Abstract
Clearance of apoptotic debris is a vital role of the innate immune system. Drawing upon principles of apoptotic clearance, convenient delivery vehicles including intrinsic anti-inflammatory characteristics and specificity to immune cells can be engineered to aid in drug delivery. In this article, we examine the use of phosphatidylserine (PtdSer), the well-known “eat-me” signal, in nanoparticle-based therapeutics making them highly desirable “meals” for phagocytic immune cells. Use of PtdSer facilitates engulfment of nanoparticles allowing for imaging and therapy in various pathologies and may result in immunomodulation. Furthermore, we discuss the targeting of the macrophages and other cells at sites of inflammation in disease. A thorough understanding of the immunobiology of “eat-me” signals is requisite for the successful application of “eat-me”-bearing materials in biomedical applications.
Keywords: Phosphatidylserine, Liposomes, Immune response, Leukocytes, Phagocytes, Imaging, Drug delivery
1. Introduction
Efficient clearance of pathogenic invaders, dead and dying cells (apoptotic and necrotic), and cellular debris is vitally important for long-term maintenance of tissue homeostasis in living systems [1–3]. The immune system plays a large role in tissue homeostasis as it is finely tuned to recognize and rapidly process both endogenous and exogenous particles [4,5]. Innate immune cells recognize and often engulf exogenous particles via recognition of “danger-associated” moieties; see Table 1. Canonically, recognition of danger-associated patterns by innate immune cells results in cellular activation and the release of chemoattractant proteins, which recruit additional inflammatory cells to the area [6–9]. In contrast, clearance of dead cells as part of constant cell deletion and renewal is by and large “immunologically silent” [10]. For instance, it is estimated that the human immune system clears about one million dead or dying cells every second without causing immune activation [11]. Understanding and adapting the mechanisms involved in these processes offer a paradigm for advanced drug delivery. Approaches that mimic apoptotic cells, utilize apoptotic cells themselves as therapeutic agents, or interfere with receptors that recognize “danger-associated” species have been shown to be effective in delivery strategies in various disease states.
Table 1.
Some of the well-described molecules that facilitate immune clearance and their proposed use in drug delivery and imaging.
| Recognition molecule | Description/mechanism of action | Therapy or imaging applications | |
|---|---|---|---|
| Danger-associated molecules | Urate crystals | [93,94] | |
| Cholesterol crystals | [95] | ||
| HMGB1 | [96,97] | ||
| HSP90 | [98] | ||
| Plasma DNA | [99,100] | ||
| Formylated peptides and Mitochondrial DNA | [101] | ||
| Neutrophil extracellular traps | [102] | ||
| Purine metabolites: ATP | [103] | ||
| IL-33 | [104] | ||
| S100 calcium binding proteins | [63,105] | [63] | |
| Extracellular matrix proteins: Hyaluronate | [106] | A number of reports, see, for example [107–109] | |
| Exhaustive review list of other DAMPs | [5,12] | ||
| “Eat-me” signals (EMS) | Phosphatidylserine (PtdSer) and oxidized PtdSer | [12,30,110–112] | See specific references further in this review |
| [113] | |||
| Calreticulin (CRT) | [114,115] | ||
| Phophoethanolamine (PE) and oxPE | [116] [117] | [118,119] | |
| Cardiolipin (CL) and oxCL | [120,121] | ||
| Other oxidized lipids | [122] | [62,123–126] |
We will discuss therapeutic strategies that harness apoptotic clearance machinery by incorporation of “eat-me” signals (EMS). An “eat-me” signal is a molecular signal expressed on the surface of a cell or a particle that is recognized by a corresponding receptor on a phagocytic cell (innate immune cell) to initiate phagocytosis (cell), endocytosis, or pinocytosis (particle) [12]. This action is usually concomitant with repressed immune activation, and recapitulates the clearance of endogenous apoptotic debris. Probably the most widely described EMS — phosphatidyserine (PtdSer) [13,14] is the main focus of this review. Although other EMS have been described (calreticulin, phosphoethanolamine, oxidized lipids and recently discovered cardiolipin), reports describing the therapeutic utility or use in in vivo imaging are scarce (Table 1). In the first part of this article we discuss various applications of nanomaterial-based vehicles decorated with PtdSer that have been used for standalone therapy or for delivery of therapeutic agents. In the second part of this article, we review imaging probe delivery, and utility of PtdSer-targeted contrast agents in various pathological conditions employing numerous imaging modalities.
We limit our discussion to nanoparticulate- based vehicles bearing EMS serving as substrates for immune cell engulfment. We will not discuss the work defining those mechanisms behind the detection of cell death by innate immune cells (Annexin V [15–18] and other PtdSer-targeted molecules [19–22]). We refer the reader to a series of excellent reviews on the biology of EMS and apoptosis [13,14,23,24]. Likewise, the approaches that take advantage of “danger-associated” molecules for drug delivery have been reviewed elsewhere [25–27].
2. Therapy with phosphatidylserine carriers
Phosphatidylserine (PtdSer) is a phospholipid component of cell membranes in eukaryotes. Under normal physiological conditions, PtdSer serves primarily as a component of the inner cell membrane of the phospholipid bilayer and is retained there by the enzyme aminophospholipid translocase [14,28]. When a cell undergoes apoptosis, however, the activity of aminophospholipid translocase is lost and phospholipid scramblases serve to both disturb the membrane’s aminophospholipid asymmetry and expose a significant amount of PtdSer to the outer leaflet [14,29]. This exposed PtdSer is a specific recognition signal for phagocytic digestion of apoptotic cells [30], with several ligand-receptor mechanisms having been elucidated for the recognition of PtdSer on the surface of apoptotic cells [13,14].
Engineered nanomaterials bearing synthetic PtdSer have been designed to mimic apoptotic cells and serve as a great resource for nanoparticle targeting and drug delivery. We highlight two approaches utilizing PtdSer nanoparticles. First, nanoparticles that mimic apoptotic cells via surface-decorated PtdSer have been shown to exhibit immuno-regulatory functions in multiple models of inflammation. Second, PtdSer decorated nanocontainers loaded with drugs and therapeutic oligonucleotides, with PtdSer, directing these agents to sites of abundant phagocytic cell accumulation and activity. The utilization of PtdSer as a targeting ligand is based on the intrinsic capacity of phagocytic cells to clear PtdSer-containing vehicles more efficiently than the bare drug or imaging probe, enhancing agent delivery to phagocytic cell types in various diseases, such as sickle cell anemia, thalassemia, and various neoplastic cells in leukemia, melanoma, and colon cancer [31–33]. Thus, there is obvious utility in applying PtdSer-containing nanoparticle in both investigative and therapeutic settings.
2.1. Anti-inflammatory therapy via PtdSer signaling
Nanoparticles (NPs) such as liposomes containing PtdSer in their bi-layer have been used to mimic cellular debris as a therapeutic strategy for inflammation resolution, these are often referred to as “synthetic apoptotic cells”. Synthetic apoptotic cells leverage signaling cascades initiated in innate immune cells during endogenous cellular apoptosis, a process that is fundamentally anti-inflammatory and “immunologically silent”. While receptors that recognize PtdSer are increasingly better-defined, the signaling through these receptors is not completely understood. It is known, however, that the engulfment of PtdSer-containing nanoparticles such as liposomes (PSL) has a direct effect on anti-inflammatory cytokine production, similar to downstream of recognition of the dead cells. PSL have therefore been explored in a number of models of systemic and local inflammation that demonstrated their utility as an anti-inflammatory and immunomodulatory agents.
2.1.1. Resolving systemic inflammation with PtdSer nanoparticles and their effects on various cell types
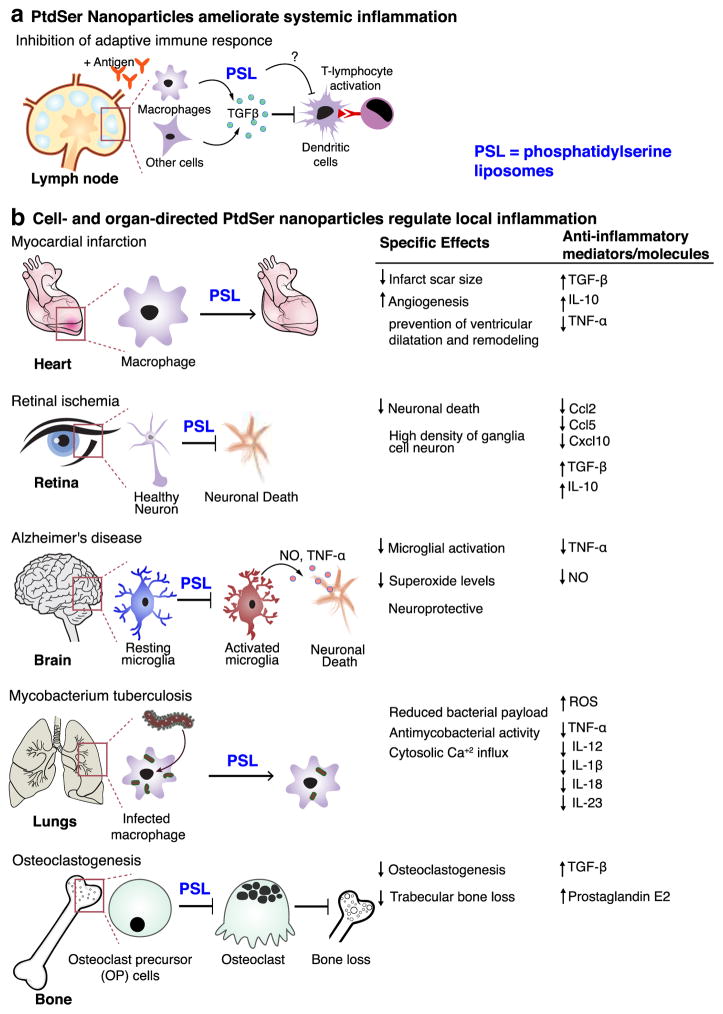
Transforming growth factor-β (TGF-β) is a key anti-inflammatory mediator implicated in apoptotic cell-driven immune modulation. Multiple studies have demonstrated that apoptotic cell administration increases production of TGF-β1. Similarly, in one example, instillation of PSL in lipopolysaccharide stimulated lungs increased production of TGF-β1 and ameliorated inflammation, the effect that was not seen when phosphatidylcholine (PC) liposomes were used [34]. A mechanism by which PSL inhibit inflammation (adaptive immune response) is thought to be dependent on TGF-β released from phagocytes and variety of cells that engulfed the liposomes in peripheral tissues including fibroblasts, epithelial cells and endothelial cells. Pre-treatment with PSL followed by antigen stimulation resulted in increased TGF-β levels in draining lymph nodes and decreased numbers of immune cells such as macrophages, dendritic cells and T-lymphocytes — an indication that PSL repress inflammation, at least in part, by stimulating secretion of TGF-β and the generation of regulatory T-cells (Fig. 1a). Interestingly, in this study PSL failed to inhibit phenotypic and functional maturation of dendritic cells in response to proinflammatory stimuli, whereas others observed an inhibitory effect [35]. In the latter study, PSL inhibited the maturation as well as pro-inflammatory cytokine release in DNCB-stimulated dendritic cells, decreasing Th1 cell-mediated immune response via effects on CD4+ T cells. It is worth noting that the same inhibitory actions were observed when dendritic cells were treated with apoptotic cells [36].
Fig. 1.
Systemic and local anti-inflammatory effects of PtdSer-based carriers. a) PtdSer-liposomes (PSL) exhibit systemic anti-inflammatory actions by means of inhibition of antigen presentation by dendritic cells (and possibly monocytes/macrophages) and inhibit T-lymphocyte proliferation in Th1 immune response. This is believed to be dependent on levels of transforming growth factor beta-1 (TGF-β1). Whether PSL directly modulate the function of dendritic cells in this process is not clear. b) PSL acted in various phagocytic and non-phagocytic cell types and elicited anti-inflammatory response. Some specific effects were also noted as indicated.
An additional mechanism of action of PSL were suggested to be partly dependent on peroxisome proliferator-activated receptor gamma (PPAR-γ) activation [37]. PPAR-γ enabled PSL to reduce the late phase of inflammation induced by carrageenan injection in mice. In this model of acute inflammation [38], PSL were effective for up to 48 h after administration of carrageenan. Treatment with PSL but not PC liposomes, decreased carrageenan-induced edema, local concentrations of inflammatory cytokine IL-1β, the expression of cell adhesion molecules and leukocyte migration for up to 48 h [37].
PtdSer-NPs have been targeted to various cell types in a number of inflammatory conditions. Investigated in vitro, applied locally or intravenously, PtdSer-NPs have been shown to efficiently target phagocytes such as macrophages and macrophage-like cells. This was concomitant with reduction in inflammatory molecules production by these cell types and the surrounding tissue, and some specific effects that further improved the beneficial therapeutic response (Fig. 1b).
PSL have been employed to reduce inflammation in infarct repair; Intravenous injection of 1 μm-sized liposomes presenting PtdSer in the lipid bilayer and entrapping iron oxide nanoparticles (imaging probe) have been shown to deposit to macrophages at the infarct site in a rat model of myocardial infarction (MI). Liposome accumulation was demonstrated by magnetic resonance imaging (MRI) and confirmed by immunohistology [39]. The treatment with PSL but not with PC liposomes increased angiogenesis that promoted infarct repair and downregulated inflammation via secretion of the anti-inflammatory cytokines, such as IL-10 and TGF-β.
A relatively novel experimental application of PSL technology showed that PSL protected against neurodegeneration in an acute model of retinal ischemia, a condition involving loss of function of neurons, including neuronal death. Dvoriantchikova et al. reported a significant reduction in proinflammatory chemokines such as Ccl2, Ccl5 and Cxcl10 after 24 h of intraperitoneal administration of PSL. Moreover, this correlated with neuronal survival and reduced retinal neuronal damage [40]. A similar strategy has been used to target Alzheimer’s microglia (brain resident macrophages) that are activated by disease-associated amyloid-β-peptide, resulting in the killing of neurons by excessive release of pro-inflammatory cytokines. This potentially beneficial treatment strategy inhibited microglial activation through the reduced production of inflammatory cytokines (TNF-α) and nitric oxide (NO), thus demonstrating putative neuroprotection [41].
A combination of PtdSer and other bioactive lipids may improve the cellular response in disease, offering a therapeutic benefit. For instance, the production of phosphatidic acid (PA) at the internal leaflet of the cell membrane by phospholipase D (PLD) contributes to the antimicrobial response, manifested through calcium signaling, phagolysosome maturation, and reactive oxygen species (ROS) production. It was observed that macrophages that ingested pathogens such as Mycobacterium tuberculosis (MTB) inhibit intracellular PLD activity resulting in lower than normal levels of PA. Applying this antimycobacterial signaling, Greco et al. incorporated macrophage-directing PtdSer and PA into asymmetric liposomes resulting in potent antibacterial activity. More specifically, PtdSer was placed in the liposome bilayer, while PA was encapsulated, creating Janus-like particles referred to as “apoptotic body–like liposomes with PA (ABL/PA)” [42]. ABL/PA were effectively engulfed by macrophages, which decreased the expression of mRNA levels of pro-inflammatory genes and promoted Ca2+-mediated phagolysosome maturation. Most importantly, the addition of the ABL/PA to MTB-infected cell lines or cells from the lungs of infected patients resulted in a reduction in bacterial load. MTB-infected mice treated intranasally showed reduction in circulating pro-inflammatory mediators and markers of tissue and liver toxicity. These results suggest that the ABLs may prove an effective mechanism to deliver bioactive lipid therapeutics that fight infection in macrophages.
Interesting findings demonstrating the possible utility of PtdSer-based therapies in bone cells have been reported by Wu and colleagues [43]. The authors demonstrated that PSL prevented bone loss via inhibition of osteoclastogenesis. Osteoclastogenesis is the differentiation of osteoclasts from osteoblasts in the bone marrow cavity in an inflammatory environment causing significant bone loss. PSL targeted mononuclear osteoclast precursor (OP) cells, in contrast to PC liposomes did not accumulate in OP cells. This resulted in the secretion of TGF-β1 and prostaglandin E2 (PGE2) that synergistically inhibited osteoclastogenesis [43]. In a follow up study, intramuscular administration of PSL in an adjuvant arthritic (AA) rat model triggered a similar cascade of anti-inflammatory responses and resulted in a significant inhibition of AA-induced trabecular bone loss. It was also suggested that PSL may contribute not only to the prevention of trabecular bone loss, but also to the facilitation of trabecular bone formation in the AA [44]. These results demonstrated that PtdSer-based therapeutic strategy might extend beyond macrophage targeting and may possibly be effective for other cell types.
2.2. Delivery through direct recognition of PtdSer on synthetic nanocarriers
Nanoparticle strategies intended to interact minimally with the cells of immune system while still containing PtdSer are particularly challenging. Such strategies are common in cancer chemotherapy, as the intention is primarily to deliver cell-killing drug to cancerous cells and secondarily to avoid the delivery of cell-killing agents to phagocytic cells of the immune system. One promising development offers a potential solution to this challenge; SapC-DOPS NPs. SapC-DOPS NPs have so-called “fusogenic” activity with particles interacting with the endogenous PtdSer on cancer cells resulting in NP-cell fusion. PtdSer is highly expressed on tumor cells of certain cancers, especially high in pancreatic cancers [45], which allows for selective targeting of SapC-DOPS. SapC-DOPS contain phospholipid dioleoylphosphatidylserine (DOPS) and Saponin C (SapC), which is an 80 amino acid multifunctional glycoprotein expressed in the lysosomal membrane of all cell types. The SapC protein selectively induces fusion of PtdSer at acidic pH of tumor environment and acts as a bridge between PtdSer on tumor cells and PtdSer on the nanocarrier. The delivery of SapC to the tumor cells by means of this mechanism, leads to the activation of lysosomal acid sphingomyelinase and rise in ceramide levels, which, in turn, activate caspases leading to apoptotic cell death [46]. This approach was also adapted for treatment of other cancers and has demonstrated promising results (Table 2).
Table 2.
PtdSer-based delivery systems and their utility in various disease conditions
| Carrier | Therapeutic agent delivered | Functional effect or mechanism of action | Indication or target | Reference |
|---|---|---|---|---|
| Liposomes | Prototypical gene (GFP plasmid) | Fusogenic property of PtdSer for enhanced DNA delivery | Gene delivery to lungs | [55] |
| PtdSer | Clearance of HIV-bound particles | Antiviral (HIV infection) | [54] | |
| Phosphatidic acid (PA) or combination PA + isoniazid | Reduction of bacterial load | Mycobacterium tuberculosis (MTB) infection | [42] | |
| SapC-DOPS | Saposin C | Tumor cell apoptosis, lysosomal cell death | Pancreatic cancer, brain tumor imaging, glioblastoma, lung cancer, neuroblastoma | [46,127–132] |
| Poly(lactic-co-glycolic acid) | LXR agonist | Reduced inflammation and number of CD68+ macrophages | Atherosclerosis | [48] |
| Carbon nanotubes | Cytochrome C | Disruption of endosomes and activation of the caspase cascade, apoptosis | Alveolar macrophages | [133] |
An important potential safety concern with SapC-DOPS nanocarriers is whether their intravenous administration involves inadvertent interaction with the innate immune cells. This was recently examined by Kaihua et al. with RAW264.7 macrophages and in wild-type mice with repeated intraperitoneal administration over 28 days. The study showed that SapC-DOPS caused an increase in inflammatory cytokines such as IFN-γ, TNF-α, IL-1β in serum and by peripheral macrophages. It was noted that the immune response elicited by chronic SapC-DOPS administration was somewhat similar to that of lipopolysaccharide (LPS) and occurred through pattern recognition receptor TLR-4 signaling mechanism [47]. Targeting of cancer cells with SapC-DOPS NP is an attractive alternative to chemotherapy, however, given its fast clearance due to PtdSer recognition by immune cells and pro-apoptotic mechanism of action, safety considerations may take precedence over anti-tumor efficiency, and present a barrier to clinical translation.
The challenge of rapid sequestration of PtdSer NPs by phagocytic cells, particularly in the liver and spleen, was also recently discussed in a drug delivery study that aimed to treat atherosclerotic plaque macrophages in mice with LXR agonist GW3965. Although the authors observed that PtdSer nanoparticles modulated key LXR genes (ABCA1, SREBP-1c) and resolved inflammation in cultured macrophages, contrasting results were observed in LDL receptor knockout mice [48]. When poly(lactic-co-glycolic acid) (PLGA) nanoparticles loaded with GW3965 and PtdSer were administered, PtdSer-containing NPs (PSNP-LXR) were not superior to NP-LXR that did not have PtdSer. When the atherosclerotic regions were stained for CD68+ macrophages NP-LXR treatment caused a 50% reduction in macrophage numbers, whereas PSNP-LXR and free GW3965 treatments were less effective demonstrating 40% and 30% reduction, respectively. Researchers pointed out that faster clearance of PSNP-LXR by liver Kupffer cells could be responsible for their decreased accumulation in the plaque, attributing to observed difference in efficacy between the two types of NPs. Consistent with this explanation, PS-LXR showed higher levels of triglycerides in liver as compared to NP-LXR (140 vs. 117 mg/dL), likely because of hepatic side-effect actions of LXR agonist, known to cause hypertriglyceridemia in the liver. Interestingly, these observations may be directly related to increased affinity of liver hepatocytes for PtdSer NPs. The hepatocyte cell line HepG2 showed dose- and time-dependent uptake of PtdSer-NPs [49].
Given the ability of EMS to ameliorate inflammation, of particular interest are EMS-composed nanocarriers that discriminate cell’s inflammatory status and allow for selective targeting of inflamed cells. Such a strategy may find therapeutic application for treatment of chronic inflammatory conditions such as atherosclerosis and obesity, where macrophage inflammation plays a key pathogenic role. Bagalkot et al. reported a new lipid-latex hybrid nanoparticles (LiLa-NP) that targeted pro-inflammatory classically-activated (“M1”-type) macrophages more efficiently than anti-inflammatory macrophages. The targeting was achieved by virtue of two EMS: PtdSer and oxidized cholesterol derivative 9-CCN. Macrophage labeling by LiLa-NP was confirmed in vitro by means of imaging flow-cytometry and in vivo by means of MRI and in-travital imaging in animal models of atherosclerosis and obesity [50]. The authors also demonstrated that LiLa-NP can deliver a payload of anti-inflammatory drug rosiglitazone, a potent PPARγ agent, that efficiently downregulated the expression of inflammatory molecules in LPS-stimulated RAW264.7 macrophages.
Another area of therapeutic potential for PtdSer NPs may be in the targeted induction of the immune response to a specific antigen. A study by Yotsumoto et al. used PSL loaded with ovalbumin (OVA) antigen in co-culture studies using OVA-specific Th1 clone 42-6 A cells with splenic adherent cells (SAC) [51]. OVA-loaded PSL induced production of key Th1 cytokines such as IL-12 and IFN-γ. When used in vivo, PSL loaded with OVA significantly increased IFN-γ levels in serum and spleen versus OVA control. These studies demonstrate that PSL loaded with antigens have the potential to facilitate T cell activation toward a specific action by targeting antigens to antigen presenting, innate immune cells populations [52].
2.3. Delivery through a combination of a targeting vector and PtdSer
Targeting moieties such as antibodies or peptides that recognize markers in disease may significantly improve the targeting of nanocarriers [53]. An attractive prospect is to combine targeted delivery with the affinity of PtdSer to phagocytic cells. Such an approach may be particularly useful when macrophage targeting is required, as the PtdSer-bearing carrier would be delivered to sites with high abundance of macrophages enabling efficient intramacrophage delivery. There are but a few examples where this targeting has been tested. Immunoliposomes decorated with anti-Env antibodies (targets an HIV surface protein) and PtdSer efficiently bound HIV-like particles. These particles were efficiently engulfed by macrophages [54]. This is important, because macrophages are believed to be the first cells encountered by the HIV-1 virus during infection and they are needed for viral replication and the subsequent spread of infection. It is known that HIV infection impairs antibody-mediated phagocytosis in macrophages. It was posited that HIV-directed carriers presenting PtdSer might represent a novel macrophage-centric approach to fighting HIV by improving macrophage-mediated clearance of HIV particles, decreasing immune system activation and stimulation of B cells, and enhancing production of antibodies against the virus.
In another report, PSL containing a seven amino acid peptide sequence specific for lung epithelium (endothelin receptors) delivered a prototypical GFP plasmid to the lung. The PtdSer enabled fusion of liposomes with the endosomal membrane at low pH causing release of the plasmid cargo into the cytoplasm. The intra-tracheal instillation of these liposomes in rat model resulted in the expression of GFP in bronchioles and alveoli within 5 days. Hence, the synergistic action of PtdSer and targeting peptide demonstrated that PSL could serve as potential gene delivery vector [55].
3. Imaging with PtdSer carriers
EMS have been used in the imaging of inflammatory environments including but not limited to atherosclerosis, cancer, arthritis, and certain neurological disorders where innate immune cells such as macrophages and dendritic cells play a primary role in disease. Targeting of macrophages using PtdSer has been successful using magnetic resonance imaging (MRI), ultrasound, fluorescence, and single photon emission computed tomography (Table 3).
Table 3.
Molecular imaging with EMS vehicles
| Imaging modality | Carrier and contrast agent | Disease/animal model | Imaging enhancement effects | References |
|---|---|---|---|---|
| Magnetic resonance imaging (MRI) | Gadolinium (Gd)-PtdSer-cholesterol-9-carboxynonanoate [9-CCN] liposomes (OxPL vesicles) | Atherosclerosis: Watanabe hereditary hyperlipidemic (WHHL) rabbit model of atherosclerosis | T1 weighted MRI: high T1 contrast in the vessel wall upto 24 h. | [62] |
| Gd-PtdSer liposomes | Atherosclerosis: ApoE−/− mice | T1 weighted MRI: rapid and significant enhancement in the aortic wall upto 4 h. | [134] | |
| Gd-PtdSer liposomes | RAW 264.7 macrophages | Cellular relaxivity (r1 = 0.8 ± 0.4 mM−1.s−1) was lower than in (r1 = 3.0 ± 0.3 mM−1.s−1) solution. Compartmentalization of PtdSer liposomes results in lower r1. |
[60] | |
| PtdSer, Ω-carboxynonanoyl-cholesteryl ester, Gd lipid and phospholipid-PEG conjugated anti-Mrp polyclonal antibody (aMrp-NP) liposomes | Atherosclerosis: ApoE−/− mice | T1 weighted MRI: enhanced contrast to noise ratio (22 fold higher) in ApoE−/− abdominal aorta. | [63] | |
| CT imaging | Iodixanol-NIR800-rhodamine-PtdSer-Liposomes | Atherosclerosis: WHHL and balloon-denuded cholesterol-fed New Zealand White (NZW) rabbits | Smaller PtdSer liposomes (d = 112 ± 4 nm) were more effective in CT signal enhancement in aorta in both animal models. | [64] |
| Fluorescence imaging | Quantum dot-PtdSer -PEG micelles | J774A.1 macrophages | 50:50 M ratio of PtdSer and PEG evaded uptake by macrophages, varying PtdSer and PEG can differentially image macrophage uptake | [135] |
| Ultrasound imaging | PtdSer micro-bubbles | Ischemia: renal ischemia–reperfusion injury C57BL/6 mice. | Signal was 2-fold higher for PtdSer -containing micro-bubbles than for standard lipid micro-bubbles. | [66] |
| Myocardial contrast echocardiography (MCE) imaging/PtdSer micro-bubbles | Myocardial Ischemia: dogs-ultrasound imaging of the left anterior descending (LAD) and left circumflex (LCx) arteries | Spatial extent of inflammation by MCE similar to radionuclide imaging by 99mTc-RP517. | [67] | |
| Perfusion imaging/PtdSer-integrin-VCAM-1 micro-bubbles | Ischemia: models of vasculogenesis (subcutaneous matrigel) or hind-limb ischemia produced by arterial occlusion in C57BL/6 and MCP-1−/− mice | CEU imaging of matrigel showed the formation of channels with flow at day3. Selective signal enhancement in ischemic limb with PtdSer, integrin, VCAM-1 targeted micro-bubbles. | [68] | |
| SPECT imaging | 111In labeled- PtdSer liposomes | Atherosclerosis: ApoE−/− mice | En face autoradiography revealed successful visualization of plaques by 111In labeled- PtdSer liposomes | [65] |
3.1. Magnetic resonance imaging (MRI)
A series of reports from our group demonstrated that liposomes and anionic vesicles decorated with PtdSer exhibited enhanced affinity for macrophages in atherosclerosis, allowing their imaging. In several experimental setting these particles modulated inflammatory pathways. A common feature of atherosclerotic plaques is the presence of inflammatory macrophages [56]. Atherosclerosis-associated macrophages commonly exhibit multiple dysfunctional features including the upregulation of pro-inflammatory cytokines and transformation into lipid-laden foam cells that may directly contribute to the progression of atherogenesis and cardiovascular disease [57]. Importantly, the genesis of foam cells is believed to relate to the engulfment of apoptotic cells (called efferocytosis) and oxidatively modified lipids formed during low-density lipoprotein (LDL) oxidation [58]. Therefore, a nanoparticulate carrier of an MRI contrast agent that mimics apoptotic cells and/or oxidized lipids may have an amplified recognition by atherosclerotic plaque macrophages enabling plaque detection.
An earlier report by our laboratory noted that PSL accumulate in the atherosclerotic plaque of ApoE−/− mice even in the absence of polyethylene glycol (PEG), whereas this targeting pattern was not seen for PC liposomes. PEG is widely used in drug delivery, particularly in nanocarriers offering “stealth” properties and prolonged circulation half-lives. Therefore, in the absence of PEG, one could envision the rapid sequestration of PtdSer particles by the reticuloendothelial system (RES). However, we noted that gadolinium-tagged PSL delineated atherosclerotic plaque for at least four hours while under continuous MRI imaging. In addition, prominent accumulation of PSL were observed (by confocal microscopy) in plaque macrophages 24 h after agent administration, suggesting that PtdSer clearly enhances liposomal accumulation in the arterial wall [59]. Interestingly, our results have been recently confirmed by other researchers using almost identical liposomal formulation [60].
Macrophage-targeting in atherosclerosis may be enhanced if an additional EMS is used in conjunction with PtdSer. Based on the known affinity of oxidized lipids to plaque macrophages [61], the oxidized cholesterol ester derivative cholesterol-9-carboxynonanoate (9-CCN) was used in combination with PtdSer to increase macrophage engulfment in a rabbit model of spontaneous atherosclerosis with features similar to humans. Watanabe hereditary hyperlipidemic (WHHL) rabbits were used to test this dual-EMS targeting strategy [62]. It was shown that this strategy not only improves the imaging of macrophages in atherosclerosis 24 h post-injection (vs. ~12 h for single-EMS PSL), but also increases the circulation residence time of the dual-EMS carrier, possibly due to its spontaneous in vivo association with serum low-density lipoprotein (LDL).
The most recent approach from our group that takes advantage of PtdSer/9-CCN-enhanced targeting involves imaging inflammatory macrophages in atherosclerotic plaque and adipose tissue through LiLa nanoparticles. In this approach, hybrid LiLa-NPs are labeled with gadolinium lipids and near-infrared dye to allow simultaneous MR and optical detection. LiLa-NP enhanced atherosclerosis imaging through MRI in high-fat fed ApoE−/− mice and were cleared much more rapidly in these mice as compared to wild-type mice, as evidenced by 3D FLASH MR imaging and confirmed by traditional pharmacokinetic studies. This was possibly due to higher uptake of LiLa-NP by inflammatory macrophages that are ubiquitously expressed in atherosclerotic ApoE−/−mice but to the lesser extent in wild-type animals [50].
LiLa-NP also allowed selective discrimination of M1 versus M2 macrophages in adipose tissue. Intravital imaging experiments utilizing AlexaFluor 647-labeled LiLa-NP as a contrast agent and genetic mouse model that expresses yellow-fluorescent protein in circulating and tissue-resident macrophages (c-fmsYFP+ mice) were performed in obese and lean mice. The time-lapse imaging of the fat pad demonstrated faster LiLa-NP uptake in M1-macrophages of obese mice (inflammatory M1 macrophages are highly expressed in obesity) as compared to uptake in M2 macrophages in lean mouse model [50].
The combination of immuno-targeting with macrophage-directing EMS-nanocarriers may additionally improve atherosclerosis targeting. We explored the use of antibodies against myeloid related protein 8/14 (MRP 8/14), a pro-inflammatory protein secreted by macrophages and neutrophils in atherosclerotic plaque in response to immune activation. MRP 8/14 is abundant in rupture-prone necrotic areas of atherosclerotic plaques and thus an attractive target for imaging and therapy. Immunonanoparticles with two EMS (PtdSer and 9-CCN) delivered MRI contrast to both plaque macrophages and endothelial cells. Endothelial cell enhancement may be due to endothelium-bound MRP 8/14. The results were confirmed by means of attenuated gadolinium enhancement in aorta of mice lacking both ApoE and MRP (ApoE−/−/Mrp 14−/−), which confirmed specificity of this targeting approach [63]. Although most of these studies show that phagocytic EMS can preferentially target inflammatory macrophages in atherosclerosis, there is a possibility of translation to other potential clinical applications where macrophage inflammation has detrimental effects. Thus, EMS targeting may be impactful for treatment of cardiovascular disease, obesity, diabetes, and cancer.
3.2. CT imaging
Clinical CT imaging is a widely used modality that offers excellent spatial resolution and tissue specificity. We have shown the applicability of PSL loaded with the FDA-approved CT contrast agent iodixanol for macrophage targeting in plaque using two large animal models of atherosclerosis. We tested these liposomes in WHHL rabbits and in balloon-injured artery New Zealand white rabbits (NZW) [64]. The development of atherosclerosis in WHHL rabbits resembles human plaques, whereas the NZW injury model recapitulates vascular inflammation. We hypothesized that changes in macrophage biology, local abundance of macrophages, and nature of the endothelium would result in uptake differences of PSL and their retention in the plaque macrophages. Surprisingly, we found no difference in targeting and accumulation of PSL between these models. We found, however, that the size of PSL influenced their uptake and retention in plaques as indicated by CT imaging. Thus, PSL with hydrodynamic diameter of ~111 nm were optimal for plaque accumulation.
3.3. SPECT imaging
Non-PEGylated, indium-111-radiolabeled liposomes (111In)-PSL have been used in SPECT imaging of atherosclerotic plaques in ApoE−/− mice and WHHL rabbits [65]. In this study, liposomes of 100 and 200 nm were first tested in cultured macrophages. These in vitro findings suggested that the smaller 111In-PS100 (100 nm) liposomes were more efficient at targeting macrophages than 111In-PS200 (200 nm) liposomes. Liposomes with unnatural D-serine PtdSer were used to test the specificity to PtdSer receptors on the macrophages, showing lower uptake in macrophages, comparable to that of PC liposomes. (111In)-PSL showed fast blood clearance in the order of several minutes (~3 min). En face autoradiography and histologic analysis demonstrated accumulation of both formulations in atherosclerosis. The authors also noted high liver accumulation for both formulations and suggested that a certain level of PEGylation of liposomes may be incorporated in order to alter blood clearance rate while maintaining macrophage targeting.
3.4. Contrast-enhanced ultrasound imaging
Microbubbles (MBs) are ubiquitous in contrast-enhanced ultrasound imaging. Usually fabricated from sugar matrices, polymeric microspheres and proteins, and filled with gases or perfluorocarbons, MBs rapidly resonate in an ultrasound beam. Their contraction and expansion depends on the pressure changes of the ultrasound wave allowing for diagnostic ultrasound imaging. Early studies demonstrated that some PtdSer-containing MBs efficiently targeted blood leukocytes. It was hypothesized that incorporation of PtdSer into MBs resulted in an negatively charged particle enhancing leukocyte targeting. However, MB phagocytosis by blood leukocytes was likely a result of “eat-me” targeting. Thus, Jonathan and colleagues used PtdSer lipid-based MBs (filled with decafluorobutane) which bound leukocytes via complement-mediated activation and also to monocytes via PtdSer receptor binding [66]. In addition, non-invasive ultrasound imaging allowed quantification of MBs in mice with ischemia–reperfusion kidney injury. Within 10 min of intravenous administration PtdSer MBs were seen attaching to leukocytes in the inflamed venules compared to standard lipid micro-bubbles. After ischemia–reperfusion, the signal from retained MBs was 2-fold higher for PtdSer-containing than for standard lipid MBs. Another study by the same research group tested these PtdSer-MBs for assessing severity in post ischemic myocardial injury by coronary artery occlusion in dogs [67]. In dogs with ischemia, the PS micro-bubble accumulation in leukocytes by myocardial contrast echocardiography (MCE) correlated with leukocyte accumulation by radionuclide imaging using 99mTc-RP517.
Multi-targeted MBs along with PtdSer, α5-integrins, and vascular adhesion molecule (VCAM-1) have been reported for contrast ultrasound perfusion imaging in murine models of vasculogenesis (subcutaneous matrigel) or hind-limb ischemia produced by arterial occlusion [68]. Incorporation of these ligands on MBs enables monitoring of recovery from ischemia and the response of inflammation in tissue healing.
PtdSer-containing MBs targeted activated neutrophils with selective signal enhancement in ischemic limb (signal correlated with staining for polymorphonuclear leukocytes). Similar enhancement was seen with MBs containing integrin and VCAM-1 receptor ligands. VCAM-1 enhanced signals persisted longer and correlated with endothelial expression of VCAM-1 in intramuscular arterioles and venules by immunohistochemistry. This study demonstrated that molecular imaging can detect inflammatory processes that coincide with the appearance of functional microvessels, the type of cells and adhesion molecules involved in healing and vascular remodeling after injury [68].
Echogenic MBs with PtdSer alone or in combination with targeting antibodies offer the opportunity to detect vascular inflammation by non-invasive ultrasound imaging, however significant challenges remain. For example, it is necessary to achieve a minimum concentration of MBs at the target site. Also, the ultrasonic signal must be capable of producing acceptable signal from the MBs that achieved their target versus the noise of the tissue. This relates to the major challenge, which is distinguishing between resident/targeted MBs and circulating MBs as well as from non-specific signal produced by the blood-intimal interface.
4. Conclusions and future prospective
Modern nanoparticle (NP) technologies are often directed to a specific cell type via a targeting ligand (e.g antibody) [53] or deliver their cargo to disease sites via synthetically modified natural lipoproteins [69–71]. These strategies are challenging requiring complex NP synthesis, expensive antigen bio-conjugation, discordance of targets between mouse and human, and NP deposition in the liver increasing potential toxicity. These issues may hamper successful application of targeted NPs in clinical settings and, possibly, the reason why currently FDA-approved NPs or those in late phase clinical trials do not rely on antibody–antigen interactions [72,73]. Intelligent surface manipulation on NPs, which are made of safe materials, presents a meaningful opportunity for successful and efficient translation to clinical applications. Here, we highlight recent advances in the field of NPs that have been targeted via “eat-me” signals (EMS), mainly PtdSer and discuss therapeutic and diagnostic potential of such strategies. It is clear that the incorporation of PtdSer not only aids in targeting inflammatory phagocytic cells but also as an anti-inflammatory signal for resolution of inflammation. Hence, PtdSer carriers equipped with an imaging agent may represent an attractive “theranostic” (therapy + diagnosis) approach when one encapsulates a therapeutic agent into PtdSer carriers. The latter is obviously important in combined imaging-therapy setting, but also could be useful for understanding mechanisms of diseases and impaired apoptotic cell clearance.
To date, most imaging studies with EMS-bearing contrast agents have focused on diagnostic detection of cardiovascular abnormalities, including atherosclerotic plaque imaging. Although landmark studies by Z. Fayad, W. Mulder and E. Fisher provided almost exhaustive perspective on imaging of plaque with nanocarriers targeted via antibodies [71,74,75] and peptides [76–79] that recognize macrophages within atherosclerotic lesions, most of the above-mentioned challenges remain and may hamper clinical translation because of the antigenic nature of targeting approach employed in these studies. At the same time, the ability to accurately predict the risk of atherosclerotic plaque rupture and its complications in the clinic is currently limited to invasive diagnostic tools such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT) [80]. Emerging “molecular imaging” approaches that are based on recognition of pathophysiologic processes such as rise in tissue pH or increased metabolic activity of cells can often provide valuable information regarding disease severity and outcomes [81]. For example, molecular imaging of macrophages that relies upon detection of their high metabolic activity in inflamed plaque can be achieved by measuring hypermetabolic uptake of 2-deoxy-2-(-18F)fluoro-D-glucose (18F-FDG) using positron emission tomography (PET) [82,83]. Interestingly, the uptake of 18F-FDG-like tracer 2-deoxy-2-(18F)fluoro-D-mannose (18F-FDM) was significantly increased in macrophages expressing mannose receptor (“M2”-phenotype) as detected in plaques of atherosclerotic rabbits [84]. The role of “M2” macrophages in tumor growth should be mentioned, with various anti-cancer nanomedicines targeting the mannose receptor receiving attention recently [85,86]. Intriguingly, molecular imaging of “M1”-type macrophages, that to the best of our knowledge has been underexplored, may be achieved through “eat-me” targeting [50].
Current clinical approaches to image plaque in patients are quantitative and are not directed at providing additional information of characteristics that could predict risk. There is no current approach that combines imaging with delivery of a therapeutic at the same time. There is extraordinary interest in assessment of lesions that can provide better risk discrimination of plaques that are likely to result in complications and importantly to be able to intervene in such lesions. The landmark PROSPECT trial using intravascular ultrasound [87], studies by Motoyoma et al. in CT [88], Rudd et al. with PET-CT [89–91] and studies by Yuan et al. using MRI [92] have demonstrated that aspects of vulnerable plaque may be identified using imaging approaches. EMS approaches have the value of being applicable to a broad range of platforms and importantly provide a modality-naïve approach of combinatorial imaging therapeutics. The field of molecular imaging and intervention has matured to a point where intelligent contrast agent and drug delivery using recent new insights in atherosclerosis may be leveraged. Recently, two first-in-human nanotherapy trials were conducted in atherosclerosis patients (ClinicalTrials.gov Identifiers NCT01647685 and NCT01601106). Thereby engineered nanotherapeutics with EMS such as PtdSer and others present an exciting new treatment paradigm for halting plaque progression and rupture.
Many challenges remain ahead such as possible immunogenicity of PtdSer carriers and very broad targeting patterns associated with the abundance of “PtdSer-hungry” phagocytes in nearly every tissue. Toxicity of PtdSer agents is another issue that needs more thorough evaluation before possible translation in humans.
Acknowledgments
This review was completed with support from American Heart Association (NCRP Scientist Development Grant 13SDG14500015) to Andrei Maiseyeu, and from National Institute of Diabetes and Digestive and Kidney Diseases (grant 5 K01 DK099475) to Jeffrey Deiuliis.
Abbreviations
- PtdSer
phosphatidylserine
- OVA
ovalbumin
- PSL
phosphatidylserine liposomes
- ABL
apoptotic body like liposomes
- PC
phosphatidylcholine
- OxLDL
oxidized low density lipoprotein
- PA
phosphatidicacid
- PE
phosphoethanolamine
- AA
adjuvant arthritis
- IL6
interleukin 6
- IL12
interleukin 12
- IL 10
interleukin 10
- IL1β
interleukin 1β
- PPAR-γ
peroxisome proliferator activated receptor
- TGFβ
transforming growth factor β
- TNFα
Tumor necrosis factor α
- Ccl5
Chemokine (C-C motif) ligand 5
- Ccl2
Chemokine (C-C motif) ligand 2\
- Cx3cl1
fractalkine chemokine (C-X3-C motif) ligand 1
- NO
nitric oxide
- EMS
eat me signal
- NPs
nanoparticles
- DNCB
2,4-dinitro, 1-chlorobenzene
- CD4+ T cells
T helper cells expressing cluster of differentiation 4
- ROS
reactive oxygen species
- PLD
phospholipase D
- MTB
Mycobacterium tuberculosis
- PGE2
prostaglandin E2
- SapC-DOPS
Saponin C dioleoylphosphatidylserine
- LPS
lipopolysaccharide
- LXR
Liver X receptor
- ABCA1
ATP-binding cassette transporter
- SREBP-1c
Sterol regulatory element-binding transcription factor 1
- PLGA
poly(lactic-co-glycolic acid)
- GW3965
LXR agonist
- HepG2
hepatocarcinoma cell line
- CD68
Cluster of Differentiation 68 expressed on macrophage
- SAC
splenic adherent cells
- HIV-1 virus
human immunodeficiency 1 virus
- GFP
green fluorescent protein
- MRI
magnetic resonance imaging
- SPECT
single photon emission computed tomography
- CT
computed tomography
- PEG
polyethylene glycol
- RES
reticuloendothelial system
- 9-CCN
cholesterol-9-carboxynonanoate
- MRP 8/14
myeloid related protein 8/14
- ApoE
apolipoprotein E
- WHHL
Watanabe hereditary hyperlipidemic
- NZW
New Zealand white
- MCE
myocardial contrast echocardiography
- VCAM-1
vascular adhesion molecule
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Non-Antigenic Regulators-Maiseyeu”.
References
- 1.Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006;27(5):244–250. doi: 10.1016/j.it.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Lauber K, et al. Clearance of Apoptotic Cells: Getting Rid of the Corpses. Mol Cell. 2004;14(3):277–287. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147(4):742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi ME. DAMPs, PAMPs and alarmins: All we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 5.Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol. 2011;22(3):416–425. doi: 10.1681/ASN.2010040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott MR, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461(7261):282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chekeni FB, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467(7317):863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauber K, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113(6):717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 9.Truman LA, et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112(13):5026–5036. doi: 10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- 10.Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: Apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5(1) doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campisi L, Cummings RJ, Blander JM. Death-defining immune responses after apoptosis. Am J Transplant. 2014;14(7):1488–1498. doi: 10.1111/ajt.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poon IKH, et al. Apoptotic cell clearance: Basic biology and therapeutic potential. Nat Rev Immunol. 2014;14(3):166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravichandran KS. Beginnings of a good apoptotic meal: The find-me and eat-me signaling pathways. Immunity. 2011;35(4):445–455. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poon IK, et al. Apoptotic cell clearance: Basic biology and therapeutic potential. Nat Rev Immunol. 2014;14(3):166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narula J, et al. Annexin-V imaging for noninvasive detection of cardiac allograft rejection. Nat Med. 2001;7(12):1347–1352. doi: 10.1038/nm1201-1347. [DOI] [PubMed] [Google Scholar]
- 16.Isobe S, et al. Noninvasive imaging of atherosclerotic lesions in apolipoprotein E-deficient and low-density-lipoprotein receptor-deficient mice with annexin A5. J Nucl Med. 2006;47(9):1497–1505. [PubMed] [Google Scholar]
- 17.Laufer EM, et al. PET and SPECT imaging of apoptosis in vulnerable atherosclerotic plaques with radiolabeled annexin A5. Q J Nucl Med Mol Imaging. 2009;53(1):26–34. [PubMed] [Google Scholar]
- 18.Kolodgie FD, et al. Targeting of apoptotic macrophages and experimental atheroma with radiolabeled annexin V: A technique with potential for noninvasive imaging of vulnerable plaque. Circulation. 2003;108(25):3134–3139. doi: 10.1161/01.CIR.0000105761.00573.50. [DOI] [PubMed] [Google Scholar]
- 19.Zhou H, et al. Phosphatidylserine-targeted molecular imaging of tumor vasculature by magnetic resonance imaging. J Biomed Nanotechnol. 2014;10(5):846–855. doi: 10.1166/jbn.2014.1851. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, et al. Phosphatidylserine-targeted bimodal liposomal nanoparticles for in vivo imaging of breast cancer in mice. J Control Release. 2014;183:114–123. doi: 10.1016/j.jconrel.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 21.Wuest M, et al. Radiopharmacological evaluation of f-labeled phosphatidylserine-binding peptides for molecular imaging of apoptosis. Nucl Med Biol. 2015 doi: 10.1016/j.nucmedbio.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Li J, et al. Characterization of (18)F-dipicolylamine (DPA) derivatives in cells infected with influenza virus. Nucl Med Biol. 2015;42(3):283–291. doi: 10.1016/j.nucmedbio.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Fischer U, Schulze-Osthoff K. Apoptosis-based therapies and drug targets. Cell Death Differ. 2005;12(Suppl 1):942–961. doi: 10.1038/sj.cdd.4401556. [DOI] [PubMed] [Google Scholar]
- 24.Peer D. Immunotoxicity derived from manipulating leukocytes with lipid-based nanoparticles. Adv Drug Deliv Rev. 2012;64(15):1738–1748. doi: 10.1016/j.addr.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Krysko O, et al. Many faces of DAMPs in cancer therapy. Cell Death Dis. 2013;4:e631. doi: 10.1038/cddis.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peer D. Immunotoxicity derived from manipulating leukocytes with lipid-based nanoparticles. Adv Drug Deliv Rev. 2012;64(15):1738–1748. doi: 10.1016/j.addr.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Krysko DV, et al. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 28.Bratton DL, et al. Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J Biol Chem. 1997;272(42):26159–26165. doi: 10.1074/jbc.272.42.26159. [DOI] [PubMed] [Google Scholar]
- 29.Hankins HM, et al. Role of flippases, scramblases and transfer proteins in phosphatidylserine subcellular distribution. Traffic. 2015;16(1):35–47. doi: 10.1111/tra.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fadok VA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148(7):2207–2216. [PubMed] [Google Scholar]
- 31.Kuypers FA, et al. Detection of altered membrane phospholipid asymmetry in subpopulations of human red blood cells using fluorescently labeled annexin V. Blood. 1996;87(3):1179–1187. [PubMed] [Google Scholar]
- 32.Kuypers FA, et al. Membrane phospholipid asymmetry in human thalassemia. Blood. 1998;91(8):3044–3051. [PubMed] [Google Scholar]
- 33.Wilson MJ, Richter-Lowney K, Daleke DL. Hyperglycemia induces a loss of phospholipid asymmetry in human erythrocytes. Biochemistry. 1993;32(42):11302–11310. doi: 10.1021/bi00093a006. [DOI] [PubMed] [Google Scholar]
- 34.Huynh MLN, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J Clin Investig. 2002;109(1):41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi D, et al. Artificial phosphatidylserine liposome mimics apoptotic cells in inhibiting maturation and immunostimulatory function of murine myeloid dendritic cells in response to 1-chloro-2,4-dinitrobenze in vitro. Arch Dermatol Res. 2007;299(7):327–336. doi: 10.1007/s00403-007-0770-9. [DOI] [PubMed] [Google Scholar]
- 36.Stuart LM, et al. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168(4):1627–1635. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]
- 37.Ramos GC, et al. Apoptotic mimicry: Phosphatidylserine liposomes reduce inflammation through activation of peroxisome proliferator-activated receptors (PPARs) in vivo. Br J Pharmacol. 2007;151(6):844–850. doi: 10.1038/sj.bjp.0707302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Posadas I, et al. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br J Pharmacol. 2004;142(2):331–338. doi: 10.1038/sj.bjp.0705650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harel-Adar T, et al. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci U S A. 2011;108(5):1827–1832. doi: 10.1073/pnas.1015623108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dvoriantchikova G, et al. Phosphatidylserine-containing liposomes promote maximal survival of retinal neurons after ischemic injury. J Cereb Blood Flow Metab. 2009;29(11):1755–1759. doi: 10.1038/jcbfm.2009.95. [DOI] [PubMed] [Google Scholar]
- 41.Hashioka S, et al. Phosphatidylserine and phosphatidylcholine-containing liposomes inhibit amyloid beta and interferon-gamma-induced microglial activation. Free Radic Biol Med. 2007;42(7):945–954. doi: 10.1016/j.freeradbiomed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Greco E, et al. Janus-faced liposomes enhance antimicrobial innate immune response in mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 2012;109(21):E1360–E1368. doi: 10.1073/pnas.1200484109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Z, et al. Phosphatidylserine-containing liposomes inhibit the differentiation of osteoclasts and trabecular bone loss. J Immunol. 2010;184(6):3191–3201. doi: 10.4049/jimmunol.0803609. [DOI] [PubMed] [Google Scholar]
- 44.Ma HM, Wu Z, Nakanishi H. Phosphatidylserine-containing liposomes suppress inflammatory bone loss by ameliorating the cytokine imbalance provoked by infiltrated macrophages. Lab Investig. 2011;91(6):921–931. doi: 10.1038/labinvest.2011.54. [DOI] [PubMed] [Google Scholar]
- 45.Olowokure O, Qi X. Pancreatic cancer: Current standards, working towards a new therapeutic approach. Expert Rev Anticancer Ther. 2014;14(5):495–497. doi: 10.1586/14737140.2014.895937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chu Z, et al. Targeting and cytotoxicity of SapC-DOPS nanovesicles in pancreatic cancer. PLoS One. 2013;8(10):e75507. doi: 10.1371/journal.pone.0075507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu K, et al. Toll-like receptor 4 can recognize SapC-DOPS to stimulate macrophages to express several cytokines. Inflamm Res. 2011;60(2):153–161. doi: 10.1007/s00011-010-0249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang XQ, et al. Nanoparticles containing a liver X receptor agonist inhibit inflammation and atherosclerosis. Adv Healthcare Mater. 2015;4(2):228–236. doi: 10.1002/adhm.201400337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romero G, et al. Lipid layer engineering of poly(lactide-co-glycolide) nanoparticles to control their uptake and intracellular co-localisation. J Mater Chem B. 2013;1(17):2252. doi: 10.1039/c3tb00284e. [DOI] [PubMed] [Google Scholar]
- 50.Bagalkot V, et al. Hybrid nanoparticles improve targeting to inflammatory macrophages through phagocytic signals. J Control Release. 2015;217:243–255. doi: 10.1016/j.jconrel.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yotsumoto S, et al. Induction of antigen-dependent interleukin-12 production by negatively charged liposomes encapsulating antigens. Vaccine. 2004;22(25–26):3503–3509. doi: 10.1016/j.vaccine.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 52.Yotsumoto S, Kakiuchi T, Aramaki Y. Enhancement of IFN-gamma production for Th1-cell therapy using negatively charged liposomes containing phosphatidylserine. Vaccine. 2007;25(29):5256–5262. doi: 10.1016/j.vaccine.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 53.Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5(2):147–159. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- 54.Gramatica A, et al. alphaEnv-decorated phosphatidylserine liposomes trigger phagocytosis of HIV-virus-like particles in macrophages. Nanomedicine. 2014;10(5):981–989. doi: 10.1016/j.nano.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 55.Allon N, et al. A new liposome-based gene delivery system targeting lung epithelial cells using endothelin antagonist. J Control Release. 2012;160(2):217–224. doi: 10.1016/j.jconrel.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 56.Khallou-Laschet J, et al. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5(1):e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: A dynamic balance. Nat Rev Immunol. 2013;13(10):709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10(1):36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maiseyeu A, et al. Gadolinium-containing phosphatidylserine liposomes for molecular imaging of atherosclerosis. J Lipid Res. 2009;50(11):2157–2163. doi: 10.1194/jlr.M800405-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geelen T, et al. Internalization of paramagnetic phosphatidylserine-containing liposomes by macrophages. J Nanobiotechnol. 2012;10:37. doi: 10.1186/1477-3155-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kunjathoor VV, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277(51):8–49982. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 62.Maiseyeu A, et al. Detection of macrophages via paramagnetic vesicles incorporating oxidatively tailored cholesterol ester: an approach for atherosclerosis imaging. Nanomedicine. 2010;5:1341–1356. doi: 10.2217/nnm.10.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maiseyeu A, et al. In vivo targeting of inflammation-associated myeloid-related protein 8/14 via gadolinium immunonanoparticles. Arterioscler Thromb Vasc Biol. 2012;32:70–962. doi: 10.1161/ATVBAHA.111.244509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kee P, et al. Noninvasive detection of macrophages in atheroma using a radiocontrast-loaded phosphatidylserine-containing liposomal contrast agent for computed tomography. Mol Imaging Biol. 2015;17:328–336. doi: 10.1007/s11307-014-0798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogawa M, et al. Development of 111In-labeled liposomes for vulnerable atherosclerotic plaque imaging. J Nucl Med. 2014;55:20–115. doi: 10.2967/jnumed.113.123158. [DOI] [PubMed] [Google Scholar]
- 66.Lindner JR, et al. Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation. 2000;102:2745–2750. doi: 10.1161/01.cir.102.22.2745. [DOI] [PubMed] [Google Scholar]
- 67.Christiansen JP, et al. Noninvasive imaging of myocardial reperfusion injury using leukocyte-targeted contrast echocardiography. Circulation. 2002;105:1764–1767. doi: 10.1161/01.cir.0000015466.89771.e2. [DOI] [PubMed] [Google Scholar]
- 68.Behm CZ, et al. Molecular imaging of endothelial vascular cell adhesion molecule-1 expression and inflammatory cell recruitment during vasculogenesis and ischemia-mediated arteriogenesis. Circulation. 2008;117:2902–2911. doi: 10.1161/CIRCULATIONAHA.107.744037. [DOI] [PubMed] [Google Scholar]
- 69.Huang H, et al. Learning from biology: Synthetic lipoproteins for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(3):298–314. doi: 10.1002/wnan.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allijn IE, et al. Gold nanocrystal labeling allows low-density lipoprotein imaging from the subcellular to macroscopic level. ACS Nano. 2013;7(11):9761–9770. doi: 10.1021/nn403258w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Briley-Saebo KC, et al. Targeted iron oxide particles for in vivo magnetic resonance detection of atherosclerotic lesions with antibodies directed to oxidation-specific epitopes. J Am Coll Cardiol. 2011;57(3):337–347. doi: 10.1016/j.jacc.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng Z, et al. Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338(6109):903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13(11):813–827. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nguyen TH, et al. Manganese G8 dendrimers targeted to oxidation-specific epitopes: In vivo MR imaging of atherosclerosis. J Magn Reson Imaging. 2015;41(3):797–805. doi: 10.1002/jmri.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mulder WJ, et al. Molecular imaging of macrophages in atherosclerotic plaques using bimodal PEG-micelles. Magn Reson Med. 2007;58(6):1164–1170. doi: 10.1002/mrm.21315. [DOI] [PubMed] [Google Scholar]
- 76.Gianella A, et al. Synthesis and in vitro evaluation of a multifunctional and surface-switchable nanoemulsion platform. Chem Commun (Camb) 2013;49(82):9392–9394. doi: 10.1039/c3cc43618g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muller A, et al. Imaging atherosclerotic plaque inflammation via folate receptor targeting using a novel 18F-folate radiotracer. Mol Imaging. 2014;13:1–11. [PubMed] [Google Scholar]
- 78.Cormode DP, et al. An ApoA-I mimetic peptide high-density-lipoprotein-based MRI contrast agent for atherosclerotic plaque composition detection. Small. 2008;4(9):1437–1444. doi: 10.1002/smll.200701285. [DOI] [PubMed] [Google Scholar]
- 79.Duivenvoorden R, et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat Commun. 2014;5:3065. doi: 10.1038/ncomms4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sinclair H, et al. OCT for the identification of vulnerable plaque in acute coronary syndrome. J Am Coll Cardiol Img. 2015;8(2):198–209. doi: 10.1016/j.jcmg.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 81.Mulder WJ, et al. Imaging and nanomedicine in inflammatory atherosclerosis. Sci Transl Med. 2014;6(239):239, sr1. doi: 10.1126/scitranslmed.3005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vucic E, et al. Pioglitazone modulates vascular inflammation in atherosclerotic rabbits noninvasive assessment with FDG-PET-CT and dynamic contrast-enhanced MR imaging. J Am Coll Cardiol Img. 2011;4(10):1100–1109. doi: 10.1016/j.jcmg.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hyafil F, et al. Quantification of inflammation within rabbit atherosclerotic plaques using the macrophage-specific CT contrast agent N1177: A comparison with 18F-FDG PET/CT and histology. J Nucl Med. 2009;50(6):959–965. doi: 10.2967/jnumed.108.060749. [DOI] [PubMed] [Google Scholar]
- 84.Tahara N, et al. 2-deoxy-2-[18F]fluoro-D-mannose positron emission tomography imaging in atherosclerosis. Nat Med. 2014;20(2):215–219. doi: 10.1038/nm.3437. [DOI] [PubMed] [Google Scholar]
- 85.Niu M, et al. Biodistribution and in vivo activities of tumor-associated macrophage-targeting nanoparticles incorporated with doxorubicin. Mol Pharm. 2014;11(12):4425–4436. doi: 10.1021/mp500565q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu SS, et al. Macrophage-specific RNA interference targeting via “click”, mannosylated polymeric micelles. Mol Pharm. 2013;10(3):975–987. doi: 10.1021/mp300434e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng B, et al. Predictors of plaque rupture within nonculprit fibroatheromas in patients with acute coronary syndromes: The prospect study. J Am Coll Cardiol Img. 2015;8(10):1180–1187. doi: 10.1016/j.jcmg.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 88.Motoyama S, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-Up. J Am Coll Cardiol. 2015;66(4):337–346. doi: 10.1016/j.jacc.2015.05.069. [DOI] [PubMed] [Google Scholar]
- 89.Joshi NV, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet. 2014;383(9918):705–713. doi: 10.1016/S0140-6736(13)61754-7. [DOI] [PubMed] [Google Scholar]
- 90.Irkle A, et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun. 2015;6:7495. doi: 10.1038/ncomms8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bucerius J, et al. Arterial and fat tissue inflammation are highly correlated: A prospective 18F-FDG PET/CT study. Eur J Nucl Med Mol Imaging. 2014;41(5):934–945. doi: 10.1007/s00259-013-2653-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cai J, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: Comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112(22):3437–3444. doi: 10.1161/CIRCULATIONAHA.104.528174. [DOI] [PubMed] [Google Scholar]
- 93.Kono H, et al. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest. 2010;120(6):1939–1949. doi: 10.1172/JCI40124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kool M, et al. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity. 2011;34(4):527–540. doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 95.Sheedy FJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating the intracellular nucleation from soluble to particulate ligands in sterile inflammation. Nat Immunol. 2013;14(8):812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Friggeri A, et al. HMGB1 inhibits macrophage activity in efferocytosis through binding to the α(v)β(3)-integrin0. Am J Physiol Cell Physiol. 2010;299(6):C1267–C1276. doi: 10.1152/ajpcell.00152.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 98.Spisek R, et al. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109(11):4839–4845. doi: 10.1182/blood-2006-10-054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rumore PM, Steinman CR. Endogenous circulating DNA in systemic lupus erythematosus. Occurrence as multimeric complexes bound to histone. J Clin Invest. 1990;86(1):69–74. doi: 10.1172/JCI114716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ueki S, et al. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. 2013;121(11):2074–2083. doi: 10.1182/blood-2012-05-432088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11(6):389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 103.Leonard CA, Schoborg RV, Borel N. Damage/danger associated molecular patterns (damps) modulate chlamydia pecorum and c. trachomatis serovar e inclusion development in vitro. PLoS One. 2015;10(8):e0134943. doi: 10.1371/journal.pone.0134943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oboki K, et al. IL-33 and airway inflammation. Allergy Asthma Immunol Res. 2011;3(2):81–88. doi: 10.4168/aair.2011.3.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Foell D, et al. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81(1):28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 106.Schaefer L. Extracellular matrix molecules: endogenous danger signals as new drug targets in kidney diseases. Curr Opin Pharmacol. 2010;10(2):185–190. doi: 10.1016/j.coph.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 107.Yu J, et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci U S A. 2015;112(27):8260–8265. doi: 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim KS, et al. Bioimaging of hyaluronate-interferon alpha conjugates using a non-interfering zwitterionic fluorophore. Biomacromolecules. 2015 doi: 10.1021/acs.biomac.5b00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lam J, et al. Delivery of iPS-NPCs to the stroke cavity within a hyaluronic acid matrix promotes the differentiation of transplanted cells. Adv Funct Mater. 2014;24(44):7053–7062. doi: 10.1002/adfm.201401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: Progress and conundrums. J Exp Med. 2010;207(9):1807–1817. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: The importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25(11):2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 112.Morelli AE, Larregina AT. Apoptotic cell-based therapies against transplant rejection: Role of recipient’s dendritic cells. Apoptosis. 2010;15(9):1083–1097. doi: 10.1007/s10495-010-0469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tyurin VA, et al. Oxidatively modified phosphatidylserines on the surface of apoptotic cells are essential phagocytic ‘eat-me’ signals: Cleavage and inhibition of phagocytosis by Lp-PLA2. Cell Death Differ. 2014;21(5):825–835. doi: 10.1038/cdd.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gardai SJ, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123(2):321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 115.Garg AD, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31(5):1062–1079. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zullig S, et al. Aminophospholipid translocase TAT-1 promotes phosphatidylserine exposure during C. elegans apoptosis. Curr Biol. 2007;17(11):994–999. doi: 10.1016/j.cub.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 117.Uderhardt S, et al. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity. 2012;36(5):834–846. doi: 10.1016/j.immuni.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 118.Audi S, et al. Understanding the in vivo uptake kinetics of a phosphatidylethanolamine-binding agent (99 m)Tc-Duramycin. Nucl Med Biol. 2012;39(6):821–825. doi: 10.1016/j.nucmedbio.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Waiczies S, et al. Anchoring dipalmitoyl phosphoethanolamine to nanoparticles boosts cellular uptake and fluorine-19 magnetic resonance signal. Sci Rep. 2015;5:8427. doi: 10.1038/srep08427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chu CT, et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15(10):1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yanamala N, et al. Structural re-arrangement and peroxidase activation of cytochrome c by anionic analogues of vitamin E, tocopherol succinate and tocopherol phosphate. J Biol Chem. 2014;289(47):32488–32498. doi: 10.1074/jbc.M114.601377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hazen SL. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J Biol Chem. 2008;283(23):15527–15531. doi: 10.1074/jbc.R700054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Briley-Saebo K, et al. Imaging of oxidation-specific epitopes with targeted nanoparticles to detect high-risk atherosclerotic lesions: Progress and future directions. J Cardiovasc Transl Res. 2014;7(8):719–736. doi: 10.1007/s12265-014-9590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lu T, et al. Near-infrared fluorescence imaging of murine atherosclerosis using an oxidized low density lipoprotein-targeted fluorochrome. Int J Cardiovasc Imaging. 2014;30(1):221–231. doi: 10.1007/s10554-013-0320-9. [DOI] [PubMed] [Google Scholar]
- 125.Miller YI, Tsimikas S. Oxidation-specific epitopes as targets for biotheranostic applications in humans: Biomarkers, molecular imaging and therapeutics. Curr Opin Lipidol. 2013;24(5):426–437. doi: 10.1097/MOL.0b013e328364e85a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wen S, et al. In vivo MRI detection of carotid atherosclerotic lesions and kidney inflammation in ApoE-deficient mice by using LOX-1 targeted iron nanoparticles. Nanomedicine. 2014;10(3):639–649. doi: 10.1016/j.nano.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 127.Chu Z, et al. In vivo optical imaging of brain tumors and arthritis using fluorescent SapC-DOPS nanovesicles. J Vis Exp. 2014;87 doi: 10.3791/51187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qi X, et al. Saposin C coupled lipid nanovesicles specifically target arthritic mouse joints for optical imaging of disease severity. PLoS One. 2012;7(3):e33966. doi: 10.1371/journal.pone.0033966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wojton J, et al. SapC-DOPS-induced lysosomal cell death synergizes with TMZ in glioblastoma. Oncotarget. 2014;5(20):9703–9709. doi: 10.18632/oncotarget.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao S, et al. SapC-DOPS nanovesicles as targeted therapy for lung cancer. Mol Cancer Ther. 2015;14(2):491–498. doi: 10.1158/1535-7163.MCT-14-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wojton J, et al. Systemic delivery of SapC-DOPS has antiangiogenic and antitumor effects against glioblastoma. Mol Ther. 2013;21(8):1517–1525. doi: 10.1038/mt.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sulaiman MK, et al. SapC-DOPS nanovesicles induce Smac- and Bax-dependent apoptosis through mitochondrial activation in neuroblastomas. Mol Cancer. 2015;14:78. doi: 10.1186/s12943-015-0336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Konduru NV, et al. Phosphatidylserine targets single-walled carbon nanotubes to professional phagocytes in vitro and in vivo. PLoS One. 2009;4(2):e4398. doi: 10.1371/journal.pone.0004398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Maiseyeu A, et al. Gadolinium-containing phosphatidylserine liposomes for molecular imaging of atherosclerosis. J Lipid Res. 2009;50:2157–2163. doi: 10.1194/jlr.M800405-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maiseyeu A, Bagalkot V. In vitro uptake of apoptotic body mimicking phosphatidylserine-quantum dot micelles by monocytic cell line. Nanoscale Res Lett. 2014;9:176. doi: 10.1186/1556-276X-9-176. [DOI] [PMC free article] [PubMed] [Google Scholar]