Abstract
The main actions of the renin-angiotensin system to control blood pressure (BP) are mediated by the angiotensin type 1 receptors (AT1Rs). The major murine AT1R isoform, AT1AR, is expressed throughout the nephron, including the collecting duct in both principal and intercalated cells. Principal cells play the major role in sodium and water reabsorption. Although aldosterone is considered to be the dominant regulator of sodium reabsorption by principal cells, recent studies suggest a role for direct actions of AT1R. In order to specifically examine the contributions of AT1AR in principal cells to BP regulation and the development of hypertension in vivo, we generated inbred 129/SvEv mice with deletion of AT1AR from principal cells (PCKO). At baseline, we found that BPs measured by radiotelemetry were similar between PCKOs and controls. During one-week of low salt diet (<0.02% NaCl), BPs fell significantly (P<0.05) and to a similar extent in both groups. On a high salt (6% NaCl) diet, BP increased but was not different between groups. During the initial phase of Ang II-dependent hypertension, there was a modest but significant attenuation of hypertension in PCKOs (163±6 mmHg) compared to controls (178±2 mmHg, P<0.05) that was associated with enhanced natriuresis and decreased αENaC activation in the medulla of PCKOs. However, from day 9 onward, BPs were indistinguishable between groups. While effects of AT1AR on baseline BP and adaptation to changes in dietary salt are negligible, our studies suggest that direct actions of AT1AR contribute to the initiation of hypertension and ENaC activation.
Keywords: AT1A receptor, collecting duct, angiotensin II, hypertension, ENaC
Introduction
The renin-angiotensin system (RAS) occupies a central role in blood pressure regulation1. The major effector peptide of the RAS, angiotensin II (Ang II), is generated by sequential cleavage of the substrate angiotensinogen and acts via the angiotensin type 1 (AT1) receptor to increase blood pressure2, 3. Studies from our laboratory using kidney cross-transplantation have demonstrated the critical role of AT1 receptors inside the kidney to control blood pressure4 and to promote hypertension5. In the kidney, AT1 receptors are ubiquitously expressed in glomeruli, vascular and renal epithelia6. Ang II-dependent hypertension is associated with enhanced renal sodium reabsorption, suggesting that AT1 receptors impact blood pressure through control of renal sodium handling3.
Sodium is reabsorbed from the glomerular ultra-filtrate sequentially as it flows along the length of the nephron. A significant proportion (~60%) of filtered sodium is normally reabsorbed by the proximal tubule, largely in iso-osmotic fashion7. On the other hand, only about 5% of the filtered sodium load is reclaimed in the collecting duct, the terminal portion of the nephron. Despite the relatively small magnitude of sodium reabsorption by the collecting duct, it has been suggested that fine-tuning of sodium reabsorption by this most distal segment of the nephron represents a key step for the final regulation of sodium balance and blood pressure8.
In the collecting duct, sodium transport is primarily mediated by the epithelial sodium channel (ENaC)8. The capacity of this pathway to impact blood pressure is highlighted by observations that gain-of-function mutations of ENaC cause hypertension9. While regulation of ENaC by aldosterone acting through the mineralocorticoid receptor has been well documented10, recent studies have suggested direct effects of angiotensin via AT1 the receptor in the collecting duct to modulate sodium reabsorption11–14. The collecting duct consists of two main epithelial cell lineages: principal and intercalated cells15. The traditional view is that principal cells play the predominant role in reabsorption of water and sodium, whereas intercalated cells are primarily responsible for urinary acidification, with limited cross-talk due to the lack of gap junctions between the two cell types16, 17. On the other hand, recent work has shown substantive sodium reabsorption by intercalated cells18, along with coordinated actions of the two collecting duct cell lineages by paracrine mediators19, 20.
The goal of the present study is to examine the contribution of direct actions of AT1A receptors in principal cells of the collecting duct to blood pressure homeostasis and hypertension pathogenesis. To this end, we utilized Cre-loxP techniques to specifically eliminate AT1A receptors from principal cells. We found that AT1A receptors in principal cells of the collecting duct have little influence on basal blood pressure or adaptation to changes in dietary salt intake, whereas they contribute modestly to the initiation phase of Ang II-induced hypertension, associated with sodium retention and regulation of αENaC protein abundance.
Material and Methods
Study Approval
All experimental mice, maintained on a 129/SvEv background, were bred in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited animal facility at the Durham Veterans Affairs Medical Center under National Institutes of Health Guidelines for Care and Use of Laboratory Animals and housed with free access to standard rodent chow and water unless otherwise specified.
Statistical Analysis
The values for each parameter within a group are expressed as the mean ± the standard error of the mean (SEM). For comparisons between two experimental groups with normally distributed data, statistical significance was assessed using unpaired T test. For comparisons within groups, a paired T test was employed. Normality was determined using the Shapiro-Wilk W test. P<0.05 was considered statistically significant.
Detailed description of procedures is available in Methods in the online-only Data Supplement.
Results
Generation of mice lacking AT1A receptor in principal cells of collecting duct
To delete the floxed Agtr1a allele only in the principal cells of the collecting duct, we used the transgenic mouse line where Cre expression is driven by the promoter of the human AQP2 gene21. Specific expression of Cre in principal cells was verified by crossing with a double-fluorescence reporter mouse (mTmG), the presence of Cre recombinase triggers green fluorescence protein (GFP) expression on a background of constitutive expression of red florescence. As shown in Figure 1, robust Cre recombinase activity was restricted to principal cells of the collecting duct. We carried out a series of crosses to generate AQP2-Cre-Agtr1aflox/flox mice lacking AT1A receptors in principal cells (PCKOs) on 129SvEv background. To verify the extent of AT1A receptor deletion in PCKO mice, we prepared purified preparations of enriched inner medullary collecting ducts (IMCDs). Using real-time RT-PCR, we found a significant ~60% reduction in AT1A receptor mRNA in PCKO IMCDs compared to controls (1 ± 0.2 vs. 0.39 ± 0.05; P=0.04). We suggest that the residual AT1AR mRNA receptor expression in these IMCDs is primarily due to AT1A receptor expression in intercalated cells, which we have found express significant levels of AT1A receptors (Stegbauer et al., Submitted). To determine whether deletion of AT1A receptors from principal cells might affect expression in other parts of the kidney, we compared AT1A receptor mRNA levels in kidney cortex of control and PCKO mice, and found no significant differences (Control 1 ± 0.3 vs. PCKO 0.85 ± 0.2, P=0.72, Figure S1).
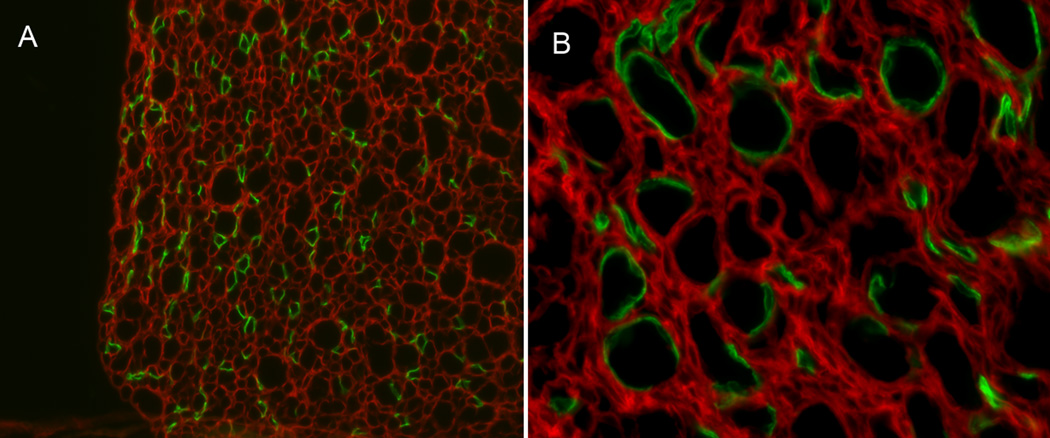
Figure 1. Photomicrographs of sections of kidney medulla in AQP2-Cre mTmG mice.
Specific expression of Cre in principal cells of the collecting duct was confirmed by crossing with a double-fluorescence reporter mouse (mTmG). Representative sections showing renal medulla from AQP2-Cre mTmG mice at: (A) 20× and (B) 40×. Expression of GFP indicates Cre recombinase expression, whereas red fluorescence indicates no presence of Cre.
The absence of AT1A receptors does not affect baseline blood pressure or sodium sensitivity
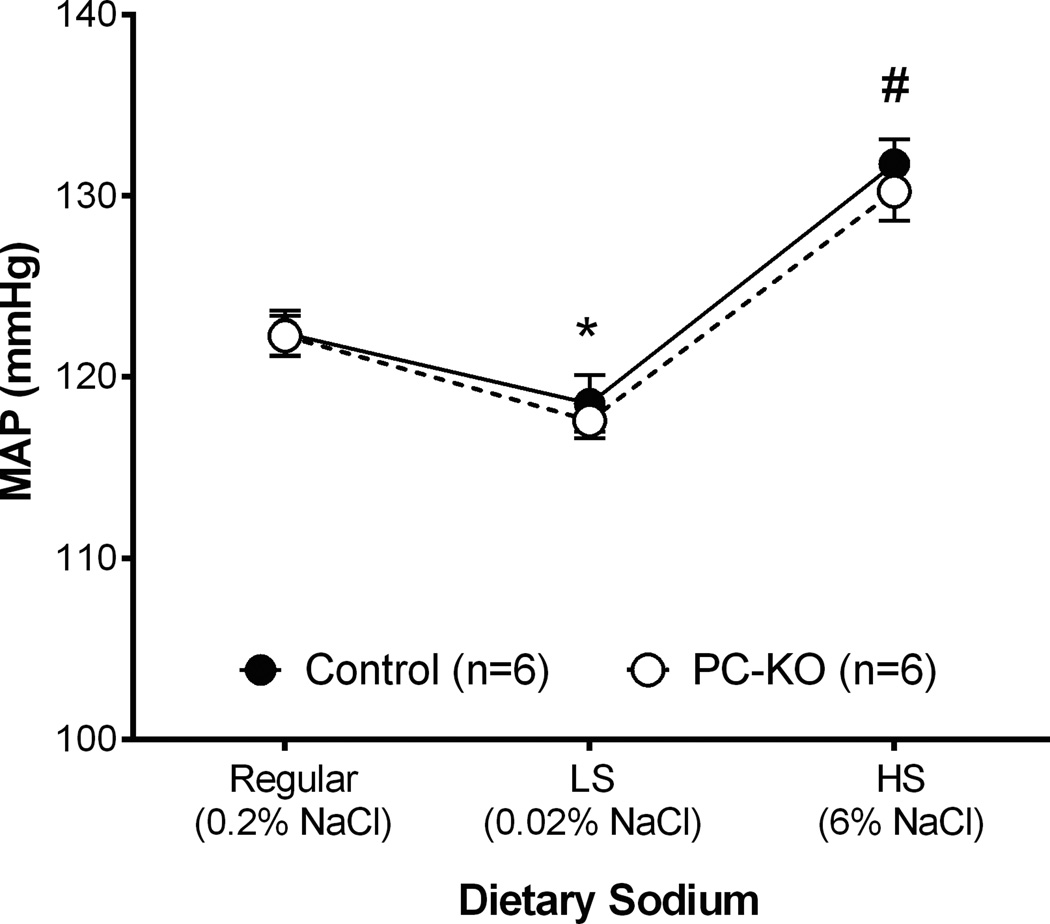
To define the impact of deleting AT1A receptors in principal cells on blood pressure, radiotelemetry units were implanted into 8–12 week old male PCKO (n=6) and control mice (n=6) to measure intra-arterial pressures. Baseline blood pressures were similar in control and PCKO mice (122 ± 2 mmHg vs. 122 ± 2 mmHg, Figure 2). We also measured cortical renin mRNA levels at baseline and found no differences between control and PCKO mice (1 ± 0.12 vs. 0.82 ± 0.08; P=0.23). Likewise, basal urinary sodium excretion was not different between PCKO and control mice (12.44 ± 1.1µmol/hr vs.11.9 ± 1.2 µmol/hr, respectively, P=0.77).
Figure 2. Mean arterial pressure (MAP) responses at baseline and to altered dietary sodium intake.
Baseline blood pressures were similar in control and PCKO mice. With one-week of low sodium (<0.02% NaCl) diet, MAP fell significantly compared to normal salt diet and to a similar extent in both PCKO and control mice (*P<0.01 vs. normal salt diet). Similarly, with a high sodium (6% NaCl) diet, MAP increased (#P<0.01 vs. normal salt diet) but was not different between groups. Values are means ± SEM.
In previous studies, we found that mice with global deficiency of AT1A receptors have exaggerated sodium sensitivity22, defined as enhanced blood pressure fluctuation in response to alterations in dietary sodium intake23. As shown in Figure 2, with one-week of low sodium (<0.02% NaCl) feeding, MAP fell significantly in the 129/SvEv control mice from 122 ± 1 mmHg to 118 ± 2 mmHg (P=0.0056), which is characteristic of the 129/SvEv strain. A similar pattern was seen in the PCKO mice, where blood pressure fell 122 ± 1 mmHg to 117±1 mmHg (P=0.0043), but blood pressures on low salt diet were not different from controls (P=0.62). Similarly, with a high sodium (6% NaCl) diet, there was a significant increase in MAP in control mice compared to the normal salt diet (132 ± 1 mmHg vs. 122 ± 1 mmHg; P=0.0002). An equivalent increase was seen in the PCKOs during high salt feeding, but once again, the blood pressures during high salt feeding were not different between the groups (PCKO: 130 ± 2 mmHg; Controls: 132 ± 2 mmHg; P=0.50). Thus, elimination of AT1A receptor from principal cells does not affect baseline blood pressures and does not recapitulate the exaggerated sodium sensitivity seen in mice with global AT1A receptor deficiency22.
Effects of AT1A receptor in principal cells on the development of hypertension
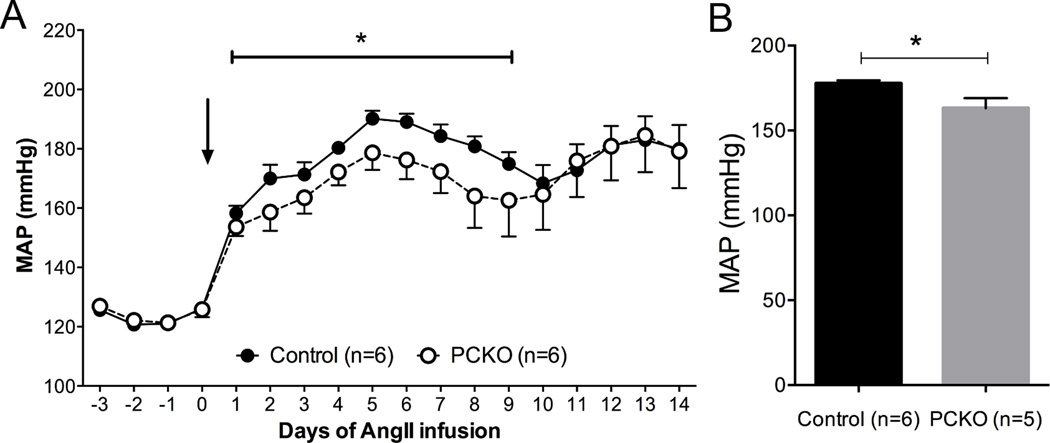
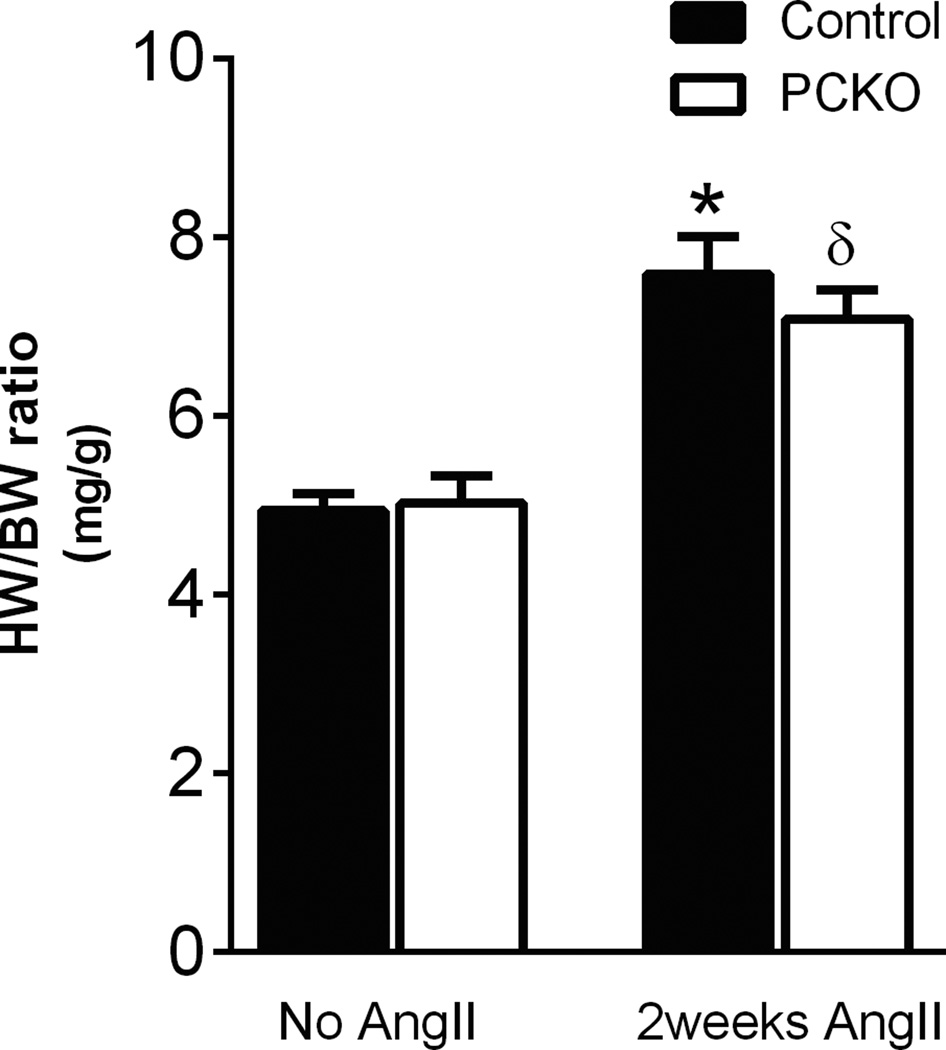
In order to determine the contribution of AT1A receptors in principal cell epithelia to the development of hypertension, osmotic minipumps were implanted into PCKO mice and controls to infuse Ang II for 2 weeks and intra-arterial pressures were measured by radiotelemetry. As shown in Figure 3A, during the initial phase of hypertension (days 2–8), there was a modest but significant attenuation of mean arterial pressure in the PCKOs (163 ± 6 mmHg) compared to controls (178 ± 2 mmHg; P<0.05; by one-way ANOVA). Similarly, as shown in Figure 3B, MAP averaged over the first 9 days of Ang II infusion was significantly attenuated in the PCKO mice (*P<0.05, t-test). However after day 10 of Ang II infusion, blood pressure levels were virtually identical between groups and there was no difference in blood pressures in this later phase of hypertension. To assess cardiac hypertrophy, an important clinical sign of end-organ damage in hypertension, we measured heart-to-body weight ratios. In mice that did not receive Ang II, heart-to-body weight ratios were similar in controls (4.94 ± 0.2 mg HW/g BW) and PCKOs (5.02 ± 0.3 mg/g; P=0.81). As shown in Figure 4, heart-to-body weight ratios were higher in both groups after Ang II infusion for 14 days compared to non-infused mice (PCKO: P=0.0055; Controls P=0.0004) but while the extent of cardiac hypertrophy induced by Ang II tended to be less in the PCKOs, this difference was not statistically significant (PCKO: 7.08 ± 0.3 mg/g; Controls: 7.6 ± 0.4 mg HW/g BW; P=0.36).
Figure 3. Blood pressure responses to chronic Angiotensin II infusion.
Infusion of Ang II (1000ng/kg/min) caused significant increases in mean arterial pressure (MAP) in both controls and PCKOs. A) Daily radiotelemetry tracings from PCKO and control mice infused with Ang II over 2 weeks. (*p<0.05 by ANOVA for days 2–8) B) Average MAP during first 9 days of Ang II infusion was significantly lower in PCKOs than controls. (*P<0.05, t-test). Values are means ± SEM.
Figure 4. Mean heart-to-body weight ratio before and after 2 weeks of chronic Angiotensin II infusions.
Basal mean heart to body weight ratio is similar between control and PCKO mice. After 2 weeks of Ang II infusion, both PCKO and control mice exhibited significant cardiac hypertrophy compared to baseline (δP=0.005 and *P=0.0004, respectively, vs. baseline) but there were no differences in the extent of cardiac hypertrophy between groups. Values are means ± SEM.
To determine whether modification of other vasoactive systems regulated by angiotensin II might be affected in the PCKOs, we measured expression of inducible cyclo-oxygenase isoform, COX-2. We found no differences in COX2 mRNA expression in renal medulla between PCKO and controls after 2 weeks of Ang II infusion (Control 1 ± 0.4 vs. PCKO 0.75 ± 0.2, P=0.55, Figure S2A). We also examined urinary prostaglandin PGE2 excretion in the experimental groups (Figure S2B). We found no difference in urinary PGE2 excretion at baseline (Control 466 ± 42 pg/24hr vs. PCKO 461 ± 38 pg/24hr, P=0.92). After 2 weeks of Ang II infusion, urinary PGE2 levels increased significantly and to a similar extent in both groups (PCKO 1482 ± 245 pg/24hr, #P=0.01 vs baseline; Controls 1330 ± 141 pg/24hr,*P=0.0017 vs baseline).
Exaggerated natriuresis in PCKO mice
In order to investigate the early separation in blood pressures between PCKO and control mice, we performed a sodium balance study. A separate group of mice were individually placed in specially designed metabolic cages24 with access to 10g/day gel diet containing nutrients, water and 0.1% w/w sodium (Nutra-Gel; Bio-Serv, Frenchtown, NJ). After 5 days of baseline collections, the mice were implanted with osmotic minipumps infusing Ang II as described above. Urinary sodium content was determined by using an IL943 Automatic Flame photometer per manufacturer’s instructions (Instrumentation Laboratory, Lexington, MA). After 7 days of Ang II infusion, urinary sodium excretion was significantly higher in the PCKO mice (511.2 ± 29µmol/day) compared to controls (338.6 ± 65µmol/day; P=0.033). This exaggerated natriuresis temporally corresponds with the attenuation in the early phase hypertension observed in the PCKOs.
After 2 weeks of Ang II infusion, serum potassium levels were similar (Control 4.50 ± 0.4 mmol/L vs. PCKO 3.97 ± 0.3 mmol/L, P=0.31, Figure S3).
Modulation of epithelial sodium channel protein abundance by AT1A receptors in principal cells of the collecting duct
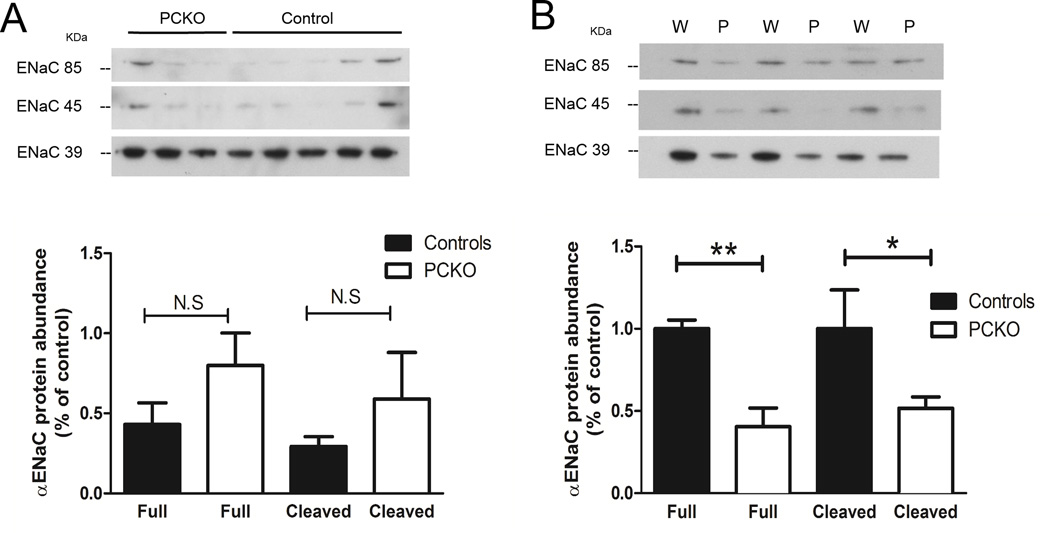
Previous studies have suggested that direct actions of Ang II on AT1 receptors in the collecting duct can affect blood pressure by directly stimulating the activity of the epithelial sodium channels25. Accordingly, to determine whether the absence of AT1A receptors in the principal cells of the collecting duct affected the abundance of the αENaC sub-unit, we measured αENaC protein by immunoblotting. The anti-αENaC antibody used in this study typically recognizes specific protein bands in kidney with apparent masses of ~85 kDa and ~40 kDa26, 27. Previous work has suggested that the ~85 kDa form is the full-length form, whereas the smaller ~40 kDa band is a proteolytic fragment of the α-subunit27–30 cleaved near the NH2 terminus31. This proteolytic cleavage enhances activity of the channel32.
Because ENaC is expressed in both connecting tubule and collecting duct in the cortex and expression of the AQP2-Cre is more robust in the renal medulla, we focused on ENaC levels in medulla. As shown in Figure 5A, relatively low levels of the full-length and cleaved forms of αENaC protein were detected at baseline in both Control and PCKO mice and there were no significant differences in total αENaC protein abundance between the groups. These data indicate that, under basal conditions, the AT1 receptor has little influence on abundance of αENaC protein in medullary collecting duct. This is consistent with our finding of similar blood pressures at baseline in control and PCKO mice. Infusion of Ang II for 7 days caused a significant increase in medullary full-length αENaC protein abundance in control mice (Basal 0.77 ± 0.1 vs. Ang II 1.88 ± 0.4; P=0.03), but had no effect on medullary full-length αENaC protein abundance in PCKO mice (Basal 1.0 ± 0.2 vs. Ang II 0.68 ± 0.2; P=0.36). As shown in Figure 5B, after Ang II infusion, levels of both full length (0.40 ± 0.1 vs. 1 ± 0.1; P=0.004) and cleaved αENaC (0.52 ± 0.1 vs. 1 ± 0.2; P<0.05) were significantly reduced in PCKOs compared to controls.
Figure 5. αENaC protein abundance in the inner region of renal medulla.
Representative blots showing protein levels the full length (FL) or cleaved (C) forms of of αENaC in inner medulla of control (n=9) and PCKO (n=7) mice. The bar graph shows quantification of band intensity. (A) At baseline, levels of full-length and cleaved ENaC protein were relatively low and similar between groups. (B) In control mice, there was a significant increase in forms of ENaC with Ang II infusion. In PCKOs, full length ENaC was reduced by 60% (0.40 ± 0.1 vs. 1 ± 0.1, respectively, P=0.0038) and the cleaved form was reduced by 48% compared to controls (0.52 ± 0.1 vs. 1 ± 0.2, respectively, P=0.046). Values are means ± SEM.
Urinary aldosterone and prostaglandin metabolite levels are similar in Ang II-infused PCKO and control mice
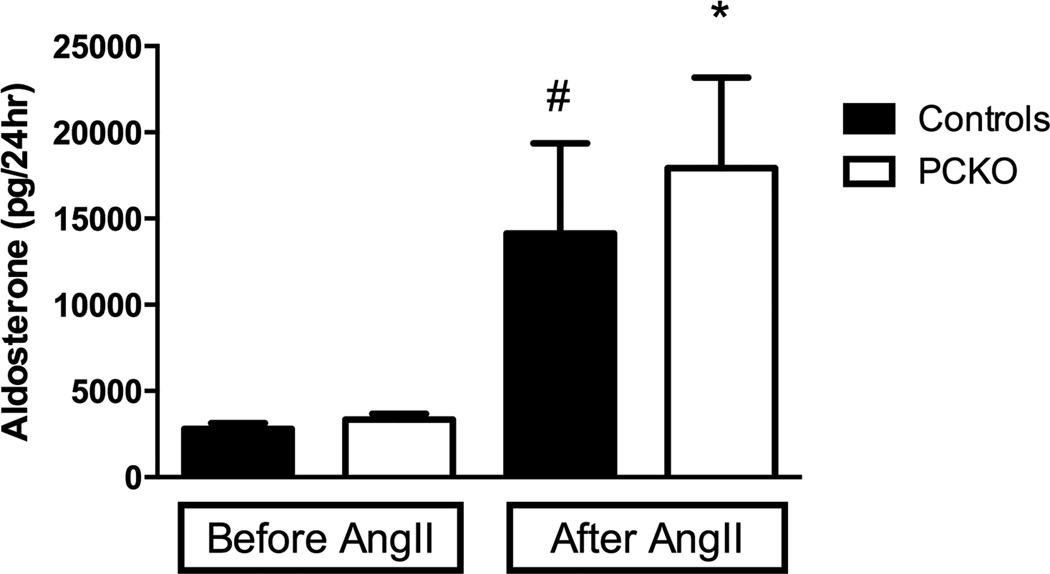
To rule out the possibility that the observed differences in αENaC abundance might be related to alterations in aldosterone levels, we compared urinary aldosterone excretion in control and PCKO mice at baseline and after 7 days of Ang II infusion. As shown in Figure 6, Ang II infusion caused significant increases in aldosterone levels in both controls and PCKOs. However, there were no significant differences in levels of urinary aldosterone between the two groups either at baseline (PCKO: 3347 ± 340 pg/24hr vs. WT: 2792 ± 334 pg/24hr, P=0.26), or with Ang II infusion (PCKO: 17920 ± 5262 pg/24hr vs. WT: 14140 ± 5213 pg/24hr, P=0.62).
Figure 6. Urinary aldosterone excretion.
At baseline, urinary aldosterone excretion was similar in controls and PCKOs. After 7 days of Ang II infusion, urinary aldosterone increased significantly in both groups (PCKO: P=0.001; Controls: P=0.0125), but there were no differences between controls and PCKOs. Values are means ± SEM.
4. Discussion
Control of sodium excretion by the kidney is a key pathway for regulating body fluid volume and blood pressure. Guyton and associates have suggested that pressure-natriuresis mechanisms where rise in blood pressure triggers enhanced sodium excretion by the kidney, provides a powerful compensatory system to prevent hypertension33. Accordingly, impairment of these pathways would be a prerequisite for sustained elevations in arterial pressure. However, recent work has suggested significant complexity in sodium handling, including immunologically modulated storage of excess sodium in the skin34. Nonetheless, substantial experimental and clinical evidence supports a key contribution of renal excretory mechanisms to blood pressure regulation, including kidney cross-transplantation experiments demonstrating the importance of the kidney as a mediator of hypertension5, 35, human genetic studies demonstrating capacity of mutations affecting renal epithelial transporters to cause chronic high or low blood pressure9 and clinical studies documenting the efficacy of diuretics in treatment of hypertension36.
The renin-angiotensin system (RAS) is a major determinant of sodium homeostasis and its actions to affect blood pressure are primarily mediated by AT1 receptors3. Our previous studies using kidney cross-transplantation showed that AT1A receptors within the kidney are crucial for regulation of blood pressure4 and for the development of hypertension5. Within the kidney, AT1 receptors are broadly expressed by epithelial cells across the nephron where they have been shown to have potent influence on sodium reabsorption3. Among the various nephron segments, actions of AT1 receptors in proximal tubule have perhaps been most clearly characterized37. In this regard, we have previously shown that cell-specific deletion of AT1A receptors from epithelia of the proximal tubule is associated with reduced baseline blood pressure and substantial resistance to hypertension38. These effects are associated with reduced tubule fluid reabsorption, decreased abundance of key sodium transporters, and enhanced natriuresis38. However, elimination of AT1A receptors from proximal tubule does not completely recapitulate the extent of blood pressure reduction or sodium sensitivity seen in the complete absence of renal AT1A receptors2, 5, indicating a significant contribution of other intra-renal AT1A receptors. Accordingly, we examined the role of AT1A receptors in collecting duct using cell-specific gene targeting.
In the classic view, aldosterone is considered to be the major regulator of sodium transport in the collecting duct through its actions to regulate the expression and activity of the epithelial sodium channel (ENaC)39. Yet, mineralocorticoid receptor blockade by spironolactone has minimal effects to reduce blood pressure in Ang II-dependent hypertension40 and previous studies in isolated tubule preparations have demonstrated significant capacity for direct actions of Ang II to influence sodium reabsorption by the collecting duct11, 12, 14. Indeed, our current studies show that AT1A receptors in the collecting duct affect sodium and blood pressure homeostasis. However, their impact on blood pressure is negligible at baseline. Instead, AT1A receptors in principal cells seem to play a modest role during the initiation phase of Ang II-dependent hypertension, where they contribute to the exaggerated renal avidity for sodium, characteristic of this disorder. In the later phases of hypertension, this distinct contribution of AT1A receptors in collecting duct wanes and blood pressures in PCKOs eventually reach elevated levels that are indistinguishable from controls. The AQP2-Cre transgene used in these studies is constitutively expressed, eliminating AT1A receptors during embryonic development and allowing for the possibility of compensatory mechanisms potentially mitigating the physiological cnsequences of loss of these receptors from principal cells. While we cannot detect compensatory changes such as changes in renin or aldosterone, or altered AT1A receptor expression in other parts of the kidney, it is possible that a more dramatic phenotype might be observed using inducible gene deletion system in adult animals.
Our studies provide evidence for a physiological role of AT1 receptors in the collecting duct in vivo, consistent with previous studies. For example, in an isolated perfused cortical collecting duct preparation, Peti-Peterdi and colleagues showed that luminal Ang II leads to an increase in ENaC activity through an AT1 receptor dependent mechanism14. Moreover, mice completely lacking AT1A receptors have reduced αENaC protein abundance despite an overall increase in circulating levels of aldosterone41. More recently, Mamenko and colleagues, using patch clamping in split-opened murine distal nephron showed that Ang II acting through AT1 receptor stimulated ENaC by affecting channel gating12, with similar findings in rat cortical collecting ducts11.
In our current study, we demonstrate significant attenuation of expression of a key sodium channel, αENaC in the renal medulla of PCKOs during Ang II infusion. This reduced expression is observed with both the full-length and cleaved (activated) forms of the protein, and is associated with enhancement of natriuresis and diminished hypertension, despite high levels of aldosterone in PCKOs that are not different from controls. These differences in ENaC protein abundance can be attributed to direct actions of AT1 receptors in principal cells based on the cell specificity of the genetic manipulation and equivalent aldosterone levels in PCKOs and controls. Our studies indicate that AT1A receptors in principal cells constitute a significant regulatory pathway for ENaC in Ang II-dependent hypertension, consistent with previous work by Gonzalez and colleagues showing that enhancement of ENaC function by chronic Ang II infusion was not extinguished by an MR inhibitor42. We have not specifically examined intra-cellular pathways linking AT1 receptors to regulation of ENaC in principal cells, but previous studies have suggested that effects of Ang II on ENaC may be mediated via WNK kinases43, whereas aldosterone affects ENaC expression and activity through distinct pathways involving Sgk144. Finally, while we have shown clear differences in ENaC protein levels between control and PCKOs during Ang II infusion, inhibitor studies with amiloride would be required to assess the impact of these differences in ENaC levels on natriuresis.
Perspectives
This study shows that while AT1A receptors in the principal cells of the collecting duct have minimal influence on blood pressure or adaptation to changes in dietary salt intake, they play a modest role in the initiation of Ang II-dependent hypertension. In the presence of high ambient levels of Ang II, AT1A receptors in the principal cells regulate αENaC protein abundance in the renal medulla and promote enhanced sodium reabsorption. In mice lacking AT1A receptors in principal cells, there is a failure to up-regulate αENaC protein in inner medulla, despite substantial elevations in aldosterone.
Supplementary Material
Novelty and Significance.
What is New?
Absence of AT1A receptors in principal cells of the collecting duct has negligible effects on baseline blood pressure or responses to dietary salt intake, but modestly attenuates the initiation phase of Ang II-induced hypertension. This diminished hypertension is associated with reduced abundance of activated αENaC protein in the renal medulla.
What is Relevant?
We demonstrate that direct actions of AT1 receptors on principal cells have effects on hypertension pathogenesis αENaC independent of aldosterone.
Summary
We describe for the first time the isolated physiological contributions of angiotensin type 1 (AT1) receptor in principal cells in vivo during hypertension pathogenesis. Deletion of AT1A receptors in principal cells of the collecting duct mice in mice (PCKO) have minimal influence on blood pressure or adaptation to changes in dietary salt intake. However, AT1 receptors in principal cells play a role in the initiation phase of Ang II-dependent hypertension. This is associated with enhanced of natriuresis and reductions in activated or cleaved αENaC in the renal medulla in PCKO mice during Ang II-induced hypertension, despite high levels of aldosterone in PCKOs that are not different from controls. These differences in cleaved αENaC protein abundance can be attributed to direct actions of AT1 receptors in principal cells. Our studies indicate that AT1A receptors in principal cells constitute a significant regulatory pathway for activating ENaC in Ang II-dependent hypertension.
Acknowledgments
Source(s) of Funding
The authors’ work in this area has been supported by funds from: 1) National Institutes of Health (HL056122, DK105049 and P30DK096493), 2) The Edna and Fred L. Mandel Center for Hypertension and Atherosclerosis Research, 3) American Heart Association Postdoctoral Fellowship (12POST11750024), and 4) Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development (IK2BX002240).
Footnotes
Conflict(s) of Interest/Disclosure(s)
The authors have no conflict of interest related to this manuscript.
References
- 1.Chen D, Coffman TM. AT1 angiotensin receptors-vascular and renal epithelial pathways for blood pressure regulation. Curr Opin Pharmacol. 2015;21:122–126. doi: 10.1016/j.coph.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1a angiotensin II receptor gene. Proc Natl Acad Sci U S A. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical renin-angiotensin system in kidney physiology. Compr Physiol. 2014;4:1201–1228. doi: 10.1002/cphy.c130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Weatherford ET, Davis DR, Keen HL, Grobe JL, Daugherty A, Cassis LA, Allen AM, Sigmund CD. Renal proximal tubule angiotensin AT1A receptors regulate blood pressure. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1067–R1077. doi: 10.1152/ajpregu.00124.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstein AM. The Kidney Physiology and Pathophysiology. Burlington, MA: Academic Press; 2008. Sodium and chloride transport: Proximal nephron; pp. 793–847. [Google Scholar]
- 8.Loffing J, Korbmacher C. Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC) Pflugers Arch. 2009;458:111–135. doi: 10.1007/s00424-009-0656-0. [DOI] [PubMed] [Google Scholar]
- 9.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 10.Rossier BC. The renal epithelial sodium channel: New insights in understanding hypertension. Adv Nephrol Necker Hosp. 1996;25:275–286. [PubMed] [Google Scholar]
- 11.Sun P, Yue P, Wang WH. Angiotensin II stimulates epithelial sodium channels in the cortical collecting duct of the rat kidney. Am J Physiol Renal Physiol. 2012;302:F679–F687. doi: 10.1152/ajprenal.00368.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, Pochynyuk O. Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J Biol Chem. 2012;287:660–671. doi: 10.1074/jbc.M111.298919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mamenko M, Zaika O, Prieto MC, Jensen VB, Doris PA, Navar LG, Pochynyuk O. Chronic angiotensin II infusion drives extensive aldosterone-independent epithelial Na+ channel activation. Hypertension. 2013;62:1111–1122. doi: 10.1161/HYPERTENSIONAHA.113.01797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 15.Madsen KM, Verlander JW, Tisher CC. Relationship between structure and function in distal tubule and collecting duct. J Electron Microsc Tech. 1988;9:187–208. doi: 10.1002/jemt.1060090206. [DOI] [PubMed] [Google Scholar]
- 16.Biemesderfer D, Stanton B, Wade JB, Kashgarian M, Giebisch G. Ultrastructure of amphiuma distal nephron: Evidence for cellular heterogeneity. The American Journal of Physiology. 1989;256:C849–C857. doi: 10.1152/ajpcell.1989.256.4.C849. [DOI] [PubMed] [Google Scholar]
- 17.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol. 2003;285:F998–F1012. doi: 10.1152/ajprenal.00067.2003. [DOI] [PubMed] [Google Scholar]
- 18.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Corniere N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R. Renal beta-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest. 2013;123:4219–4231. doi: 10.1172/JCI63492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacques T, Picard N, Miller RL, Riemondy KA, Houillier P, Sohet F, Ramakrishnan SK, Busst CJ, Jayat M, Corniere N, Hassan H, Aronson PS, Hennings JC, Hubner CA, Nelson RD, Chambrey R, Eladari D. Overexpression of pendrin in intercalated cells produces chloride-sensitive hypertension. J Am Soc Nephrol. 2013;24:1104–1113. doi: 10.1681/ASN.2012080787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eladari D, Chambrey R, Peti-Peterdi J. A new look at electrolyte transport in the distal tubule. Annu Rev Physiol. 2012;74:325–349. doi: 10.1146/annurev-physiol-020911-153225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson RD, Stricklett P, Gustafson C, Stevens A, Ausiello D, Brown D, Kohan DE. Expression of an AQP2 cre recombinase transgene in kidney and male reproductive system of transgenic mice. Am J Physiol. 1998;275:C216–C226. doi: 10.1152/ajpcell.1998.275.1.C216. [DOI] [PubMed] [Google Scholar]
- 22.Oliverio MI, Best CF, Smithies O, Coffman TM. Regulation of sodium balance and blood pressure by the AT(1a) receptor for angiotensin II. Hypertension. 2000;35:550–554. doi: 10.1161/01.hyp.35.2.550. [DOI] [PubMed] [Google Scholar]
- 23.Weinberger MH. Sodium sensitivity of blood pressure. Curr Opin Nephrol Hypertens. 1993;2:935–939. doi: 10.1097/00041552-199311000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Francois H, Athirakul K, Mao L, Rockman H, Coffman TM. Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertension. 2004;43:364–369. doi: 10.1161/01.HYP.0000112225.27560.24. [DOI] [PubMed] [Google Scholar]
- 25.Zaika O, Mamenko M, Staruschenko A, Pochynyuk O. Direct activation of ENaC by angiotensin II: Recent advances and new insights. Curr Hypertens Rep. 2013;15:17–24. doi: 10.1007/s11906-012-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999;104:R19–R23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen MT, Yang LE, Fletcher NK, Lee DH, Kocinsky H, Bachmann S, Delpire E, McDonough AA. Effects of K+-deficient diets with and without NaCl supplementation on Na+, K+, and H2O transporters' abundance along the nephron. Am J Physiol Renal Physiol. 2012;303:F92–F104. doi: 10.1152/ajprenal.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frindt G, Houde V, Palmer LG. Conservation of Na+ vs. K+ by the rat cortical collecting duct. Am J Physiol Renal Physiol. 2011;301:F14–F20. doi: 10.1152/ajprenal.00705.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ergonul Z, Frindt G, Palmer LG. Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol. 2006;291:F683–F693. doi: 10.1152/ajprenal.00422.2005. [DOI] [PubMed] [Google Scholar]
- 30.Christensen BM, Perrier R, Wang Q, Zuber AM, Maillard M, Mordasini D, Malsure S, Ronzaud C, Stehle JC, Rossier BC, Hummler E. Sodium and potassium balance depends on alphaenac expression in connecting tubule. J Am Soc Nephrol. 2010;21:1942–1951. doi: 10.1681/ASN.2009101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR. Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J Biol Chem. 2003;278:37073–37082. doi: 10.1074/jbc.M307003200. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Ray EC, Yates ME, Buck TM, Brodsky JL, Kinlough CL, Winarski KL, Hughey RP, Kleyman TR, Sheng S. Functional roles of clusters of hydrophobic and polar residues in the epithelial Na+ channel knuckle domain. J Biol Chem. 2015;290:25140–25150. doi: 10.1074/jbc.M115.665398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 34.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-c-dependent buffering mechanism. Nature Medicine. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 35.Rettig R, Grisk O. The kidney as a determinant of genetic hypertension: Evidence from renal transplantation studies. Hypertension. 2005;46:463–468. doi: 10.1161/01.HYP.0000178189.68229.8a. [DOI] [PubMed] [Google Scholar]
- 36.Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). ALLHAT collaborative research group. JAMA. 2000;283:1967–1975. [PubMed] [Google Scholar]
- 37.Cogan MG. Angiotensin II: A powerful controller of sodium transport in the early proximal tubule. Hypertension. 1990;15:451–458. doi: 10.1161/01.hyp.15.5.451. [DOI] [PubMed] [Google Scholar]
- 38.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13:469–475. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: Interaction between genetic and environmental factors. Annu Rev Physiol. 2002;64:877–897. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- 40.Crowley SD, Zhang J, Herrera M, Griffiths R, Ruiz P, Coffman TM. Role of AT(1) receptor-mediated salt retention in angiotensin II-dependent hypertension. Am J Physiol Renal Physiol. 2011;301:F1124–F1130. doi: 10.1152/ajprenal.00305.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brooks HL, Allred AJ, Beutler KT, Coffman TM, Knepper MA. Targeted proteomic profiling of renal Na(+) transporter and channel abundances in angiotensin II type 1a receptor knockout mice. Hypertension. 2002;39:470–473. doi: 10.1161/hy02t2.102959. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC. Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension. 2011;57:594–599. doi: 10.1161/HYPERTENSIONAHA.110.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zietse R, Hoorn EJ. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int. 2011;79:66–76. doi: 10.1038/ki.2010.290. [DOI] [PubMed] [Google Scholar]
- 44.Pearce D, Kleyman TR. Salt, sodium channels, and sgk1. J Clin Invest. 2007;117:592–595. doi: 10.1172/JCI31538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.