Abstract
Psychological stress contributes to the development of hypertension in humans. The ovarian hormone, estrogen, has been shown to prevent stress-induced pressor responses in females by unknown mechanisms. Here we showed that the anti-hypertensive effects of estrogen during stress were blunted in female mice lacking estrogen receptor-α (ERα) in the brain medial amygdala (MeA). Deletion of ERα in MeA neurons also resulted in increased excitability of these neurons, associated with elevated ionotropic glutamate receptor expression. We further demonstrated that selective activation of MeA neurons mimicked effects of stress to increase blood pressure in mice. Together, our results support a model where estrogen acts upon ERα expressed by MeA neurons to prevent stress-induced activation of these neurons, and therefore prevents pressor responses to stress.
Keywords: estrogen, receptor, blood pressure, stress, amygdala
Introduction
The prevalence of hypertension has continued to rise for the last 20 years. In the United States only 1/3 of patients with hypertension have their blood pressure (BP) controlled. Interventions that treat and prevent hypertension are urgently needed1. The etiology of essential hypertension remains poorly understood. Genetic and behavioral factors do not fully explain the development of hypertension. Increasing evidence suggests that psychological stress leads to the development of hypertension in humans2, 3. For example, acute stress exposure triggers rapid increases in BP and heart rate (HR)4, 5. Chronic stress and particularly the non-adaptive response to stress are associated with sustained elevation of BP6. Dysfunctions of central nervous system (CNS) and neuroendocrine system are thought to contribute to the pathophysiological process of stress-induced hypertension4, but the critical CNS networks and the neuro-hormonal systems that regulate BP during psychological stress remain to be unraveled.
The ovarian hormone, estrogen, is long believed to prevent development of hypertension. Before menopause, BP has been typically lower in women than in age-matched men; after menopause, however, the incidence of hypertension in women increases dramatically7. In particular, psychological stress causes greater pressor responses in post-menopausal women than in pre-menopausal women, and this effect is blunted by 17β-estradiol replacement8. Multiple clinical trials have reported anti-hypertensive benefits of estrogen replacement in post-menopausal women9–12. Unfortunately, higher risk of thromboembolism was also reported in post-menopausal women receiving estrogen replacement13, likely due to the pro-coagulation effects of estrogen14. Better understanding specific mechanisms for the anti-hypertensive effects of 17β-estradiol may facilitate developing new estrogen replacement therapies that only produce cardiovascular benefits with no or fewer side effects.
The amygdala, a neural complex near the temporal pole of the mammalian cerebral hemisphere, plays important roles in mediating the emotional and hormonal responses to stress15. Recent evidence indicates that the amygdala is involved in development of stress-induced pressor responses in humans16. In particular, neural activities in the medial amygdala (MeA), one subdivision of the amygdala, are positively correlated with stress-induced pressor responses in mice17, 18. Thus, increased MeA neural activities may mediate stress-induced pressor responses.
MeA neurons express abundant estrogen receptor-α (ERα), one of estrogen receptors19. Here we tested a hypothesis that ERα expressed by MeA neurons is a key site where 17β-estradiol acts to prevent stress-induced pressor effects. To this end, we generated two mouse models with ERα deleted in the MeA. Using these tools, we examined the physiological functions of MeA ERα on stress-induced pressor responses. We also explored mechanisms by which ERα signals may regulate firing activities of MeA neurons. Finally, we used the Designer Receptors Exclusively Activated by Designer Drugs (DREADD) approach20 to examine effects of MeA neural activity on BP balance in mice.
Methods
Mice
All mouse strains were backcrossed onto C57BL/6 background for more than 12 generations. SIM1-Cre transgene21 was bred onto Esr1f/f mice22 to generate Esr1f/f/SIM1-Cre and Esr1f/f littermates, which were referred as SIM1-ERα-KO and control mice, respectively. These mice were used for BP studies, qPCR and Western Blotting, as described below. In parallel, Esr1f/+/SIM1-Cre mice were crossed to Esr1f/+/rosa26:GFP mice to generate Esr1f/f/SIM1-Cre/rosa26GFP (SIM1-ERα-KO) mice and Esr1+/+/SIM1-Cre/rosa26GFP (control) mice. These mice were used for histology validation. In addition, the rosa26:tdTOMATO allele was crossed onto SIM1-Cre transgenic mice (control) or Esr1f/f/SIM1-Cre mice (SIM1-ERα-KO), which were used for electrophysiological recordings. In addition, Esr1f/+ mice were crossed to Esr1f/+ to generate Esr1f/f mice, which received AAV-Cre-GFP/AAV-GFP stereotaxic injections into the MeA. SIM-Cre transgenic mice were crossed to C57BL/6 mice to produce SIM-Cre transgenic mice, which were used for DREADD study.
All mice were weaned on chow diet (6.5% fat, #2920, Harlan) at 3–4 weeks of age and were grouped housed (2–5 mice per cage). For mice used in BP recording, they were singly housed after surgeries; other mice (e.g. for electrophysiological studies) were grouped housed till study. All mice were housed in a 12-h light, 12-h dark cycle. Care of all animals and procedures were conformed to the Guide for Care and Use of Laboratory Animals of the US National Institutes of Health and approved by the Animal Subjects Committee of Baylor College of Medicine.
Co-localization of SIM1 and ERα
Female Esr1f/f/SIM1-Cre/rosa26:GFP and SIM1-Cre/rosa26:GFP mice were perfused with 10% formalin. To avoid influence of estrous cycles on expression pattern, female mice were all perfused at diestrus. Briefly, mice for this study were subjected to daily vaginal smear followed by cytology evaluation. Exclusive leukocytes in the vaginal smear samples were used as a determinant for the stage of diestrus. Brain sections were cut at 25 μm (1:5 series) and the sections were incubated in the primary rabbit anti-ERα antibody (1:10000; #C1355, Millipore, Billerica, MA) overnight, followed by donkey anti-rabbit AlexaFluor594 (1:500; #A21207, Invitrogen, Grand Island, NY) for 1.5 hr. Then, the sections were incubated in primary chicken anti-GFP antibody (1:5000; #GFP-1020, Aves Labs, Inc., Tigard, OR) overnight, followed by the goat anti-chicken AlexaFluor 488 (1:250; #A11039, Invitrogen, Grand Island, NY) for 1.5 hr. The slides were cover-slipped and images were analyzed using the Leica 5500 fluorescent microscope.
Effects of subcutaneous 17β-estradiol on stress-induced pressor responses
In order to examine the effects of estrogen, 12-week old female control and SIM1-ERα-KO mice were anesthetized with inhaled isoflurane. As previously described23, 24, bilateral ovariectomy was performed, followed by subcutaneous implantations of pellets containing 17β-estradiol (0.5 μg/day lasting for 30 days, OVX+E) or empty pellets (OVX+V). These pellets were purchased from Innovative Research of America. Under the same anesthesia, the left carotid artery was isolated, and the catheter of the telemetry transmitter (TA11PA-C10, Data Sciences International, St. Paul, MN) was inserted into the vessel. The body of the transmitter was slipped in a subcutaneous pocket along the right flank. The mice were allowed to recover for 7 days and then followed by recording for BP and HR using DSI Physio Tel Receivers (Data Sciences International, St. Paul, MN). On the recording day, BP and HR were be recorded continuously for 2 phases: the acclimation phase (8am–11am) and the restraint phase (11am–12am). Twenty min prior to the restraint phase (10:40am), the experimenter entered the room and turned on the telemetry probe with a magnetic switch. At 11am, the experimenter re-entered the room to restrain mice with a mouse plastic restraining cone which immobilized the mice. Importantly, mice were maintained at the normal position and were able to breathe normally; there were no pain or discomfort except that mice could not move. BP and HR data during the last 20 min during the acclimation phase were used as the baseline.
Because the stress-induced pressor responses may be influenced by different baseline BP/HR levels, we first performed pilot studies in a small cohort of wild type OVX+V and OVX+E mice to compare their 24-hour baseline MAP and HR. We found that OVX+E mice showed significantly reduced MAP compared to OVX+V mice during the entire dark cycle and the early phase of light cycle; however, during 10am to 2pm, there was not significant difference in MAP between the two groups (Figure S1A). On the other hand, HR was not significantly different between OVX+V and OVX+E mice throughout the entire 24-hour period (Figure S1B). Based on these observations, we chose to perform the restraint stress during 11am to 12pm, as described above.
Note that hypertension is often associated with obesity25. We chose to perform BP/HR recording 7 days after OVX+V/E treatment, because body weight is comparable within at this time point23. At the end of recordings, mice were sacrificed. Fat pads were weighed to ensure comparable adiposity between the groups (data not shown). Uteruses were weighted to confirm successful 17β-estradiol depletion in OVX+V mice and sufficient 17β-estradiol supplement in OVX+E mice.
Deletion of ERα in the MeA
Female Esr1f/f mice were anesthetized by isoflurane and received bilateral stereotaxic injections of AAV-Cre-GFP (AAV9.CMV.HI.GFP-Cre.WPRE.SV40, Penn Vector Core at University of Pennsylvania, Philadelphia, PA) into the MeA (200 nl/side), and these mice were referred as MeA-ERα-KO. Esr1f/f mice that received AAV-GFP in the MeA were used as controls. The coordinates for the MeA were 1.7 mm posterior and 2.5 mm lateral to the Bregma, and 5 mm ventral to the dura. After the recovery from the stereotaxic surgeries (7 days), these mice received the OVX+E treatment and implantation of the telemetry transmitters, followed by recordings of BP and HR under the baseline condition and the restrained condition as described above.
At the end of experiment, all mice were perfused with 10% Formalin. Brain sections were collected, and expression of GFP was checked in the MeA. Only those with expression of GFP exclusively in both MeA sides were included in data analyses. To further validate the deletion of ERα, some brain sections with accurate GFP expression were also subjected to ERα immunofluorescent staining, as described above.
DREADD activation of MeA SIM1 neurons
Female SIM1-Cre mice (at 14–16 weeks of age) were anesthetized by isoflurane and received bilateral stereotaxic injections of AAV-hM3Dq-mCherry (AAV-hSyn-DIO-hM3D(Gq)-mCherry in AAV2, UNC Vector Core, Chapel Hill, NC) into the MeA (200 nl/side), followed by a second surgery to receive the implantation of the telemetry transmitters as described above. After recovery, BP and HR were recorded continuously during 9am–11am, with an i.p. injection of saline or CNO (3 mg/kg) at 10am. Both saline and CNO were tested in the same mice at two different trials (at the same time of the day) with a one-week interval; the order of the two injections was randomized to avoid any “sequence” effect.
At the end of experiment, all mice were perfused with 10% Formalin. Brain sections were collected and subjected to mCherry immunohistochemistry, as described before26. Briefly, brain sections were first incubated in 0.3% H2O2 in PBS for 30 minutes to abolish endogenous peroxidase activity. After several washes, sections were incubated overnight at room temperature with the primary rabbit Living Colors® DsRed polyclonal antibody (1:1000, Clontech Laboratories, Inc., Mountain View, CA). After several washes, sections were incubated with the biotinylated secondary antibody (1:1000, anti-rabbit, Jackson Immuno Research, West Grove, PA) for 1 hour. Sections were then washed and visualized by incubation with the VECTASTAIN® ABC kit (Vector Laboratories, Burlingame, CA) according to the manufacture’s instruction. Sections were washed and treated with diaminobenzidine (Sigma, St. Louis, MO) for 5 minutes, followed by dehydration in a graded ethanol series from 50% to 100% and a final wash in xylene. The slides were cover-slipped and images were analyzed using the Leica 5500 microscope under the brightfield. Only those with mCherry immunoreactivity selectively in both MeA sides were included in data analyses.
To prove the concept that CNO does activate hM3Dq-mCherry-expressing MeA SIM1 neurons, some SIM1-Cre mice were used for electrophysiological recordings after the stereotaxic AAV-hM3Dq-mCherry injections, as described below.
Electrophysiology
Whole-cell patch clamp recordings were performed on TOMATO-labeled SIM1 neurons in the acute MeA slices from SIM1-Cre/rosa26:tdTOMATO mice and Esr1f/f/SIM1-Cre/rosa26:tdTOMATO (SIM1-ERα-KO) mice. As we described before26, 27, six to twelve week old female mice were deeply anesthetized with isoflurane and transcardially perfused with a modified ice-cold sucrose-based cutting solution (adjusted to pH 7.3) containing (in mM) 10 NaCl, 25 NaHCO3, 195 Sucrose, 5 Glucose, 2.5 KCl, 1.25 NaH2PO4, 2 Na pyruvate, 0.5 CaCl2, 7 MgCl2 bubbled continuously with 95% O2 and 5% CO2. The mice were then decapitated, and the entire brain was removed and immediately submerged in ice-cold sucrose-based cutting solution. Coronal sections containing the MeA (250 μm) were cut with a Microm HM 650V vibratome (Thermo Scientific). The slices were recovered for 1 hour at 34 °C in artificial cerebrospinal fluid (aCSF, adjusted to pH7.3) containing (in mM) 126 NaCl, 2.5 KCl, 2.4 CaCl2, 1.2 NaH2PO4, 1.2 MgCl2, 11.1 glucose, and 21.4 NaHCO3)28 saturated with 95% O2 and 5% CO2 before recording.
Slices were transferred to the recording chamber and allowed to equilibrate for at least 10 min before recording. The slices were perfused at 34 °C in oxygenated aCSF at a flow rate of 1.8–2 ml/min. tdTOMATO-positive SIM1 neurons in MeA were visualized using epifluorescence and IR-DIC imaging on an upright microscope (Eclipse FN-1, Nikon) equipped with a moveable stage (MP-285, Sutter Instrument). Patch pipettes with resistances of 3–5 MΩ were filled with intracellular solution (adjusted to pH 7.3) containing (in mM) 128 K gluconate, 10 KCl, 10 HEPES, 0.1 EGTA, 2 MgCl2, 0.05 Na-GTP, 3 Mg-ATP and 0.1 lucifer yellow dye. Recordings were made using a MultiClamp 700B amplifier (Axon Instrument), sampled using Digidata 1440A and analyzed offline with pClamp 10.3 software (Axon Instrument). Series resistance was monitored during the recording, and the values were generally < 10 MΩ and were not compensated. The liquid junction potential (LJP) was +12.5 mV, and was corrected after the experiment. Data would be excluded if the series resistance increased more than 20% during the experiment or without overshoot for action potential.
Neural firing frequency and resting membrane potential (RM) were measured under the current clamp mode. For the miniature excitatory postsynaptic current (mEPSC) recordings, the internal recording solution contained (in mM): CsCH3SO3 125; CsCl 10; NaCl 5; MgCl2 2; EGTA 1; HEPES 10; (Mg)ATP 5; (Na)GTP 0.3 (pH 7.3 with NaOH). mEPSC was measured in the voltage clamp mode with a holding potential of −60 mV in the presence of 1 μM tetrodotoxin (TTX)29 and 50 μM bicuculline30. For the miniature inhibitory postsynaptic current (mIPSC) recordings, patch electrodes were filled with a recording solution that contained (in mM): 153.3 CsCl, 1.0 MgCl2, 5.0 EGTA, and 10.0 HEPES, pH of 7.20 with CsOH31. CsCl was included to block potassium currents. Mg-ATP (3mM) was added to the intracellular solution before recording. Glutamate receptor-mediated synaptic currents were blocked by 30 μM D-AP-5 and 30 μM CNQX in the external solution, along with 1 μM tetrodotoxin in the external solution blocking action potentials. Neurons were voltage-clamped at −70 mV during the recording. Slices were fixed with 4% formalin in PBS at 4°C overnight and then subjected to post hoc identification of the anatomical location of the recorded neurons within the MeA.
To validate that hM3Dq-mCherry-expressing MeA SIM1 neurons can be activated by CNO treatment, the MeA-containing brain slices were prepared from SIM1-Cre mice receiving stereotaxic AAV-hM3Dq-mCherry injections. Effects of CNO (10 μM, bath perfusion 6 min) on the firing frequency and resting membrane potential were recorded in mCherry-labelled neurons under the current clamp mode.
Real time RT-PCR
Female SIM1-ERα-KO and control littermates were sacrificed at diestrus, and the amygdala was quickly microdissected and stored at −80°C. Real time RT-PCR was performed as described previously32. Primer sequences were listed in Table S1.
Western Blot
Female SIM1-ERα-KO and control littermates were sacrificed at diestrus, and the amygdala was quickly microdissected. Subsequently, the fresh whole amygdala tissue was homogenized in cell lysis buffer that was supplemented with protease inhibitors and phosphatase inhibitors. Then cell lysates were combined with 1X Laemmli buffer, and boiled for 5 minutes. The precipitated proteins were separated by SDS-8% PAGE and the samples were transferred onto nitrocellulose membranes (BioRad Transfer equipment). Dry powder milk was dissolved into TBST (50 mM Tris-HCL, 150 mM NaCl [pH 7.5], 0.1% Tween 20) to have a final 3% concentration and added to the nitrocellulose membranes for 1 hour. The membranes were washed in TBST for 5 minutes three times. The nitrocellulose membranes were incubated with anti-GluN1 antibody (1:1,000; Cat#4204, Cell Signaling Technology) overnight, followed by incubation with the anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:10,000; Cat#7074, Cell Signaling Technology) for 1 hour at the room temperature. Similarly, as a loading control, the nitrocellulose membranes were incubated with mouse anti-beta actin (1:5,000; Cat#A3853, Sigma-Aldrich), followed by the anti-mouse horseradish peroxidase-conjugated secondary antibody (1:10,000; Cat#7076P2, Cell Signaling Technology). Western blots were visualized with Thermo Fisher Scientific SuperSignal West Pico Chemoluminescence substrate.
Serum corticosterone
To measure stressed levels of corticosterone, mice were restrained for 15 min, and blood was collected from the tail vein; for the measurements of basal corticosterone, mice were rapidly decapitated at 9:00 am and trunk blood was collected. Blood samples were processed to measure serum corticosterone using a corticosterone EIA kit (900-097, Assay Designs, Ann Arbor, MI).
Statistical analyses
The data are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism to evaluate normal distribution and variations within and among groups. Methods of statistical analyses were chosen based on the design of each experiment and are indicated in figure legends. P<0.05 was considered to be statistically significant.
Study approval
Care of all animals and procedures were conformed to the Guide for Care and Use of Laboratory Animals of the US National Institutes of Health and were approved by the Animal Subjects Committee of Baylor College of Medicine.
Results
Deletion of ERα from SIM1 neurons blunts estrogenic actions to prevent stress-induced pressor responses
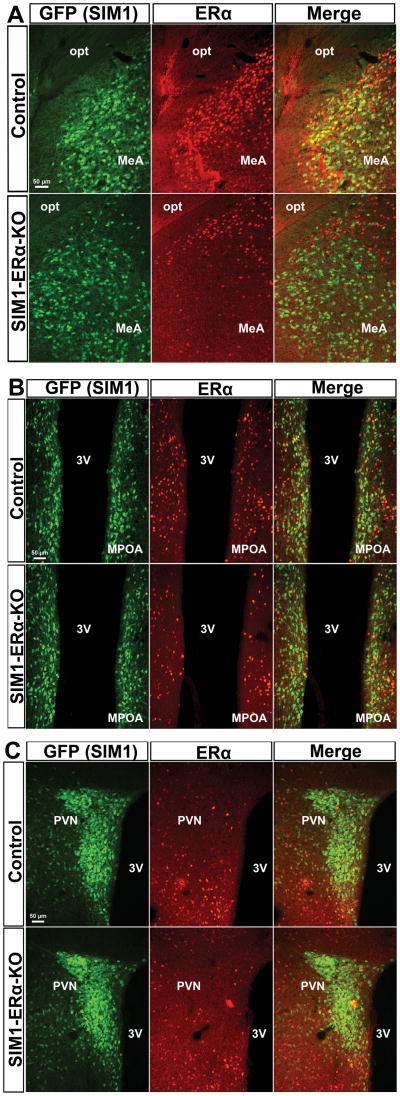
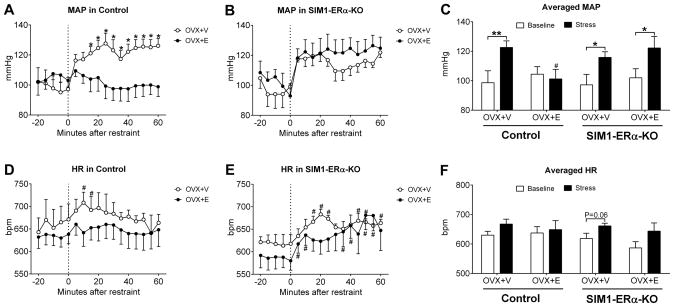
We have generated a SIM1-ERα-KO (Esr1f/f/SIM1-Cre) mouse line, in which ERα (encoded by the Esr1 gene) is selectively deleted in single-minded-1 (SIM1) neurons26. As we reported previously26, SIM1-ERα-KO mice have ERα deleted primarily in the MeA (Figure 1A), with modest deletion in the medial pre-optic area (MPOA, Figure 1B) and the paraventricular nucleus of the hypothalamus (PVN, Figure 1C). Here we used female SIM1-ERα-KO mice and their control (Esr1f/f) littermates to examine the role of ERα in SIM1 neurons in the regulation of BP during stress. To this end, we measured BP and HR in conscious control and SIM1-ERα-KO female mice that received either OVX+V or OVX+E treatment, at the unstressed condition (baseline) followed by a one-hour restraint that caused stress. We found that in OVX+V control mice, the restraint stress provoked strong and sustained increases in mean arterial pressure (MAP) compared to the baseline (Figure 2A and 2C). Importantly, these stress-induced pressor responses were significantly blunted in OVX+E control females (Figure 2A and 2C). These findings indicate that 17β-estradiol supplement in female mice prevents pressor responses induced by stress. We then tested the stress-induced pressor effects in female SIM1-ERα-KO mice. Interestingly, regardless whether SIM1-ERα-KO mice were treated with OVX+V or OVX+E, the restraint stress provoked robust increases in MAP, responses that were comparable to those seen in OVX+V control mice (Figure 2B and 2C). Notably, the levels of HR were significantly elevated by the restraint stress at multiple time points in OVX+V control mice, OVX+V or OVX+E SIM1-ERα-KO mice, but not in OVX+E control mice (Figure 2D and 2E); however, the averaged HR at the baseline and during the 60-min stress period were not significantly different presumably due to big variations among each mouse (Figure 2F). Changes in systolic arterial pressure (SAP) and in diastolic arterial pressure (DAP) were similar as MAP (Figure S2A–S2F). Terminal analyses revealed that OVX+V mice displayed expected uterine atrophy which confirmed successful 17β-estradiol depletion, while OVX+E mice showed heavier uterus than OVX+V mice, indicating sufficient 17β-estradiol supplement (Figure S2G). Together, these results indicate that ERα expressed by SIM1 neurons is required to mediate estrogenic actions to prevent stress-induced pressor responses in female mice.
Figure 1. Deletions of ERα in SIM1 neurons.
Dual immunofluorescence for GFP (green) and ERα (red) in the MeA (A), MPOA (B) and PVN (C) in control (SIM1-Cre/Rosa26-GFP, upper panel) and SIM1-ERα-KO (Esr1f/f/SIM1-Cre/Rosa26-GFP mice, lower panel) mice. 3V, third ventricle; MPOA, medial pre-optic area; opt, optic tract. Scale bars = 50 μm.
Figure 2. Deletion of ERα in SIM1 neurons blunted 17β-estradiol effects on MAP and HR.
(A–B) Temporal changes in mean arterial pressure in OVX+V or OVX+E-treated control (A) and SIM1-ERα-KO (B) mice at the baseline (−20 to 0 min) and the restrained condition (0 to 60 min). Data are presented as mean±SEM. N=6 to 9/group. *, P<0.05 between OVX+V and OVX+E in two-way ANOVA analysis followed by post hoc Sidak tests.
(C) Averaged mean arterial pressure at the baseline or at the restrained condition in OVX+V or OVX+E-treated control and SIM1-ERα-KO mice. Data are presented as mean±SEM. N=6 to 9/group. *, P<0.05 or **, P<0.01 between baseline and stress condition within the same mice; #, P<0.05 between OVX+V and OVX+E within the same genotype in two-way ANOVA analysis followed by post hoc Sidak tests.
(D–E) Temporal changes in heart rate in OVX+V or OVX+E-treated control (D) and SIM1-ERα-KO (E) mice at the baseline (−20 to 0 min) and the restrained condition (0 to 60 min). Data are presented as mean±SEM. N=6 to 9/group. #, P<0.05 vs. the baseline within the same group of mice in t-tests.
(F) Averaged heart rate at the baseline or at the restrained condition in OVX+V or OVX+E-treated control and SIM1-ERα-KO mice. Data are presented as mean±SEM. N=6 to 9/group.
Deletion of ERα selectively in the MeA blunts estrogenic actions to prevent stress-induced pressor responses
It is worth noting that in SIM1-ERα-KO mice, ERα was deleted primarily in the MeA, with modest ERα deletions in the MPOA and the PVN. Therefore, BP phenotypes observed in SIM1-ERα-KO mice may result not only from loss of ERα in the MeA, but also to some degree from loss of ERα in the MPOA/PVN. To further confirm the physiological functions of ERα in MeA neurons, we used the stereotaxic AAV-Cre-GFP injections to the MeA (bilaterally) of female Esr1f/f mice to selectively delete ERα in the MeA during the adulthood (MeA-ERα-KO mice); female Esr1f/f mice receiving AAV-GFP stereotaxic injections into the MeA were used as controls (see Figure 3A for validation). Both MeA-ERα-KO and control mice were treated with OVX+E, and we assessed stress-induced pressor responses in these mice. In OVX+E control mice, the restraint stress failed to induce elevations in MAP (Figure 3B and 3C). On the other hand, in MeA-ERα-KO mice treated with OVX+E, the restraint stress evoked strong and sustained pressor responses (Figure 3B and 3C). Notably, the restraint stress did not alter HR of OVX+E control females, whereas in OVX+E MeA-ERα-KO mice, HR was significantly increased (Figure 3D and 3E). Changes in SAP and DAP were similar as MAP (Figure S3). Thus, these results are consistent with our observations from SIM1-ERα-KO mice, and further pinpoint ERα in the MeA as one critical site where 17β-estradiol acts to prevent stress-induced pressor responses in female mice.
Figure 3. Deletion of ERα in the MeA blunted 17β-estradiol effects on MAP and HR.
(A) Immunofluorescence for ERα in the MeA (upper panel) and the hypothalamus (lower panel) in Esr1f/f mice receiving AAV-GFP (control) or AAV-Cre-GFP (MeA-ERα-KO) injections in the MeA. 3V, 3rd ventricle; ARH, arcuate nucleus of the hypothalamus; MeA, medial amygdala; opt, optic tract; VMH, ventromedial hypothalamic nucleus. Scale bar = 100 μm.
(B) Temporal changes in mean arterial pressure in OVX+E-treated control and MeA-ERα-KO mice at the baseline (−20 to 0 min) and the restrained condition (0 to 60 min). Data are presented as mean±SEM. N=5/group. *, P<0.05 in two-way ANOVA analysis followed by post hoc Sidak tests.
(C) Averaged mean arterial pressure at the baseline or at the restrained condition in OVX+E-treated control and MeA-ERα-KO mice. Data are presented as mean±SEM. N=5/group. **, P<0.01 between baseline and stress condition within the same mice; #, P<0.05 between control and MeA-ERα-KO mice in two-way ANOVA analysis followed by post hoc Sidak tests.
(D–E) Temporal changes in heart rate in OVX+E-treated control and MeA-ERα-KO mice at the baseline (−20 to 0 min) and the restrained condition (0 to 60 min). Data are presented as mean±SEM. N=5/group. *, P<0.05 in two-way ANOVA analysis followed by post hoc Sidak tests.
(F) Averaged heart rate at the baseline or at the restrained condition in OVX+E-treated control and MeA-ERα-KO mice. Data are presented as mean±SEM. N=5/group. *, P<0.05 between baseline and stress condition within the same mice in two-way ANOVA analysis followed by post hoc Sidak tests.
17β-estradiol potentiates stress-induced corticosterone
In order to explore how 17β-estradiol regulates BP during stress, we first measured the stress hormone, corticosterone. As expected, in OVX+V mice, stress increased plasma corticosterone levels compared to the baseline. However, this stress-induced corticosterone response was further potentiated, instead of inhibited, in OVX+E mice (Figure S4). Indeed, similar phenomenon has been reported in female rats treated with 17β-estradiol33. Thus, it is not likely that 17β-estradiol prevents stress-induced pressor responses via its actions on corticosterone levels.
Loss of ERα increases excitability of MeA SIM1 neurons
It has been shown that 17β-estradiol supplement attenuates stress-induced c-fos immunoreactivity in the MeA34, indicating that estrogen prevents neural activation of MeA neurons during stress. However, mechanisms underlying these estrogenic actions were not known. Here we tested if ERα expressed by MeA neurons is involved in the excitability of these neurons. To this end, we used the whole-cell patch clamp electrophysiology to record firing activities of identified MeA SIM1 neurons from female control vs. SIM1-ERα-KO mice (Figure 4A). We found that MeA SIM1 neurons in SIM1-ERα-KO mice showed significantly increased firing frequency compared to those in control mice, while the resting membrane potential was comparable between the two groups (Figure 4B–4D). We further analyzed the miniature excitatory post-synaptic currents (mEPSC) in these neurons, and found that both the amplitude and frequency of mEPSC in MeA SIM1 neurons from SIM1-ERα-KO mice were significantly increased compared to those from control mice (Figure 4E–4G). In contrast, the amplitude and frequency of miniature inhibitory post-synaptic currents (mIPSC) were not significantly different between the two groups (Figure 4H–4J). Further, we found that mRNA levels of the AMPA receptor subunits (GluA1 and GluA2) and the NMDA receptor subunit (GluN1) were significantly elevated in the amygdala of female SIM1-ERα-KO mice compared to control littermates, while expression of other estrogen receptors (including ERβ and GPR30) was comparable between the two groups (Figure 4K). We further confirmed that the protein levels of GluN1 in the amygdala of female SIM1-ERα-KO mice were significantly higher than those from control mice (Figure 4L and 4M). Compared to the ionotropic AMPA and NMDA glutamate receptors, mRNAs of metabotropic glutamate receptors (mGluR1-mGluR8) were less abundant in the amygdala of control mice, and were not significantly changed in the amygdala of SIM1-ERα-KO mice (Figure 4K). In summary, these results indicate that loss of ERα in MeA SIM1 neurons leads to increased expression of ionotropic glutamate receptors, which is associated with increased excitability of these neurons.
Figure 4. Effects of ERα deletion on excitability of MeA SIM1 neurons.
(A) Electrophysiological recording from an identified MeA SIM1 neuron in the brain slice from a SIM1-Cre/rosa26:tdTOMATO mouse. Illuminations for TOMATO, injected lucifer yellow dye, and the brightfield. Scale bars=20 μm.
(B) Representative action potential traces in MeA SIM1 neurons from control and SIM1-ERα-KO mice. (C–D) Averaged firing frequency (C) and resting membrane potential (D). Data are presented as mean±SEM. N=9 or 16/group. *, P<0.05 in t-tests.
(E) Representative mEPSC traces in MeA SIM1 neurons from control and SIM1-ERα-KO mice. (F–G) Averaged mEPSC amplitude (F) and frequency (G). Data are presented as mean±SEM. N=25 or 29/group. *, P<0.05 and ***, P<0.001 in t-tests.
(H) Representative mIPSC traces in MeA SIM1 neurons from control and SIM1-ERα-KO mice. (I–J) Averaged mIPSC amplitude (I) and frequency (J). Data are presented as mean±SEM. N=17 or 14/group.
(K) Relative mRNA levels of indicated genes in the amygdala from control and SIM1-ERα-KO mice. Data are presented as mean±SEM. N=7/group. *, P<0.05 in t-tests. Note that mGluR6 were undetectable in all samples and were not shown here.
(I) Western blot showing protein levels of GluN1 and actin in the amygdala from control and SIM1-ERα-KO mice. (M) Summary quantification for the ratios of GluN1/actin. Data are presented as mean±SEM. N=4 or 5 in each group. *, P<0.05 in t-tests.
Selective activation of MeA SIM1 neurons increases BP
It has been shown that lesions of the MeA reduce BP in mice35 and rats36, which suggests that MeA neurons are vasopressors. Here we used DREADD approach20 to selectively activate MeA SIM1 neurons in free-moving mice (Figure 5A). To prove the concept that the designers’ drug, CNO, does activate these hM3Dq-mCherry-expresing SIM1 neurons, we used the whole-cell patch clamp electrophysiology to record effects of CNO on the firing activities of mCherry-labelled neurons in brain slices prepared from AAV-hM3Dq-mCherry-infected SIM1-Cre mice (Figure 5B). We showed that CNO (10 μM, bath 6 min) increased the firing frequency of all recorded mCherry-labelled MeA SIM1 neurons, and caused depolarization (Figure 5C–5E). Importantly, we showed in vivo that CNO injections (3 mg/kg, i.p.) significantly increased MAP and HR compared to saline treatment in the same mice (Figure 5F and 5G). Similar changes in SAP and DAP were observed (data not shown). Together, these data demonstrate that selective activation of MeA SIM1 neurons provokes pressor responses in mice.
Figure 5. Effects of selective activation of MeA SIM1 neurons.
(A) mCherry immunoreactivity in the MeA of SIM-Cre mice receiving stereotaxic AAV-hM3Dq–mCherry infection.
(B) Electrophysiological recording from a mCherry-labelled MeA SIM1 neuron in the brain slice from a SIM1-Cre mouse receiving stereotaxic AAV-hM3Dq–mCherry infection. Illuminations for mCherry and the brightfield. Scale bars=10 μm.
(C) Representative action potential traces in mCherry-labelled MeA SIM1 neurons in response to bath application of CNO (10 μM). (D–E) Firing frequency (D) and resting membrane potential (E) in each neuron at the baseline and after CNO treatment.
(F–G) Temporal changes in mean arterial pressure (F) and heart rate (G) in AAV-hM3Dq-mCherry-infected SIM1-Cre mice received i.p. injections of saline or CNO (3 mg/kg). Data are presented as mean±SEM. N=7/group. *, P<0.05 in two-way ANOVA analysis followed by post hoc Sidak tests.
Discussion
The MeA has been implicated as a key brain structure that links stress and development of hypertension. First, various physical and psychological stressors (e.g. noise, restraint and forced swim) activate neurons in the MeA37–39. Interestingly, increased MeA neural activities are correlated with stress-induced pressor responses in animals13, 14 and in humans16. Supporting a physiological role of MeA neurons in the regulation of BP, lesions of the MeA lower BP in BPH/2J genetically hypertensive mice35 and in spontaneously hypertensive rats36. Here we provided the direct functional evidence that the DREADD-mediated activation of a subset of MeA neurons (SIM1 neurons) results in increases in BP and HR. Collectively, these results indicate that elevated neural activities in the MeA mediate stress-induced pressor responses. Notably, activation of MeA neurons has been reported to trigger multiple behaviors, including grooming40, aggression40, 41, social recognition and anxiety42. Thus, we suggest that the increased BP evoked by MeA neural activation could represent an important component of the complex adaptive behaviors in response to stressful challenges.
In various hypertension animal models, OVX exacerbates the course of hypertension, while 17β-estradiol supplement prevents hypertension43–49. In particular, 17β-estradiol supplement prevents pressor responses induced by restraint stress in rodents50, 51 and in post-menopausal women8. However, the mechanisms for this protective effect remain unknown. Here we demonstrated that genetic deletion of ERα selectively in SIM1 neurons blunted the effects of 17β-estradiol to prevent stress-induced pressor responses in female mice. Given that most of SIM1-Cre-mediated ERα deletion is restricted in the MeA (with minor deletions in the MPOA and PVN), we suggest that this phenotype results primarily from loss of ERα in the MeA. Consistently, Xue at al. has reported that selective knock-down of ERα in the PVN does not affect BP in rats52. The specific role of MeA ERα was further confirmed in mice lacking ERα only in the MeA (through the stereotaxic AAV-Cre-GFP approach). Thus, we provided compelling evidence to highlight ERα expressed by MeA neurons as the key mediator for the anti-hypertensive effects of 17β-estradiol during stress. Of course, it is worth noting that other estrogen receptors, including estrogen receptor-β (ERβ)52, 53 and G protein-coupled receptor 30 (GPR30)54, are both implicated in the regulation of BP. Both these receptors are highly expressed in the MeA55, 56. Functions of ERβ and GPR30 in the regulation of stress-induced pressor responses warrant further investigations.
The mechanisms by which estrogen-ERα signals prevent stress-induced pressor responses at least partly involve an inhibition of MeA neurons during stress. Supporting this notion, it has been reported that chronic 17β-estradiol supplement attenuates MeA neural activation (c-fos immunoreactivity) evoked by the restraint stress34. Consistently, we found that MeA SIM1 neurons lacking ERα showed increased firing frequency associated with increased mEPSC, indicating that ERα-mediated signals in these neurons inhibit their firing activity. Notably, we recently reported that a selective ERα agonist, propyl pyrazole triol (PPT), rapidly (in the time scale of mini-seconds) activates MeA neurons in an ERα-dependent manner26. This PPT-induced rapid activation appears to be in contrast to the activation of MeA neurons caused by chronic loss of ERα. Similar discrepancy also exists in animals receiving acute vs. chronic 17β-estradiol treatment. While acute injections of 17β-estradiol rapidly induce c-fos immunoreactivity in the MeA57, chronic 17β-estradiol supplement prevents stress-induced c-fos immunoreactivity in the same brain region34. We suggest that these opposite rapid vs. chronic effects are mediated by segregated rapid signals vs. genomic effects of ERα. On one hand, estrogen can act upon ERα to rapidly activate neural activity, phenomena that have been widely observed in multiple neural populations27, 58, 59. On the other hand, in a more chronic setting, ERα functions as a classic nuclear receptor to regulate expression of target genes. Indeed, it has been reported that chronic 17β-estradiol treatment in ovariectomized rodents decreases expression of ionotropic glutamate receptors in the amygdala and hypothalamus60, 61. Consistently, we showed that deletion of ERα increased levels of ionotropic glutamate receptors in the MeA, which may have contributed to the increased mEPSC and firing frequency. Collectively, we suggest that estrogen inhibits expression of ionotropic glutamate receptors in amygdalar neurons, presumably through genomic ERα actions as a nuclear receptor. Thus, low levels of glutamate receptors decrease the excitability of these MeA neurons and prevent them from stress-induced activation.
Notably, changes in HR were largely correlated with changes in BP in most cases. For example, we showed that DREADD-mediated stimulation of MeA neurons increased BP and HR. Similarly, restraint stress caused increases in both BP and HR. These results are consistent with a number of previous reports in rats50 or in mice62. Importantly, 17β-estradiol treatment in control mice prevented stress-induced increases in both BP and HR, while these effects were both blunted in mice lacking ERα in MeA neurons. This pattern suggests that the regulations on BP are at least partly through modulations on cardiac functions. However, we could not fully exclude the possibility that MeA neurons may also regulate BP via actions on the vascular tone.
How ERα-expressing MeA neurons regulate BP and HR during stress is unknown. Stress is well known to activate the hypothalamus-pituitary-adrenal (HPA) axis and leads to elevated corticosterone levels in the circulation, which may partly contribute to the stress-induced pressor responses. However, it has been shown that 17β-estradiol supplement potentiates, instead of inhibits, stress-induced corticosterone release in female rats33. Similar potentiated corticosterone levels were observed in our OVX+E mice compared to OVX+V mice under the stressed condition, although the corticosterone level at a single time point during the stress may not fully reflect responses of the HPA axis. Given the anti-hypertensive effects of ERα-expressing MeA neurons during stress, we suggest that estrogenic actions on the HPA responses and on BP responses to stress are likely mediated by distinct neural circuits. The neural circuits by which MeA neurons regulate BP balance remain to be elucidated.
Perspectives
In summary, our studies provide evidence that MeA neurons are vasopressors and activation of these MeA neurons mediates the elevations in BP during restraint stress. Further, we demonstrate that estrogen acts upon ERα expressed by MeA neurons to prevent stress-induced pressor responses in female mice. These anti-hypertensive effects of MeA ERα appear to be mediated through transcription control of ionotropic glutamate receptors in MeA neurons. Thus, we identified MeA ERα as a potential therapeutic target for hypertension, especially in post-menopausal women.
Supplementary Material
Novelty and Significance.
(1) What is new?
While the roles of the hypothalamus and brainstem in the regulation of BP have been well documented, the effects of higher brain structures on BP control are certainly less appreciated. These higher neural centers (e.g. the amygdala) are well positioned to integrate psychological/emotional (stress), neural and hormonal inputs, and may provide a coordinated control of BP. Our studied added to the emerging literature to establish a crucial role of one amygdalar subdivision in the control of BP.
(2) What is relevant?
Since the ERα gene variants are associated with hypertension in humans, our studies focusing on the role of MeA ERα in BP control revealed one potential mechanism for hypertension in patients with the ERα gene variants. Even for women with normal ERα gene, ERα functions will undergo monthly changes (within the menstrual cycles) and eventually diminish (after menopause) due to changes in 17β-estradiol levels. Thus, our studies are significant in that we may demonstrate the cardiovascular consequences induced by these fluctuations that affect all women.
(3) Summary
17β-estradiol is known to produce anti-hypertensive benefits in women, but its application has been hampered due to detrimental effects in peripheral tissues (e.g. venous thrombosis and breast cancer). Our studies provided the proof of the concept that selective activation of MeA ERα can lower BP. Thus, from a therapeutic perspective, our results may identified a novel drug target for the treatment of hypertension.
Acknowledgments
The authors thank the Mouse Phenotyping Core at Baylor College of Medicine for all the measurements of BP and HR in mice.
Source of Funding: This work was supported by grants from the NIH (1F31HL128054 2T32GM008231, and IMSD R25 GM56929 to AHJ; R01DK093587 and R01DK101379 to YX; P01 DK088761 to DJC; T32CA059268 to SAK; R01DK092605 to QT), American Diabetes Association (1-11-BS-180 to YX and #7-13-JF-61 to QW), and American Heart Association awards to PX and to QT.
Footnotes
Conflict of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1.Hajjar I, Kotchen JM, Kotchen TA. Hypertension: Trends in prevalence, incidence, and control. Annu Rev Public Health. 2006;27:465–490. doi: 10.1146/annurev.publhealth.27.021405.102132. [DOI] [PubMed] [Google Scholar]
- 2.Spruill TM. Chronic psychosocial stress and hypertension. Curr Hypertens Rep. 2010;12:10–16. doi: 10.1007/s11906-009-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to blood pressure in the cardia study. Health Psychol. 2009;28:338–346. doi: 10.1037/a0013785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 5.Kario K, McEwen BS, Pickering TG. Disasters and the heart: A review of the effects of earthquake-induced stress on cardiovascular disease. Hypertens Res. 2003;26:355–367. doi: 10.1291/hypres.26.355. [DOI] [PubMed] [Google Scholar]
- 6.Sparrenberger F, Cichelero FT, Ascoli AM, Fonseca FP, Weiss G, Berwanger O, Fuchs SC, Moreira LB, Fuchs FD. Does psychosocial stress cause hypertension? A systematic review of observational studies. J Hum Hypertens. 2009;23:12–19. doi: 10.1038/jhh.2008.74. [DOI] [PubMed] [Google Scholar]
- 7.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the us adult population. Results from the third national health and nutrition examination survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 8.Lindheim SR, Legro RS, Bernstein L, Stanczyk FZ, Vijod MA, Presser SC, Lobo RA. Behavioral stress responses in premenopausal and postmenopausal women and the effects of estrogen. Am J Obstet Gynecol. 1992;167:1831–1836. doi: 10.1016/0002-9378(92)91783-7. [DOI] [PubMed] [Google Scholar]
- 9.Cacciatore B, Paakkari I, Hasselblatt R, Nieminen MS, Toivonen J, Tikkanen MI, Ylikorkala O. Randomized comparison between orally and transdermally administered hormone replacement therapy regimens of long-term effects on 24-hour ambulatory blood pressure in postmenopausal women. Am J Obstet Gynecol. 2001;184:904–909. doi: 10.1067/mob.2001.111246. [DOI] [PubMed] [Google Scholar]
- 10.Manhem K, Ahlm H, Milsom I, Svensson A. Transdermal oestrogen reduces daytime blood pressure in hypertensive women [see comment] J Hum Hypertens. 1998;12:323–327. doi: 10.1038/sj.jhh.1000563. [DOI] [PubMed] [Google Scholar]
- 11.Seely EW, Walsh BW, Gerhard MD, Williams GH. Estradiol with or without progesterone and ambulatory blood pressure in postmenopausal women. Hypertension. 1999;33:1190–1194. doi: 10.1161/01.hyp.33.5.1190. [DOI] [PubMed] [Google Scholar]
- 12.Reckelhoff JF, Fortepiani LA. Novel mechanisms responsible for postmenopausal hypertension. Hypertension. 2004;43:918–923. doi: 10.1161/01.HYP.0000124670.03674.15. [DOI] [PubMed] [Google Scholar]
- 13.Collins P, Rosano G, Casey C, Daly C, Gambacciani M, Hadji P, Kaaja R, Mikkola T, Palacios S, Preston R, Simon T, Stevenson J, Stramba-Badiale M. Management of cardiovascular risk in the perimenopausal women: A consensus statement of european cardiologists and gynecologists. Climacteric. 2007;10:508–526. doi: 10.1080/13697130701755213. [DOI] [PubMed] [Google Scholar]
- 14.Rosano GM, Vitale C, Fini M. Hormone replacement therapy and cardioprotection: What is good and what is bad for the cardiovascular system? Ann N Y Acad Sci. 2006;1092:341–348. doi: 10.1196/annals.1365.031. [DOI] [PubMed] [Google Scholar]
- 15.Van de Kar LD, Blair ML. Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrinol. 1999;20:1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- 16.Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci. 2008;28:990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davern PJ, Jackson KL, Nguyen-Huu TP, La Greca L, Head GA. Cardiovascular reactivity and neuronal activation to stress in schlager genetically hypertensive mice. Neuroscience. 2010;170:551–558. doi: 10.1016/j.neuroscience.2010.07.040. [DOI] [PubMed] [Google Scholar]
- 18.Davern PJ, Head GA. Role of the medial amygdala in mediating responses to aversive stimuli leading to hypertension. Clin Exp Pharmacol Physiol. 2011;38:136–143. doi: 10.1111/j.1440-1681.2010.05413.x. [DOI] [PubMed] [Google Scholar]
- 19.Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: In vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 20.Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved g protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 22.Feng Y, Manka D, Wagner KU, Khan SA. Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc Natl Acad Sci U S A. 2007;104:14718–14723. doi: 10.1073/pnas.0706933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L, Zou F, Yang Y, Xu P, Saito K, Othrell Hinton A, Jr, Yan X, Ding H, Wu Q, Fukuda M, Sun Z, Tong Q, Xu Y. Estrogens prevent metabolic dysfunctions induced by circadian disruptions in female mice. Endocrinology. 2015;156:2114–2123. doi: 10.1210/en.2014-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu L, Yang Y, Xu P, Zou F, Yan X, Liao L, Xu J, O’Malley BW, Xu Y. Steroid receptor coactivator-1 mediates estrogenic actions to prevent body weight gain in female mice. Endocrinology. 2013;154:150–158. doi: 10.1210/en.2012-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: New insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 26.Xu P, Cao X, He Y, Zhu L, Yang Y, Saito K, Wang C, Yan X, Hinton AO, Jr, Zou F, Ding H, Xia Y, Yan C, Shu G, Wu SP, Yang B, Feng Y, Clegg DJ, DeMarchi R, Khan SA, Tsai SY, DeMayo FJ, Wu Q, Tong Q, Xu Y. Estrogen receptor-alpha in medial amygdala neurons regulates body weight. J Clin Invest. 2015;125:2861–2876. doi: 10.1172/JCI80941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao X, Xu P, Oyola MG, Xia Y, Yan X, Saito K, Zou F, Wang C, Yang Y, Hinton A, Jr, Yan C, Ding H, Zhu L, Yu L, Yang B, Feng Y, Clegg DJ, Khan S, DiMarchi R, Mani SK, Tong Q, Xu Y. Estrogens stimulate serotonin neurons to inhibit binge-like eating in mice. J Clin Invest. 2014;124:4351–4362. doi: 10.1172/JCI74726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 29.Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013;152:612–619. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Liu H, Sauvey C, Yao L, Zarnowska ED, Zhang SC. Directed differentiation of forebrain gaba interneurons from human pluripotent stem cells. Nat Protoc. 2013;8:1670–1679. doi: 10.1038/nprot.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rannals MD, Kapur J. Homeostatic strengthening of inhibitory synapses is mediated by the accumulation of gaba(a) receptors. J Neurosci. 2011;31:17701–17712. doi: 10.1523/JNEUROSCI.4476-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Y, Hill JW, Fukuda M, Gautron L, Sohn JW, Kim KW, Lee CE, Choi MJ, Lauzon DA, Dhillon H, Lowell BB, Zigman JM, Zhao JJ, Elmquist JK. Pi3k signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell Metab. 2010;12:88–95. doi: 10.1016/j.cmet.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 34.Ueyama T, Kasamatsu K, Hano T, Tsuruo Y, Ishikura F. Catecholamines and estrogen are involved in the pathogenesis of emotional stress-induced acute heart attack. Ann N Y Acad Sci. 2008;1148:479–485. doi: 10.1196/annals.1410.079. [DOI] [PubMed] [Google Scholar]
- 35.Jackson KL, Palma-Rigo K, Nguyen-Huu TP, Davern PJ, Head GA. Major contribution of the medial amygdala to hypertension in bph/2j genetically hypertensive mice. Hypertension. 2014;63:811–818. doi: 10.1161/HYPERTENSIONAHA.113.02020. [DOI] [PubMed] [Google Scholar]
- 36.Fukumori R, Nishigori Y, Goshima Y, Kubo T. Contribution of the medial amygdaloid nucleus to the development of hypertension in spontaneously hypertensive rats. Neurosci Lett. 2004;365:128–131. doi: 10.1016/j.neulet.2004.04.066. [DOI] [PubMed] [Google Scholar]
- 37.Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 38.Beckett SR, Duxon MS, Aspley S, Marsden CA. Central c-fos expression following 20khz/ultrasound induced defence behaviour in the rat. Brain Res Bull. 1997;42:421–426. doi: 10.1016/s0361-9230(96)00332-2. [DOI] [PubMed] [Google Scholar]
- 39.Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: Acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- 40.Hong W, Kim DW, Anderson DJ. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell. 2014;158:1348–1361. doi: 10.1016/j.cell.2014.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unger EK, Burke KJ, Jr, Yang CF, Bender KJ, Fuller PM, Shah NM. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep. 2015;10:453–462. doi: 10.1016/j.celrep.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, Agmo A. The role of the estrogen receptor alpha in the medial amygdala and ventromedial nucleus of the hypothalamus in social recognition, anxiety and aggression. Behav Brain Res. 2010;210:211–220. doi: 10.1016/j.bbr.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 43.Crofton JT, Share L. Gonadal hormones modulate deoxycorticosterone-salt hypertension in male and female rats. Hypertension. 1997;29:494–499. doi: 10.1161/01.hyp.29.1.494. [DOI] [PubMed] [Google Scholar]
- 44.Haywood JR, Hinojosa-Laborde C. Sexual dimorphism of sodium-sensitive renal-wrap hypertension. Hypertension. 1997;30:667–671. doi: 10.1161/01.hyp.30.3.667. [DOI] [PubMed] [Google Scholar]
- 45.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of dahl hypertension. Hypertension. 2000;35:484–489. doi: 10.1161/01.hyp.35.1.484. [DOI] [PubMed] [Google Scholar]
- 46.Ouchi Y, Share L, Crofton JT, Iitake K, Brooks DP. Sex difference in the development of deoxycorticosterone-salt hypertension in the rat. Hypertension. 1987;9:172–177. doi: 10.1161/01.hyp.9.2.172. [DOI] [PubMed] [Google Scholar]
- 47.Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen protection from angiotensin ii-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol. 2007;292:H1770–1776. doi: 10.1152/ajpheart.01011.2005. [DOI] [PubMed] [Google Scholar]
- 48.Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female dahl salt-sensitive rats. Hypertension. 2004;44:405–409. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- 49.Chappell MC, Yamaleyeva LM, Westwood BM. Estrogen and salt sensitivity in the female mren(2). Lewis rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1557–1563. doi: 10.1152/ajpregu.00051.2006. [DOI] [PubMed] [Google Scholar]
- 50.Cherney A, Edgell H, Krukoff TL. No mediates effects of estrogen on central regulation of blood pressure in restrained, ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R842–849. doi: 10.1152/ajpregu.00035.2003. [DOI] [PubMed] [Google Scholar]
- 51.Morimoto K, Kurahashi Y, Shintani-Ishida K, Kawamura N, Miyashita M, Uji M, Tan N, Yoshida K. Estrogen replacement suppresses stress-induced cardiovascular responses in ovariectomized rats. Am J Physiol Heart Circ Physiol. 2004;287:H1950–1956. doi: 10.1152/ajpheart.00341.2004. [DOI] [PubMed] [Google Scholar]
- 52.Xue B, Zhang Z, Beltz TG, Johnson RF, Guo F, Hay M, Johnson AK. Estrogen receptor-beta in the paraventricular nucleus and rostroventrolateral medulla plays an essential protective role in aldosterone/salt-induced hypertension in female rats. Hypertension. 2013;61:1255–1262. doi: 10.1161/HYPERTENSIONAHA.111.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295:505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- 54.Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the g protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 55.Canonaco M, Giusi G, Madeo A, Facciolo RM, Lappano R, Canonaco A, Maggiolini M. A sexually dimorphic distribution pattern of the novel estrogen receptor g-protein-coupled receptor 30 in some brain areas of the hamster. J Endocrinol. 2008;196:131–138. doi: 10.1677/JOE-07-0392. [DOI] [PubMed] [Google Scholar]
- 56.Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: In vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 57.Lehmann ML, Erskine MS. Glutamatergic stimulation of the medial amygdala induces steroid dependent c-fos expression within forebrain nuclei responsive to mating stimulation. Neuroscience. 2005;136:55–64. doi: 10.1016/j.neuroscience.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 58.Malyala A, Zhang C, Bryant DN, Kelly MJ, Ronnekleiv OK. Pi3k signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol. 2008;506:895–911. doi: 10.1002/cne.21584. [DOI] [PubMed] [Google Scholar]
- 59.Park CJ, Zhao Z, Glidewell-Kenney C, Lazic M, Chambon P, Krust A, Weiss J, Clegg DJ, Dunaif A, Jameson JL, Levine JE. Genetic rescue of nonclassical eralpha signaling normalizes energy balance in obese eralpha-null mutant mice. J Clin Invest. 2011;121:604–612. doi: 10.1172/JCI41702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian Z, Wang Y, Zhang N, Guo YY, Feng B, Liu SB, Zhao MG. Estrogen receptor gpr30 exerts anxiolytic effects by maintaining the balance between gabaergic and glutamatergic transmission in the basolateral amygdala of ovariectomized mice after stress. Psychoneuroendocrinology. 2013;38:2218–2233. doi: 10.1016/j.psyneuen.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 61.Gu G, Varoqueaux F, Simerly RB. Hormonal regulation of glutamate receptor gene expression in the anteroventral periventricular nucleus of the hypothalamus. J Neurosci. 1999;19:3213–3222. doi: 10.1523/JNEUROSCI.19-08-03213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andreev-Andrievskiy AA, Popova AS, Borovik AS, Dolgov ON, Tsvirkun DV, Custaud M, Vinogradova OL. Stress-associated cardiovascular reaction masks heart rate dependence on physical load in mice. Physiol Behav. 2014;132:1–9. doi: 10.1016/j.physbeh.2014.03.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.