Abstract
Background
Epidemiologic data suggest a protective effect of Helicobacter pylori infection against the development of autoimmune disease. Laboratory data illustrate H. pylori's ability to induce immune tolerance and limit inflammatory responses. Numerous observational studies have investigated the association between H. pylori infection and inflammatory bowel disease (IBD). Our aim was to perform a systematic review and meta-analysis of this association.
Methods
Medline, EMBASE, bibliographies, and meeting abstracts were searched by 2 independent reviewers. Of 369 abstracts reviewed, 30 promising articles were reviewed in detail. Twenty-three studies met our inclusion criteria (subject N = 5903). Metaanalysis was performed with the metan command in Stata 10.1.
Results
Overall, 27.1% of IBD patients had evidence of infection with H. pylori compared to 40.9% of patients in the control group. The estimated relative risk of H. pylori infection in IBD patients was 0.64 (95% confidence interval [CI]: 0.54–0.75). There was significant heterogeneity in the included studies that could not be accounted for by the method of IBD and H. pylori diagnosis, study location, or study population age.
Conclusions
These results suggest a protective benefit of H. pylori infection against the development of IBD. Heterogeneity among studies and the possibility of publication bias limit the certainty of this finding. Further studies investigating the effect of eradication of H. pylori on the development of IBD are warranted. Because environmental hygiene and intestinal microbiota may be strong confounders, further mechanistic studies in H. pylori mouse models are also necessary to further define the mechanism of this negative association.
Keywords: Helicobacter pylori, inflammatory bowel disease, Crohn's disease, ulcerative colitis
Inflammatory bowel disease (IBD) is a growing worldwide health burden.1,2 Specifically, many developing countries have seen a dramatic rise in the incidence of IBD since 1990.2–7 This rise may partially be accounted for by the implementation of improved diagnostic methods and heightened awareness of IBD.1,3 Furthermore, improved access to a cleaner environment and the resulting decreased incidence of common childhood infections may be contributing as well by altering one's susceptibility to certain diseases with an autoimmune component, such as IBD.8,9 Importantly, this suggests a possible protective benefit of microbial infection during childhood.
Helicobacter pylori has coexisted with the human race for over 50,000 years.10,11 It is an infection acquired early in childhood, and if not eliminated by antimicrobial therapy, is carried throughout life, producing symptoms in only a minority.12,13 Recent epidemiological data suggests a possible protective benefit of H. pylori colonization against the development of certain diseases with an autoimmune component, such as asthma.14,15 Furthermore, there is emerging laboratory evidence illustrating H. pylori's role in the regulation of the immune system. Specifically, H. pylori has been associated with increased gastric mucosal expression of Foxp3 (a T-regulatory cell marker) and has shown the ability to skew the host immunologic tone away from inflammatory Th1/Th17 responses (Fig. 1).16–21 Finally, IBD is more prevalent in areas with lower rates of H. pylori colonization, such as in the United States.22 In fact, there is a steady rise in the incidence of IBD in H. pylori endemic regions that corresponds to the beginning of anti-H. pylori therapy for peptic ulcer disease.2
Figure 1.
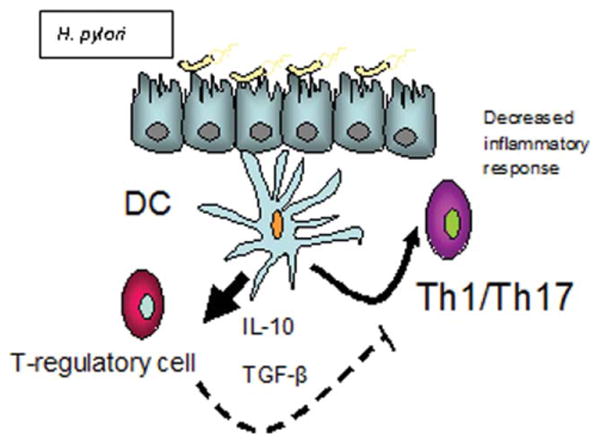
A proposed model of H. pylori's effect on host immune regulation. H. pylori, through its interaction with dendritic cells (DC), is able to upregulate regulatory T-cells. This upregulation leads to decreased production of proinflammatory cytokines. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To further investigate the possible association between H. pylori infection and IBD, we conducted a systematic review and meta-analysis to estimate the relative risk of H. pylori infection in patients with and without IBD. Given the epidemiological and laboratory data previously cited, we hypothesized an inverse relationship between H. pylori infection and IBD.
Materials and Methods
Search Strategy
This review was performed according to the standard guidelines for meta-analyses and systematic reviews of observational studies.23 To find relevant articles for this review, we searched the following databases (from inception to March 2009): MEDLINE, EMBASE, Google Scholar, the Cochrane Central Register of Controlled Trials, ACP Journal Club, DARE, CMR, and HTA. The search strategy used free-text words and MeSH terms to increase the sensitivity of the search. The following search terms were used: “inflammatory bowel disease,” “crohn's disease,” “colitis, ulcerative,” “IBD,” “CD,” “UC,” “ulcerative colitis,” “Crohn's,” “Helicobacter pylori,” “H. pylori,” and “HP.” Boolean operators (AND, OR, NOT) were used to narrow and widen the search results. The titles and abstracts from the search results were examined closely for potential inclusion in the study. Additionally, the references from selected articles were examined as a further search tool. We also consulted experts in the field to identify additional published and unpublished studies. Last, we searched the abstracts presented at Digestive Disease Week, United European Gastroenterology Week, and the American College of Gastroenterology Annual Scientific Meeting from 2003–2007.
Study Selection
For inclusion in the systematic review, a study had to meet the following criteria established by the study team: 1) H. pylori infection diagnosed by serology (IgG antibody), urea breath test (UBT), fecal antigen test (FAT), rapid urease test (RUT), or histology; 2) inclusion of a control group; 3) IBD and control groups were similar in age, sex, and from the same catchment area; 4) studies of human; and 5) data were reported that were sufficient to calculate H. pylori infection rates in both the IBD and control groups. Studies were excluded if they used data from a previously published study.
Data Extraction
To reduce reporting bias and error in data collection, 2 independent reviewers (J.L. and M.D.) extracted data from selected studies using standardized data extraction forms. These forms, created by the study team, included the: a) authors; b) title; c) year of publication; d) journal; e) study design; f) inclusion and exclusion criteria; g) method by which H. pylori infection was diagnosed; h) method by which IBD was diagnosed; i) number of patients with Crohn's disease (CD) and within this group, the number who were H. pylori-positive and -negative; j) number of patients with ulcerative colitis (UC) and within this group, the number who were H. pylori positive and negative; k) number of patients in the control group and within this group, the number of patients who were H. pylori-positive and -negative; 1) reported previous use of antibiotics, and specifically antibiotics used to treat H. pylori, in the IBD and control groups; and m) reported previous use of immunosuppressive agents in the IBD group, specifically steroids, 5-aminosalicylates (5-ASAs), and tumor necrosis factor alpha (TNF-α) antibody medications. If needed, authors were contacted regarding specific questions relating to their study. The independent reviewers conferred after data extraction was complete, discrepancies were identified, and review of the relevant article led to consensus.
Statistical Analysis
The primary outcome of this analysis was the relative risk (RR) of H. pylori infection in IBD versus controls. RR was used to describe the ratio of the probability of the H. pylori infection occurring in IBD patients versus the controls. We calculated the RR with a 95% confidence interval (CI) based on a random-effects model as described by Mantel–Haenszel. Meta-analysis was performed with the metan command in Stata 10.1 (StataCorp, College Station, TX). Analysis with a funnel plot, Begg's test, and Egger's test were used to assess publication bias. Subgroup analyses were also performed. An I2 statistic was used to measure the proportion of inconsistency in individual studies that could not be explained by chance.24 Any heterogeneity identified would prompt subgroup analysis in an attempt to explain these findings.
Assessment of Study Quality
Each study chosen for review was carefully assessed for study quality by the study team. Study quality was assessed using the following criteria: 1) study design; 2) method of H. pylori diagnosis; 3) method of IBD diagnosis; 4) method of patient enrollment (consecutive versus selected); and 5) whether H. pylori infection rate was the primary or secondary outcome of the study.
Results
Search Results
Our initial search strategy yielded 369 potential articles for inclusion. After detailed analysis of selected articles, 29 articles were reviewed in detail. Subsequently. 6 articles ∼ did not meet inclusion criteria. The reasons for exclusion included: 1 study included a control group from a different catchment area from the IBD group, and also differed significantly in mean age25, 1 study examined a control group with known H. pylori infection,26 1 study was published in abstract form only and the H. pylori infection rates in the control and IBD groups could not be calculated,27 2 studies did not provide data on H. pylori infection rates,28,29 and 1 study used IgA serology as a diagnostic method for H. pylori infection.30 Therefore, 23 studies31-53 with 5903 patients fulfilled the inclusion criteria for the review (Fig. 2).
Figure 2. Flow diagram of studies identified in the systematic review.

Study Characteristics
The characteristics of the included studies are summarized in Tables 1 and 2. The results of each study are in Table 3. The largest study examining the relationship between H. pylori infection and incidence of IBD was conducted in the Netherlands by Wagtmans et al.31 The authors recovered frozen sera from 386 patients with known CD and 277 controls, and the sera was tested for the presence of IgG and IgA antibodies. Interestingly, the sera from the patients with Crohn's were recovered from frozen storage and in some instances had been there for 20 years. Unlike Halme et al,30 which was excluded from our analysis, the authors provided data on the number of IgG-positive, IgA-positive, and IgG/IgA-positive patients. Therefore, we were able to exclude the IgA-positive patients in our analysis. Overall, 12.1% of IBD patients were infected with H. pylori, while 35.4% of the control group were found to have H. pylori infection.
Table 1. Characteristics of the Included Studies.
| Author | Year | Location | Single vs. Multicenter | n, Total | n, IBD (C/UC) | n, Control | Mean Age, IBD (CD/UC) | Mean Age, Control |
|---|---|---|---|---|---|---|---|---|
| el-Omar et al. (32) | 1994 | Poland | Single | 210 | 110(63/47) | 100 | 38.7/47.3 | NR |
| Mantzaris et al. (35) | 1995 | Greece | Single | 210 | 90 (0/90) | 120 | NR | NR |
| Meining et al. (50) | 1997 | Germany | Multi | 72 | 36 (36/0) | 36 | 34.3 | 34.4 |
| Oberhuber et al. (41) | 1997 | Germany | Single | 275 | 82 (75/7) | 193 | NR | NR |
| Parente et al. (33) | 1997 | Italy | Single | 432 | 216 (123/93) | 216 | 38.6/39.9 | NR |
| Wagtmans et al. (31) | 1997 | Netherlands | Single | 663 | 386 (386/0) | 277 | NR | NR |
| Duggan et al. (52) | 1998 | U.K. | Single | 431 | 257 (87/170) | 174 | NR | NR |
| Corrado et al. (43) | 1998 | Italy | Single | 90 | 30 (NR/NR) | 60 | 12.2 | 7.3 |
| D'Inca et al. (42) | 1998 | Italy | Single | 151 | 108 (67/41) | 43 | 40/37 | 38 |
| Pearce et al. (34) | 2000 | U.K. | Single | 133 | 93 (42/51) | 40 | 42/46 | 43 |
| Parente et al. (46) | 2000 | Italy | Single | 361 | 220 (141/79) | 141 | NR | NR |
| Parlak et al. (37) | 2001 | Turkey | Single | 188 | 111 (45/66) | 77 | 37.2/41.9 | 37 |
| Vare et al. (40) | 2001 | Finland | Single | 349 | 279 (94/185) | 70 | 43 | NR |
| Feeney et al. (39) | 2002 | U.K. | Single | 552 | 276 (139/137) | 276 | NR | NR |
| Furusu et al. (51) | 2002 | Japan | Single | 75 | 50 (25/25) | 25 | NR | NR |
| Guslandi et al. (38) | 2002 | Italy | Single | 90 | 60 (60/0) | 30 | NR | NR |
| Pascasio et al. (44) | 2003 | U.S.A. | Single | 486 | 56 (56/0) | 382 | NR | NR |
| Piodi et al. (47) | 2003 | Italy | Single | 144 | 72 (32/40) | 72 | 48/49 | NR |
| Triantafillidis et al. (36) | 2003 | Greece | Single | 243 | 116(39/77) | 127 | 42 | 44 |
| Pronai et al. (48) | 2004 | Hungary | Single | 333 | 133 (51/82) | 200 | 34.2/38.4 | 36.3 |
| Oliveira et al. (53) | 2004 | Brazil | Single | 116 | 42 (0/42) | 74 | 38.9 | 49.4 |
| Oliveira et al. (49) | 2006 | Brazil | Single | 117 | 43 (43/0) | 74 | 40.9 | 49.4 |
| Sladek et al. (45) | 2007 | Poland | Single | 198 | 94 (50/44) | 194 | 12.9 | 13.6 |
CD, Crohn's disease; UC, ulcerative colitis; NR, not reported.
Table 2. Quality Assessment of the Included Studies.
| Author | H.P.a Diagnosis | IBD Diagnosis | Study Type | Patient Enrollment | Outcome |
|---|---|---|---|---|---|
| el-Omar et al. (32) | IgG | Chart review | Prospective | Random | Primary |
| Mantzaris et al. (35) | Histology/RUT | Not reported | Prospective | Consecutive | Primary |
| Meining et al. (50) | Histology | Histology | Prospective | Consecutive | Secondary |
| Oberhuber et al. (41) | Histology | Histology | Prospective | Consecutive | Secondary |
| Parente et al. (33) | IgG/histology | Chart review | Prospective | Consecutive | Primary |
| Wagtmans et al. (31) | IgG | Chart review | Retrospective | Random | Primary |
| Duggan et al. (52) | IgG | Chart review | case-series | Consecutive | Primary |
| Corrado et al. (43) | IgG | Histology | Prospective | Consecutive | Secondary |
| D'Inca et al. (42) | Histology | Chart review | Prospective | Consecutive | Primary |
| Pearce et al. (34) | IgG/UBT | Radiology/histology | Prospective | Consecutive | Primary |
| Parente et al. (46) | UBT/histology | Chart review | Prospective | Consecutive | Primary |
| Parlak et al. (37) | Histology | Not reported | Prospective | Not reported | Primary |
| Vare et al. (40) | IgG | Chart review | Prospective | previous study | Primary |
| Feeney et al. (39) | IgG | Clinical criteria | Case-series | Matched | Primary |
| Furusu et al. (51) | IgG/histology | Histology | Prospective | Not reported | Secondary |
| Guslandi et al. (38) | IgG | Not reported | Not reported | Not reported | Primary |
| Pascasio et al. (44) | Histology | Histology | Retrospective | Consecutive | Secondary |
| Piodi et al. (47) | UBT | Chart review | Prospective | Consecutive | Primary |
| Triantafillidis et al. (36) | IgG | Chart review | Prospective | Not reported | Primary |
| Pronai et al. (48) | UBT | Histology | Prospective | Not reported | Primary |
| Oliveira et al. (53) | IgG/UBT | Histology | Prospective | Not reported | Primary |
| Oliveira et al. (49) | UBT | Histology | Prospective | Not reported | Primary |
| Sladek et al. (45) | Histology/RUT | Clinical/histology/serology | Not reported | Consecutive | Primary |
H.P., Helicobacter pylori.
Clinical criteria: authors state diagnoses were made according to “conventional clinical criteria.”
Table 3. Study Results.
| Author | % IBD Patients H.P. Positive (% CD/%UC) |
% Controls H.P. Positive |
|---|---|---|
| el-Omar et al. (32) | 21.8 (14.9/27.0) | 52 |
| Mantzaris et al. (35) | 30.0 (NR/30.0) | 52.5 |
| Meining et al. (50) | 8.3 (8.3/NR) | 36.1 |
| Oberhuber et al. (41) | 30.5 (33.3/0.0) | 35.2 |
| Parente et al. (33) | 48.1 (40.7/55.9) | 58.8 |
| Wagtmans et al. (31) | 12.2 (12.2/NR) | 35.4 |
| Duggan et al. (52) | 34.2 (33.3/34.7) | 36.2 |
| Corrado et al. (43) | 0.0 (NR/NR) | 23.3 |
| D'Inca et al. (42) | 28.7 (28.4/29.3) | 39.5 |
| Pearce et al. (34) | 17.2 (11.9/21.6) | 25 |
| Parente et al. (46) | 38.2 (33.3/46.8) | 50.3 |
| Parlak et al. (37) | 66.7 (62.2/69.7) | 63.6 |
| Vare et al. (40) | 24.4 (12.9/29.7) | 37.1 |
| Feeney et al. (39) | 0.05 (0.05/NR) | 15.8 |
| 13.9 (NR/13.9) | 15.3 | |
| Furusu et al. (51) | 29.4 (34.6/24.0) | 52 |
| Guslandi et al. (38) | 15.0 (15.0/NR) | 36.7 |
| Pascasio et al. (44) | 32.1 (32.1/NR) | 46.1 |
| Piodi et al. (47) | 47.2 (53.1/42.5) | 61.1 |
| Triantafillidis et al. (36) | 34.5 (NR/NR) | 55.1 |
| Pronai et al. (48) | 12.8 (13.7/12.2) | 39 |
| Oliveira et al. (53) | 52.4 (NR/52.4) | 51.4 |
| Oliveira et al. (49) | 51.2 (51.2/NR) | 70.3 |
| Sladek et al. (45) | 9.6 (14.0/0.05) | 38.5 |
NR, not reported; H.P., Helicobacter pylori.
The earliest study examining H. pylori infection rates and IBD was published in 1994 by el-Omar et al.32 In this Polish study, el-Omar et al investigated 110 patients with IBD and 100 age- and sex-matched controls. H. pylori was diagnosed by the presence of IgG serologic antibodies. Prior to the study the authors studied serum samples from patients from their hospital with known H. pylori infection diagnosed by UBT and histology. By performing IgG antibody titers in these patients, they were able to show an IgG titer of 15 U/mL or above had a sensitivity and specificity of 96% and 84%, respectively, for H. pylori infection, thereby increasing the specificity of the serologic test. Therefore, they used this value as the cutoff for diagnosing H. pylori infection in the IBD and control groups. Overall, 22% of the IBD patients were positive for H. pylori, while 52% of the patients in the control group were H. pylori-positive. The authors, in a post-hoc analysis, did report a possible relationship between the lower prevalence of H. pylori infection in the IBD groups and current or previous use of sulfasalazine. This inverse relationship between previous or current sulfasalazine use and H. pylori infection was reported by 3 other included studies.33–35 One of these studies35 was a letter to the editor (one of 3 letters to the editor included in our analysis).36,37 In this study, Mantzaris et al reported H. pylori infection rates, based on histological analysis, of 30% in UC patients versus 53% in the control group (patients with irritable bowel syndrome). However, 7 of the included studies found no relationship between sulfasalazine use and incidence of H. pylori infection.36–42
Three of the included studies examined the pediatric population exclusively.43–45 Among these is the only study conducted in North America. Pascasio et al44 identified 56 cases of CD through retrospective analysis of 438 consecutive gastric biopsies with evidence of inflammation. In a secondary analysis, the authors examined each specimen for the presence of H. pylori and found that 32.1 % of IBD patients were H. pylori-positive, while 34.0% of the specimens with no evidence of IBD had evidence for H. pylori infection.
Five of the included studies commented on previous H. pylori treatment.39,42,46–48 Of these, 4 studies excluded any patients who had been previously tested or treated for H. pylori.42,46–48 Parlak et al37 excluded any patients who had ever received proton-pump inhibitors or antibiotics; therefore, one can assume these patients had never received treatment for H. pylori. Feeney et al, in an attempt to assess different childhood risk factors for the development of IBD, included patients who had previously been treated for H. pylori. However, in a subgroup analysis, they could not account for the difference in H. pylori infection rates between the 2 groups based on previous H. pylori treatment. Two studies excluded any patients who had ever received certain antibiotics such as flagyl, ciprofloxacin, or clarithromycin, yet they did not specify previous treatment for H. pylori.36,38 Oliveira et al49 and Sladek et al45 excluded patients who received any antibiotics 3 months prior to H. pylori testing, yet the authors did not comment on antimicrobial exposure prior to this. Meining et al50 excluded patients taking proton-pump inhibitors, antibiotics, or bismuth at the time of diagnosis, yet no mention of previous use was made.
Meta-Analysis of RR
Overall, a total of 27.1% of IBD patients had evidence of H. pylori infection, while 40.9% of patients in the control group were found to have H. pylori infection. The RR of H. pylori in IBD patients compared to controls was 0.64 (95% CI: 0.54–0.75) (Fig. 3). Subgroup analyses revealed a trend toward a greater effect for CD (RR: 0.60, 95% CI: 0.49–0.72) when compared to UC (RR: 0.75, 95% CI: 0.62–0.90). There was significant heterogeneity in the included studies (I2 = 75.8%). Furthermore, analysis of the funnel for publication bias suggested a possible bias against small studies demonstrating high RR (Fig. 4).
Figure 3. Forest plot of rate of H. pylori infection in patients with IBD versus controls.

Figure 4. Funnel plot analysis.

We conducted multiple subgroup analyses in an attempt to explain the observed heterogeneity. We divided the data based on the method of H. pylori diagnosis (serology versus UBT, FAT, RUT, or culture), method of IBD diagnosis (clinical versus pathological), study location (Eastern versus Western hemisphere), and study population age (pediatrics versus adult). None of these subgroup analyses were able to account for the observed heterogeneity. Subsequently, we separated the dataset into CD and UC and reperformed each of the aforementioned subgroup analyses. This analysis revealed a statistically significant reduction in the RR of H. pylori infection in CD patients diagnosed with H. pylori by nonserologic methods (RR: 0.71, 95% CI: 0.58–0.87; I2: 54%)
Discussion
Our systematic review of the literature has identified numerous studies examining the relationship between H. pylori infection and IBD, the majority of which find a lower rate of H. pylori infection in IBD patients as compared to controls. Thirteen of the 23 studies found a statistically significant RR less than 1 for H. pylori infection in IBD patients versus controls, while none of the included studies found a statistically significant RR greater than 1. Our meta-analysis suggests a potential protective benefit of H. pylori infection against the development of IBD; however, significant heterogeneity and the possibility of publication bias limit our certainty in this association.
Mechanistic support for the association between the possible protective benefit of H. pylori infection against IBD is emerging. Rad et al20 demonstrated that H. pylori-infected individuals expressed higher levels of Foxp3, a T-cell regulatory (Treg) marker, and that the depletion of Tregs resulted in a higher degree of gastric inflammation and reduced bacterial colonization. Furthermore, the importance of Tregs in the pathogenesis of IBD can be illustrated by the development of spontaneous colitis in mice deficient of IL-10, a key regulatory cytokine for Treg function.54 It has also been shown that adoptive transfer of Tregs inhibits the development of experimental colitis in several models,55,56 suggesting that Tregs play an integral role in preventing the development of colitis.57 Further work attempting to define the possible role of Tregs on colitis is needed.
The data on the incidence of H. pylori infection and IBD found in the literature has several limitations. Most of the studies did not comment on the participants' previous history of treatment for H. pylori infection. It is therefore possible that study participants had been treated for H. pylori prior to entering the study, thereby producing a falsely low H. pylori infection rate. Additionally, our analysis included studies that used IgG serological antibodies as the diagnostic method for H. pylori. Given the high sensitivity and lower specificity of serologic testing, our results may include false-positives. Furthermore, many of the studies did not clearly identify the criteria for establishing an IBD diagnosis. Many studies referred to chart review and characteristic clinical and radiological findings associated with IBD as the standard for inclusion, yet few commented on personal review of the endoscopic findings or histology. Last, as with any study examining the association between 2 entities, causality cannot be inferred from the results.
Ideally, future studies should address these limitations. An ideal study examining the relationship between H. pylori infection and IBD would be conducted at the time of IBD diagnosis. After confirming the diagnosis of IBD through review of the endoscopic and histological findings, diagnostic testing for H. pylori with UBT, RUT, or histology would be initiated. In patients found to be H. pylori-positive, the presence or absence of cagA+ would be investigated, as the possible protective benefits of H. pylori against other autoimmune diseases come from cagA+ strains.15,58,59 The mechanism for the inverse association between cagA+ strains of H. pylori and the lower incidence of autoimmune disease has yet to be defined. Chen and Blaser59 suggest the intense host responses to specifically cagA+ H. pylori strains may further alter TH1- and TH2-type immune responses with subsequent induction of immunoregulatory lymphocytes. Controls who are age- and sex-matched to the IBD group would be selected from the same area as the IBD group and tested for H. pylori by the same method. In both groups, a thorough history examining previous H. pylori treatment would be obtained.
In summary, our review suggests a possible protective benefit of H. pylori against the development of IBD. However, significant variation among the studies and the possibility of publication bias limit the certainty of this association. Therefore, further clinical studies investigating the effect of H. pylori eradication on the development of IBD are warranted. Because environmental hygiene and intestinal microbiota may be strong confounders, further mechanistic studies in H. pylori mouse models are also necessary to further define the mechanism of this negative association. If it is found that H. pylori does indeed protect against IBD, this will have profound effects not only on the way we approach H. pylori testing and treatment, but also the way we approach the treatment of IBD.
References
- 1.Loftus EV. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Thia KT, Loftus EV, Sandborn WJ, et al. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167–3182. doi: 10.1111/j.1572-0241.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 3.Sood A, Midha V. Epidemiology of inflammatory bowel disease in Asia. Indian J Gastroenterol. 2007;26:285–289. [PubMed] [Google Scholar]
- 4.Morita N, Toki S, Hirohashi T, et al. Incidence and prevalence of inflammatory bowel disease in Japan: nationwide epidemiological survey during the year 1991. J Gastroenterol. 1995;30:1–4. [PubMed] [Google Scholar]
- 5.Yang SK, Hong WS, Min YI, et al. Incidence and prevalence of ulcerative colitis in the Songpa-Kangdong District, Seoul, Korea, 1986-1997. J Gastroenterol Hepatol. 2000;15:1037–1042. doi: 10.1046/j.1440-1746.2000.02252.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee YM, Fock KM, See SJ, et al. Racial differences in the prevalence of ulcerative colitis and Crohn's disease in Singapore. J Gastroenterol Hepatol. 2000;15:622–625. doi: 10.1046/j.1440-1746.2000.02212.x. [DOI] [PubMed] [Google Scholar]
- 7.Sood A, Midha V, Sood, et al. Incidence and prevalence of ulcerative colitis in Punjab, North India. Gut. 2003;52:1587–1590. doi: 10.1136/gut.52.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloomfield SF, Stanwell-Smith R, Crevel RW, et al. Too clean, or not too clean: the hygiene hypothesis and home hygiene. Clin Exp Allergy. 2006;36:402–425. doi: 10.1111/j.1365-2222.2006.02463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koloski NA, Bret L, Radford-Smith G. Hygiene hypothesis in inflammatory bowel disease: a critical review of the literature. World J Gastroenterol. 2008;14:165–173. doi: 10.3748/wjg.14.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linz B, Balloux F, Moodley Y, et al. An African origin for the intimate association between human and Helicobacter pylori. Nature. 2007;445:915–918. doi: 10.1038/nature05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falush D, Wirth T, Linz B, et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299:1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 12.Malaty HM, El Kasabany A, Graham DY, et al. Age at acquisition of Helicobacter pylori infection: a follow-up study from infancy to childhood. Lancet. 2002;359:931–935. doi: 10.1016/S0140-6736(02)08025-X. [DOI] [PubMed] [Google Scholar]
- 13.Kuipers EJ, Pena AS, van Kamp G, et al. Seroconversion for Helicobacter pylori. Lancet. 1993;42:328–331. doi: 10.1016/0140-6736(93)91473-y. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198:553–560. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reibman J, Marmor M, Filner J, et al. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS One. 2008;2:e4060. doi: 10.1371/journal.pone.0004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Amsterdam K, van Vliet AH, Kusters JG. Of microbe and man: determinants of Helicobacter pylori-related diseases. FEMS Microbiol Rev. 2006;30:131–156. doi: 10.1111/j.1574-6976.2005.00006.x. [DOI] [PubMed] [Google Scholar]
- 17.Kao JY, Rathinavelu S, Eaton KA. Helicobacter pylori-secreted factors inhibit dendritic cell IL-12 secretion: a mechanism of ineffective host defense. Am J Physiol Gastrointest Liver Physiol. 2006;291:G73–81. doi: 10.1152/ajpgi.00139.2005. [DOI] [PubMed] [Google Scholar]
- 18.Paziak-Domanska B, Chmiela M, Jarosinska A. Potential role of CagA in the inhibition of T cell reactivity in Helicobacter pylori infections. Cell Immunol. 2000;202:136–139. doi: 10.1006/cimm.2000.1654. [DOI] [PubMed] [Google Scholar]
- 19.Gerbert B, Fischer W, Haas R. The Helicobacter pylori vacuolating cytotoxin: from cellular vacuolation to immunosuppressive activites. Rev Physiol Biochem Pharmacol. 2004;152:205–202. doi: 10.1007/s10254-004-0027-3. [DOI] [PubMed] [Google Scholar]
- 20.Rad R, Brenner L, Bauer S, et al. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology. 2006;131:525–537. doi: 10.1053/j.gastro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Lundgren A, Suri-Payer E, Enarsson K, et al. Helicobacter pylori-specific CD4+ CD25+ high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect Immun. 2003;1:1755–1762. doi: 10.1128/IAI.71.4.1755-1762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atherton JC, Blaser MJ. Harrison's Principles of Internal Medicine. 16th. New York: McGraw-Hill; 2005. Helicobacter pylori infections; p. 886. [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumura M, Matsui T, Hatakeyama S, et al. Prevalence of Helicobacter pylori infection and correlation between severity of upper gastrointestinal lesions and H. pylori infection in Japanese patients with Crohns disease. J Gastroenterol. 2001;36:740–747. doi: 10.1007/s005350170015. [DOI] [PubMed] [Google Scholar]
- 26.Sharif F, McDermott M, Dillon M, et al. Focally enhanced gastritis in children with Crohn's disease and ulcerative colitis. Am J Gastroenterol. 2002;97:1415–1420. doi: 10.1111/j.1572-0241.2002.05785.x. [DOI] [PubMed] [Google Scholar]
- 27.Triantafillidis JK, Tzourmakliotis D, Peros G, et al. Serum gastrin levels in patients with inflammatory bowel disease. Hepatogastroenterology. 2003;50(suppl 2):cccxv–cccxvii. [PubMed] [Google Scholar]
- 28.Koloski NA, Bret L, Radford-Smith G. Hygiene hypothesis in inflammatory bowel disease: a critical review. World J Gastroenterol. 2008;14:165–173. doi: 10.3748/wjg.14.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrara M, Coppola L, Coppola A, et al. Iron deficiency in childhood and adolescence: a retrospective review. Hematology. 2006;11:183–186. doi: 10.1080/10245330600775105. [DOI] [PubMed] [Google Scholar]
- 30.Halme L, Rautelin H, Leidenius M, et al. Inverse correlation between Helicobacter pylori infection and inflammatory bowel disease. J Clin Pathol. 1996;49:65–67. doi: 10.1136/jcp.49.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagtmans MJ, Witte AMC, Taylor DR, et al. Low seroprevalence of Helicobacter pylori antibodies in historical sera of patients with Crohn's disease. Scand J Gastroenterol. 1997;32:712–718. doi: 10.3109/00365529708996523. [DOI] [PubMed] [Google Scholar]
- 32.el-Omar E, Penman I, Cruikshank G, et al. Low prevalence of Helicobacter pylori in inflammatory bowel disease: association with sulfasalazine. Gut. 1994;35:1385–1388. doi: 10.1136/gut.35.10.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parente F, Molteni P, Bollani S, et al. Prevalence of Helicobacter pylori infection and related upper gastrointestinal lesions in patients with inflammatory bowel disease. A cross-sectional study with matching. Scand J Gastroenterol. 1997;32:1140–1146. doi: 10.3109/00365529709002994. [DOI] [PubMed] [Google Scholar]
- 34.Pearce CB, Duncan HD, Timmis L, et al. Assessment of the prevalence of infection with Helicobacter pylori in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000;12:439–443. doi: 10.1097/00042737-200012040-00012. [DOI] [PubMed] [Google Scholar]
- 35.Mantzaris GJ, Archavlis E, Zografos C, et al. Low prevalence of Helicobacter pylori in inflammatory bowel disease: association with sulfasalazine. Am J Gastroenterol. 1995;90:1900. [PubMed] [Google Scholar]
- 36.Triantafillidis JK, Gikas A, Apostolidiss N, et al. The low prevalence of Helicobacter infection in patients with inflammatory bowel disease could be attributed to previous antibiotic treatment. Am J Gastroenterol. 2003;98:1213–1214. doi: 10.1111/j.1572-0241.2003.07434.x. [DOI] [PubMed] [Google Scholar]
- 37.Parlak E, Ulker A, Disibeyaz S, et al. There is no significant increase in the incidence of Helicobacter pylori infection in patients with inflammatory bowel disease in Turkey. J Clin Gastroenterol. 2001;33:87–88. doi: 10.1097/00004836-200107000-00025. [DOI] [PubMed] [Google Scholar]
- 38.Guslandi M, Fanti L, Testoni PA. Helicobacter pylori seroprevalence in Crohn's disease: lack of influence by pharmacological treatment. Hepatogastroenterology. 2002;49:1296–1297. [PubMed] [Google Scholar]
- 39.Feeney MA, Murphy F, Clegg AJ, et al. A case-control study of childhood environmental risk factors for the development of inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000;14:529–534. doi: 10.1097/00042737-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Vare PO, Heikius B, Silvennoinen R, et al. Seroprevalence of Helicobacter pylori infection in inflammatory bowel disease: is Helicobacter pylori infection a protective factor. Scand J Gastroenterol. 2001;36:1295–1300. doi: 10.1080/003655201317097155. [DOI] [PubMed] [Google Scholar]
- 41.Oberhuber G, Puspok A, Oesterreicher C, et al. Focally enhanced gastritis: a frequent type of gastritis in patients with Crohn's disease. Gastroenterology. 1997;112:698–706. doi: 10.1053/gast.1997.v112.pm9041230. [DOI] [PubMed] [Google Scholar]
- 42.D'Inca R, Sturniolo G, Cassaro M, et al. Prevalence of upper gastrointestinal lesions and Helicobacter pylori infection in Crohn's disease. Dig Dis Sci. 1998;43:988–992. doi: 10.1023/a:1018870415898. [DOI] [PubMed] [Google Scholar]
- 43.Corrado G, Luzzi I, Lucarelli S, et al. Positive association between Helicobacter pylori infection and food allergy in children. Scand J Gastroenterol. 1998;33:1135–1139. doi: 10.1080/00365529850172467. [DOI] [PubMed] [Google Scholar]
- 44.Pascasio JM, Hammond S, Quaknan SJ. Recognition of Crohn disease on incidental gastric biopsy in childhood. Pediatr Dev Pathol. 2003;6:209–214. doi: 10.1007/s10024-002-0601-0. [DOI] [PubMed] [Google Scholar]
- 45.Sladek M, Jedynak-Wasowicz U, Wedrychowicz A, et al. The low prevalence of Helicobacter pylori gastritis in newly diagnosed inflammatory bowel disease children and adolescent. Przegl Lek. 2007;64(suppl 3):65–67. [PubMed] [Google Scholar]
- 46.Parente F, Cucino C, Bollani S, et al. Focal gastric inflammatory infiltrates in inflammatory bowel diseases: prevalence, immunohistochemical characteristics, and diagnostic role. Am J Gastroenterol. 2000;95:705–711. doi: 10.1111/j.1572-0241.2000.01851.x. [DOI] [PubMed] [Google Scholar]
- 47.Piodi LP, Bardella M, Rocchia C, et al. Possible protective effect of 5-aminosalicylic acid on Helicobacter pylori infection in patients with inflammatory bowel disease. J Clin Gastroenterol. 2003;36:22–25. doi: 10.1097/00004836-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Pronai L, Schandl L, Orosz Z, et al. Lower prevalence of Helicobacter pylori infection in patients with inflammatory bowel disease but not with chronic obstructive pulmonary disease—antibiotic use in the history does not play a significant role. Helicobacter. 2004;9:278–283. doi: 10.1111/j.1083-4389.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- 49.Oliveira AG, Rocha GA, Rocha AMC, et al. Isolation of Helicobacter pylori from the intestinal mucosa of patients with Crohn's disease. Helicobacter. 2006;11:2–9. doi: 10.1111/j.0083-8703.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 50.Meining A, Bayerdorffer E, Bastlein E, et al. Focal inflammatory infiltrations in gastric biopsy specimens are suggestive of Crohn's disease. Scand J Gastroenterol. 1997;32:813–818. doi: 10.3109/00365529708996539. [DOI] [PubMed] [Google Scholar]
- 51.Furusu H, Murase K, Nishida Y, et al. Accumulation of mast cells and macrophages in focal active gastritis of patients with Crohn's disease. Hepatogastroenterology. 2002;49:639–643. [PubMed] [Google Scholar]
- 52.Duggan AE, Usmani I, Neal KR, et al. Appendicectomy, childhood hygiene, Helicobacter pylori status, and risk of inflammatory bowel disease: a case control study. Gut. 1998;43:494–498. doi: 10.1136/gut.43.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliveira AG, Sanna MGP, Rocha GA, et al. Helicobacter species in the intestinal mucosa of patients with ulcerative colitis. J Clin Microbiol. 2004;41:384–386. doi: 10.1128/JCM.42.1.384-386.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leach MW, Davidson NJ, Fort MM, et al. The role of IL-10 in inflammatory bowel disease: “of mice and men”. Toxicol Pathol. 1999;27:123–133. doi: 10.1177/019262339902700124. [DOI] [PubMed] [Google Scholar]
- 55.De Winter H, Cheroutre H, Kronenberg M. Mucosal immunity and inflammation. II. The yin and yang of T cells in intestinal inflammation: pathogenic and protective roles in a mouse colitis model. Am J Physiol. 1999;276:G1317–1321. doi: 10.1152/ajpgi.1999.276.6.G1317. [DOI] [PubMed] [Google Scholar]
- 56.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 57.Gad M. Regulatory T cells in experimental colitis. Curr Top Microbiol Immunol. 2005;293:179–208. doi: 10.1007/3-540-27702-1_9. [DOI] [PubMed] [Google Scholar]
- 58.Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136:1863–1873. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821–827. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]


