Abstract
Aims
To present a synopsis of the presentations and discussions from Think Tank I, “Implications for afferent–urothelial bidirectional communication” of the 2014 International Consultation on Incontinence-Research Society (ICI-RS) meeting in Bristol, UK.
Methods
The participants presented what is new, currently understood or still unknown on afferent–urothelial signaling mechanisms. New avenues of research and experimental methodologies that are or could be employed were presented and discussed.
Results
It is clear that afferent–urothelial interactions are integral to the regulation of normal bladder function and that its disruption can have detrimental consequences. The urothelium is capable of releasing numerous signaling factors that can affect sensory neurons innervating the suburothelium. However, the understanding of how factors released from urothelial cells and afferent nerve terminals regulate one another is incomplete. Utilization of techniques such as viruses that genetically encode Ca2+ sensors, based on calmodulin and green fluorescent protein, has helped to address the cellular mechanisms involved. Additionally, the epithelial–neuronal interactions in the urethra may also play a significant role in lower urinary tract regulation and merit further investigation.
Conclusion
The signaling capabilities of the urothelium and afferent nerves are well documented, yet how these signals are integrated to regulate bladder function is unclear. There is unquestionably a need for expanded methodologies to further our understanding of lower urinary tract sensory mechanisms and their contribution to various pathologies.
Keywords: dorsal root ganglia (DRG), GCaMP3, interstitial cystitis/bladder pain syndrome (IC/BPS), pseudorabies virus (PRV)
INTRODUCTION
For many years, the urothelium was thought to be merely a barrier to urine with research interests focusing on tight junctions and transepithelial resistances, aquaporins and water and urea permeability, and uroplakins and membrane trafficking. However, a publication by the Birder and Kanai labs demonstrated adrenergic-mediated nitric oxide production by urothelial umbrella cells that established one-way communication from the urothelium to the underlying nerves and other cellular tissues.1 They coined the term “neuronal-like” properties for the urothelium and opened up a new area of research. We now present data, obtained using novel viral probes, demonstrating that afferent nerves also directly communicate with the urothelium (i.e., bidirectional communication). Moreover, there is a unique relationship between afferent nerves and the urothelium that may underlie a role for viruses in some forms of interstitial cystitis/bladder pain syndrome (IC/BPS).
AFFERENT–UROTHELIAL SIGNALING
The influence that the urothelium exerts on underlying tissues in the bladder wall is now well investigated but there has been much less attention directed as to how such tissues may influence urothelial function. Three such cell types are afferent nerves, interstitial cells (IC) and detrusor smooth muscle.
The concept of signaling between sensory nerves and the urothelium is largely derived from the demonstration that urothelial cells release various signaling factors.2 Afferent nerves innervating the bladder express many of the appropriate receptors that would allow them to respond to these signals, and their close proximity to the urothelium makes them likely targets.3 However, until recently there has not been a clear demonstration of direct afferent nerve communication with the urothelium, which required new experimental approaches.4
The development of genetically encoded Ca2+-sensitive fluorescent indicators offers a new methodology5 to study cellular interactions within the bladder wall by monitoring afferent–urothelial activity in response to stretch or agonists. These probes can be introduced into L6-S2 dorsal root ganglia (DRG) using pseudorabies,6 herpes simplex or adeno-associated viruses.7 These viruses transfect sensory neurons that innervate the pelvic viscera thereby labeling them with Ca2+-dependent fluorescent indicator.4 These studies have demonstrated that mouse L6-S1 DRG injected with pseudorabies virus (PRV-468, a Becker strain that travels anterograde/retrograde) expressed the circularly permutated Ca2+ sensing probe, GCaMP3 (λex = 470 nm) and that after 3 days, there was effective expression of virus and GCaMP in sensory axons throughout the bladder wall of the injected mice, with particularly intense labeling of the urothelium due to direct infection by the viral vector. However, surprisingly there was no labeling of IC in the lamina propria or detrusor and also no labeling of detrusor smooth muscle cells. There was also labeling of the L6-S1 DRG, but not ones above or below (i.e., L5 and S2) indicating the accuracy of the injections. As further proof of the unique relationship between afferent nerves and urothelial cells, the authors injected the tail muscle (i.e., abductor caudalis dorsalis) and descending colon of a mouse with PRV-468 and isolated and imaged L6-S1 DRG and the bladder wall after 6 days, which resulted in a similar pattern of GCaMP expression to that of DRG injections.4 One of the first studies to inject viruses into the tail muscle of rodents demonstrated that this resulted in bladder inflammation based on H&E assessment of RBC/WBC infiltration.8 They argued that this was solely neurogenic inflammation as viruses could not be isolated from the bladder or the urine and it was preventable by the cutting the afferent innervation. However, they injected a Bartha strain of PRV that can only travel retrogradely. Assuming that the virus entered the DRG first, only viral protein, but not the virus, could travel anterogradely to the bladder. In the more recent study, a Becker strain that also travels anterogradely was used so that virus entered the urothelium.
Accordingly, one of the consequences of afferent–urothelial communication is the possibility that virus transfecting the colon could take up residence in the DRG and periodically flare up leading to bladder inflammation not unlike what happens to those suffering from cold sores or interstitial cystitis. Recently, a study reported positive BK polyomavirus titers in urine samples of 11 of 15 IC/BPS patients and none of the controls,9 thus supporting our theory.
EFFERENT–UROTHELIAL INTERACTIONS
The urothelium releases modulators (e.g., ATP and acetylcholine) when subjected to physical strain and it is possible that active detrusor contractions can set up strain vectors that impact on the urothelium. This could lead to vicious cycles between the two tissues, as ATP and acetylcholine are contractile agonists. The fact that high-KCl solution has no effect on ATP release highlights that urothelial cells lack voltage-gated Ca2+ channels and are not influenced by membrane depolarization. However, it is not known if detrusor-related urothelium strain changes could influence the release of other mediators. For example, release of efferent nerve transmitters may also influence urothelial function, as ATP, in particular, has a positive feedback effect on further urothelial ATP release10 and its breakdown product, adenosine, which suppresses ATP release.11 This interaction has not been investigated and is amenable to further study.
Nerve fiber terminals in the suburothelium, that are presumed to convey afferent information, contain clear or dense-cored vesicles that are associated with interstitial cells.12 These excitable cells respond to ATP with a large depolarization and have been proposed to modulate afferent nerve activity because of this close association. However, this potential two-way inter-relationship has not been investigated on a functional basis. It would be of interest to determine the contents of the nerve vesicles and characterize their effects on Interstitial cells. If they inhibit interstitial cell function, it offers a negative feedback route to regulate afferent signaling from the bladder wall.
URETHRAL EPITHELIUM
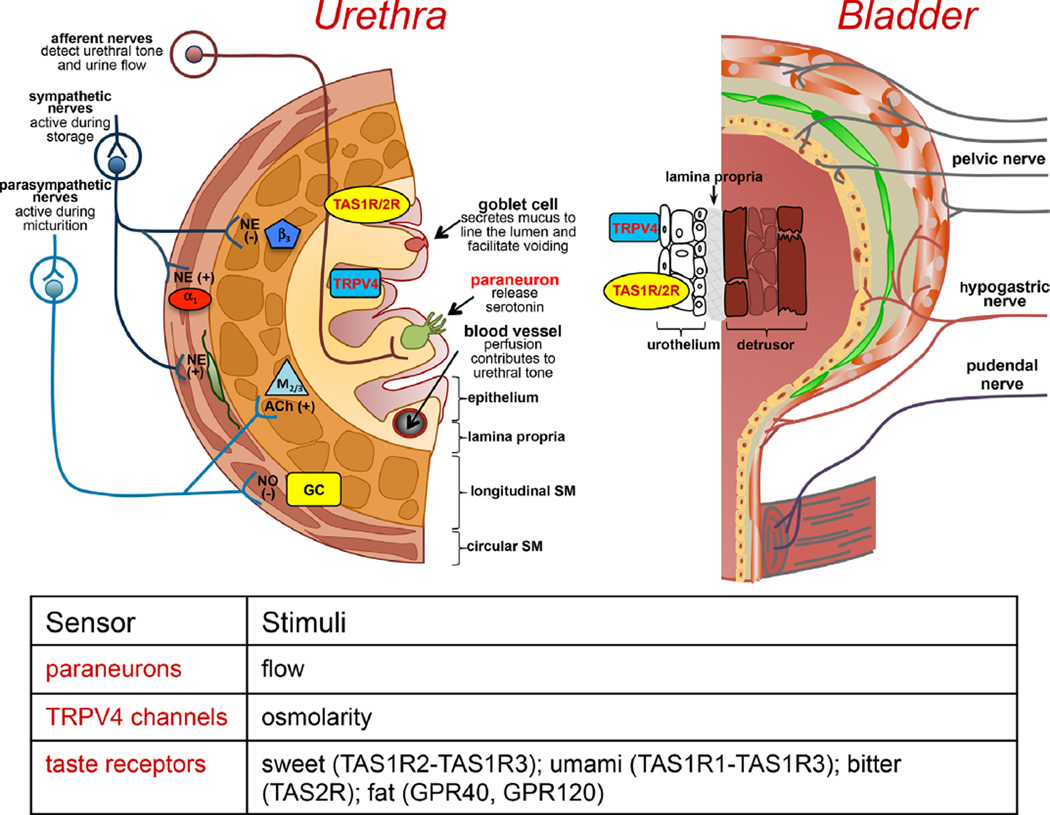
The urothelium that lines the bladder and proximal urethra is a stratified squamous epithelium with a differentiated apical layer containing uroplakin plaques. This results in a highly impermeable barrier suitable for urine storage. Conversely, the urethra is lined predominantly by a columnar epithelium which does not express many of the surface markers observed in bladder urothelium13 and does not develop a tight barrier which is not critical at this region of the lower urinary tract. Differences in the structure, function and innervation of the bladder and urethra are highlighted in Figure 1.
Fig. 1.
Schematic of neural, sensory, and urothelial/epithelial regulation of the bladder and urethra. Although there are significant structural differences between the bladder urothelium and urethral epithelium, they appear to share similar sensory receptors to detect changes in the urine. Notably, taste (TAS1/2) receptors are expressed in both bladder and urethra which are able to detect bitter, sweet, and umami chemical stimuli and may influence afferent sensitivity in response to the presence of bacterial infections19 or artificial sweeteners.38 Transient receptor potential channels, especially TRPV4, have been implicated in urothelial/epithelial sensory function in both the bladder and urethra.39 The specific roles of each channel under normal and pathological conditions are an area of active investigation by many labs. However, there are certain differences between the two tissues, including the specialized paraneuron cells located within the urethral epithelium that project microvilli into the lumen and can release serotonin.20 They are also in close approximation to sensory axons raising the possibility they are involved in detecting changes in urine content or flow within the urethra.
Sensory input from the urethra (e.g., flow of urine, irritation) modulates bladder function via peripheral and CNS mediated reflexes and contributes to bladder sensation including bladder pain as demonstrated in animal14–17 and clinical15,18 studies. However, how information from the lumen of the urethra is detected and transmitted to the nervous system is not well understood. Here we introduce a novel concept for the detection and transmission of sensory information, via paraneuron-mediated communication. The paraneurons (also termed neuroendocrine cells or brush-like cells) are specialized cells embedded in the urethral epithelium, which share similar features with neurons, including the presence of synaptic vesicles and the ability to synthetize and release neurotransmitters. Several paraneuron populations, positive for acetylcholine (ACh), serotonin (5-HT) and somatostatin, can be identified based on morphological and structural features. Some cells possess an apical tuft of microvilli protruding into the urethral lumen; others have dendritic-like processes extending through the epithelium.19,20 While their functions are relatively unknown, a recent report that characterized a population of ACh-positive (ACh+) paraneurons hypothesized that these cells act as “chemosensory sentinels” that monitor the urethral lumen for potential hazardous content19. These cells are located in close proximity to nerve fibers expressing nAChRs, possess the classical taste signal transduction cascade (used presumably to detect potential noxious compounds such as uropathogenic bacteria), and, in response to noxious stimulation may release ACh which activates muscarinic receptors on neighboring epithelial cells. These studies provide evidence for communication between paraneurons and epithelial cells. While there is no direct evidence for interactions between paraneurons and sensory nerves, ACh+ cells were located in close proximity to nerve fibers, suggesting that the anatomical substrate for communication exists. Additionally, functional in vivo studies in urethane anesthetized rats indicated that bitter stimuli delivered into the urethra alter bladder contractility.19
Multi-directional communication between paraneurons, epithelial cells and nerves may take place in the epithelium and may play a role in information processing. For example, upon detection of sensory information (urine flow, irritants, inflammation) paraneurons may release transmitters (e.g., ACh, 5-HT) that can either act directly on nerves or stimulate the nerves via an indirect action on epithelial cells. It is known that afferent nerve fibers possess receptors for potential transmitters such as ACh, 5-HT, or ATP. Conversely, nerves release transmitters including CGRP, SubP, and NKA that may act on paraneurons. It is unknown what specific neurotransmitter receptors the paraneurons possess.
UROTHELIAL DISRUPTION
The urothelium is known to have specialized sensory and signaling properties that allow it to respond to chemical and mechanical stimuli.2,21,22 Consistent with this role, afferent nerves have been identified in close proximity to the abluminal or inner surface of the urothelium with axons that extend into the epithelial layer.23,24 It is increasingly recognized that afferent outflow from the bladder may be modulated within the bladder wall itself, through regulation of sensitivity of the systems that generate afferent activity.25 Unsurprisingly, modification of the urothelium and/or loss of its epithelial integrity have been implicated in hypersensitivity disorders of the lower urinary tract such as IC/BPS and overactive bladder syndrome. The literature suggests that altered urothelial differentiation, increased urothelial permeability, and augmented urothelial “transducer-sensor” function contribute to the development and/or persistence of the sensory symptoms that characterize these conditions.26 However, it is yet to be established whether these urothelial aberrations are primary etiological defects or secondary compensatory changes related to neural plasticity and cellular adaptations.
Urothelial disruption may result from direct physical or chemical damage or be indirectly mediated by neural or hormonal mechanisms.27 In keeping with the latter, spinal cord injury has been shown to be accompanied by a rapid loss in barrier function, which is due in part to an interaction with bladder nerve as shown in rodent studies.28 Loss of urothelial integrity allows leakage of urine constituents and toxic substances in to the underlying cell layers leading to changes in the properties of afferent nerves and the development of sensory symptoms such as urgency, frequency and pain, during bladder filling, and voiding. Following injury, the urothelium undergoes both functional and structural changes in order to restore barrier integrity as shown in rats.29 Regeneration requires a precise coordination of cell proliferation, migration and differentiation, but the mechanisms that regulate normal repair have yet to be fully established. The initiation of urothelial proliferation involves upregulation of growth factors such as fibroblast growth factor, heparin-binding epidermal growth factor and nerve growth factors in urothelial cells from human surgical explants30 and mice.31 The clinical relevance of these factors is suggested by the finding of decreased levels of heparin-binding epidermal growth factor in the urine from IC/BPS patients compared to asymptomatic controls.26,32 Expression of the protein called antiproliferative factor (APF) was reported to inhibit epithelial proliferation and impair restoration of barrier function in IC/BPS.33 However, the utilization of APF as a diagnostic IC/BPS biomarker has been largely discounted. This is attributed to the minimal correlation of urine and tissue protein levels for most putative IC/BPS biomarkers34 and technical difficulty associated with the clinical use of the APF assay.35 Additionally, there has been a recent study which did not find increased APF expression in IC/BPS patients.36
Given that conditions that cause urothelial damage are common, knowledge about urothelium repair mechanisms and the role of non-epithelial factors may provide important insights into the pathophysiology of different forms of cystitis and how to treat them. Future studies are required to define the process by which barrier function fails, the chemical mediators involved and how it can be recovered. There is also a limited understanding about the factors that influence an individual’s response to injury and how this affects the chronicity/natural history of the resulting condition. The effect of gender, age, and hormone status has also not been adequately explored.
RESEARCH RECOMMENDATIONS
Release of urothelial factors that act on afferent nerves has been extensively studied. However, the influence of afferent excitable neuropeptides on urothelial cells needs further investigation. Moreover, the mechanism by which neurotropic viruses take advantage of bidirectional communication is important to investigate. For example, is there an urothelial binding protein that could be a pharmacological target to prevent urothelial infections as recent findings suggest occur in IC/BPS.9,37
The release of efferent neurotransmitters such as ACh, ATP, norepinephrine, and 5-HT, like afferent excitatory neuropeptides, may also influence urothelial function. ATP in particular has a powerful positive feedback on urothelial ATP release and its breakdown product, adenosine, suppresses ATP release. These interactions have not been extensively investigated and are amenable to further study.
Future studies are needed to investigate the different populations of paraneurons, their communication with nerves and their roles in bladder function and dysfunction. The availability of genetically engineered mice such as eGFP-ChAT, which provides easy identification of ACh releasing paraneurons, may help differentiate discrete populations of these cells.
SUMMARY
There is ample evidence that the urothelium and associated afferent nerves are capable of influencing each other’s activity through the release of various factors. Augmentation of signaling activity appears to play a major role in sensory pathologies of the lower urinary tract (e.g., IC/BPS and overactive bladder syndrome). However, the key mediators and signaling pathways involved are unclear and further investigation of afferent–urothelial communication mechanisms is necessary. This is especially imperative for the identification of therapeutic targets and development of treatment options.
Footnotes
Potential conflicts of interest: Nothing to disclose.
REFERENCES
- 1.Birder LA, Apodaca G, De Groat WC, et al. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol. 1998;275:F226. doi: 10.1152/ajprenal.1998.275.2.F226. [DOI] [PubMed] [Google Scholar]
- 2.Birder L, Andersson KE. Urothelial signaling. Physiol Rev. 2013;93:653. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun B, Li Q, Dong L, et al. Ion channel and receptor mechanisms of bladder afferent nerve sensitivity. Auton Neurosci. 2010;153:26. doi: 10.1016/j.autneu.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda Y, Zabbarova I, Kanai A. Bidirectional communication between afferent neurons and urothelial cells in the mouse urinary bladder. J Urol. 2014;191:E142. [Google Scholar]
- 5.Broussard GJ, Liang R, Tian L. Monitoring activity in neural circuits with genetically encoded indicators. Front Mol Neurosci. 2014;7:97. doi: 10.3389/fnmol.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Granstedt AE, Szpara ML, Kuhn B, et al. Fluorescence-based monitoring of in vivo neural activity using a circuit-tracing pseudorabies virus. PLoS ONE. 2009;4:e6923. doi: 10.1371/journal.pone.0006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodward JJ, Pava M. Ethanol inhibition of up-states in prefrontal cortical neurons expressing the genetically encoded calcium indicator GCaMP3. Alcohol Clin Exp Res. 2012;36:780. doi: 10.1111/j.1530-0277.2011.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doggweiler R, Jasmin L, Schmidt RA. Neurogenically mediated cystitis in rats: An animal model. J Urol. 1998;160:1551. [PubMed] [Google Scholar]
- 9.Van der Aa F, Beckley I, de Ridder D. Polyomavirus BK-a potential new therapeutic target for painful bladder syndrome/interstitial cystitis? Med Hypotheses. 2014;83:317. doi: 10.1016/j.mehy.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Chai TC. Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Physiol Cell Physiol. 2006;290:C27. doi: 10.1152/ajpcell.00552.2004. [DOI] [PubMed] [Google Scholar]
- 11.Dunning-Davies BM, Fry CH, Mansour D, et al. The regulation of ATP release from the urothelium by adenosine and transepithelial potential. BJU Int. 2013;111:505. doi: 10.1111/j.1464-410X.2012.11421.x. [DOI] [PubMed] [Google Scholar]
- 12.Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int. 2003;91:89. doi: 10.1046/j.1464-410x.2003.03802.x. [DOI] [PubMed] [Google Scholar]
- 13.Sun TT. Altered phenotype of cultured urothelial and other stratified epithelial cells: implications for wound healing. Am J Physiol Renal Physiol. 2006;291:F9. doi: 10.1152/ajprenal.00035.2006. [DOI] [PubMed] [Google Scholar]
- 14.Jung SY, Fraser MO, Ozawa H, et al. Urethral afferent nerve activity affects the micturition reflex; implication for the relationship between stress incontinence and detrusor instability. J Urol. 1999;162:204. doi: 10.1097/00005392-199907000-00069. [DOI] [PubMed] [Google Scholar]
- 15.Birder LA, De Wachter S, Gillespie J, et al. Urethral sensation: Basic mechanisms and clinical expressions. Int J Urol. 2014;21:13. doi: 10.1111/iju.12349. [DOI] [PubMed] [Google Scholar]
- 16.Woock JP, Yoo PB, Grill WM. Intraurethral stimulation evokes bladder responses via 2 distinct reflex pathways. J Urol. 2009;182:366. doi: 10.1016/j.juro.2009.02.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robain G, Combrisson H, Mazieres L. Bladder response to urethral flow in the awake ewe. Neurourol Urodyn. 2001;20:641. doi: 10.1002/nau.1014. [DOI] [PubMed] [Google Scholar]
- 18.Shafik A, Shafik AA, El-Sibai O, et al. Role of positive urethrovesical feedback in vesical evacuation. The concept of a second micturition reflex: The urethrovesical reflex. World J Urol. 2003;21:167. doi: 10.1007/s00345-003-0340-5. [DOI] [PubMed] [Google Scholar]
- 19.Deckmann K, Filipski K, Krasteva-Christ G, et al. Bitter triggers acetylcholine release from polymodal urethral chemosensory cells and bladder reflexes. Proc Natl Acad Sci USA. 2014;111:8287. doi: 10.1073/pnas.1402436111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vittoria A, La Mura E, Cocca T, et al. Serotonin-, somatostatin- and chromogranin A-containing cells of the urethro-prostatic complex in the sheep. An immunocytochemical and immunofluorescent study. J Anat. 1990;171:169. [PMC free article] [PubMed] [Google Scholar]
- 21.de Groat WC. The urothelium in overactive bladder: Passive bystander or active participant? Urology. 2004;64:7. doi: 10.1016/j.urology.2004.08.063. [DOI] [PubMed] [Google Scholar]
- 22.Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int. 2007;72:1057. doi: 10.1038/sj.ki.5002439. [DOI] [PubMed] [Google Scholar]
- 23.Gabella G, Davis C. Distribution of afferent axons in the bladder of rats. J Neurocytol. 1998;27:141. doi: 10.1023/a:1006903507321. [DOI] [PubMed] [Google Scholar]
- 24.Wiseman OJ, Brady CM, Hussain IF, et al. The ultrastructure of bladder lamina propria nerves in healthy subjects and patients with detrusor hyperreflexia. J Urol. 2002;168:2040. doi: 10.1016/S0022-5347(05)64291-7. [DOI] [PubMed] [Google Scholar]
- 25.Eastham JE, Gillespie JI. The concept of peripheral modulation of bladder sensation. Organogenesis. 2013;9:224. doi: 10.4161/org.25895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keay SK, Birder LA, Chai TC. Evidence for bladder urothelial pathophysiology in functional bladder disorders. Biomed Res Int. 2014;2014:865463. doi: 10.1155/2014/865463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birder LA, de Groat WC. Mechanisms of disease: Involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol. 2007;4:46. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apodaca G, Kiss S, Ruiz W, et al. Disruption of bladder epithelium barrier function after spinal cord injury. Am J Physiol Renal Physiol. 2003;284:F966. doi: 10.1152/ajprenal.00359.2002. [DOI] [PubMed] [Google Scholar]
- 29.Lavelle J, Meyers S, Ramage R, et al. Bladder permeability barrier: Recovery from selective injury of surface epithelial cells. Am J Physiol Renal Physiol. 2002;283:F242. doi: 10.1152/ajprenal.00307.2001. [DOI] [PubMed] [Google Scholar]
- 30.Zhang D, Kosman J, Carmean N, et al. FGF-10 and its receptor exhibit bidirectional paracrine targeting to urothelial and smooth muscle cells in the lower urinary tract. Am J Physiol Renal Physiol. 2006;291:F481. doi: 10.1152/ajprenal.00025.2006. [DOI] [PubMed] [Google Scholar]
- 31.Bassuk JA, Cochrane K, Mitchell ME. Induction of urothelial cell proliferation by fibroblast growth factor-7 in RAG1-deficient mice. Adv Exp Med Biol. 2003;539:623. doi: 10.1007/978-1-4419-8889-8_40. [DOI] [PubMed] [Google Scholar]
- 32.Keay SK, Zhang CO, Shoenfelt J, et al. Sensitivity and specificity of antiproliferative factor, heparin-binding epidermal growth factor-like growth factor, and epidermal growth factor as urine markers for interstitial cystitis. Urology. 2001;57:9. doi: 10.1016/s0090-4295(01)01127-x. [DOI] [PubMed] [Google Scholar]
- 33.Keay SK, Szekely Z, Conrads TP, et al. An antiproliferative factor from interstitial cystitis patients is a frizzled 8 protein-related sialoglycopeptide. Proc Natl Acad Sci USA. 2004;101:11803. doi: 10.1073/pnas.0404509101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erickson DR, Tomaszewski JE, Kunselman AR, et al. Urine markers do not predict biopsy findings or presence of bladder ulcers in interstitial cystitis/painful bladder syndrome. J Urol. 2008;179:1850. doi: 10.1016/j.juro.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilkinson DR, Erickson AD. Urinary and serologic markers for interstitial cystitis: An update. Curr Urol Rep. 2006;7:414. doi: 10.1007/s11934-006-0013-1. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa T, Homma T, Igawa Y, et al. CXCR3 binding chemokine and TNFSF14 over expression in bladder urothelium of patients with ulcerative interstitial cystitis. J Urol. 2010;183:1206. doi: 10.1016/j.juro.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Eisen DP, Fraser IR, Sung LM, et al. Decreased viral load and symptoms of polyomavirus-associated chronic interstitial cystitis after intravesical cidofovir treatment. Clin Infect Dis. 2009;48:e86. doi: 10.1086/597827. [DOI] [PubMed] [Google Scholar]
- 38.Elliott RA, Kapoor S, Tincello DG. Expression and distribution of the sweet taste receptor isoforms T1R2 and T1R3 in human and rat bladders. J Urol. 2011;186:2455. doi: 10.1016/j.juro.2011.07.083. [DOI] [PubMed] [Google Scholar]
- 39.Birder L, Kullmann FA, Lee H, et al. Activation of urothelial transient receptor potential vanilloid 4 by 4alpha-phorbol 12,13-didecanoate contributes to altered bladder reflexes in the rat. J Pharmacol Exp Ther. 2007;323:227. doi: 10.1124/jpet.107.125435. [DOI] [PubMed] [Google Scholar]