The importance of the central nervous system in cardiovascular regulation is well established (1;2). The brain is able to sense changes that occur in the peripheral circulation through several mechanisms including afferent neural reflexes and humoral signals. The brain in turn can adjust various components of the cardiovascular system to maintain homeostasis by modulating the release of critical hormonal factors, chemical messengers, and neurotransmitters in concert with changing the activity of the sympathetic and parasympathetic branches of the autonomic nervous system subserving different organs and tissues throughout the body.
It has long been recognized that brain regulation of the cardiovascular system involves complex networks of neurons located in the brainstem, midbrain, and forebrain (1). Among the various brain structures involved in circulatory regulation the hypothalamus plays a central role by integrating the information conveyed via various signal inputs with the neural networks involved to determine and coordinate the appropriate responses to maintain homeostasis (3). Much of the investigation of the cardiovascular regulation by the hypothalamus has focused on the paraventricular, dorsomedial, lateral, and posterior nuclei. Consequently, there is a wealth of information about the anatomical, cellular, and molecular processes underlying the control of autonomic and cardiovascular functions by these hypothalamic nuclei, particularly the paraventricular nucleus (3;4).
The arcuate nucleus of the hypothalamus (ARC) is emerging as a major player in cardiovascular and sympathetic regulation (5). I will review the neuroanatomical and cellular characteristics of the ARC before discussing the evidence that implicates the ARC in the control of blood pressure and sympathetic outflow.
Linking the ARC to Cardiovascular Homeostasis
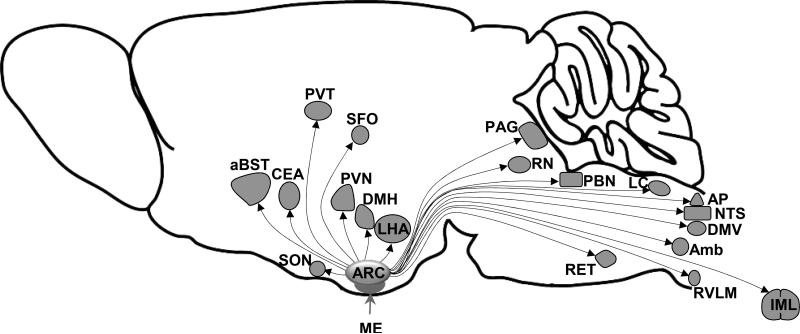
The ARC, located at the bottom of the hypothalamus, surrounds the ventral part of the third ventricle extending from retrochiasmatic to premamillary regions (6). The ventral surface of the ARC is sited on the top of the endocrine median eminence (Figure 1), a circumventricular organ that lacks the blood–brain barrier. The dense projections from the ARC to the median eminence have long been known as the route through which the hypothalamus regulates the secretory activity of the anterior pituitary gland (7). In addition, presence in the ependyma of the ARC of tanycytes, special polarized ependymoglial cells, that project to the fenestrated capillaries of the portal vessels in the palisade zone of the median eminence is thought to represent a “window” for the ARC neurons to monitor changes in circulating factors and hormonal signals, particularly with regard to the control of energy homeostasis (8).
Figure 1.
Schematic of the connections between the arcuate nucleus of the hypothalamus (ARC) and the various autonomic and cardioregulatory nuclei. Abbreviations: aBST, anterior bed nucleus of the stria terminalis; Amb, nucleus ambiguus; AP, area postrema; CEA, central nucleus of the amygdala; DMH, dorsomedial hypothalamus; DMV, dorsal motor nucleus of the vagus; IML, intermediolateral cell column; LC, locus coeruleus; LHA, lateral hypothalamic area; ME, median eminence; PAG, periaqueductal gray; PBN, parabrachial nucleus; PVN, paraventricular nucleus of the hypothalamus; PVT, paraventricular thalamus; RET, reticular nucleus; RN, raphe nuclei, RVLM; rostral ventrolateral medulla; SFO, subfornical organ; SON, supraoptic nucleus.
ARC neurons project directly to multiple areas of the hypothalamus, but with variable intensity (9;10). The highest fiber density from the ARC terminates in selected nuclei such as the paraventricular nucleus, the dorsomedial hypothalamus, and the lateral hypothalamus (Figure 1). Moreover, anatomical and electrophysiological studies have demonstrated that fibers from the ARC have widespread projections to many extra-hypothalamic areas including those involved in cardiovascular regulation such as the subfornical organ, the nucleus tractus solitarii, the parabrachial nucleus, the dorsal raphe nucleus, the locus coeruleus, the nucleus ambiguus, and the rostral ventrolateral medulla (11). Direct projections from the ARC to the sympathetic preganglionic neurons at the level of intermediolateral cell column of the thoracic spinal cord have also been documented (12). In addition to sending projections to different brain nuclei, the ARC neurons receive synaptic inputs from various brains regions, especially the hypothalamic nuclei such as the posterior hypothalamus, ventromedial hypothalamus, and paraventricular nucleus (13;14). Such reciprocal relationship may enable the ARC to integrate neuronal signals with information conveyed by circulating hormones and other factors.
Retrograde tracing studies have linked the ARC to key cardiovascular organs. For instance, pseudorabies viruses injected into the kidney can be detected in the ARC with the infection occurring at later stages after the inoculation (15). Virally infected neurons in the ARC were also reported to appear late after pseudorabies inoculation of the left ventricular myocardium or hindlimb skeletal muscle (16). The late appearance of the infections in the ARC combined with the temporal distribution of viral labeling throughout the central nervous system following inoculation of the kidney, heart, or muscle is consistent with neurocircuitries involving multiple synaptic connections, with the ARC being three or forth orders removed from these tissues.
Additional evidence linking the kidney to the ARC derives from studies that interfered with the activity of the renal nerves. Electrical stimulation of the afferent renal nerves increased ARC neuronal activity as indicated by induction of Fos expression (17). Interestingly, assessment of hexokinase activity revealed altered metabolic activity in the ARC following renal denervation (18), but not after aortic baroreceptor denervation (19). These studies raise the possibility that the ARC is among the brain structures that receive the information carried by the afferent nerves. It is interesting to note that prolonged decrease in arterial pressure induced by sodium nitroprusside was found to cause a strong increase in Fos-positive neurons in the ARC along with other brain cardioregulatory nuclei (20). Alterations in blood volume also evoked robust Fos induction in the ARC (21), and chronic water deprivation affected gene expression and protein synthesis in the ARC (22;23). Furthermore, ARC neurons were found to be sensitive to hypertonic saline given systemically or centrally (22;24), and lesioning the ARC caused chronic hypovolemia and exaggerated hypernatremia during water deprivation (17). Altogether, these findings implicate the ARC in the compensatory responses to changes in arterial pressure and balance of body fluid and electrolytes.
Neuronal Populations of the ARC
The ARC contains several populations of neurons that are defined by virtue of the transmitters or neuropeptides they express and their function. Neuroendocrine neurons of the ARC have long been the focus of neuroendocrinologists due to their proven influence on the anterior pituitary gland hormone secretion (7). These ARC neuroendocrine neurons include those expressing dopamine, which regulate secretion of prolactin and gonadotropin-releasing hormone, and those making growth hormone–releasing hormone that control the secretion of growth hormone. More recently, ARC neurons expressing kisspeptins have emerged as potent stimulators of the gonadotropic axis, with important implications in puberty onset and the control of gonadotropin secretion (25).
Apart from the neuroendocrine neurons regulating the pituitary gland, the ARC contains several neuronal populations involved in various physiological functions. The discovery of leptin in 1994 and subsequent identification of the ARC as one of its major targets brought this nucleus to the front stage of the neural control of energy homeostasis. The ARC was recognized as the site of “first order” neurons in the neural circuits regulating metabolism. The information about the nutritional status of the organism received by these neurons from various sources is conveyed to different regions of the central nervous system.
The anorexigenic proopiomelanocortin (POMC) neurons and orexigenic Agouti-related protein (AgRP) neurons of the ARC have been studied extensively in the context of central control of food intake and energy expenditure. POMC is a large protein that does not appear to have any biological activity, but its breakdown produces β-endorphins and melanocortins that have various functions as hormones and neuropeptides. The melanocortin peptides consist of α-, β-, and γ-melanocyte stimulating hormone (MSH), and adrenal corticotrophin hormone (ACTH). α-MSH is a potent stimulator of the melanocortin-3 (MC3R) and melanocortin-4 (MC4R) receptors located predominantly in the second-order neurons. On the other hand, AgRP neurons produce the strong MC3R and MC4R antagonist AgRP. Thus, POMC and AgRP neurons are considered a critical component of the melanocortin system (26).
It should be noted, however, that the relationship between POMC and AgRP neurons is more complex than a simple modulation of MC3R and MC4R located in second-order neurons. This is due to the ability of AgRP neurons to directly influence the activity of POMC neurons (27). In addition, ARC AgRP and POMC neurons are able to modulate the activity of second-order neurons beyond the release of AgRP (28) and α-MSH (29), respectively. This divergent capacity of ARC neurons is due to the fact that often they contain more than one biologically active molecule. Indeed, early studies have shown co-localization in the ARC of dopamine and neurotensin and sparse co-existence of dopamine and the inhibitory neurotransmitter γ-aminobutyric acid (GABA) and neuropeptide Y (NPY) with somatostatin (6). We also know that the majority of POMC neurons express cocaine- and amphetamine-regulating transcript, whereas a subset of these neurons produces GABA or the neurotransmitter acetylcholine. On the other hand, most AgRP neurons contain NPY and/or GABA. This divergent signaling capacity allows ARC neurons to engage different transmitters for neuronal communication to control specific functions. For instance, AgRP neurons engage mainly GABA signaling to control nearby POMC neurons (27). In addition, loss of GABAergic transmission, but not AgRP or NPY, was shown to underlie the dramatic starvation phenotype caused by ablation of ARC AgRP neurons in mice (30). This opens up the interesting possibility of targeting selective neurotransmitters in disease conditions implicating ARC neurons.
Recently, additional ARC neuronal populations expressing insulin-2 promoter (31), prodynorphin (32), or amylin precursor, amyloid polypeptide (33), have been identified as targets of leptin and implicated in the regulation of energy homeostasis, but the underlying molecular and cellular processes involved remain largely unknown. Other subsets of ARC neurons whose function is still unclear include those that produce ghrelin (34) and those sensing glucose or lipids (35). Future studies are needed to determine the exact identity of these newly identified ARC neurons and how distinct they are from other well defined cell types residing in this nucleus, such as those expressing dopamine, kisspeptins, POMC, or AgRP.
Significance of the ARC in Cardiovascular Regulation
An involvement of the ARC in cardiovascular regulation has long been suspected based on the anatomical connections between this nucleus and many brain structures influencing the cardiovascular system discussed above. This was further supported by early studies describing the hemodynamic and renal effects evoked by the melanocortins (Reviewed in (36;37)). However, the first direct evidence implicating the ARC in blood pressure control derives from the work of Michael Brody and colleagues (38) demonstrating that direct electrical stimulation of the ARC evoked a frequency-dependent increase in arterial pressure and in renal, mesenteric, and hindquarter vascular resistance in anesthetized and conscious rats. Chemical stimulation of the ARC by microinjection of the excitotoxin kainic acid, which activates glutamate receptors, also produced significant pressor and regional vasoconstrictor effects. These pressor and vasoconstrictor responses evoked by electrical or chemical stimulation of the ARC were substantially enhanced after removal of the baroreflex by sinoaortic denervation and were abolished following vasopressin receptor blockade. A decrease in heart rate that was observed only in the conscious rats that had their ARC electrically stimulated was eliminated after sinoaortic denervation (38).
Subsequent reports have shown that electrical stimulation of the ARC elicits a biphasic change in arterial pressure, depressor response followed by a pressor response, and bradycardia (39;40). The reasons behind this biphasic arterial pressure response is not clear, but may involve different parts of the ARC with the depressor and bradycardic components postulated to originate from the caudal ARC and its projections to the dorsal motor nucleus of the vagus causing parasympathetic activation (17;40). Alternatively, this could be due to the stimulation of both excitatory (e.g., POMC neurons) and inhibitory (e.g., NPY neurons) cells of the ARC.
In addition to the hemodynamic responses, it is established that broad stimulation or inhibition of the ARC alters sympathetic nerve activity (SNA). Studies from the Sapru laboratory have shown that when baseline arterial pressure is low the pressor response and bradycardia evoked by nonspecific activation of the ARC with N-methyl-d-aspartic acid (NMDA) was associated with an increase in renal and splanchnic SNA in anesthetized rats (41). However, in subsequent studies this group found that at normal baseline values of arterial pressure ARC stimulation with NMDA led to sympathetic inhibition and a depressor response (Reviewed in (5)). Baroreceptor unloading was proposed as a potential explanation for these contrasting changes in arterial pressure and SNA after ARC stimulation with NMDA (5).
Cardiovascular and sympathetic responses were also elicited by pharmacologic modulation of GABAergic signaling in the ARC. Administration of a GABAA receptor antagonist, gabazine, into the ARC of rats increased arterial pressure, heart rate, and splanchnic SNA (5). Conversely, inhibition of the ARC with GABAA receptor agonist, muscimol, was recently reported to decrease regional SNA, arterial pressure, and heart rate in rats (42). Taken together, the studies discussed above highlighted the fundamental significance of the ARC in cardiovascular regulation. This is fostered by the substantial evidence accumulated over the last several years demonstrating that a vast number of peripheral signals affect the cardiovascular function through action in the ARC.
Hemodynamic and Sympathetic Effects of Leptin Signaling in the ARC
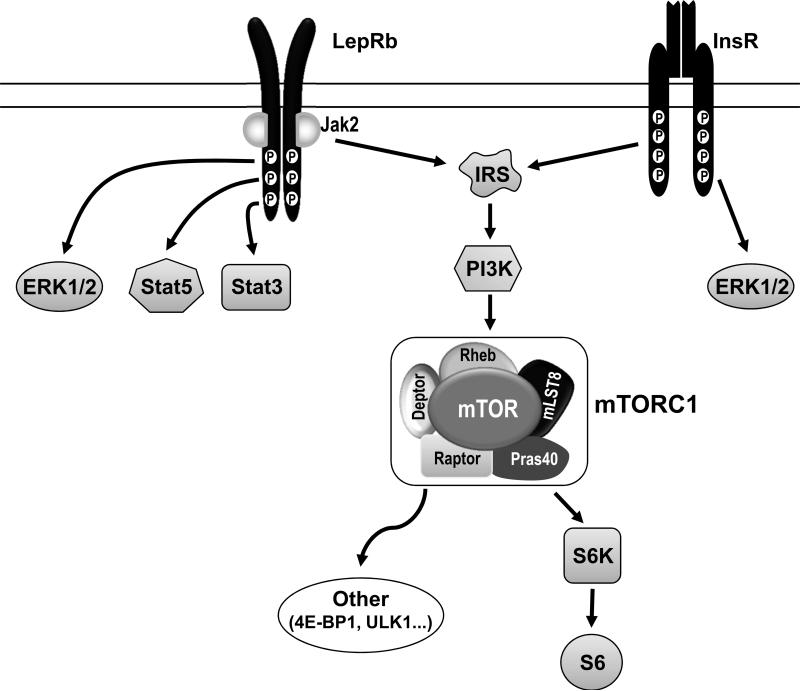
The documentation of the ability of leptin to increase arterial pressure and SNA, and its role in coupling obesity with hypertension and sympathetic overdrive, has led to intense effort to understand its mechanism of action. Our interest in the role of the ARC in mediating the sympathetic and cardiovascular effects of leptin was derived from the well documented high density in this nucleus of the long signaling form of the leptin receptor (LepRb) (43). This receptor belongs to the tyrosine kinase family of receptors and has a long intracellular domain able to activate several intracellular signaling pathways (Figure 2).
Figure 2.
Signaling pathways associated with the leptin receptor (LepRb) and insulin receptor (InsR). mTORC1 components and downstream signaling pathways are also depicted. Abbreviations: Deptor, DEP-domain-containing mTOR-interacting protein; 4EBP1, 4E binding protein 1; ERK1/2, extracellular signal-regulated kinase 1 and 2; mLST8, mammalian lethal with Sec13 protein 8; mTOR, mechanistic target of rapamycin; mTOTC1, mTOR complex 1; PI3K, phosphatidylinositol-3-kinase; Pras40, proline rich Akt substrate 40 kDa; S6, ribosomal protein S6; S6K, S6 kinase; Stat3 and 5, Signal transducer and activator of transcription 3 and 5; Raptor, regulator-associated protein of mTOR; Rheb, rat sarcoma (Ras) homolog enriched in brain; ULK1, Unc-51 like autophagy activating kinase.
We reasoned that if the cardiovascular actions of leptin emanate from the ventromedial and dorsomedial nuclei as reported by Marsh et al. (44), then injection of leptin into the ARC should spare the renal SNA and arterial pressure. On the other hand and given the predominant role of the ARC in underlying the metabolic actions of leptin (43), we postulated that this nucleus may underlie the SNA subserving thermogenic brown adipose (BAT). To test this, we combined site-specific microinjection of leptin into the ARC with simultaneous recording of SNA subserving the kidney and BAT as well as measurement of hemodynamic parameters in anesthetized rats (45).
In contrast to our expectation, we observed that microinjection of leptin (0.5 μg/0.5 μL) into the ARC increased sympathetic nerve outflow to both the kidney and thermogenic BAT. This was associated with arterial pressure elevation. Montanaro et al. (16) reported that microinjection of leptin (0.1 μg/0.1 nL) into the ARC caused lumbar sympathetic nerve activation and arterial pressure increase although these responses displayed large variabilities that could be due to the relatively low dose of leptin or the erraticism of the injection sites within the ARC. Interestingly, our data indicated that the sympathetic and pressor responses induced by ARC microinjection of leptin (0.5 μg) were qualitatively and quantitatively comparable to the responses obtained after cerebroventricular administration (10 μg) of this hormone (45). Thus, leptin action in the ARC is sufficient to cause regional sympathetic activation and pressor response. More recently, we found that leptin signaling in the ARC increases parasympathetic outflow to the liver (46), suggesting that ARC leptin regulates both arms of the autonomic nervous system. However, the cardiovascular consequences of ARC leptin–induced increase in the parasympathetic outflow are not clear.
To address the necessity of the ARC for the sympathetic and pressor actions of leptin, we examined the effect of selective deletion of the LepR from the ARC. This was achieved by ARC-restricted delivery of an adenovirus-expressing Cre recombinase (Ad-Cre) to mice expressing floxed alleles of the LepR (LepRflox/flox) (47). The selectivity and efficiency of this strategy was verified through the demonstration of strong suppression of LepR expression and signaling capacity (using signal transducer and activator of transcription 3 (Stat3) as read out) in the ARC, but not in the adjacent nuclei, after Ad-Cre microinjection in LepRflox/flox mice. Notably, deleting leptin signaling in the ARC completely abolished leptin-induced sympathetic activation to both the kidney and BAT, whereas Ad-Cre microinjections that missed the ARC failed to alter the regional SNA responses evoked by leptin (47). These findings demonstrate that LepRs in the hypothalamic ARC are necessary for the renal and BAT sympathoexcitatory effects of leptin.
POMC neurons have emerged as the likely mediator of the cardiovascular effects of leptin action in the ARC. This is based on the work of John Hall and colleagues demonstrating that selective deletion of the LepR from POMC neurons abolished the ability of leptin to increase arterial pressure (48). This is further supported by the demonstration that genetic ablation from POMC neurons of different components of the LepRb signaling pathways such as Stat3 interferes with the pressor effects evoked by leptin (49). Implications of POMC neurons as first-order neurons in mediating the action of leptin on blood pressure is consistent with the requirement of downstream MC4R and melanocortinergic drive from ARC to the paraventricular nucleus for the sympathetic and pressor effects of leptin (50–53). Neuropeptide Y signaling and glutamatergic transmission in the paraventricular nucleus have also been implicated in the sympathoexcitarory and pressor actions of leptin (53).
Insulin Acts in the ARC to Increase Sympathetic Outflow
Insulin action in the brain has been implicated in the regulation of various physiological processes. Pancreatic-derived insulin reaches the brain through a specific mechanism that involves active and saturable transport through the blood–brain barrier. Converging evidence from animal and human studies have shown that insulin is an important determinant of the activity of the sympathetic nervous system (54;55). Moreover and in line with the broad spectrum of physiological effects evoked by brain action of insulin, cerebroventricular administration of this hormone causes sympathetic nerve activation to numerous beds including the hindlimb, BAT, kidney, and adrenal gland (56;57). In addition, central action of insulin increases baroreflex control of both heart rate and lumbar SNA (58) and causes a modest rise in arterial pressure (57).
The earlier finding that lesioning the anteroventral third ventricle eliminated the ability of insulin to induce sympathetic nerve activation implicated a key role for the hypothalamus in the sympathoexcitatory effects of this hormone (59). This was further substantiated by the elimination of insulin-induced sympathetic nerve activation after inhibition of insulin receptor signaling pathways in the hypothalamus (57) and the capacity of insulin to increase SNA when administered into the lateral ventricle, but not the fourth ventricle (58).
More recent studies have identified the ARC as the main site that mediates the sympathetic responses evoked by insulin (60;61). Cassaglia et al. (60) demonstrated that muscimol-mediated inhibition of the ARC or paraventricular nucleus completely reversed the lumbar sympathetic activation as well as the increase in the gain of the baroreflex control of lumbar SNA caused by peripheral hyperinsulinemia. On the other hand, these authors found that direct administration of insulin into the ARC, but not the paraventricular nucleus or dorsomedial hypothalamus, yielded a dose-dependent increase in lumbar SNA and baroreflex sensitivity. Together, these findings point to the sufficiency of insulin action in the ARC to increase sympathetic SNA and baroreflex sensitivity through a neural network that involves the paraventricular nucleus.
Luckett et al. (61) used a different strategy taking advantage of the availability of a small molecule anti-insulin affibody to implicate the ARC in the sympathoexcitatory effects of insulin. They demonstrated that pre-treatment with the anti-insulin affibody into the ARC interfered with the lumbar sympathetic activation induced by insulin when administered directly into the ARC, intracerebroventricularly, or systemically. Interestingly, microinjection of the anti-insulin affibody into the ventromedial hypothalamus did not impede the lumbar sympathetic activation to insulin. The rise in arterial pressure following systemic insulin was also eliminated when the anti-insulin affibody was microinjected into the ARC, but not into the ventromedial hypothalamus. Notably, pretreatment with the ARC anti-insulin affibody failed to interfere with the pressor and regional sympathetic responses evoked by gabazine, indicating that the effects observed with insulin are specific. Thus, insulin signaling in the ARC is necessary to affect sympathetic traffic and arterial pressure.
The insulin receptor is a member of the tyrosine kinase family consisting of two extracellularα- and two transmembrane β-subunits. Notably, although it is present throughout the central nervous system, the hypothalamus contains the highest density the insulin receptor. This was demonstrated by in situ hybridization for insulin receptor messenger (13), binding assay with isotope-labeling insulin (11;12), and immunohistochemical labeling of the β- (10) or α-subunit (62) of the insulin receptor. Notably, among the hypothalamic nuclei the ARC displayed the highest expression of the insulin receptor (62). Within the ARC, POMC and AgRP neurons are known to be equipped with the insulin receptor (63). These neurons may provide the melanocortinergic drive and/or glutamatergic neurotransmission to the paraventricular nucleus that have been implicated in mediating the sympathoexcitatory effects of insulin (64;65).
Novel Mechanisms Underlying Cardiovascular Regulation by the ARC
The mechanistic target of rapamycin (mTOR) is an evolutionary conserved protein that belongs to the phosphatidylinositol kinase–related kinase family (66). mTOR is critically involved in the regulation of a number of cellular functions, including gene transcription, protein synthesis, cell growth and proliferation, ribosome and mitochondria biogenesis, cytoskeleton organization, and autophagy (67–69). mTOR activity is modulated by various cues arising from growth factors, hormones, stress, nutrients, and energy status. mTOR functions largely as the catalytic subunit of large protein kinase complexes (66;69). mTOR interaction with several other proteins leads to the formation of a functionally distinct large complex, termed mTOR complex 1 (mTORC1, Figure 2). Well characterized downstream targets of mTORC1 are 70S ribosomal protein S6 kinase (S6K) and its downstream effector, the ribosomal protein S6 (66).
Recently, we demonstrated that hypothalamic mTORC1 signaling is involved in the control of the sympathetic nerve drive and arterial pressure (70). We took advantage of the unique ability of leucine to activate mTORC1 signaling to show that leucine-mediated stimulation of mTORC1 signaling in the mediobasal hypothalamus increased arterial pressure and SNA subserving the kidney in rats (70). This was associated with a parallel, but uneven, decrease in blood flows recorded from the aortic, iliac, mesenteric, and renal arteries. Importantly, rapamycin-mediated hypothalamic mTORC1 inhibition abolished the increase in renal SNA and arterial pressure as well as the vasoconstriction induced by leucine (70). These sympathetic and cardiovascular responses evoked by mTORC1 appear to emanate from the ARC as inhibition of mTORC1 signaling selectively in this nucleus with an adenovirus expressing a dominant-negative S6K decreased renal SNA and arterial pressure (70). Conversely, ARC-specific activation of mTORC1 signaling with an adenovirus expressing a constitutively active S6K (Ad-S6K) increases renal SNA and arterial pressure (K.R., unpublished data). Collectively, these findings establish mTORC1 signaling in the ARC as a critical regulator of SNA and arterial pressure.
ARC mTORC1 has also emerged as a key downstream pathway mediating the regulation of sympathetic nerve traffic by leptin and insulin. Both leptin and insulin are able to stimulate mTORC1 signaling in the ARC through phosphatidylinositol-3-kinase (PI3K, Figure 2). Blockade of ARC mTORC1 virtually eliminated the increase in renal SNA and arterial pressure caused by leptin (70). On the other hand, insulin requires ARC mTORC1 signaling to stimulate lumbar SNA (62). Thus, leptin and insulin engage selectively mTORC1 signaling in the ARC to cause sympathetic nerve activation to the kidney and hindlimb, respectively. However, the cellular and neuronal pathways implicated in ARC mTORC1 control of cardiovascular sympathetic function remain to be determined.
Conclusions and Perspectives
The ARC plays a prominent role in coordinating the regulation of multiples physiological functions to maintain homeostasis. Evidence discussed above demonstrates that this nucleus is an integral part of the neural network governing cardiovascular function. Hemodynamic and sympathetic changes can be evoked from ARC, and neurons in this nucleus appear to play a critical role in sensing and relying signals emanating from the circulation to neuroendocrine and autonomic systems to mediate cardiovascular responses. The ARC neurons also receive neuronal signals from many brain regions and perhaps from peripheral organs including those involved in cardiovascular control. Human studies implicating the melanocortin system in blood pressure regulation (71) and obesity-associated hypertension and sympathetic overdrive (71;72) highlight the relevance of the ARC for cardiovascular health in humans. These findings also emphasize the need for studies in human subjects to translate laboratory discoveries regarding the cardiovascular regulation by the ARC to clinical applications.
Presence in the ARC of several components of the renin–angiotensin system—including angiotensinogen, renin, angiotensin (Ang)-converting enzyme, and Ang type 1 receptor—points to a possible contribution to arterial pressure homeostasis of Ang II produced locally in the ARC (73–75). This is further supported by the increase in arterial pressure and heart rate after direct ARC application of Ang II (74). Assessing the significance of the ARC renin–angiotensin system may provide clues about the role of this brain nucleus in cardiovascular health and disease, especially in disorders that compromise the integrity of the blood–brain barrier rendering the ARC accessible to circulating Ang II (76).
Many of the details of the molecular and cellular processes, as well as the downstream neuronal pathways by which the ARC regulates the cardiovascular system, are emerging. Investigations of these processes and pathways have been hindered by the high degree of overlap in the mechanisms and networks underlying ARC control of various physiological processes. Dissection of the neural network emanating from the ARC that influences the cardiovascular system is central to our understanding of the role of the ARC in the cardiovascular complications of disease states such as obesity (49;77). Finally, the contribution of ARC cells other than neurons such as microglia (78) to cardiovascular regulation remain largely untapped. Obtaining insights into the molecular and cellular mechanisms underlying cardiovascular regulation by the ARC will enhance our understanding how the brain controls circulation.
Acknowledgments
The author wishes to thank Paul Casella for English-language editing of the manuscript.
Sources of Funding
The author's research is supported by the US National Institutes of Health (HL084207), the American Heart Association (14EIA18860041), the University of Iowa Fraternal Order of Eagles Diabetes Research Center, and the University of Iowa Center for Hypertension Research.
Footnotes
Disclosures: None
Supplemental Information: None
References
- 1.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 2.Malpas SC. Sympathetic Nervous System Overactivity and Its Role in the Development of Cardiovascular Disease. Physiol Rev. 2010;90:513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 3.Carmichael CY, Wainford RD. Hypothalamic signaling mechanisms in hypertension. Curr Hypertens Rep. 2015;17:39. doi: 10.1007/s11906-015-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dampney RAL, Horiuchi J, Killinger S, Sheriff MJ, Tan PSP, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: Some critical questions. Clin Exp Pharmacol Physiol. 2005;32:419–425. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 5.Sapru HN. Role of the hypothalamic arcuate nucleus in cardiovascular regulation. Auton Neurosci. 2013;175:38–50. doi: 10.1016/j.autneu.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chronwall BM. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides. 1985;6:1–11. doi: 10.1016/0196-9781(85)90128-7. [DOI] [PubMed] [Google Scholar]
- 7.Everitt BJ, Meister B, Hokfelt T, Melander T, Terenius L, Rokaeus A, Theodorsson-Norheim E, Dockray G, Edwardson J, Cuello C. The hypothalamic arcuate nucleus-median eminence complex: immunohistochemistry of transmitters, peptides and DARPP-32 with special reference to coexistence in dopamine neurons. Brain Res. 1986;396:97–155. doi: 10.1016/s0006-8993(86)80192-5. [DOI] [PubMed] [Google Scholar]
- 8.Langlet F. Tanycytes: a gateway to the metabolic hypothalamus. J Neuroendocrinol. 2014;26:753–760. doi: 10.1111/jne.12191. [DOI] [PubMed] [Google Scholar]
- 9.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- 10.Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci. 2004;24:2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 12.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 13.Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH --> arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- 14.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, Liberles SD, Lowell BB. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cano G, Card JP, Sved AF. Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J Comp Neurol. 2004;471:462–481. doi: 10.1002/cne.20040. [DOI] [PubMed] [Google Scholar]
- 16.Montanaro MS, Allen AM, Oldfield BJ. Structural and functional evidence supporting a role for leptin in central neural pathways influencing blood pressure in rats. Exp Physiol. 2005;90:689–696. doi: 10.1113/expphysiol.2005.030775. [DOI] [PubMed] [Google Scholar]
- 17.Solano-Flores LP, Rosas-Arellano MP, Ciriello J. Fos induction in central structures after afferent renal nerve stimulation. Brain Res. 1997;753:102–119. doi: 10.1016/s0006-8993(96)01497-7. [DOI] [PubMed] [Google Scholar]
- 18.Simon JK, Ciriello J. Contribution of afferent renal nerves to the metabolic activity of central structures involved in the control of the circulation. Can J Physiol. Pharmacol. 1989;67:1130–1139. doi: 10.1139/y89-180. [DOI] [PubMed] [Google Scholar]
- 19.Turton WE, Ciriello J, Calaresu FR. Changes in forebrain hexokinase activity after aortic baroreceptor denervation. Am J Physiol. 1986;251:R274–R281. doi: 10.1152/ajpregu.1986.251.2.R274. [DOI] [PubMed] [Google Scholar]
- 20.Li YW, Dampney RA. Expression of Fos-like protein in brain following sustained hypertension and hypotension in conscious rabbits. Neuroscience. 1994;61:613–634. doi: 10.1016/0306-4522(94)90439-1. [DOI] [PubMed] [Google Scholar]
- 21.Potts PD, Ludbrook J, Gillman-Gaspari TA, Horiuchi J, Dampney RA. Activation of brain neurons following central hypervolaemia and hypovolaemia: contribution of baroreceptor and non-baroreceptor inputs. Neuroscience. 2000;95:499–511. doi: 10.1016/s0306-4522(99)00426-1. [DOI] [PubMed] [Google Scholar]
- 22.Lepetit P, Grange E, Gay N, Bobillier P. Comparison of the effects of chronic water deprivation and hypertonic saline ingestion on cerebral protein synthesis in rats. Brain Res. 1992;586:181–187. doi: 10.1016/0006-8993(92)91625-o. [DOI] [PubMed] [Google Scholar]
- 23.O'Shea RD, Gundlach AL. Preproneuropeptide Y Messenger Ribonucleic Acid in the Hypothalamic Arcuate Nucleus of the Rat is Increased by Food Deprivation or Dehydration. J Neuroendocrinol. 1991;3:11–14. doi: 10.1111/j.1365-2826.1991.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 24.Simon JK, Kasting NW, Ciriello J. Afferent renal nerve effects on plasma vasopressin and oxytocin in conscious rats. Am J Physiol. 1989;256:R1240–R1244. doi: 10.1152/ajpregu.1989.256.6.R1240. [DOI] [PubMed] [Google Scholar]
- 25.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosc. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 27.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 28.Ghamari-Langroudi M, Digby GJ, Sebag JA, Millhauser GL, Palomino R, Matthews R, Gillyard T, Panaro BL, Tough IR, Cox HM, Denton JS, Cone RD. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature. 2015;520:94–98. doi: 10.1038/nature14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koch M, Varela L, Kim JG, Kim JD, Hernandez-Nuno F, Simonds SE, Castorena CM, Vianna CR, Elmquist JK, Morozov YM, Rakic P, Bechmann I, Cowley MA, Szigeti-Buck K, Dietrich MO, Gao XB, Diano S, Horvath TL. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519:45–50. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choudhury AI, Heffron H, Smith MA, Al-Qassab H, Xu AW, Selman C, Simmgen M, Clements M, Claret M, Maccoll G, Bedford DC, Hisadome K, Diakonov I, Moosajee V, Bell JD, Speakman JR, Batterham RL, Barsh GS, Ashford ML, Withers DJ. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J Clin Invest. 2005;115:940–950. doi: 10.1172/JCI24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allison MB, Patterson CM, Krashes MJ, Lowell BB, Myers MG, Jr., Olson DP. TRAP-seq defines markers for novel populations of hypothalamic and brainstem LepRb neurons. Mol Metab. 2015;4:299–309. doi: 10.1016/j.molmet.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Kelly L, Heiman M, Greengard P, Friedman JM. Hypothalamic Amylin Acts in Concert with Leptin to Regulate Food Intake. Cell Metab. 2015;22:1059–1067. doi: 10.1016/j.cmet.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Guan JL, Wang QP, Kageyama H, Takenoya F, Kita T, Matsuoka T, Funahashi H, Shioda S. Synaptic interactions between ghrelin- and neuropeptide Y-containing neurons in the rat arcuate nucleus. Peptides. 2003;24:1921–1928. doi: 10.1016/j.peptides.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Levin BE. Metabolic sensing neurons and the control of energy homeostasis. Physiol Behav. 2006;89:486–489. doi: 10.1016/j.physbeh.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Gruber KA, Callahan MF. ACTH-(4-10) through gamma-MSH: evidence for a new class of central autonomic nervous system-regulating peptides. Am J Physiol. 1989;257:R681–R694. doi: 10.1152/ajpregu.1989.257.4.R681. [DOI] [PubMed] [Google Scholar]
- 37.Versteeg DH, Van BP, Adan RA, De Wildt DJ. Melanocortins and cardiovascular regulation. Eur J Pharmacol. 1998;360:1–14. doi: 10.1016/s0014-2999(98)00615-3. [DOI] [PubMed] [Google Scholar]
- 38.Brody MJ, O'Neill TP, Porter JP. Role of Paraventricular and Arcuate Nuclei in Cardiovascular Regulation. In: Magro A, Osswald W, Reis D, Vanhoutte P, editors. Central and Peripheral Mechanisms of Cardiovascular Regulation. Plenum Publishing Corporation; 1986. pp. 443–464. [Google Scholar]
- 39.Mastrianni JA, Palkovits M, Kunos G. Activation of brainstem endorphinergic neurons causes cardiovascular depression and facilitates baroreflex bradycardia. Neuroscience. 1989;33:559–566. doi: 10.1016/0306-4522(89)90408-9. [DOI] [PubMed] [Google Scholar]
- 40.Li SJ, Varga K, Archer P, Hruby VJ, Sharma SD, Kesterson RA, Cone RD, Kunos G. Melanocortin antagonists define two distinct pathways of cardiovascular control by alpha- and gamma-melanocyte-stimulating hormones. J Neurosci. 1996;16:5182–5188. doi: 10.1523/JNEUROSCI.16-16-05182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura T, Bhatt S, Sapru HN. Cardiovascular Responses to Hypothalamic Arcuate Nucleus Stimulation in the Rat Role of Sympathetic and Vagal Efferents. Hypertension. 2009;54:1369–1375. doi: 10.1161/HYPERTENSIONAHA.109.140715. [DOI] [PubMed] [Google Scholar]
- 42.Shi Z, Cassaglia PA, Gotthardt LC, Brooks VL. Hypothalamic Paraventricular and Arcuate Nuclei Contribute to Elevated Sympathetic Nerve Activity in Pregnant Rats: Roles of Neuropeptide Y and alpha-Melanocyte-Stimulating Hormone. Hypertension. 2015;66:1191–1198. doi: 10.1161/HYPERTENSIONAHA.115.06045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 44.Marsh AJ, Fontes MA, Killinger S, Pawlak DB, Polson JW, Dampney RA. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension. 2003;42:488–493. doi: 10.1161/01.HYP.0000090097.22678.0A. [DOI] [PubMed] [Google Scholar]
- 45.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49:647–652. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 46.Tanida M, Yamamoto N, Morgan DA, Kurata Y, Shibamoto T, Rahmouni K. Leptin receptor signaling in the hypothalamus regulates hepatic autonomic nerve activity via phosphatidylinositol 3-kinase and AMP-activated protein kinase. J Neurosci. 2015;35:474–484. doi: 10.1523/JNEUROSCI.1828-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the Leptin Receptor in the Hypothalamic Arcuate Nucleus Abrogates Leptin-Induced Sympathetic Activation. Circ Res. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.do Carmo JM, da Silva AA, Cai ZW, Lin SY, Dubinion JH, Hall JE. Control of Blood Pressure, Appetite, and Glucose by Leptin in Mice Lacking Leptin Receptors in Proopiomelanocortin Neurons. Hypertension. 2011;57:918–926. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23:5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.da Silva AA, Kuo JJ, Hall JE. Role of hypothalamic melanocortin 3/4-receptors in mediating chronic cardiovascular, renal, and metabolic actions of leptin. Hypertension. 2004;43:1312–1317. doi: 10.1161/01.HYP.0000128421.23499.b9. [DOI] [PubMed] [Google Scholar]
- 52.Zhang ZH, Felder RB. Melanocortin receptors mediate the excitatory effects of blood-borne murine leptin on hypothalamic paraventricular neurons in rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R303–R310. doi: 10.1152/ajpregu.00504.2003. [DOI] [PubMed] [Google Scholar]
- 53.Shi Z, Li B, Brooks VL. Role of the Paraventricular Nucleus of the Hypothalamus in the Sympathoexcitatory Effects of Leptin. Hypertension. 2015;66:1034–1041. doi: 10.1161/HYPERTENSIONAHA.115.06017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landsberg L. Feast or famine: The sympathetic nervous system response to nutrient intake. Cell Mol Neurobiol. 2006;26:497–508. doi: 10.1007/s10571-006-9010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia Produces Both Sympathetic Neural Activation and Vasodilation in Normal Humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muntzel MS, Anderson EA, Johnson AK, Mark AL. Mechanisms of Insulin Action on Sympathetic-Nerve Activity. Clin Exp Hypertens. 1995;17:39–50. doi: 10.3109/10641969509087053. [DOI] [PubMed] [Google Scholar]
- 57.Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest. 2004;114:652–658. doi: 10.1172/JCI21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pricher MP, Freeman KL, Brooks VL. Insulin in the brain increases gain of baroreflex control of heart rate and lumbar sympathetic nerve activity. Hypertension. 2008;51:514–520. doi: 10.1161/HYPERTENSIONAHA.107.102608. [DOI] [PubMed] [Google Scholar]
- 59.Muntzel M, Beltz T, Mark AL, Johnson AK. Anteroventral 3Rd Ventricle Lesions Abolish Lumbar Sympathetic Responses to Insulin. Hypertension. 1994;23:1059–1062. doi: 10.1161/01.hyp.23.6.1059. [DOI] [PubMed] [Google Scholar]
- 60.Cassaglia PA, Hermes SM, Aicher SA, Brooks VL. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol. 2011;589:1643–1662. doi: 10.1113/jphysiol.2011.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luckett BS, Frielle JL, Wolfgang L, Stocker SD. Arcuate nucleus injection of an anti-insulin affibody prevents the sympathetic response to insulin. Am J Physiol Heart Circ Physiol. 2013;304:H1538–H1546. doi: 10.1152/ajpheart.00081.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muta K, Morgan DA, Rahmouni K. The role of hypothalamic mTORC1 signaling in insulin regulation of food intake, body weight, and sympathetic nerve activity in male mice. Endocrinology. 2015;156:1398–1407. doi: 10.1210/en.2014-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plum L, Belgardt BF, Bruning JC. Central insulin action in energy and glucose homeostasis. J Clin Invest. 2006;116:1761–1766. doi: 10.1172/JCI29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension. 2011;57:435–441. doi: 10.1161/HYPERTENSIONAHA.110.160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stocker SD, Gordon KW. Glutamate receptors in the hypothalamic paraventricular nucleus contribute to insulin-induced sympathoexcitation. J Neurophysiol. 2015;113:1302–1309. doi: 10.1152/jn.00764.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 68.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–6422. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- 69.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;127:5–19. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 70.Harlan SM, Guo DF, Morgan DA, Fernandes-Santos C, Rahmouni K. Hypothalamic mTORC1 signaling controls sympathetic nerve activity and arterial pressure and mediates leptin effects. Cell Metab. 2013;17:599–606. doi: 10.1016/j.cmet.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O'Rahilly S, Farooqi IS. Modulation of Blood Pressure by Central Melanocortinergic Pathways. N Engl J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 72.Sayk F, Heutling D, Dodt C, Iwen KA, Wellhoner JP, Scherag S, Hinney A, Hebebrand J, Lehnert H. Sympathetic Function in Human Carriers of Melanocortin-4 Receptor Gene Mutations. J Clin Endocrinol Metabol. 2010;95:1998–2002. doi: 10.1210/jc.2009-2297. [DOI] [PubMed] [Google Scholar]
- 73.Healy DP, Printz MP. Distribution of immunoreactive angiotensin II, angiotensin I, angiotensinogen and renin in the central nervous system of intact and nephrectomized rats. Hypertension. 1984;6:I130–I136. doi: 10.1161/01.hyp.6.2_pt_2.i130. [DOI] [PubMed] [Google Scholar]
- 74.Arakawa H, Chitravanshi VC, Sapru HN. The hypothalamic arcuate nucleus: a new site of cardiovascular action of angiotensin-(1-12) and angiotensin II. Am J Physiol Heart Circ Physiol. 2011;300:H951–H960. doi: 10.1152/ajpheart.01144.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, Rahmouni K, Sigmund CD, Mark AL. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2012;303:H197–H206. doi: 10.1152/ajpheart.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood–brain barrier. Hypertension. 2014;63:572–579. doi: 10.1161/HYPERTENSIONAHA.113.01743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rahmouni K. Obesity-associated hypertension: recent progress in deciphering the pathogenesis. Hypertension. 2014;64:215–221. doi: 10.1161/HYPERTENSIONAHA.114.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reis WL, Yi CX, Gao Y, Tschop MH, Stern JE. Brain innate immunity regulates hypothalamic arcuate neuronal activity and feeding behavior. Endocrinology. 2015;156:1303–1315. doi: 10.1210/en.2014-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]