.
The percent of unstable patients seen weekly. We developed a behavior-specific 8-item checklist specifically for the learning collaborative (OBOT Stability Checklist [OS Checklist]; available upon request) as a practice improvement aid to determine acuity and stability in addiction severity, social-environmental problems, and treatment compliance. Indicators of instability include missed appointments, reports of stolen or lost medication, self-reported illicit substance use, and positive urine drug screens. For patients who had 1 or more indicators of instability, more frequent contact and shorter-term prescriptions were indicated. Although not required, practices were encouraged to use the OS Checklist to identify unstable patients. Some practices utilized their own method of identifying unstable patients; those methods were not reported to the learning collaborative.
The percent of patients prescribed more than 16 milligrams (mg). Acting out of a desire to reduce buprenorphine diversion, state officials requested tracking the percent of patients receiving more than 16 mg of buprenorphine daily. Although the science is unsettled regarding the matter of optimal or maximum daily dosing, based on the research literature and meta-analyses, the maximum recommended daily dose was set at 16 mg to reduce diversion. Dosing was not capped at 16 mg, but higher doses required clinical review by clinicians employed by the state government.
The percent of urine drug screens analyzed at least monthly. Urine drug screen monitoring is routine in specialty addiction treatment programs, but is not typically utilized in primary care. The clinical benefits of urine drug screens include objective monitoring of medication compliance (and possible diversion), other substance use (eg, cocaine, benzodiazepines), and increased patient accountability.
The percent of patients for whom the state prescription monitoring system was accessed at admission and quarterly thereafter. The Vermont Prescription Monitoring System (VPMS) is a statewide web-based system that provides pharmacy data on a patient level. By accessing the VPMS, practices were able to view all prescriptions filled at pharmacies within the state. This allowed buprenorphine prescribers to know whether a patient had recently filled a prescription for medication that was potentially harmful in combination with buprenorphine. It also allowed providers to investigate whether a patient was using illicit medication or filling prescriptions for buprenorphine earlier than was intended by the prescriber.
The percent of patients continuing in care for at least 6 months. Although there is no specific scientific evidence on the indications for the duration a patient might benefit from buprenorphine, this benchmark was set to establish a measure of acceptable retention (Thomas et al., 2013, Center for Substance Abuse Treatment, 2005). This measure was also designed to encourage patients and physicians to avoid repeated induction phases, which are both costly and associated with increased risk for relapse. Practices identified patients from their panels who began MAT 6 or more months ago, and then indicated the percent of those patients who were currently continuing in treatment.
The percent of patients for whom comanagement of other substance use disorders, mental health disorders, or primary care is documented. This measure examined the proportion of patients referred for additional mental health or specialty care. As specialty care was not necessary for all patients, the denominator of this measure only included patients with specialty care needs, such as mental health diagnoses or untreated chronic health conditions. Practices were encouraged to comanage care with local providers. For primary care practices, this measure encouraged the coordination of care with specialty behavioral health providers for patients in need of additional services. For psychiatry practices, this measure encouraged coordination of patient care with primary care, OB/GYN, infectious disease, or other specialist practices. To meet this QI measure, practices had to have documentation in the patient's medical record of both the referral and ongoing contact with the comanaging provider. If patients received specialty care within a primary care practice, we also considered this to be a referral.
Number of patients prescribed buprenorphine. Although not a formal quality measure, we hypothesized that with increased clinical support and self-efficacy, physician practices may take on more patients with opioid use disorders.
Statistical Analyses
The QI measures data were gathered on site by each practice. No other patient or practice data were collected. Deidentified practice-level data were then transferred to Dartmouth for analysis. Descriptive statistics (frequencies and percentages) were used to aggregate the data by practice and in total. Standard deviations were used to identify variation within measures at each time point. Bivariate logistic regression models were used to investigate whether statistically significant changes occurred in adherence to the QI measures between the first and final data collection periods. Unlike usual logistic regression which use binary outcome data at the individual level, we conducted logistic regression analysis using aggregated data, that is, the numerator and denominator used to compute the aforementioned percent using an “event/trial” type of syntax (Allison, 2012). For instance, the percent of unstable patients seen weekly was computed as the number of unstable patients seen on a weekly basis divided by total number of unstable patients.
Statistical process control p-charts were also created to examine and track changes in QI measures (Duclos and Voirin, 2010). Variable control limits were used, as sample sizes changed over time. Points falling above or below the control limits were considered special cause variation, and indicated a potentially significant change. For cross-validation of findings, both the logistic regression and p-chart approaches were used to test for convergence in statistically significant change by measures over time. SAS software was used for statistical analyses (SAS Institute Inc, 2006).
Results
Twenty-eight practices were recruited and 24 (85.7%) attended all in-person and teleconference sessions. Twenty-two (78.5%) practices gathered and shared QI data. Among only those practices who attended any learning collaborative sessions, 91.6% (22 practices) gathered and shared QI data. Across the 2 cohorts and 4 learning collaborative groups, there were no obvious variations in learning collaborative continuation (χ 2 [3, N = 28]= 3.27, P = 0.25) or in data reporting (χ2 [3, N = 28] = 5.35, P = 0.15; see Fig. 1).
Figure 1.
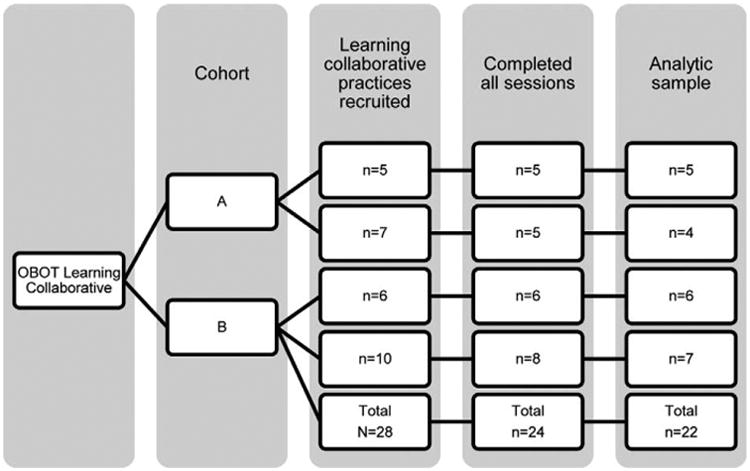
Flow diagram of practices participating in office-based opioid treatment (OBOT) learning collaborative.
As depicted in Figure 2, there was considerable reduction in practice variation across all 7 QI measures, as measured by standard deviations. There was nearly a 50% reduction in variation from month 2 to month 8 in the percent of patients with documented opioid use disorder and the percent of patients getting monthly urine drug screens. Variation in the percent of unstable patients seen weekly, specialty care comanagement, patients continuing care for 6 months, and doses over 16mg was reduced by roughly 30%. Contrary to our hypothesis, there was only a slight (3.4%) increase in patient volume (1493 to 1544) across all practices reporting data.
Figure 2.
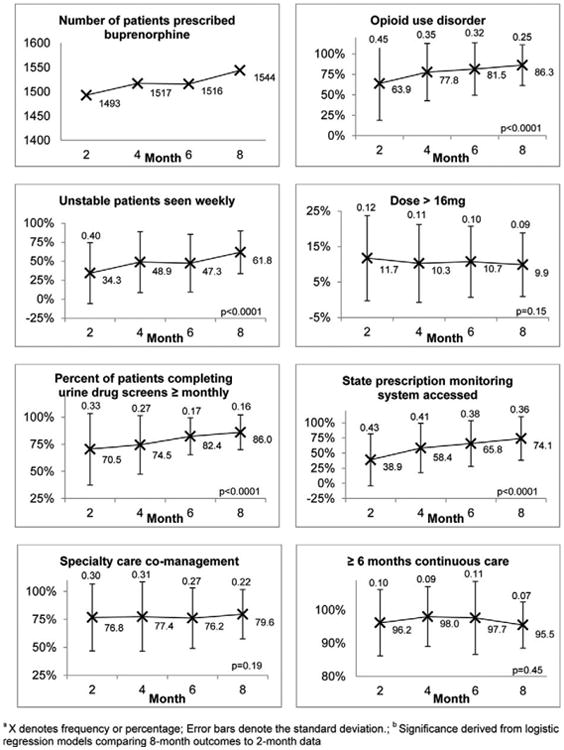
Changes in QI measures during the Learning Collaborative across 22 office-based opioid treatment (OBOT) practicesa,b.
Based on results of logistic regression analysis, the practices had statistically significant improvements on 4 of the QI measures (Fig. 2). Compared with the initial data collection period, there was a 3-fold increase in the odds of having opioid use disorder documented in patient charts during the final data collection period (odds ratio [OR] 3.55; 95% confidence interval [CI], 2.92, 4.32). There was also a 3-fold increase in the odds of seeing unstable patients weekly during the final data collection period, compared with the initial collection period (OR 3.10; 95% CI, 2.28, 4.22). The practices were also more likely to perform monthly urine drug screens (OR 2.57; 95% CI, 2.11, 3.12) and access the state prescription monitoring system quarterly (OR 4.50; 95% CI, 3.78, 5.36) at the final data collection period. Changes in the likelihood of specialty care comanagement (OR 1.18,; 95% CI, 0.92, 1.52), 6-month patient retention (OR 0.85; 95% CI, 0.56, 1.30), and prescribing over 16 mg of buprenorphine (OR 0.82; 95% CI, 0.64, 1.07) were not statistically significant.
The p-chart analytic approach suggested that the percentages of opioid use disorder diagnosis documentation, monthly urine screens, unstable patients seen weekly, and state prescription drug system monitoring increased significantly from baseline to 8 months. No special cause signals were present in the p-charts tracking other outcomes. Thus, both statistical approaches converged to find the same four QI measures statistically significant.
Discussion
Much as variation is often found in clinical practice, variation in the real world application of implementation strategies is common (Proctor et al., 2013; Powell et al., 2014). Whereas the Institute of Healthcare Improvement (IHI) recommends 18-sessions for a learning collaborative approach, the 9-session duration of our learning collaborative was 50% of the recommended dose. Nonetheless, despite this abbreviated timeline, high levels of participation and significant practice changes were found. Eighty-six percent of recruited practices attended all teleconference and face-to-face sessions. Only 6 of the 28 recruited practices ultimately did not collect QI data. This level of commitment was impressive for practices located in distant, rural regions. On the QI data, statistically significant improvement occurred on 4 of the 7 QI measures. The percent of patients with opioid use disorder diagnosis documented in charts increased, and practices became more likely to access the prescription drug monitoring system. Improvements also occurred in the percent of unstable patients seen weekly and the percent of patients receiving monthly urine drug screens.
Importantly, practice variation decreased on all measures over time. Practice variation in documenting opioid use disorders and administering monthly urine drug screens decreased by half, whereas variation in the proportion of patients receiving specialty care comanagement and 6 months of continuous care decreased by a third. Practices were also more consistent about seeing unstable patients weekly. Therefore, practices became more uniform in adhering to these best practice guidelines after participating in the learning collaborative. The modest increase in patients prescribed the medication during the relatively short interval was less than expected, as patient volume only increased by 3.4%. However, it is possible that these numbers may simply be slower to rise.
There are several limitations to this study. Because we were unable to collect data after the first year of the learning collaborative, we had no data on changes in measures during the second year. This also prevented us from using the first cohort as a contemporaneous control group, against which to compare the second cohort. As a result, we cannot say whether the trends we observed in improved care and reduced practice variation were secular, or a result of our learning collaborative intervention. In addition, given the nature and scope of our data collection, we could not control for clustering of providers and patients. Provider experience also was not assessed in this study. Providers may benefit by overcoming isolation and self-doubt by engaging with other physicians and teams, and might also develop increased self-confidence in the practice of addiction medicine. Future research should examine how learning collaboratives might impact these outcomes. Finally, some of the patients included in the 4, 6, and 8-month QI data were also included in the baseline group, whereas some were not. Thus, the observations for some individuals may be independent, which may therefore somewhat inflate the results of the logistic regression models. Despite this, the results of the p-chart analysis were consistent with results of the regression models, providing additional support for our findings.
Conclusions
There is an overlap between traditional QI and strategies that have been derived from the new field of implementation science (Mittman, 2004). QI approaches such as Lean and Six-Sigma include aspects of industrial engineering and a detailing of workflow and work process relative to enhanced efficiency and achieving targeted objectives. Focused awareness and measurement of processes and outcomes are core to systematic quality improvement approaches in health care. An exemplar learning collaborative is the Northern New England Cardiovascular Study Group, which assisted interventional cardiologists in significantly decreasing mortality and infection rates across geographically dispersed hospital surgical and operating room teams (O'Connor et al., 1996). This learning collaborative approach, like the one used in the present project, had a significant impact on practice variation. Using common data gathered across practices, and having high outcome performers share their clinical strategies, workflow and work processes with the group had a dramatic useable impact on the other practices. Unlike many QI approaches, the learning collaborative model is not top-down, not driven by research-based findings exclusively, but rather draws upon systematic evidence and the practice-based experience and reciprocal feedback of frontline practitioners. In our project, the learning collaborative capitalized on a commitment to address a complex clinical problem (prescribing a medication and managing addictive disease), within diverse and highly motivated practice teams. To date, there has been no systematic study of an implementation strategy or QI approach specifically for OBOT.
Results of the present study demonstrate that the learning collaborative method is feasible and beneficial to reduce practice variation and improve care for opioid-dependent patients prescribed buprenorphine. When considering the challenges of a rural region with geographically dispersed practices separated by mountains and by inclement weather during winter months, the level of participation and engagement was remarkable. A learning collaborative approach may be especially effective for dispersed providers and their teams who are experienced, but are wrestling with complex clinical presentations. Given that opioid addiction persists and is increasing, patient access is limited, and physician capacity and care quality vary widely, the learning collaborative model may be worthwhile for other systems, states, and regions to consider.
Acknowledgments
The authors wish to acknowledge the participation of the physicians and their affiliated practice teams. This project would not have been successful without the support of the Department of Vermont Health Access Blueprint for Health practice managers and facilitators, as well as the Spoke nurses and behavioral health clinicians. Special thanks to Jenny Samuelson, Dennis Charles, Dana Noble, and Dr Craig Jones from the Blueprint for Health, and Anthony Folland.
Funding was provided by the State of Vermont Department of Health and Human Services Alcohol and Drug Abuse Programs (Barbara Cimaglio, Deputy Commissioner) and the Department of Vermont Health Access (Dr. Mark Larson, Commissioner).
Footnotes
The authors declare no conflicts of interest.
References
- Allison PD. Logistic regression using SAS: theory and application. SAS Institute. 2012 [Google Scholar]
- Arfken CL, Johanson CE, di Menza S, Schuster CR. Expanding treatment capacity for opioid dependence with office-based treatment with buprenorphine: national surveys of physicians. J Subst Abuse Treatment. 2010;39:96–104. doi: 10.1016/j.jsat.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Barry DT, Irwin KS, Jones ES, et al. Integrating buprenorphine treatment into office-based practice: a qualitative study. J Gen Internal Med. 2009;24(2):218–225. doi: 10.1007/s11606-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DR, Drake RE, Bond GR, et al. Best practices: a national mental health learning collaborative on supported employment. Psychiatric Serv. 2011;62(7):704–706. doi: 10.1176/ps.62.7.pss6207_0704. [DOI] [PubMed] [Google Scholar]
- Becker DR, Drake RE, Bond GR. The IPS supported employment learning collaborative. Psychiatr Rehabil J. 2014;37(2):79–85. doi: 10.1037/prj0000044. [DOI] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment. Treatment Improvement Protocol (TIP) series 42. Rockville MD: Substance Abuse and Mental Health Services Administration; 2005. Substance abuse treatment for persons with co-occurring disorders. [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821–826. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- Clarke JR. The use of collaboration to implement evidence-based safe practices. J Public Health Res. 2013;2(3):e26. doi: 10.4081/jphr.2013.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan A. Buprenorphine: the basic pharmacology revisited. J Addict Med. 2007;1:68–72. doi: 10.1097/ADM.0b013e31806c9202. [DOI] [PubMed] [Google Scholar]
- Ducharme LJ, Abraham AJ. State policy influence on the early diffusion of buprenorphine in community treatment programs. Subst Abuse Treatment Prevent Policy. 2008;3(17) doi: 10.1186/1747-597X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care. 2010;22(5):402–407. doi: 10.1093/intqhc/mzq037. [DOI] [PubMed] [Google Scholar]
- Gustafson DH, Quanbeck AR, Robinson JM, et al. Which elements of improvement collaboratives are most effective? A cluster-randomized trial Addiction. 2013;108(6):1145–1157. doi: 10.1111/add.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulscher ME, Schouten LM, Grol RP, Buchan H. Determinants of success of quality improvement collaboratives: what does the literature show? BMJ Qual Safety. 2013;22(1):19–31. doi: 10.1136/bmjqs-2011-000651. [DOI] [PubMed] [Google Scholar]
- Institute for Healthcare Improvement. The Breakthrough Series: IHI's Collaborative Model for Achieving Breakthrough Improvement. Cambridge, MA: Institute for Healthcare Improvement; 2003. [Google Scholar]
- Jones CM. Frequency of prescription pain reliever nonmedical use: 2002–2003 and 2009–2010. Arch General Med. 2012:E1–E2. doi: 10.1001/archinternmed.2012.2533. [DOI] [PubMed] [Google Scholar]
- Knudsen HK, Ducharme LJ, Roman PM. Early adoption of buprenorphine in substance abuse treatment centers: data from the private and public sectors. J Subst Abuse Treatment. 2006;30:363–373. doi: 10.1016/j.jsat.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Knudsen HK, Ducharme LJ, Roman PM. The adoption of medications in substance abuse treatment: associations with organizational characeristics and technology clusters. Drug Alcohol Depend. 2007;87:164–174. doi: 10.1016/j.drugalcdep.2006.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenane for opioid dependence. Cochrane Library. 2008;(3):1–33. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- McGovern MP, Matzkin AL, Giard J. Assessing the dual diagnosis capability of addiction treatment services: The Dual Diagnosis Capability in Addiction Treatment (DDCAT) Index. J Dual Diagn. 2007;3(2):111–123. [Google Scholar]
- Mittman BS. Creating the evidence base for quality improvement collaboratives. Ann Internal Med. 2004;140(11):897–901. doi: 10.7326/0003-4819-140-11-200406010-00011. [DOI] [PubMed] [Google Scholar]
- Mold JW, Peterson KA. Primary care practice-based research networks: working at the interface between research and quality improvement. Ann Fam Med. 2005;3(S1):S12–S20. doi: 10.1370/afm.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherland J, Botsko M, Egan JE, et al. BHIVES Collaborative. Factors affecting willingness to provide buprenorphine treatment. J Subst Abuse Treatment. 2009;36:244–251. doi: 10.1016/j.jsat.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor GT, Plume SK, Olmstead EM, et al. A regional intervention to improve the hospital mortality associated with coronary artery bypass graft surgery: The Northern New England Cardiovascular Disease Study Group. J Am Med Assoc. 1996;275(11):841–846. [PubMed] [Google Scholar]
- Office of National Drug Control Policy. Epidemic: RespondingtoAmerica's Prescription Drug Abuse Crisis. Washington, DC: 2011. Report #NCJ 234164. [Google Scholar]
- Paulozzi LJ, Jones C, Mack K, Rudd R. Vital signs: overdoses of prescription opioid pain relievers: United States, 1999–2008. Morbid Mortal Wkly Rep. 2011;60(43):1487–1492. [PubMed] [Google Scholar]
- Powell BJ, Proctor EK, Glass JE. A systematic review of strategies for implementing empirically supported mental health interventions. Res Soc Work Pract. 2014;24(2):192–212. doi: 10.1177/1049731513505778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013;8:139, 1–11. doi: 10.1186/1748-5908-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckitt Benkiser Pharmaceuticals. Medication Guide: Suboxone. SBF-0092-o. Warren, NJ: MonoSol Rx; Apr, 2014. 2014. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) TIP 40: clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Rockville, MD: U.S. Department of Health and Human Services; 2004. [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT User's Guide. 9.1.3. Cary, NC: SAS Institute Inc.; 2006. [Google Scholar]
- Schouten LM, Hulscher ME, van Everdingen JJ, et al. Evidence for the impact of quality improvement collaboratives: systematic review. Br J Med. 2008;336(7659):1491–1494. doi: 10.1136/bmj.39570.749884.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BD, Gordon AJ, Sorbero M, et al. The impact of buprenorphine on treatment of opioid dependence in a Medicaid population: recent service utilization trends in the use of buprenorphine and methadone. Drug Alcohol Depend. 2012;123(1–3):72–78. doi: 10.1016/j.drugalcdep.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Office of Applied Statistics, Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services, editor. Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2013. [Google Scholar]
- Thomas CP, Garnick DW, Horgan CM, et al. Establishing the feasibility of measuring performance in use of addiction pharmacotherapy. J Subst Abuse Treatment. 2013;45(1):11–18. doi: 10.1016/j.jsat.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannoy SD, Mauer B, Kern J, et al. A learning collaborative of CMHCs and CHCs to support integration of behavioral health and general medical care. Psychiatric Serv. 2011;62(7):753–758. doi: 10.1176/ps.62.7.pss6207_0753. [DOI] [PubMed] [Google Scholar]
- Walley AY, Alperen JK, Cheng DM, et al. Office-based management of opioid dependence with buprenorphine: clinical practices and barriers. J Gen Internal Med. 2008;23(9):1393–1398. doi: 10.1007/s11606-008-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


