Abstract
IL-6 is a pleiotropic cytokine increased in CRC and known to directly promote tumor growth. Colonic myofibroblasts/fibroblasts (CMFs or stromal cells) are CD90+ innate immune cells representing up to 30% of normal colonic mucosal lamina propria cells. They are expanded in CRC tumor stroma, where they also known as a cancer associated fibroblasts (CAFs). Cells of mesenchymal origin, such as normal myofibroblasts/fibroblasts, are known to secrete IL-6; however their contribution to the increase in IL-6 in CRC and to tumor-promoting inflammation is not well defined. Using in situ, ex vivo and co-culture analyses we have demonstrated that the number of IL-6 producing CMFs is increased in CRC (C-CMFs) and they represent the major source of IL-6 in T2-T3 CRC tumors. Expression of stem cell markers-aldehyde dehydrogenase (ALDH) and LGR5- was significantly up-regulated in colon cancer cells (SW480, Caco-2 or HT29) cultured in the presence of conditioned medium from tumor isolated C-CMFs in an IL-6 dependent manner. C-CMF and its derived condition medium, but not normal CMF isolated from syngeneic normal colons, induced differentiation of tumor promoting inflammatory Th17 cell responses in an IL-6 dependent manner. Our study suggests that CD90+ fibroblasts/myofibroblasts may be the major source of IL-6 in T2-T3 CRC tumors, which supports the stemness of tumor cells and induces an immune adaptive inflammatory response (a.k.a. Th17) favoring tumor growth. Taken together our data supports the notion that IL-6 producing CAFs (a.k.a. C-CMFs) may provide a useful target for treating or preventing CRCs.
Keywords: CD90+ fibroblasts, colorectal cancer, inflammation, IL-6, tumor stem cells
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related death in the US.1 The optimal approach for prevention, screening and therapy of CRC has yet to be determined. Despite significant progress in the field, the major processes with promoting tumor growth and cancer-associated inflammation are poorly determined. Identification of such factors is critical to developing effective treatments of CRC.
Among the cytokines with pleiotropic functions, IL-6 has been implicated in a wide range of cancers, including CRC.2 Elevated levels of serum IL-6 is associated with a decrease in survival of patients with CRC.3
IL-6 is a protein with four straight helices (A, B, C, D), which binds to IL-6R, a type I transmembrane protein. The IL-6-IL6R requires an interaction with a second transmembrane protein, gp130. This interaction results in homodimerization of gp130, which then initiates signaling trough Janus kinases (JAKs).4, 5 IL-6 is known to act in a paracrine and autocrine manner on diverse target cells and has been suggested to protect target cells from apoptotic death, thus functioning as a survival factor.4, 6-8
IL-6 has been shown to enhance stemness in prostate tumor cells in a STAT3 dependent manner7 and to induce expansion of CD44+ cancer stem-like cells in hepatocellular carcinoma.9 An increase in epithelial-mesenchymal transition (EMT) has been suggested to be among the key mechanisms contributing to the IL-6-mediated increase in tumor invasiveness and metastasis in CRC.10 Through stimulation of VEGF production, IL-6 plays a key role in CRC associated angiogenesis.11
Inflammatory immune responses also play an important role in cancer development and progression. IL-6 induces tumor promoting inflammatory responses in both the innate and adaptive components of immune system.2, 6, 9 IL-6 has been shown to cooperate with hypoxia to enhance generation of M2-like tumor promoting macrophages in Non-Small Cell Lung Cancer and CRC.12, 13 More recently, IL-6 was also suggested to be implicated in the increase of tumor-promoting, IL-17A-producing T-helper (Th17) cells. 14, 15
The source of IL-6 within colorectal tumors is currently not well defined. Studies based on the murine colitis-associated CRC model suggest that IL-6 within the tumors is produced by hematopoietic-derived cells.6, 16, 17 According to Grivennikov et al6, who used the AOM-DSS colitis CRC model, ~ 40% of IL-6 producing cells are dendritic cells, macrophages, and lymphocytes. However, the lineage of the remaining IL-6 producing cells remains unclear. The source of IL-6 in sporadic human CRC remains even less defined. Although the basal level of IL-6 is low in CRC cancer epithelial cell lines11, they are able to produce IL-6 in culture in response to commensal bacteria and its products.18
Mucosal fibroblasts/myofibroblasts (CMFs or stromal cells) account for up to 30% of lamina propria cells in normal human colon19 and are increased in CRC tumor stroma.20, 21 These cells express the mesenchymal marker CD90+ and upon activation become α-smooth actin (α-SMA) myofibroblasts, also known as a cancer associated fibroblasts or CAFs. We and other have observed that in normal colonic mucosa these CD90+ CMFs produce IL-6, which is increased upon stimulation with microbial ligands and several inflammatory cytokine.22, 23
Several independent research teams have shown that cancer mesenchymal stromal cells can contribute to tumorigenesis via IL-6 dependent processes.11, 24, 25 Murine bone marrow-derived gastric myofibroblasts have been shown to promote cancer stem cell spheroids in an IL-6/JAK2/STAT3 dependent manner.24 Further, a recent study by Nagasaki et al11 suggests that CAFs can produce IL-6, which is critical for tumor angiogenesis.
While it is established that cells of mesenchymal origin are able to secrete IL-6, their contribution to the upregulated level of IL-6 within the CRC tumor microenvironment is still unclear. In order to determine this, in human tissue we investigated CRC expression of IL-6 in situ and we isolated CD90+ stromal cells (CMFs) from CRC and from adjacent normal tissue (controls) to study ex vivo and in culture. We show that the number of IL-6 producing cancer derived colonic CD90+ cells (C-CMFs or CAFs) is increased in CRC tumors and they represent the major source of IL-6 in T2-T3 CRC tumors. Further, the C-CMF isolates produced higher level of IL-6 when compared to its matched normal CMF. IL-6 was the critical soluble mediator in C-CMF's capacity to support “stem-like” early progenitor cells from human CRC cancer cell lines. We also found that C-CMFs, but not its matched peritumoral CMF control, promote generation of Th17 cells from activated CD4+ T cells in an IL-6 dependent manner.
Material and methods
Antibodies
Fluorochrome-conjugated murine anti-α-smooth muscle actin (α-SMA, clone 1A4) monoclonal antibodies (Abs) were purchased from Sigma (St Louis, MO). Fluorochromeconjugated, biotinilated or unconjugated forms of IgG1κ, IgG2a, isotype controls and monoclonal Abs directed against human CD90 (clone 5E10), CD4 (clone RPA-T4), EpCAM (clone 1B7), RORγτ (clone AFKJS-9), IL-6 (clone MQ2-13A5), IL-17A (clone eBio64DEC17), gp130 (clone AN-G30) were purchased from eBioscience (San Diego, CA). Goat anti-human IL-6Rα biotinylated polycolonal Abs were purchased from R&D Systems, Inc. (Mineapolis, MN). Zenon Mouse IgG labeling kits were purchased from Life Technology (Grand Island, NY).
Human tissue and cells
All human samples were collected from patients undergoing colectomy for colon cancer were studied under IRB-approved human protocols at the University of Texas Medical Branch, University of New Mexico Health Sciences Center, and Legacy Research Tumor Bank (Portland, OR). For CMF isolation, full thickness fresh human colonic tissue samples were obtained from discarded colonic surgical resection material from both the CRC tumor and its adjacent uninvolved normal colonic tissue. T2-T3 tumors were used in this study. Total mucosal cell preparations were performed as described previously.26 CMFs were isolated according to the protocol of Mahida et al. 27, which is routinely used in our laboratory.19 The purity of isolated CD90+ CMFs (98-99%) was confirmed by flow cytometry, as previously described.19 CaCo-2, HT29 and SW480 colorectal cancer epithelial cell lines were purchased from ATCC (Manassas, VA). Studies were performed with primary CMFs isolates at passages 4-10. Cells were cultured at 37°C in 5% CO2 atmosphere in complete Modified Eagle Medium (MEM) as described previously.19
CMF-T cell co-cultures
Assays were performed using allogeneic CMF: T cell co-cultures. Human CD4+ CD45RA+ T cells were purified from peripheral blood mononuclear cell (PBMCs) of healthy donors by negative selection using Naïve CD4+ T Cell Isolation Magnetic Bead Kit II (Miltenyi Biotec., Auburn, CA). Purity of isolated T cells was examined by flow cytometry. The naïve CD4+ T cells were then preactivated for 1 hour with anti-CD3/anti-CD28- coupled T cell activation beads (Life Technologies) and co-cultured with normal (N)- or C-CMFs for 5 days. Neutralizing anti-IL-6 Ab or isotype control was added when needed at a concentration of 2.5 μg/mL 30 minutes before the addition of T cells. Co-cultures were incubated for 5 days at 37°C. CD3/CD28 activation beads were removed from the T cells prior to immunostaining followed by flow cytometry analysis as recommended by the beads manufacturer.
Flow cytometry
Flow cytometry on freshly digested CMFs and CMF primed T cells was performed as previously described.19 Briefly, single- and multi-color immunostaining was performed according to standard surface and intracellular FACS staining protocols (eBioscience). Cells were analyzed by flow cytometry using LSRII cytometer (BD Biosciences, Franklin Lakes, NJ) according to the manufacturer's protocol. Area, height and width parameters for forward and side scatters were used to discriminate single live cells. An additional gate was set up based on the negativity for the fixable viability dye eFluor® 780 (eBIoscience), which was used to exclude dead cells from the analysis. Flow cytometry data were analyzed with software FACSDiva 6.2 (Becton Dickenson) and FlowJo (Tree Star, Inc., OR, USA) softwares.
Confocal microscopy
Confocal microscopy on frozen human colonic tissue sections (10 μm thickness) was performed as previously described19 with minor modifications. Briefly, freshly frozen human colon tissue sections were fixed at room temperature in 1% paraformaldehyde for 20 minutes, blocked with normal murine serum (2.5% in PBS) for 15 min, and then incubated with mix of AF®546 conjugated anti-human IL-6 mouse mAbs (1 μg/mL) and AF488-conjugated anti-α-SMA monoclonal Abs (0.2 μg/mL) for 1h. Each staining step was followed by six washes with PBS with Ca++/Mg++. Isotype controls were included in the analyses. The sections were then mounted in SlowFade® Gold antifade reagent with DAPI (Life Technology, Inc.). Confocal microscopy was performed with a Zeiss LSM510 META laser-scanning confocal microscope (Carl Zeiss, Thornwood, NY).
Real-time RT-PCR
Real-time RT-PCR analysis was performed as previously described22 according to the Applied Biosystems's two-step RT real-time PCR protocol (Applied Biosystems, Foster City, CA). All reagents were purchased from Life Science Technology Inc. The appropriate assayson-demand™ gene expression assay mix (Life Science Technology Inc) for human 18S RNA and the gene of interest (a 20X mix of unlabeled PCR primers and TaqMan® MGB probe, FAM™ dye-labeled) and 2 μL of cDNA were added to the PCR reaction step. The reactions were carried out in a 20 μL final volume using a Bio-Rad Q5 real-time PCR machine according to the following protocol: 2 min at 50°C, 10 min at 95°C (1 cycle) and 15 sec at 95°C and one min at 60°C (40 cycles).
Western Blot analysis
Western blot analysis was used to determine the protein expression of IL-6Rα and gp130 by cancer epithelial cells using Abs described above. Analysis was performed on 10 μg of protein as previously described.19
ALDH analysis
Cancer stem-like populations within carcinoma cell lines were examined by staining with the Aldefluor™ kit (Stem Cell Technologies, Vancouver, BC) according the manufacturer's Aldefluor staining protocol. The Aldefluor™ reagent system is a non-immunological method to identify human stem/progenitor cells on the basis of their aldehyde dehydrogenase (ALDH) activity. Briefly, the uncharged ALDH-substrate, BAAA (BODIPY® aminoacetaldehyde), is taken up by living cells through passive diffusion. BAAA is converted by intracellular ALDH into a negatively charged reaction product BAA-(BODIPY®- aminoacetate), which is retained inside cells, causing the cells expressing high levels of ALDH to become brightly fluorescence. These cells can then detected and enumerated in the green fluorescence channel (520-540 nm) of a standard flow cytometer. All samples were analyzed on a LSRII flow cytometer (Millipore, Bellerica, MA), and analyzed using FACSDiva 6.2 (Becton Dickenson) software.
Luminex Arrays
Production of cytokines was measured by Milliplex cytokine array assay (EMD Millipore, Billerica, MA) according to the manufacturer's protocol and as previously published.28 All samples were analyzed on a Luminex 200 machine (Bio-Rad).
Statistical analysis
Unless otherwise indicated, the results were expressed as the mean ± SE of data obtained from at least three independent experiments done with duplicate sets in each experiment. Differences between means were evaluated by one–way ANOVA using Tukey's test for multiple comparisons. Values of P <0.05 were considered statistically significant. Association between gene expressions was examined using Spearman correlation analysis.
Results
IL-6 is increased in CRC tumors
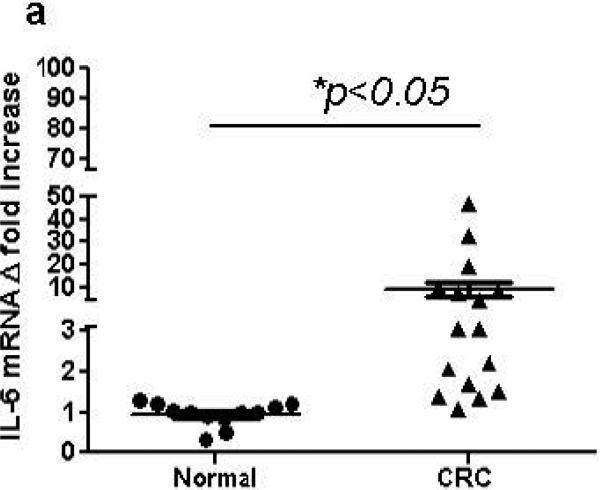
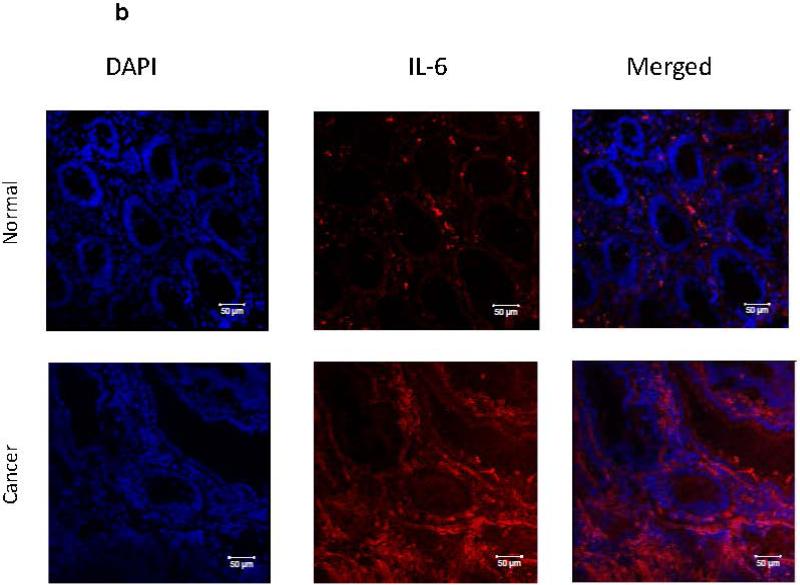
Despite recent evidence pointing to a pro-tumorigenic function of IL-6 during CRC development, its expression and compartmentalization within the CRC tumors remains contradictory. Thus, we first determined the level of IL-6 expression in CRC tumors from patients with sporadic CRC T2-T3 tumors. We observed a significant 2-47 fold increase in IL-6 mRNA expression in 11 out of 16 CRC tumor tissues when compare to the matched normal adjacent colonic mucosa (Figure 1a). In situ analysis of CRC tumors and adjacent normal colonic mucosa with immunostaining followed by confocal microscopy confirmed the observations above and demonstrated that IL-6 protein expression is increased within the CRC tumors when compared to the matched normal colonic mucosa (Figure 1b, in red).
Figure 1.
IL-6 is increased is tumor stroma in CRC. (a) IL-6 mRNA levels in colon tumors was compared to the normal tissue controls (real time RT-PCR analysis). The means ± SEM are shown as the results of duplicates of each paired tumors and normal tissues (n=16 per group),* = p<0.05. (b) IL-6 protein expression is increased in CRC tumors in situ. In these experiments CRC tumor and its surrounding matched normal colonic tissue sections were stained with DAPI to identify cell nuclei (blue) and anti-IL-6 mAb (clone MQ2-13A5, in red) and analyzed by confocal microscopy. Low power resolution confocal images of representative sections from eight matched sets of normal and CRC tumor tissue are shown.
Stromal fibroblasts are the major cell phenotype producing IL-6 in CRC tumors
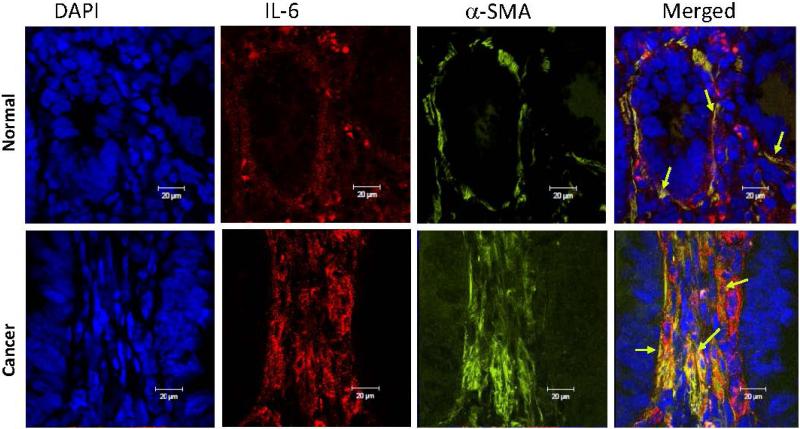
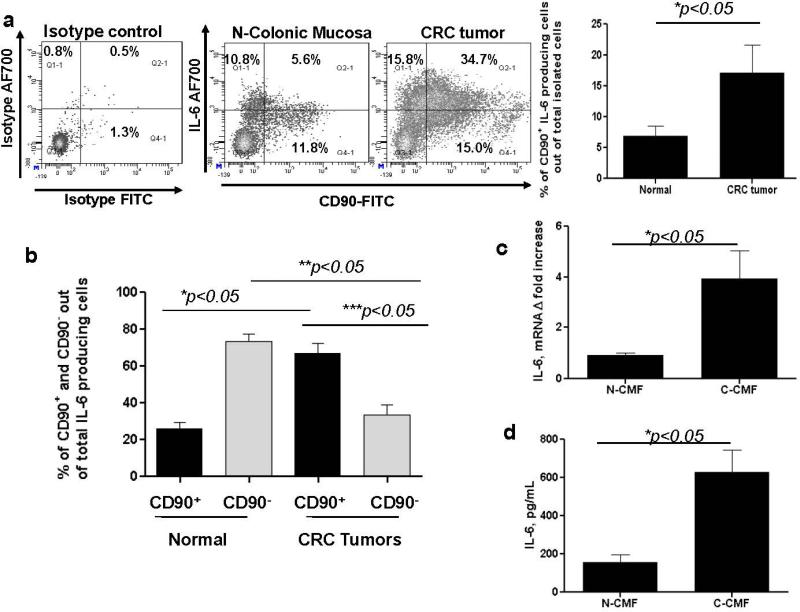
As demonstrated on Figure 1b, we noted that the major increase in IL-6 production was seen within the CRC tumor stroma, but not in cancer epithelial cells. Cancer-associated CMFs play a key role in tumor progression and are the major cell phenotype within the tumor stroma in CRC.21, 29 Expression of myofibroblasts marker α-smooth actin (α-SMA) is highly increased by CMFs during CRC development.21, 29 We and others previously observed that intestinal fibroblasts might be an important source of IL-6.11, 22, 23 Thus, we analyzed the contribution of this major tumor stromal cellular subset to the increase in IL-6 production in CRC. Coimmunostaining for α-SMA and IL-6 demonstrated that IL-6 expression was mostly co-localized with α-SMA+ CMFs (formation of yellow-orange color on merged images, Figure 2). The majority of the CMFs in epithelial cancers are reported to be activated and to bear the myofibroblast marker α-SMA. However, CD90, which marks hematopoietic cells and fibroblasts in mice, marks only mesenchymal cells such as fibroblasts and myofibroblasts (stromal cells) in humans.21, 29 Thus, using immunostaining followed by flow cytometry we analyzed the contribution of the CD90+ cells to the IL-6 production in fresh single cell suspension preparation of human CRC tumors. We observed a significant increase in the total IL-6 producing cells from the CRC tumors when compare to normal matched controls. CD90+ C-CMFs were the major cell population producing IL-6 within the CRC tumors: up to 35 % of the total cells isolated from the tumor itself (Figure 3a). Next, IL-6-positive cells were gated out of total live cells freshly isolated from tumor or matched normal mucosa and analyzed for the expression of CD90. The number of IL-6-producing CD90+ CMFs was increased by 2-3 folds, representing up to 70% of IL-6-producing cells in CRC tumors when compare to 20-30% of IL-6 producing cells in normal adjacent (peritumoral) colonic mucosa (Figure 3b). Additionally, some increase in the IL-6 producing cells expressing Th17 transcriptional factor RORγτ was observed at tumor site. However, it did not reach significant level when compare to the normal tissue (Supplement, Figure S1).
Figure 2.
The increase in IL-6 protein expression in CRC tumors is mostly seen in tumor stroma and co-localizes with activated a-SMA+ CMFs (also known as CAFs). High power resolution confocal images from representative sections of eight matched sets of normal and CRC tumor tissue from the same subject are shown. In these experiments immunostaining of CRC tumor and its surrounding normal colonic tissue sections was performed, followed by confocal microscopy. DAPI was used to stain cell nuclei (blue); activated CMFs were detected by anti-α-SMA mAb (green, clone A4) and stained for IL-6 with mAb (red, clone MQ2-13A5). A yellow-orange color on merged images indicates co-localization of α-SMA and IL-6, and thus the expression of IL-6 by CMFs (indicated by arrows).
Figure 3.
IL-6 production is strongly increased by CD90+ CMFs in CRC tumor stroma. (a) Freshly digested normal colonic mucosal and CRC tumor cell preparations from the same subject were prepared, stimulated for 4 h with PMA/Iono and co-stained with CD90 and IL-6 or isotype controls and analyzed by flow cytometry. One representative experiment and the summary of the collective results of 12 separate experiments are shown, * = p < 0.05. (b) IL-6 producing cells were gated out of total live events in freshly digested normal colonic mucosal and its matched CRC tumor cell preparations and analyzed for the expression of CD90. One representative experiment and the summary of the collective results of 12 separate experiments are shown,* = p < 0.05. (c) IL-6 mRNA (real time RT-PCR analysis) and (d) IL-6 secretion (singleplex cytokine analysis) is increased by primary C-CMFs when compare to matched N-CMFs. The means ± SE are shown as the results of duplicates of ten tested CMF pairs,* = p<0.05.
Taken together these data indicate that IL-6 production is strongly increased in stage T2-T3 sporadic CRC tumors, and the IL-6 is predominantly expressed by CD90+ tumor stroma fibroblasts/myofibroblasts.
CRC derived CD90+ stromal cells retain IL-6high phenotype in culture
In order to study IL-6 expression and function by colonic CD90+ CMFs independently from other cell types, we have established primary cultures of these cells from T2-T3 CRC tumors (C) and adjacent normal tissue to serve as normal (N) controls. Using real-time RT-PCR we demonstrated that C-CMF expression of IL-6 is higher when compare to the matched NCMF pair (Figure 3c). We also observed higher level of the IL-6 production in the C-CMF condition medium as detected by IL-6 single-plex analysis (Figure 3d). Thus, we established that primary C-CMF cultures constitutively produce higher IL-6 level when compare to the matched N-CMFs.
CRC-derived CD90+ stromal cells support stem-like population within the colon cancer cells in an IL-6 dependent manner
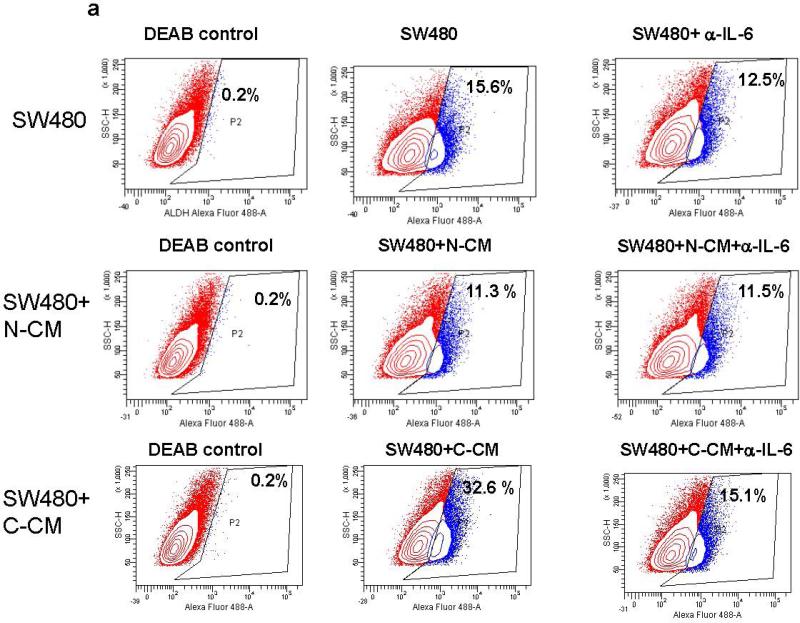
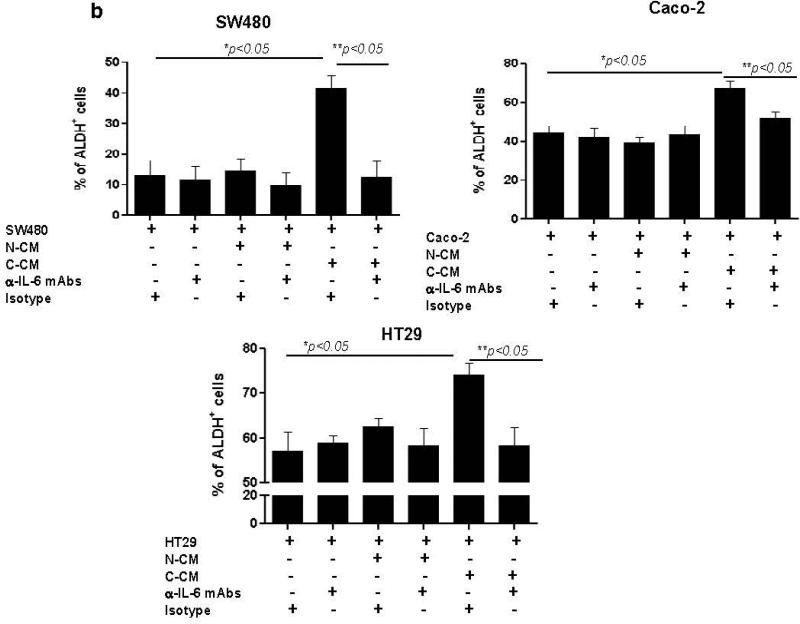
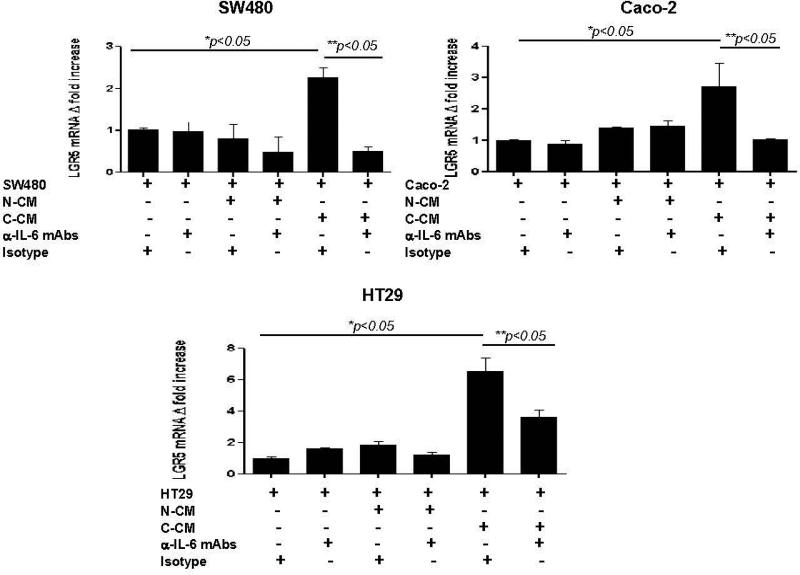
IL-6 induces the proliferation of cancer epithelial cells in vitro and promotes tumorigenesis in vivo.10, 16, 30 Likewise, intestinal myofibroblasts are reported to support growth of normal intestinal epithelial stem cells.31 Recently, sub-populations of cells within CRC tumors and cell lines have been shown to express stemness markers such as aldehyde dehydrogenase 1(ALDH1)32 and LGR5.33 Previous finding by others demonstrated bone morrow derived murine and human gastric myofibroblasts isolated from dysplastic tissue were shown to stimulate spheroid formation in cancer epithelial cell lines and IL-6 was involved in this processes.24 Thus, we determined if increased IL-6 production by C-CMFs enhances generation of stem-like population within the colon cancer epithelial cell lines SW480, Caco-2 and HT29. We first demonstrated the expression of IL-6Rα and gp130 receptor on these cancer epithelial cells (Supplement online, Figure S2). These receptor molecules are required for the initiation of IL-6 signaling.4 Epithelial cancer cell lines were treated with condition media from C- or N-CMF cultures for 1 week and then ALDH activity and LGR5 expression was analyzed. Although the basal number of ALDH+ cells was variable between tested cancer cell lines, exposure of cancer cells to the conditioned media from C-CMFs, but not matched N-CMFs, significantly increased the number of ALDH+ cells (Figure 4a-b) and expression of LGR5, a well-defined epithelial stem cell marker, in all three tested colonic cancer cell line (Figure 5). Further, addition of anti-IL-6 neutralizing mAbs to the condition media derived from C-CMFs abrogated increases in the stem-like ALDH+ population and LGR5 expression within the tested cancer cell lines (Figure 4-5).
Figure 4.
IL-6 derived from C-CMF condition media contributes to the increase in stem-like ALDH activity by SW480, Caco-2 and HT-29 CRC cells. 72 h condition media (CM) derived from C- and matched N-CMF was added to the SW480, Caco-2 or HT-29 cancer epithelial cell line culture in a ratio of 2:1 (2 parts of CM: 1 part freshly prepared cell growth media) in presence/absence of IL-6 neutralizing mAbs or isotype control. The cancer epithelial cells were subjected to the normal or cancer-derived CMF-CM treatment every second day for one week and then ALDH activity was measured. (a) One representative experiment and (b) summary of the obtained results is shown. The mean ± SE are the results of duplicates of four experiment per cell line, * =p < 0.05.
Figure 5.
IL-6 contained in C-CMF condition media (CM) upregulates stem cell marker LGR5 mRNA expression in cancer cell lines. Matched C-CMF- and N-CMF-72 h CM was added to the cancer epithelial cell lines SW480, Caco-2 and HT-29. CM and Cancer cell lines were cultured in a ratio of 2:1 (2 parts of CM: 1 part fresh cell growth media) in presence/absence of IL-6 neutralizing mAbs or isotype control. The cancer epithelial cells were subjected to the CM treatment every second day for one week and then LGR5 mRNA expression was analyzed by using real time RT-PCR. The mean ± SE are shown as the results of duplicates of five experiment per cell line, * =p < 0.05.
CRC-derived CD90+ stromal cells enhance generation of Th17 cells in IL-6-dependent manner
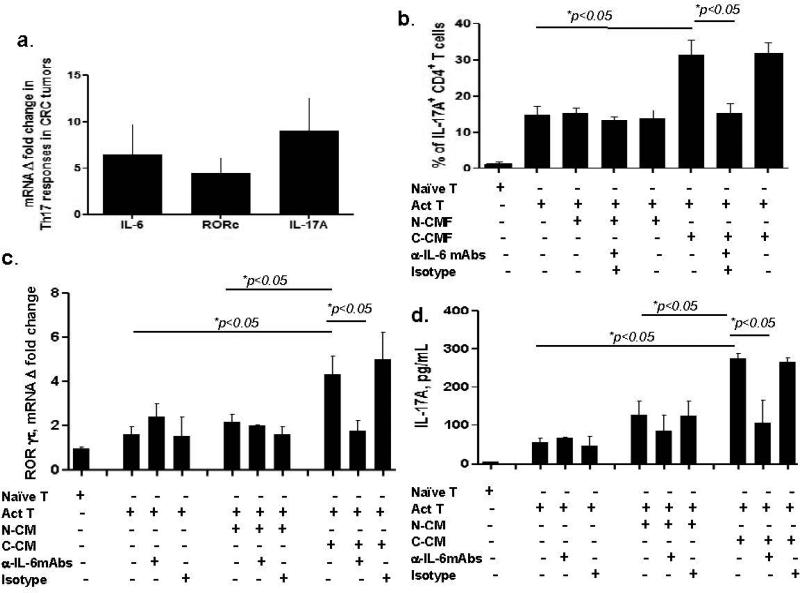
CD4+ Th17 cells are thought to be the key adaptive immunity component of tumor promoting inflammation.14, 15 IL-6 is required for Th17 development.14, 15 Therefore, we examined the expression of the Th17 transcription factor RORγτ (gene alias RORC) and its major cytokine IL-17A mRNA expression in T2-T3 CRC tumors previously analyzed for IL-6 expression. An increase in ROR γτ and IL-17A mRNA was observed in ~ 50 % and ~80% respectively of tested CRC tumors (Figure 6a). By using Spearman correlation analysis, a strong association (alpha=0.05) between IL-6 and IL-17A expression (Spearman r =0.8500) and to a lesser extent between IL-6 and RORγτ (Spearman r=0.6084) was observed in CRC tumors with P summary values of 0.0061 and 0.0124, respectively. Because our data suggests that in T2-T3 CRC tumors C-CMFs are major source of IL-6, we determined the role of IL-6 production by C-CMF in the generation of Th17 cells. We observed that, in contrast to N-CMFs, co-culturing of CCMFs with CD3/CD28 activated CD4+ T cells for five days resulted in an increased production of IL-17A producing RORγτ+ CD4+ T cells. This response was abrogated by anti-IL-6 neutralizing mAbs (Figure 6b). Further, we reproduced the same increase in Th17 responsesIL-17A by applying only condition media derived from C-CMF. As shown on Figure 6c-d stimulation of preactivated T cells with C-CMF derived condition media resulted in a significant increase of both, expression of the Th17 transcriptional factor ROR γτ by CD4+ T cells, as well production of the Th17 cytokine IL-17A. Further, these effects were abrogated in a presence of anti-IL-6 neutralizing Abs, but not isotype controls. Taken together these data suggest that production of the IL-6 by C-CMFs in cancer stroma might be among the key processes contributing to the increase in inflammation promoting Th17 cells in CRC tumors.
Figure 6.
Th17 cells are increased in CRC tumors and C-CMF-derived IL-6 is a contributor to the proliferation of Th17 cells. (a) IL-6 mRNA, Th17 cell transcription factor RORC mRNA and IL-17A gene mRNA expression are simultaneously increased in CRC tumors as determined by real-time RT-PCR. The mean ± SE are shown as the results of duplicates of 16 tested CRC tumors (stage II-III), * p < 0.05. (b) C-CMFs induce IL-17 producing RORγτ+CD4+ Th17 cells in an IL-6 dependent manner from CD3/CD28-preactivated CD4+ T cells (Act T). CMFs were co-cultured with Act T cells at a ratio 1:3 for 4 days in 24-well plates. T cell monocultures were included as experimental controls. Anti-IL-6 neutralizing mAbs or isotype controls were used at concentration 2.5 μg/mL. Th17 transcription factor RORγτ and cytokine IL-17A was analyzed by immunostaining followed by flow cytometry, n=5 allogeneic donor pairs were tested twice. The mean ± SE, * =p < 005. Condition media derived from C-CMFs induces (c) RORγτ mRNA expression and (d) strong IL-17 production by CD4+ T cells in an IL-6 dependent manner. 72 h condition media (CM) from matched N- or C-CMFs were added to the Act T cells at a ratio 2:1 (2 parts of CM: 1 part fresh MEM media) for 4 days in 24-well plates. Anti-IL-6 neutralizing mAbs or isotype controls were used at concentration 2.5 μg/mL. Th17 transcription factor RORγτ was analyzed using real time RT-PCR and cytokine IL-17A levels were analyzed by luminex, n=4 allogeneic donor pairs were tested. The mean ± SE, * =p < 005.
Discussion
IL-6 is among the inflammatory cytokines upregulated in solid cancers, including CRC, and it is thought to be critically involved in the cancerogenesis.2, 6 Our data presented herein support the idea that due to its pleiotropic functions, IL-6 plays a major role in tumor promoting inflammation and expansion of cancer stem-like cells in CRC.
While much evidence supports the idea that IL-6 signaling is involved in cancer development and progression, the cellular source of the IL-6 within the solid epithelial cancers, and in particularly CRC, remains debatable. Based on the studies of the murine colitis associated cancer AOM-DSS model, a hematopoietic origin (e.g., macrophages) of the increase in IL-6 within the CRC has been suggested.6, 16 An increase in the IL-6 producing M2-like macrophage has been noted.34 On the other hand studies using the AOM-DSS model by Shaker A. et al30 suggested that tumor stromal myofibroblasts were the source of IL-6 during inflammation associated colon cancer development. Finally, more recent publications support the idea that cells bearing mesenchymal markers, such as myofibroblasts/fibroblasts and mesenchymal stem cells, can be important sources of IL-6 within the human sporadic CRC.11, 24, 25
The importance of CAFs as a source of IL-6 has been recently published by the Nakasaki et al.11. Our data extends this study and clearly supports the conclusion that tumor stromal C-CMFs (a.k.a. CAFs) that bear marker of activated fibroblast α-SMA and mesenchymal marker CD90 are the major cell phenotype producing IL-6 in sporadic CRC T2-T3 tumors. Importantly, we observed that high IL-6 production was sustained by primary cultured C-CMFs over multiple passages when compared to cultures of normal matched control CMFs, indicating a hardwired modification of phenotype. Nagasaki T. et al also found that LPS and co-culture with CRC cell lines HT29 and COLM5 stimulated increases in IL-6 in CAFs, suggesting that microbial and cancer cell derived stimuli are involved in the IL-6 increase in C-CMFs.11 Using a broad panel of human matched N- and C-CMF primary isolates, we have confirmed that LPS is a strong inducer of IL-6 in CMFs (Supplement online Figure S3). However, as previously shown for HT29 and COLN511, we observed no increase in IL-6 production in Caco-2 co-cultured with N-CMFs or C-CMFs when compare to N- or C-CMFs alone. Because Caco-2, HT29 and COLM5 CRC cell line have different mutations, it is possible that induction of the IL-6 expression by CMF in cancer epithelial cells and vise versa will depend on epithelial cancer cell mutations.
While exact mechanism(s) responsible for the IL-6 increase within the CRC tumor stroma remains to be elucidated, we and others have suggested that persistence of the inflammatory processes that contributes to cancer development may also initiate fibroblast apoptosis, and that bone morrow-derived mesenchymal stem cells may migrate to the tumor and contribute to the formation of the C-CMF phenotype.21, 35 Mesenchymal stem cells (MSC) are also known to be strong producers of IL-6.25 Further, IL-6 has been demonstrated to enhance mesenchymal stem cell proliferation,36 and to increase the recruitment of MSC to the CRC tumor site.35, 37 Thus, it is possible that increased migration and proliferation of MSC together with a decrease in MSC differentiation in the tumor site may be responsible for the formation of IL-6high C-CMF phenotype. In fact IL-6 producing cells exhibiting a fibroblastic shape, expressing mesenchymal lineage markers CD90 and vimentin, and preserving expression of the stem cell marker Oct 4 were recently isolated from CRC.25 Further studies are needed to evaluate this concept.
Fibroblast derived IL-6 may support tumor growth through induction of cancer cell proliferation, invasiveness and angiogenesis,11, 24 and cancer associated fibroblasts have been reported to be an essential component of the cancer stem cell niche.38 Aldehyde dehydrogenase (ALDH) activity is a marker of cancer stem-like cells and early progenitors in CRC, 32, 39 and the intestinal stem cell signature marker LGR5 identifies normal and CRC epithelial stem cells.33 We demonstrated that the increase in the IL-6 production by C-CMFs increases stem-like cell markers ALDH and LGR5 in all three tested CRC cell lines. Although novel, this data is not without precedence for cells of mesenchymal lineage. Recently colon cancer-derived mesenchymal stem cells and murine bone marrow derived fibroblasts have been demonstrated to support tumor spheroid formation, another marker for cancer stem-like cell population, and IL-6 was involved in this process.24, 25
IL-17A-producing T-helper (Th17) cell responses have emerged recently as an important component of adaptive immunity supporting CRC tumor growth.14, 15 Conversely, decreased IL-17 production by tumor-infiltrating, dually expressing Th1/Th17 T effector cells enabled TNF-α-mediated killing of cancer cells both in vitro and in vivo.40 An increase in the inflammatory Th17 cell responses has been shown to promote tumorigenesis in an animal model41 and to be associated with a poor prognosis in human CRC.42, 43 Further, IL-17A has been shown to enhance angiogenesis in cancer43 and to play a role in the desmoplasia44 which occurs during CRC progression.45 IL-6 is critical for the differentiation of T cells to Th17 cell in healthy individuals.16 We demonstrate here the existence of a significant association between the increase in the expression of IL-6 and markers for Th17 cell responses (IL-17A and RORγτ) in CRC T2-T3 tumor tissue. We also demonstrate with our co-cultures experiments that IL-6 is critically involved in the C-CMF mediated induction of Th17 cells. The concept of fibroblasts as active regulators of adaptive immune responses at gut mucosal sites is strongly supported by reports from our laboratory and others.19, 21, 46-50 However, the regulatory effect of cancer associated fibroblasts on adaptive immunity in general and Th17 cells in particular remains poorly described.
Taken together with already published data by ourselves and others, the present studies suggest that, through increased IL-6 production, tumor stromal CD90+ fibroblasts/myofibroblasts not only directly support growth of stem-like population within CRC cancer cell lines, but also induce adaptive immune responses (a.k.a. Th17 cells) which favors tumor growth and expansion. Thus, our data strongly support the significance of IL-6 production by cancer associated fibroblast as a potential therapeutic target in CRC.
Supplementary Material
What's new?
IL-6 is increased in colorectal cancer (CRC), but its source remains poorly defined. We found that cancer derived colonic CD90+ stromal fibroblasts/myofibroblasts (C-CMFs or CAFs) are the major source of IL-6 in T2-T3 CRC tumors. Through secretion of IL-6, C-CMFs support increases in cancer stem like cells, and induces inflammatory Th17 cell response known to support tumor growth. Our data suggests that IL-6 production by C-CMFs may represent a potential target for treating CRC.
Acknowledgements
We thank Dr. David Konkel for critically editing the manuscript.
Grant support: NIDDK (1R01DK103150-01A1), NCATS (KL2TR000072 and ILTR000072), NCATS (TR000071), NCI (3R01-CA97959), NCI P30CA118100, NCAT 8UL1TR000041, ACS RSG-10-159-01-LIB.
Abbreviations
- α-SMA
α-smooth muscle actin
- CMF
colonic myofibroblasts/fibroblasts
- CM
colonic mucosal cells
- CRC
colorectal cancer
- Th17
T helper 17 cells
Footnotes
Disclosures: No conflict of interest exists.
References
- 1.Gill MD, Bramble MG, Hull MA, Mills SJ, Morris E, Bradburn DM, Bury Y, Parker CE, Lee TJ, Rees CJ. Screen-detected colorectal cancers are associated with an improved outcome compared with stage-matched interval cancers. Br J Cancer. 2014;111:2076–81. doi: 10.1038/bjc.2014.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waldner MJ, Neurath MF. Master regulator of intestinal disease: IL-6 in chronic inflammation and cancer development. Semin Immunol. 2014;26:75–9. doi: 10.1016/j.smim.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Belluco C, Nitti D, Frantz M, Toppan P, Basso D, Plebani M, Lise M, Jessup JM. Interleukin-6 blood level is associated with circulating carcinoembryonic antigen and prognosis in patients with colorectal cancer. Ann Surg Oncol. 2000;7:133–8. doi: 10.1007/s10434-000-0133-7. [DOI] [PubMed] [Google Scholar]
- 4.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calabrese LH, Rose-John S. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat Rev Rheumatol. 2014 doi: 10.1038/nrrheum.2014.127. [DOI] [PubMed] [Google Scholar]
- 6.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han Z, Wang X, Ma L, Chen L, Xiao M, Huang L, Cao Y, Bai J, Ma D, Zhou J, Hong Z. Inhibition of STAT3 signaling targets both tumor-initiating and differentiated cell populations in prostate cancer. Oncotarget. 2014;5:8416–28. doi: 10.18632/oncotarget.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin J, Lin G, Huang H, Xu D, Yu H, Ma X, Zhu L, Ma D, Jiang H. Capsaicin mediates cell cycle arrest and apoptosis in human colon cancer cells via stabilizing and activating p53. Int J Biol Sci. 2014;10:285–95. doi: 10.7150/ijbs.7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, Simeone DM, Zou W, Welling TH. Tumor-Associated Macrophages Produce Interleukin 6 and Signal via STAT3 to Promote Expansion of Human Hepatocellular Carcinoma Stem Cells. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rokavec M, Oner MG, Li H, Jackstadt R, Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, Slotta-Huspenina J, Bader FG, et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2014;124:1853–67. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. 2014;110:469–78. doi: 10.1038/bjc.2013.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Cao J, Ma S, Dong R, Meng W, Ying M, Weng Q, Chen Z, Ma J, Fang Q, He Q, Yang B. Tumor hypoxia enhances Non-Small Cell Lung Cancer metastasis by selectively promoting macrophage M2 polarization through the activation of ERK signaling. Oncotarget. 2014;5:9664–77. doi: 10.18632/oncotarget.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Oh SH, Kim EJ, Park SJ, Hong SP, Cheon JH, Kim TI, Kim WH. The role of myofibroblasts in upregulation of S100A8 and S100A9 and the differentiation of myeloid cells in the colorectal cancer microenvironment. Biochem Biophys Res Commun. 2012;423:60–6. doi: 10.1016/j.bbrc.2012.05.081. [DOI] [PubMed] [Google Scholar]
- 14.De Simone V, Franze E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald TT, Pallone F, Monteleone G, Stolfi C. Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2014 doi: 10.1038/onc.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Xu K, Wu J, Luo C, Li Y, Wu X, Gao H, Feng G, Yuan BZ. The changes of Th17 cells and the related cytokines in the progression of human colorectal cancers. BMC Cancer. 2012;12:418. doi: 10.1186/1471-2407-12-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto S, Hara T, Mitsuyama K, Yamamoto M, Tsuruta O, Sata M, Scheller J, Rose-John S, Kado S, Takada T. Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J Immunol. 2010;184:1543–51. doi: 10.4049/jimmunol.0801217. [DOI] [PubMed] [Google Scholar]
- 18.Rakhesh M, Cate M, Vijay R, Shrikant A, Shanjana A. A TLR4-interacting peptide inhibits lipopolysaccharide-stimulated inflammatory responses, migration and invasion of colon cancer SW480 cells. Oncoimmunology. 2012;1:1495–506. doi: 10.4161/onci.22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saada JI, Pinchuk IV, Barrera CA, Adegboyega PA, Suarez G, Mifflin RC, Di Mari JF, Reyes VE, Powell DW. Subepithelial myofibroblasts are novel nonprofessional APCs in the human colonic mucosa. J Immunol. 2006;177:5968–79. doi: 10.4049/jimmunol.177.9.5968. [DOI] [PubMed] [Google Scholar]
- 20.Gong Y, Scott E, Lu R, Xu Y, Oh WK, Yu Q. TIMP-1 promotes accumulation of cancer associated fibroblasts and cancer progression. PLoS One. 2013;8:e77366. doi: 10.1371/journal.pone.0077366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol. 2011;73:213–37. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinchuk IV, Beswick EJ, Saada JI, Suarez G, Winston J, Mifflin RC, Di Mari JF, Powell DW, Reyes VE. Monocyte chemoattractant protein-1 production by intestinal myofibroblasts in response to staphylococcal enterotoxin a: relevance to staphylococcal enterotoxigenic disease. J Immunol. 2007;178:8097–106. doi: 10.4049/jimmunol.178.12.8097. [DOI] [PubMed] [Google Scholar]
- 23.Rogler G, Gelbmann CM, Vogl D, Brunner M, Scholmerich J, Falk W, Andus T, Brand K. Differential activation of cytokine secretion in primary human colonic fibroblast/myofibroblast cultures. Scand J Gastroenterol. 2001;36:389–98. doi: 10.1080/003655201300051216. [DOI] [PubMed] [Google Scholar]
- 24.Zhu L, Cheng X, Ding Y, Shi J, Jin H, Wang H, Wu Y, Ye J, Lu Y, Wang TC, Yang CS, Tu SP. Bone marrow-derived myofibroblasts promote colon tumorigenesis through the IL-6/JAK2/STAT3 pathway. Cancer Lett. 2014;343:80–9. doi: 10.1016/j.canlet.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Lin JT, Wang JY, Chen MK, Chen HC, Chang TH, Su BW, Chang PJ. Colon cancer mesenchymal stem cells modulate the tumorigenicity of colon cancer through interleukin 6. Exp Cell Res. 2013;319:2216–29. doi: 10.1016/j.yexcr.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Beswick EJ, Johnson JR, Saada JI, Humen M, House J, Dann S, Qiu S, Brasier AR, Powell DW, Reyes VE, Pinchuk IV. TLR4 activation enhances the PD-L1-mediated tolerogenic capacity of colonic CD90+ stromal cells. J Immunol. 2014;193:2218–29. doi: 10.4049/jimmunol.1203441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahida YR, Beltinger J, Makh S, Goke M, Gray T, Podolsky DK, Hawkey CJ. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol. 1997;273:G1341–8. doi: 10.1152/ajpgi.1997.273.6.G1341. [DOI] [PubMed] [Google Scholar]
- 28.Morris KT, Khan H, Ahmad A, Weston LL, Nofchissey RA, Pinchuk IV, Beswick EJ. G-CSF and G-CSFR are highly expressed in human gastric and colon cancers and promote carcinoma cell proliferation and migration. Br J Cancer. 2014;110:1211–20. doi: 10.1038/bjc.2013.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adegboyega PA, Mifflin RC, DiMari JF, Saada JI, Powell DW. Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps, and adenomatous colorectal polyps. Arch Pathol Lab Med. 2002;126:829–36. doi: 10.5858/2002-126-0829-ISOMIN. [DOI] [PubMed] [Google Scholar]
- 30.Shaker A, Swietlicki EA, Wang L, Jiang S, Onal B, Bala S, DeSchryver K, Newberry R, Levin MS, Rubin DC. Epimorphin deletion protects mice from inflammation-induced colon carcinogenesis and alters stem cell niche myofibroblast secretion. J Clin Invest. 2010;120:2081–93. doi: 10.1172/JCI40676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lei NY, Jabaji Z, Wang J, Joshi VS, Brinkley GJ, Khalil H, Wang F, Jaroszewicz A, Pellegrini M, Li L, Lewis M, Stelzner M, et al. Intestinal subepithelial myofibroblasts support the growth of intestinal epithelial stem cells. PLoS One. 2014;9:e84651. doi: 10.1371/journal.pone.0084651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shenoy A, Butterworth E, Huang EH. ALDH as a marker for enriching tumorigenic human colonic stem cells. Methods Mol Biol. 2012;916:373–85. doi: 10.1007/978-1-61779-980-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Wei B, Han X, Zheng Z, Huang J, Liu J, Huang Y, Wei H. LGR5 is required for the maintenance of spheroid-derived colon cancer stem cells. Int J Mol Med. 2014;34:35–42. doi: 10.3892/ijmm.2014.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu H, Lai W, Zhang Y, Liu L, Luo X, Zeng Y, Wu H, Lan Q, Chu Z. Tumor-associated macrophage-derived IL-6 and IL-8 enhance invasive activity of LoVo cells induced by PRL-3 in a KCNN4 channel-dependent manner. BMC Cancer. 2014;14:330. doi: 10.1186/1471-2407-14-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worthley DL, Si Y, Quante M, Churchill M, Mukherjee S, Wang TC. Bone marrow cells as precursors of the tumor stroma. Exp Cell Res. 2013;319:1650–6. doi: 10.1016/j.yexcr.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei H, Shen G, Deng X, Lou D, Sun B, Wu H, Long L, Ding T, Zhao J. The role of IL-6 in bone marrow (BM)-derived mesenchymal stem cells (MSCs) proliferation and chondrogenesis. Cell Tissue Bank. 2013;14:699–706. doi: 10.1007/s10561-012-9354-9. [DOI] [PubMed] [Google Scholar]
- 37.Hogan NM, Dwyer RM, Joyce MR, Kerin MJ. Mesenchymal stem cells in the colorectal tumor microenvironment: recent progress and implications. Int J Cancer. 2012;131:1–7. doi: 10.1002/ijc.27458. [DOI] [PubMed] [Google Scholar]
- 38.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, Friedman R, Varro A, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–72. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–9. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizzo A, De Mare V, Rocchi C, Stolfi C, Colantoni A, Neurath MF, Macdonald TT, Pallone F, Monteleone G, Fantini MC. Smad7 induces plasticity in tumor-infiltrating Th17 cells and enables TNF-alpha-mediated killing of colorectal cancer cells. Carcinogenesis. 2014;35:1536–46. doi: 10.1093/carcin/bgu027. [DOI] [PubMed] [Google Scholar]
- 41.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–8. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L, Moss T, Mangala LS, Marini J, Zhao H, Wahlig S, Armaiz-Pena G, Jiang D, Achreja A, Win J, Roopaimoole R, Rodriguez-Aguayo C, et al. Metabolic shifts toward glutamine regulate tumor growth, invasion and bioenergetics in ovarian cancer. Mol Syst Biol. 2014;10:728. doi: 10.1002/msb.20134892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407:348–54. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 44.Ray S, De Salvo C, Pizarro TT. Central role of IL-17/Th17 immune responses and the gut microbiota in the pathogenesis of intestinal fibrosis. Curr Opin Gastroenterol. 2014;30:531–8. doi: 10.1097/MOG.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asayama N, Oka S, Tanaka S, Hayashi N, Arihiro K, Chayama K. Endoscopic submucosal dissection as total excisional biopsy for clinical t1 colorectal carcinoma. Digestion. 2015;91:64–9. doi: 10.1159/000368866. [DOI] [PubMed] [Google Scholar]
- 46.Pinchuk IV, Mifflin RC, Saada JI, Powell DW. Intestinal mesenchymal cells. Curr Gastroenterol Rep. 2010;12:310–8. doi: 10.1007/s11894-010-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinchuk IV, Morris KT, Nofchissey RA, Earley RB, Wu JY, Ma TY, Beswick EJ. Stromal cells induce Th17 during Helicobacter pylori infection and in the gastric tumor microenvironment. PLoS One. 2013;8:e53798. doi: 10.1371/journal.pone.0053798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinchuk IV, Saada JI, Beswick EJ, Boya G, Qiu SM, Mifflin RC, Raju GS, Reyes VE, Powell DW. PD-1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T-cell activity. Gastroenterology. 2008;135:1228–37. 37, e1–2. doi: 10.1053/j.gastro.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Owens BM, Simmons A. Intestinal stromal cells in mucosal immunity and homeostasis. Mucosal Immunol. 2013;6:224–34. doi: 10.1038/mi.2012.125. [DOI] [PubMed] [Google Scholar]
- 50.Owens BM, Steevels TA, Dudek M, Walcott D, Sun MY, Mayer A, Allan P, Simmons A. CD90(+) Stromal Cells are Non-Professional Innate Immune Effectors of the Human Colonic Mucosa. Front Immunol. 2013;4:307. doi: 10.3389/fimmu.2013.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.