Abstract
Intrauterine growth restriction induced via placental insufficiency programs a significant increase in blood pressure at 12 months of age in female growth-restricted rats that is associated with early cessation of estrous cyclicity indicative of premature reproductive senescence. In addition, female growth-restricted rats at 12 months of age exhibit a significant increase in circulating testosterone with no change in circulating estradiol. Testosterone is positively associated with blood pressure after menopause in women. Thus, we tested the hypothesis that androgen receptor blockade would abolish the significant increase in blood pressure that develops with age in female growth-restricted rats. Mean arterial pressure was measured in animals pretreated with and without the androgen receptor antagonist, flutamide (8 mg/kg/day, sc for 2 weeks). Flutamide abolished the significant increase in blood pressure in growth-restricted rats relative to control at 12 months of age. To examine the mechanism(s) by which androgens contribute to increased blood pressure in growth-restricted rats, blood pressure was assessed in rats untreated or treated with enalapril (250 mg/L for 2 weeks). Enalapril eliminated the increase in blood pressure in growth-restricted relative to vehicle- and flutamide-treated controls. Furthermore, the increase in medullary angiotensin type 1 receptor mRNA expression was abolished in flutamide-treated growth-restricted relative to untreated counterparts and controls; cortical angiotensin converting enzyme mRNA expression was reduced in flutamide-treated growth-restricted versus untreated counterparts. Thus, these data indicate that androgens, via activation of the renin angiotensin system, are important mediators of increased blood pressure that develops by 12 months of age in female growth-restricted rats.
Keywords: low birth weight, women’s health, testosterone, blood pressure, renin angiotensin system
Introduction
Men have higher blood pressure compared to age-matched women in young adulthood; however, this sex difference is lost with the onset of menopause when the risk for cardiovascular disease in women surpasses their age-matched male counterparts (1). The prevalence of hypertension is greater in postmenopausal women relative to men of similar age (2, 3) and the age-related decline in blood pressure control is greater in women relative to men (4) despite women being more compliant with their therapeutic regiment (5). Yet, the mechanisms responsible for the decrease in blood pressure control in women after menopause are unknown.
Numerous physiological systems are implicated in the pathogenesis of postmenopausal hypertension including the renin angiotensin system (RAS) (6), the endothelin system (7), and alterations in sex steroids (8). Circulating testosterone levels are elevated in postmenopausal women compared to premenopausal women (9), and testosterone is positively associated with blood pressure after menopause (10). Thus, these studies suggest that androgens play a contributory role in the development of hypertension in women as they age and pass through the menopausal transition. However, the exact mechanism(s) by which a hyper-androgenic state results in an increase in blood pressure in women is not well-defined.
The experimental model of the aged female spontaneously hypertensive rat (SHR) mimics many of the characteristics reported for women with postmenopausal hypertension including cessation of cycling, age-dependent increases in blood pressure and significant increases in circulating testosterone levels compared to young female SHRs (11). The mechanism responsible for the increase in blood pressure in the aged female SHR is multifactorial and involves the renin angiotensin system (12), endothelin system (13), and eicosanoids (14). However, in these studies, monotheraphy to block each of these individual pathways fails to normalize blood pressure in the aged female SHR relative to young female SHR counterparts; an observation also noted with triple therapy (15). Testosterone is implicated in the activation of each of these pathways (16, 17, 18). Therefore, these findings suggest that blockade of the androgen receptor might be a potential therapeutic option for postmenopausal hypertension.
Numerous epidemiological studies indicate that low birth weight individuals exposed to intrauterine growth restriction (IUGR) are at an increased risk for cardiovascular disease and high blood pressure in later life (19, 20, 21). In addition to the increased risk for cardiovascular disease, low birth weight women exhibit an increased prevalence for early age at menopause compared to normal birth weight counterparts (22, 23). To investigate the long-term effects of IUGR on later cardiovascular risk our laboratory utilizes an experimental model of IUGR induced by placental insufficiency at day 14 of gestation in the Sprague Dawley rat (24). Female growth-restricted rats are normotensive in young adulthood (25); however, they develop a significant age-related increase in blood pressure by 12 months of age (26). In addition, female growth-restricted rats enter reproductive senescence at an earlier rage compared to their female normal birth weight counterparts, control offspring of sham operated dams (27). Circulating testosterone levels are significantly elevated in female growth-restricted rats at 12 months of age that have ceased cycling whereas serum estradiol levels do not differ relative to age-marched female controls (27). Since an elevation in testosterone is observed in female growth-restricted rats at 12 months of age in association with a significant elevation in blood pressure, the aim of this study was to test the hypothesis that chronic blockade of the androgen receptor would abolish the significant age-related increase in blood pressure in female growth-restricted offspring. Based on studies suggesting that testosterone is elevated in postmenopausal women compared to premenopausal women (28), and that testosterone may function as an activator of the RAS in postmenopausal women (29), we also tested the hypothesis that involvement of testosterone in the etiology of age-related increases in blood pressure in female growth-restricted rats at 12 months of age incorporates androgen receptor-dependent activation of the RAS.
MATERIALS AND METHODS
All experimental procedures were conducted in accordance with National Institutes of Health guidelines with all protocols approved by the Animal Care and Use Committee at the University of Mississippi Medical Center. In brief, female IUGR offspring exposed to reduced uterine perfusion at day 14 of gestation or female control offspring from sham operated dams were divided into 2 groups: daily injections of androgen receptor antagonist, flutamide (8 mg/kg SC, n=9/10) (Sigma Aldrich, Saint Louis, Missouri, USA) or daily injections of vehicle (ethanol in castor oil, n=11/12) for two weeks. Mean arterial pressure was measured in conscious, chronically instrumented rats followed by collection of plasma and kidneys for further analysis. In a separate cohort of animals, a sub-group of female offspring were pretreated with angiotensin converting enzyme (ACE) inhibitor, enalapril (250mg/L) in the drinking water for two weeks (n=6–7) followed by measurement of mean arterial pressure (MAP). A more detailed Methods section is available in the online-only Data Supplement.
Statistics
Data are presented as mean values ± SE, with n representing the number of female offspring with different mothers per group. MAP, serum testosterone and estradiol levels within the different study groups were compared using two-way repeated-measures ANOVA (Prism 5.0, GraphPad, San Diego, CA). Post hoc testing was performed using Bonferoni’s post hoc test to utilize multiple comparisons where appropriate. Differences were reported as significant when P < 0.05.
Results
Birth weight and body weight in female control and growth-restricted rats at 12 months of age
Birth weight was significantly reduced in growth-restricted females compared to same-sex controls (5.70±0.07 versus 4.87±0.09g; P<0.05, control versus IUGR, respectively). Body weight did not differ between vehicle-treated control and vehicle-treated growth-restricted offspring at 12 months of age (288±11 versus 267±9g, P>0.05, control versus IUGR, respectively). Flutamide treatment had no effect on body weight in control or growth-restricted offspring (269±3 versus 263±6g; P>0.05, control versus IUGR, respectively) at 12 months of age.
Mean arterial pressure (MAP) and sex steroids in female control and growth-restricted rats at 12 months of age: the effect of blockade of the androgen receptor
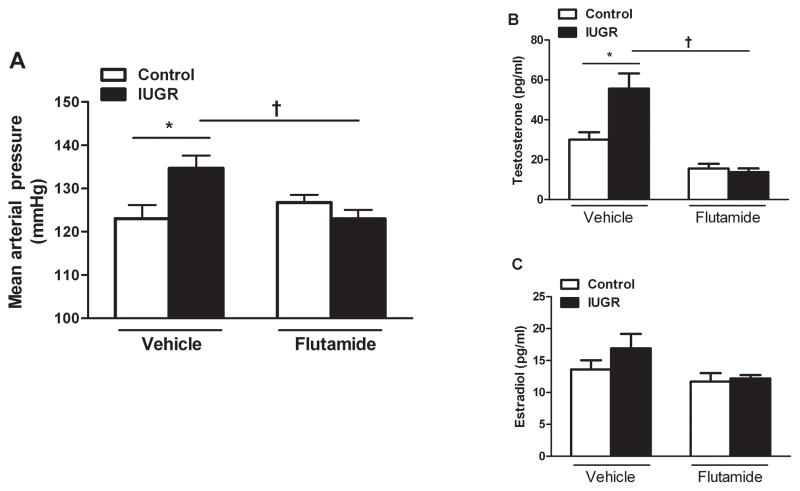
MAP was significantly elevated in vehicle-treated growth-restricted offspring compared to control counterparts at 12 months of age (P<0.05) (Figure 1a). Chronic blockade of the androgen receptor with flutamide abolished the increase in MAP in flutamide- treated growth-restricted relative to flutamide- treated control offspring at 12 months of age (P>0.05) (Figure 1a). Circulating testosterone levels were significantly elevated in vehicle-treated growth-restricted offspring compared to controls (P<0.05) (Figure 1b). Chronic androgen receptor blockade significantly reduced circulating testosterone levels in female growth-restricted offspring relative to vehicle-treated growth-restricted offspring (P<0.05, flutamide-treated IUGR versus vehicle-treated IUGR, respectively) (Figure 1b). Estradiol levels were comparable between vehicle-treated controls and growth-restricted offspring (P>0.05), and flutamide treatment had no effect on estradiol levels in either group (Figure 1c). As observed previously, female growth-restricted offspring were predominantly in persistent estrous at 12 months of age while age-matched control offspring retained a normal pattern of estrous cyclicity. At 18 months of age, chronic flutamide did not significantly reduce blood pressure in control or growth-restricted offspring at 18 months of age (Figure S1).
Figure 1. Effect of androgen receptor antagonism on mean arterial pressure (MAP) and sex steroids in female intrauterine growth-restricted (IUGR) rats that develop an increase in blood pressure at 12 months of age.
Female control and intrauterine growth-restricted (IUGR) rats received vehicle or were treated for 2 weeks with the androgen receptor antagonist flutamide (8 mg · kg−1 · d−1; Vehicle-treated control n=11, Vehicle-treated IUGR n=12, flutamide-treated control n=9, flutamide-treated IUGR n=10). MAP was measured at 12 months of age in chronically instrumented, conscious animals via carotid catheter (Figure 1a). Circulating testosterone and estradiol levels were measured in control and IUGR offspring at 12 months of age (Figure 1b, 1c). *P<0.05 versus vehicle-treated control. †P<0.05 versus vehicle-treated IUGR. Data values represent mean±SEM.
Intrarenal mRNA expression of the renin angiotensin system in female control and growth-restricted rats at 12 months of age
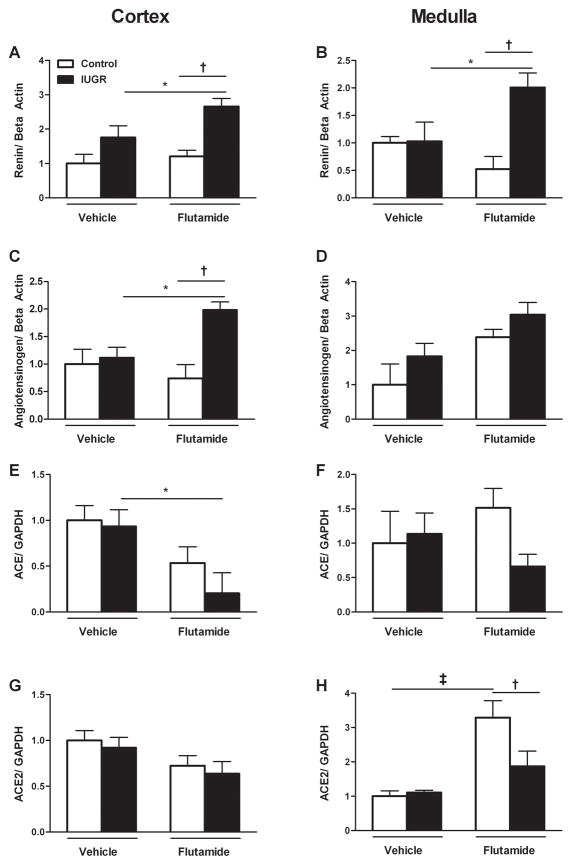
Renin mRNA expression did not differ upon comparison of vehicle-treated control and growth-restricted offspring in the cortex or medulla at 12 months of age. However, renin mRNA expression was significantly increased three-fold in cortex and two-fold in medulla of flutamide-treated growth-restricted offspring compared to their flutamide-treated control counterpart and two-fold relative to their vehicle-treated growth-restricted counterpart (Figure 2a and 2b). Cortical and medullary angiotensinogen mRNA expression was comparable between vehicle-treated control and growth-restricted offspring but renal cortical angiotensinogen mRNA expression was significantly elevated two-fold in flutamide-treated growth-restricted compared to vehicle-treated growth-restricted and flutamide-treated controls; an observation not observed within the renal medulla (Figure 2c and 2d). Renal cortical and medullary angiotensin converting enzyme (ACE) mRNA did not differ between vehicle-treated control and growth-restricted offspring (Figure 2e and 2f). Yet, there was a significant two-fold reduction in renal cortical ACE mRNA expression in flutamide-treated growth-restricted compared to vehicle-treated growth-restricted (Figure 2g). Chronic blockade of the androgen receptor also did not alter medullary ACE mRNA expression in control offspring but cortical ACE mRNA expression was significantly decreased two-fold in flutamide-treated growth-restricted offspring relative to vehicle-treated counterparts (Figure 2g and 2h). There was no difference in cortical or medullary ACE2 mRNA expression between vehicle-treated control and growth-restricted offspring (Figure 2g and 2h); however, medullary expression of ACE2 mRNA was significantly increased three-fold in flutamide-treated control offspring compared to vehicle-treated control and two-fold relative to flutamide-treated growth-restricted (Figure 2h). Flutamide-treated growth-restricted offspring did not exhibit a significant change in ACE2 mRNA expression compared to vehicle-treated groups.
Figure 2. mRNA levels of kidney RAS components from vehicle-treated and flutamide-treated female control and intrauterine growth-restricted (IUGR) rats by real-time RT-PCR at 12 months of age.
Cortical renin mRNA expression (2a), medullary renin mRNA expression (2b), cortical angiotensinogen mRNA expression (2c), medullary angiotensinogen mRNA expression (2d), cortical ACE mRNA expression (2e), medullary ACE mRNA expression (2f), cortical ACE2 mRNA expression (2g), medullary ACE2 mRNA expression (2h) in female vehicle-treated control (n=8) and IUGR offspring (n=7) and flutamide-treated control (n=9) and IUGR offspring (n=8). Data are expressed as fold changes relative to the mean expression level of the vehicle-treated control rats that were arbitrarily defined as 1. *P<0.05 versus vehicle-treated IUGR. †P<0.05 versus flutamide-treated control. ‡P<0.05 versus vehicle-treated control.
Mean arterial pressure and plasma renin activity in female control and growth-restricted rats at 12 months of age: the effect of blockade of the renin angiotensin system
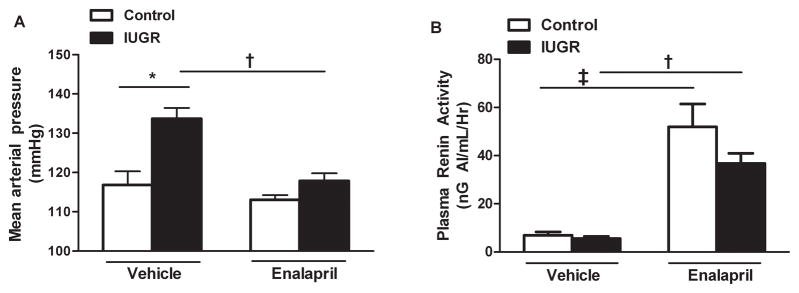
MAP was significantly elevated in vehicle treated growth-restricted offspring relative to control counterparts at 12 months of age (P<0.05, IUGR vs. Control) (Figure 3a). Blockade of the RAS with the ACE inhibitor, enalapril, abolished the significant increase in MAP in growth-restricted relative to vehicle-treated growth-restricted (P<0.05, IUGR vs. Control) (Figure 3a). Plasma renin activity (PRA) did not differ between vehicle-treated control and growth-restricted offspring (P>0.05) (Figure 3b). However, following chronic blockade of the RAS with enalapril, PRA was significantly elevated in control and growth-restricted offspring to comparable levels (P<0.05, vehicle-treated versus enalapril-treated counterparts) (Figure 3b).
Figure 3. The effect of renin-angiotensin system (RAS) blockade with the angiotensin converting enzyme (ACE) inhibitor, enalapril (250mg/L for 2 weeks), on mean arterial pressure (MAP) and plasma renin activity (PRA) measured in female control and intrauterine growth-restricted (IUGR) rats at 12 months of age.
MAP was measured at 12 months of age in chronically instrumented, conscious animals via carotid catheter. PRA was measured using radioimmunoassay. Vehicle-treated control n=7, vehicle-treated IUGR n=6, enalapril-treated control n=6, enalapril-treated IUGR n=6. *P<0.05 versus vehicle-treated control. †P<0.05 versus vehicle-treated IUGR. ‡P<0.05 versus vehicle-treated control. Data are expressed as mean±SEM.
Intrarenal androgen and angiotensin receptor mRNA expression i in female control and growth-restricted rats at 12 months of age
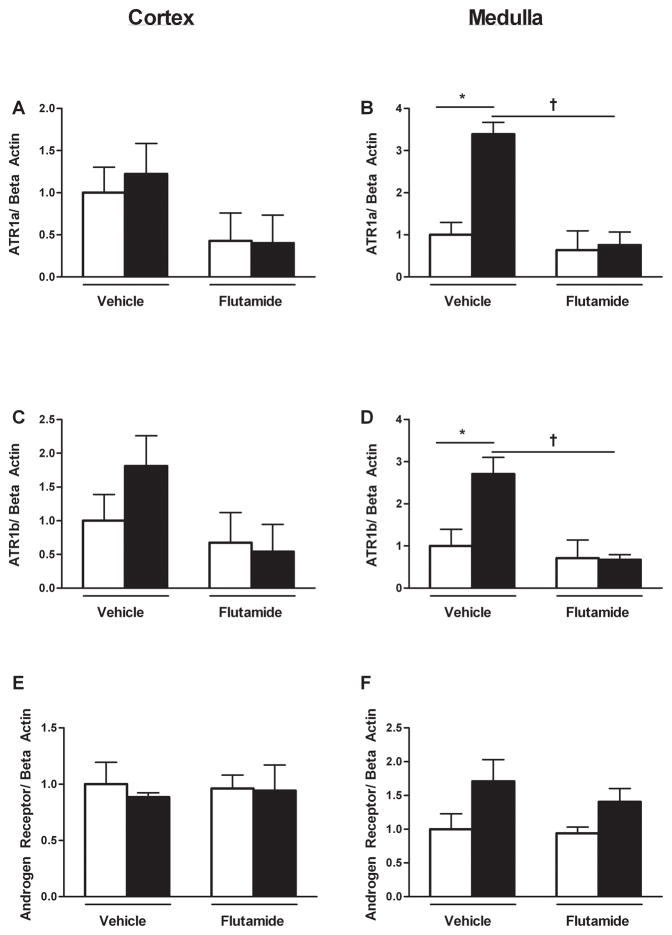
Cortical ATR1a expression did not differ upon comparison of control and growth-restricted offspring; flutamide treatment had no effect on expression level (Figure 4a). ATR1a expression was significantly increased three-fold within the medulla of growth-restricted relative to control; chronic flutamide abolished this increase (Figure 4b). Cortical ATR1b expression did not differ between control and growth-restricted offspring. Furthermore, flutamide treatment did not alter expression (Figure 4c). Medullary ATR1b expression was significantly increased two-fold in growth-restricted offspring compared to controls; chronic flutamide abolished this increase (Figure 4d). Androgen receptor mRNA expression in both the cortical and medullary regions did not differ upon comparison of vehicle-treated control and growth-restricted offspring; intrarenal androgen receptor mRNA expression was not altered by treatment with flutamide (Figure 4e and 4f).
Figure 4. Angiotensin and androgen receptor mRNA expression in the kidney from vehicle-treated and flutamide-treated female control and intrauterine growth-restricted (IUGR) rats at 12 months of age by real-time RT-PCR.
Cortical ATR1a mRNA expression (4a), medullary ATR1a mRNA expression (4b), cortical ATR1b mRNA expression (4c), medullary ATR1b mRNA expression (4d), cortical androgen receptor mRNA expression (4e), medullary androgen receptor mRNA expression (4f) in female vehicle-treated control (n=8) and IUGR offspring (n=7) and flutamide-treated control (n=9) and IUGR offspring (n=8). Data are expressed as fold changes relative to the mean expression level of the vehicle-treated control rats that were arbitrarily defined as 1. *P<0.05 versus vehicle-treated control. †P<0.05 versus vehicle-treated IUGR.
Discussion
Previously, we reported that female growth-restricted rats are normotensive in young adulthood relative to age-matched female controls (24). However, a more recent study from our laboratory demonstrates that female growth-restricted rats exhibit a significant increase in blood pressure relative to age-matched female controls at 12 months of age (26) that is associated with cessation of estrous cyclicity and a significant increase in circulating levels of testosterone (27). The main finding from this study indicated that the increase in blood pressure in female growth-restricted offspring at 12 months of age relative to age-matched controls was abolished by chronic treatment with the androgen receptor antagonist, flutamide, with no effect on blood pressure in age-matched female controls. Furthermore, flutamide had no effect on blood pressure in female control offspring at 18 months of age that were in persistent estrous. Therefore, these results suggest that the reduction in blood pressure in response to chronic blockade of the androgen receptor is specific to female rats that exhibit early cessation of estrous cyclicity following fetal exposure to IUGR, not reproductive senescence per se. This study also determined the importance of the RAS in the etiology of androgen-dependent increased blood pressure in female growth-restricted rats at 2 months of age. Intrarenal expression of components of the RAS including renin, angiotensinogen and ACE were not significantly increased in vehicle-treated female growth-restricted offspring compared to vehicle-treated controls at 12 months of age. Yet, cortical ACE mRNA expression was significantly reduced in flutamide-treated growth-restricted rats in conjunction with a significant increase in renin and angiotensinogen mRNA expression at 12 months of age; perhaps indicative of feedback from the reduction in renal cortical ACE expression. Medullary ATR1a and AT1b mRNA expression were increased in female growth-restricted offspring relative to control at 12 months of age; this increase was not present in rats treated with flutamide implicating modulation of the intrarenal RAS by the androgen receptor in female growth-restricted rats. The importance of the RAS in the etiology of increased blood pressure in female growth-restricted offspring at 12 months of age was determined by the effect of RAS blockade. Blockade of the RAS using the ACE inhibitor, enalapril, completely abolished the increase in blood pressure in enalapril-treated growth-restricted offspring relative to enalapril- and vehicle-treated controls. Therefore, these results indicate that the RAS is also a contributory factor in the etiology of increased blood pressure at 12 months of age in female growth-restricted offspring.
Numerous experimental models of developmental programming induced by exposure to a mild developmental insult such as moderate maternal protein restriction (9% versus 18% protein), fetal exposure to increased glucocorticoids or placental insufficiency report a sex difference in blood pressure with male offspring in young adulthood exhibiting a higher blood pressure relative to age-matched female counterparts (24, 30, 31, 32) whereas female offspring are normotensive in young adulthood relative to their control counterparts (24, 30, 31, 32, 33). However, female rat offspring exposed to a moderate (9% versus 18% protein) reduction in maternal protein intake during gestation (34, 35) or placental insufficiency induced via a reduction in uterine perfusion (26) during fetal life develop a significant increase in blood pressure by one to two years of age relative to their control counterparts suggesting that females do not remain protected against increased cardiovascular risk. The etiology for the age-related increase in blood pressure in female offspring in models of developmental insult that mimic the pathophysiological causes for low birth weight is not clear. Hypertension in male growth-restricted offspring relative to male control in young adulthood is abolished by castration suggesting that hypertension in male rats is testosterone-dependent (36). Ovariectomy induces a significant increase in blood pressure in female growth-restricted offspring in young adulthood that is abolished by estrogen replacement (25) implicating that estrogen may be protective. Therefore, previous studies from our laboratory indicate that sex steroids contribute to the sexual dimorphism of blood pressure in growth-restricted offspring in young adulthood. Although testosterone levels are increased in female growth-restricted offspring at 12 months of age, circulating estradiol levels do not differ relative to age-matched female control (27). Female growth-restricted rats at 12 months of age exhibit persistent estrous compared to age-matched female controls which retain a normal pattern of estrous cyclicity (27). A reduction in ovarian follicle number (37) and early disruption in estrous cyclicity associated with an increase in circulating levels of testosterone (38) is reported in female rats exposed to a moderate reduction (9% versus 18% protein) in maternal protein intake. Earlier age at menopause is reported in low birth weight women (22, 23) and in women exposed in utero to famine (39). Therefore, these studies suggest that insults during early life program early reproductive senescence.
It is well established that early onset menopause increases the risk for cardiovascular disease (40). While the mechanisms involved are not yet known, testosterone is indicated as a potential mediator (41). Testosterone is positively associated with blood pressure in women across their lifespan (42) with the association significant in postmenopausal women (42, 43) and in women with polycystic ovary syndrome (PCOS) (44, 45), a syndrome often characterized by a state of hyperandrogenemia. Although Huang et al. report that administration of exogenous testosterone in women with endogenous low testosterone levels does not alter blood pressure (45), administration of exogenous testosterone (46) or 5alpha-dihydrotestosterone (DHT) (47) increases blood pressure in female rats in experimental models of increased blood pressure. The detrimental effect of exogenous testosterone on cardiovascular health in women is not well-defined (48) but the use of exogenous testosterone for treatment of numerous disorders in women is currently contraindicated (49).
The effect of androgen deprivation therapy on cardiovascular health in women is also inconclusive (50). Experimental studies indicate that blockade of androgen receptor is beneficial against increases in blood pressure in the female rat. The transgenic TGR(mREN2)27 rat overexpresses the mouse renin transgene and spontaneously develops hypertension in association with inappropriate activation of the rat RAS (51). Endogenous testosterone levels are not elevated in the female TGR(mREN2)27 rat. Yet, blockade of the androgen receptor with flutamide abolishes hypertension in conjunction with a decrease in plasma renin expression and PRA in the absence of an effect on testosterone (51). Thus, these findings suggest that endogenous testosterone is the mediator of inappropriate activation of the RAS in the female TGR(mREN2)27 rat. Treatment with flutamide also reduces blood pressure in female rats exposed to a severe (5% versus 18% protein) reduction in maternal protein intake during gestation (52). Unlike more physiologically relevant models of developmental insult, blood pressure is significantly elevated as early as 3 months of age in female offspring exposed to a severe maternal protein restriction during fetal life (52). Like the female TGR(mREN2)27 rat, circulating levels of testosterone are not elevated in female rats in this model indicating an androgen independent effect of flutamide on blood pressure (52). The mechanism by which flutamide reduces blood pressure in female protein-restricted rats was not investigated; however, estrogen levels are decreased and flutamide reverses this effect (53). Sex steroids mediate their actions via genomic and non-genomic pathways. Circulating levels of testosterone and estradiol were not altered by blockade of the androgen receptor in our study suggesting a lack of a non-genomic vascular effect on blood pressure. Thus, we tested the hypothesis that blockade of the androgen receptor modulated expression of the intrarenal RAS to reduce blood pressure in female growth-restricted rats at 12 months of age.
Postmenopausal women have higher risk for cardiovascular risk compared to premenopausal women (54). The exact mechanisms responsible for postmenopausal hypertension are unknown, but evidence shows it is a multifactorial disease (55). Blood pressure increases with age after estrous cycling ceases in the female SHR (11) suggesting that aged female SHRs may be a model for the study of postmenopausal hypertension. Serum testosterone levels are increased in aged female SHRs relative to young same-sex counterparts (11). PRA is also elevated relative to the young female SHR implicating a role for the RAS (12). In the present study, blockade of the androgen receptor abolished the increase in blood pressure in female growth-restricted rats relative to control at 12 and 18 months of age with no effect on blood pressure in control offspring at either age. Only female control rats at 12 months of age exhibited different phases of the estrous cycle. Thus, the decrease in blood pressure by flutamide was specific to rats exposed to IUGR and not related to the presence of persistent estrous. ACE inhibition also abolished the increase in blood pressure in female growth-restricted rats at one year of age suggesting an important role for the RAS. Intrarenal ACE mRNA expression did not differ in vehicle-treated female control in this study relative to vehicle-treated female growth-restricted offspring. Yet, intrarenal ACE mRNA expression was decreased in flutamide-treated growth-restricted rats relative to flutamide-treated control suggesting that the effect of flutamide on expression of renal ACE was specific to the female growth-restricted rat. Renal ACE activity was not examined. However, exogenous estradiol can modulate ACE activity in the female Sprague Dawley rat by reducing ACE mRNA (56). Intrarenal mRNA expression of renin and angiotensinogen mRNA expression did not differ in vehicle-treated rats but were significantly increased in flutamide-treated growth-restricted offspring relative to untreated counterparts. The increase in expression of these factors may involve feedback from inhibition of ACE by the actions of flutamide. ACE2 mRNA expression was increased in flutamide-treated female control relative to untreated control and ACE2 was the only component of the RAS altered in female control offspring by flutamide. A direct test of flutamide on renal ACE2 mRNA expression or activity is not yet been reported. However, Gupte et al. report that renal ACE2 mRNA expression is increased in male C57Bl/6 mice relative to females suggesting a correlative role (57). Estrogen increases adipocyte ACE2 mRNA expression in female C57Bl/6 mice (57) and we previously demonstrated that ovariectomy reduces ACE2 mRNA expression and activity in female growth-restricted offspring in young adulthood (25). Thus, the mechanism by which ACE2 was increased by chronic blockade of the androgen receptor in the absence of a change in estradiol is not clear. The increase in renal AT1R expression was abolished by flutamide in correlation with a loss of increased blood pressure in female growth-restricted offspring relative to control. Expression of the AT1R is androgen dependent (58) suggesting increased expression of the AT1R in female growth-restricted offspring is androgen-dependent. Therefore, our study indicated a direct role for the RAS in the etiology of increased blood pressure that develops in female growth-restricted rats that exhibit early reproductive senescence at 12 months of age. Yet, diuretics are the most common treatment for hypertension in postmenopausal women while men are reported to respond better to ACE inhibitors (59). ACE inhibitors are also the most prescribed antihypertensive medication in for low birth weight Medicaid male recipients whereas low birth weight is associated with a greater use of calcium channel blockers in low birth weight women (60).
Experimental models of hypertension exhibit a sex difference in the blood pressure response to different anti-hypertensive therapies (61, 62, 63, 64, 65). Angiotensin receptor blockade produces a greater decrease in blood pressure in aged male SHRs compared to aged female SHRs (65) despite no sex difference in intrarenal mRNA expression of ACE or the AT1R. Oxidative stress may play a more important role in blood pressure control in male rats in some experimental models of hypertension relative to their female counterparts (61, 64). Furthermore, male rats in models of developmental insult exhibit a blood pressure response to blockade of the endothelin type A receptor that is absent in female littermates (62, 63). Therefore, understanding the etiology of hypertension following reproductive senescence is needed in order to prescribe the appropriate therapeutic approach for postmenopausal women. Future studies will address the specificity of enalapril on blood pressure in female growth-restricted offspring. Understanding the etiology of hypertension that develops following a developmental insult in women as they age and are also at risk for earlier age of reproductive senescence is also necessitated.
Clinical Perspectives
Low birth weight women are at an increased risk for cardiovascular disease and earlier age for onset of menopause compared to normal birth weight counterparts; yet, less is known about the etiology of hypertension in individuals, in particular women, exposed to a prenatal insult. This study demonstrates an important role for androgens in the development of age-related increases in blood pressure in female growth-restricted rats and also implicates the RAS as a contributor to the developmental programming of increased blood pressure that develops with age in the female growth-restricted rat.
Supplementary Material
NOVELTY AND SIGNFICANCE.
What is new?
Our study demonstrates that testosterone acting through the androgen receptor contributes to the age-related increase in blood pressure in female growth-restricted rats. Our study further reveals that the renin angiotensin system is also a contributor to the development of increased blood pressure in female growth-restricted rat at 12 months of age via the actions of the androgen receptor.
What is Relevant?
The risk for hypertension in women greatly increases after the onset of menopause, yet the etiology of postmenopausal hypertension is still unclear. The etiology of increased blood pressure in women born low birth weight is also unknown and studies looking at the effect of age, and the pathophysiological mechanisms that contribute to a greater prevalence of hypertension in low birth weight women as they age, are very limited. Findings from our study that indicate a role for androgens in the etiology of increased blood pressure that develops with age in female growth-restricted rats should encourage further examination into the relevance of testosterone and its detrimental effect on blood pressure in women, in particular in low birth weight women in later life.
Summary
Further studies are necessary to discern the impact of age and reproductive status on effectiveness of an antihypertensive regimen in low birth weight women.
Acknowledgments
SOURCES OF FUNDING
Dr. Alexander is supported by the American Heart Grant GRNT19900004 and NIH grants HL074927 and HL51971. Dr. Intapad is supported by funding from the AHA, 12POST1198002, and the NIH P20GM104357. Mr. Dasinger is supported by funding from the AHA, 15PRE24700010, and the NIH T32HL105324.
Footnotes
DISCLOSURES
None.
References
- 1.Mozaffarian D, et al. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Ong KL, Tso AWK, Lam KSL, Cheung BMY. Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension. 2008;51:1142–1148. doi: 10.1161/HYPERTENSIONAHA.107.105205. [DOI] [PubMed] [Google Scholar]
- 3.Taddei S. Blood pressure through aging and menopause. Climacteric. 2009;12(Suppl 1):36–40. doi: 10.1080/13697130903004758. [DOI] [PubMed] [Google Scholar]
- 4.Kim JK, Alley D, Seeman T, Karlamangla A, Crimmins E. Recent changes in cardiovascular risk factors among women and men. J Womens Health. 2006;15:734–746. doi: 10.1089/jwh.2006.15.734. [DOI] [PubMed] [Google Scholar]
- 5.Wassertheil-Smoller S, Anderson G, Psaty BM, Black HR, Manson J, Wong N, Francis J, Grimm R, Kotchen T, Langer R, Lasser N. Hypertension and its treatment in postmenopausal women: baseline data from the Women’s Health Initiative. Hypertension. 2000;36:780–789. doi: 10.1161/01.hyp.36.5.780. [DOI] [PubMed] [Google Scholar]
- 6.Schunkert H, Danser AH, Hense HW, Derkx FH, Kurzinger S, Riegger GA. Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women. Circulation. 1997;95:39–45. doi: 10.1161/01.cir.95.1.39. [DOI] [PubMed] [Google Scholar]
- 7.Lekontseva O, Chakrabarti S, Davidge ST. Endothelin in the female vasculature: a role in aging? Am J Physiol Regul Integr Comp Physiol. 2010;298:R509–R516. doi: 10.1152/ajpregu.00656.2009. [DOI] [PubMed] [Google Scholar]
- 8.Krug E, Berga AL. Postmenopausal hyperthecosis: functional dysregulation of androgenesis in climacteric ovary. Obstet Gynecol. 2002;99:893–897. doi: 10.1016/s0029-7844(01)01588-5. [DOI] [PubMed] [Google Scholar]
- 9.Janssen I, Powell LH, Jasielec MS, Kazlauskaite R. Covariation of change in bioavailable testosterone and adiposity in midlife women. Obesity. 2015;23:488–494. doi: 10.1002/oby.20974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rexrode KM, Manson JE, Lee IM, Ridker PM, Sluss PM, Cook NR, Buring JE. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108:1688–1693. doi: 10.1161/01.CIR.0000091114.36254.F3. [DOI] [PubMed] [Google Scholar]
- 11.Fortepinai LA, Zhang H, Racusen L, Roberts LJ, 2nd, Reckelhoff JF. Characterization of an animal model of postmenopausal hypertension in spontaneously hypertensive rats. Hypertension. 2003;41:640–645. doi: 10.1161/01.HYP.0000046924.94886.EF. [DOI] [PubMed] [Google Scholar]
- 12.Yanes LL, Romero DG, Iliescu R, Zhang H, Davis D, Reckelhoff JF. Postmenopausal hypertension: role of the renin-angiotensin system. Hypertension. 2010;56:359–363. doi: 10.1161/HYPERTENSIONAHA.110.152975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanes LL, Romero DG, Cucchiarelli VE, Fortepiani LA, Gomez-Sanchez CE, Santacruz F, Reckelhoff JF. Role of endothelin in mediating postmenopausal hypertension in a rat model. Am J Physiol Regul Integr Comp Physiol. 2005;288:R229–233. doi: 10.1152/ajpregu.00697.2003. [DOI] [PubMed] [Google Scholar]
- 14.Yanes LL, Lima R, Moulana M, Romero DG, Yuan K, Ryan MJ, Baker R, Zhang H, Fan F, Davis DD, Roman RJ, Reckelhoff JF. Postmenopausal hypertension: role of 20-HETE. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1543–R1548. doi: 10.1152/ajpregu.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lima R, Yanes LL, Davis DD, Reckelhoff JF. Roles played by 20-HETE, angiotensin II and endothelin in mediating the hypertension in aging female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2013;304:R348–R351. doi: 10.1152/ajpregu.00380.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen regulation of rat renal angiotensinogen messenger RNA expression. J Clin Invest. 1989;83:1941–1945. doi: 10.1172/JCI114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kientiz T, Quinkler M. Testosterone and blood pressure regulation. Kidney Blood Press Res. 2008;31:71–79. doi: 10.1159/000119417. [DOI] [PubMed] [Google Scholar]
- 18.Reckelhoff JF, Roman RJ. Androgens and hypertension: Role in both males and females? Hypertension. 2011;57:681–682. doi: 10.1161/HYPERTENSIONAHA.110.162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huxley R, Neil A, Collins R. Unravelling the fetal origins hypothesis: is there really an inverse association between birthweight and subsequent blood pressure. Lancet. 2002;360:659–665. doi: 10.1016/S0140-6736(02)09834-3. [DOI] [PubMed] [Google Scholar]
- 20.Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Favers PM, Martyn CN, de Swiet M. Fetal, infant, and childhood growth and adulthood blood pressure: a longitudinal study from birth to 22 years old. Circulation. 2002;105:1088–1092. doi: 10.1161/hc0902.104677. [DOI] [PubMed] [Google Scholar]
- 21.Smith CJ, Ryckman KK, Barnabei VM, Howard BV, Isasi CR, Sarto GE, Tom SE, Van Horn LV, Wallace RB, Robinson JG. The impact of birth weight on cardiovascular disease risk in the Women’s Health Initiative. Nutr Metab Cardiovasc Dis. 2016;26:239–245. doi: 10.1016/j.numecd.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tom SE, Cooper R, Kuh D, Guralnik JM, Hardy R, Power C. Fetal environment and early age at natural menopause in a British birth cohort study. Hum Reprod. 2010;25:791–798. doi: 10.1093/humrep/dep451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steiner AZ, D’Aloisio AA, DeRoo LA, Sandler DP, Baird DD. Association of intrauterine and early-life exposures with age at menopause in the Sister Study. Am J Epidemiol. 2010;172:140–148. doi: 10.1093/aje/kwq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41:457–462. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 25.Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension. 2007;50:679–685. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Intapad S, Tull FL, Brown AD, Dasinger JH, Ojeda NB, Fahling JM, Alexander BT. Renal denervation abolished the age-dependent increase in blood pressure in female intrauterine growth-restriction rats at 12 months of age. Hypertension. 2013;61:828–834. doi: 10.1161/HYPERTENSIONAHA.111.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Intapad S, Dasinger JH, Brown AD, Fahling JM, Esters J, Alexander BT. Glucose intolerance develops prior to increased adiposity and accelerated cessation of estrous cyclicity in female growth-restricted rats. Ped Research. 2016 doi: 10.1038/pr.2016.14. Published online: March 2, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiroutek MR, Chen MH, Johnston CC, Longcope C. Changes in reproductive hormones and sex hormone-binding globulin in a group of postmenopausal women measured over 10 years. Menopause. 1998;5:90–94. [PubMed] [Google Scholar]
- 29.Fernandez-Vega F, Abellan J, Vegazo O, De Vinuesa SG, Rodriguez JC, Maceira B, de Castro SS, Nicolas RR, Luno J. Angiotensin II type 1 receptor blockade to control blood pressure in postmenopausal women: influence of hormone replacement therapy. Kidney Int Suppl. 2002:S36–41. doi: 10.1046/j.1523-1755.62.s82.8.x. [DOI] [PubMed] [Google Scholar]
- 30.Moritz KM, Mazzuca MQ, Siebel AL, Mibus A, Arena D, Tare M, Owens JA, Wlodek ME. Uteroplacental insufficiency causes a nephron deficit, modest renal insufficiency but no hypertension with ageing in female rats. J Physiol. 2009;587:2635–2646. doi: 10.1113/jphysiol.2009.170407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1131–R1136. doi: 10.1152/ajpregu.00037.2003. [DOI] [PubMed] [Google Scholar]
- 33.Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension. 2003;41:328–334. doi: 10.1161/01.hyp.0000049763.51269.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Black MJ, Lim K, Zimanyi MA, Sampson AK, Bubb KJ, Flower RL, Parkington HC, Tare M, Denton KM. Accelerated age-related decline in renal and vascular function in female rats following early-life growth restriction. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1153–R1161. doi: 10.1152/ajpregu.00403.2014. [DOI] [PubMed] [Google Scholar]
- 35.Pijacka W, Clifford B, Tilburgs C, Joles JA, Langley-Evans S, McMullen S. Protective role of female gender in programmed accelerated renal aging in the rat. Physiol Rep. 2015;3:e12342. doi: 10.14814/phy2.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, Alexander BT. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2007;292:R758–R763. doi: 10.1152/ajpregu.00311.2006. [DOI] [PubMed] [Google Scholar]
- 37.Bernal AB, Vickers MH, Hampton MB, Poynton RA, Sloboda DM. Maternal undernutrition significantly impacts ovarian follicle number and increases ovarian oxidative stress in adult rat offspring. PLoS One. 2010;13:e15558. doi: 10.1371/journal.pone.0015558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khorram O, Keen-Rinehart E, Chuang TD, Ross MG, Desai M. Maternal undernutrition induces premature reproductive senescence in adult female rat offspring. Fertility and Sterility. 2015;103:291–298. doi: 10.1016/j.fertnstert.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarde F, Broekmans FJ, van der Pal-de Bruin KM, Schonbeck Y, te Velde ER, Stein AD, Lumey LH. Prenatal famine, birthweight, reproductive performance and age at menopause: the Dutch hunger winter families study. Hum Reprod. 2013;28:3328–3336. doi: 10.1093/humrep/det331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lubiszewska B, Kruk M, Broda G, Ksiezycka E, Piotrowski W, Kurjata P, Zielinski T, Ploski R. The impact of early menopause on risk of coronary artery disease (PREmature Coronary Artery Disease In Women--PRECADIW case-control study) Eur J Prev Cardiol. 2012;19:95–101. doi: 10.1177/1741826710394269. [DOI] [PubMed] [Google Scholar]
- 41.Maturana MA, Breda V, Lhullier F, Spritzer PM. Relationship between endogenous testosterone and cardiovascular risk in early postmenopausal women. Metabolism. 2008;57:961–965. doi: 10.1016/j.metabol.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Ziemens B, Wallaschofski H, Volzke H, Rettig R, Dorr M, Nauck M, Keevil BG, Brabant G, Haring R. Positive association between testosterone, blood pressure, and hypertension in women: longitudinal findings from the Study of Health in Pomerania. J Hypertens. 2013;31:1106–1113. doi: 10.1097/HJH.0b013e3283603eb1. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Szklo M, Folsom AR, Cook NR, Gapstur SM, Ouyang P. Endogenous sex hormones, blood pressure change, and risk of hypertension in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2012;224:228–234. doi: 10.1016/j.atherosclerosis.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen MJ, Yang WS, Yang JH, Chen CL, Ho HN, Yang YS. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension. 2007;49:1442–1447. doi: 10.1161/HYPERTENSIONAHA.106.083972. [DOI] [PubMed] [Google Scholar]
- 45.Huang G, Tang E, Aakil A, Anderson S, Jara H, Davda M, Stroh H, Travison TG, Bhasin S, Basaria S. Testosterone dose-response relationships with cardiovascular risk markers in androgen-deficient women: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2014;99:E1287–E1293. doi: 10.1210/jc.2013-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu B, Ely D. Testosterone increases: sodium reabsorption, blood pressure, and renal pathology in female spontaneously hypertensive rats on a high sodium diet. Adv Pharmacol Sci. 2011;2011:817–835. doi: 10.1155/2011/817835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yanes LL, Romero DG, Moulana M, Lima R, Davis DD, Zhang H, Lockhart R, Racusen LC, Reckelhoff JF. Cardiovascular-renal and metabolic characterization of a rat model of polycystic ovary syndrome. Gend Med. 2011;8:103–115. doi: 10.1016/j.genm.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis SR, Wahlin-Jacobsen S. Testosterone in women-the clinical significance. Lancet Diabetes Endocrinol. 2015;3:980–992. doi: 10.1016/S2213-8587(15)00284-3. [DOI] [PubMed] [Google Scholar]
- 49.Wierman ME, Arlt W, Basson R, Davis SR, Miller KK, Murad MH, Rosner W, Santoro N. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3489–3510. doi: 10.1210/jc.2014-2260. [DOI] [PubMed] [Google Scholar]
- 50.Domecq JP, Prutsky G, Mullan RJ, Sundaresh V, Wang AT, Erwin PJ, Welt C, Ehrmann D, Montori VM, Murad MH. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4646–4654. doi: 10.1210/jc.2013-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baltatu O, Cayla C, Iliescu R, Andreev D, Bader M. Abolition of end-organ damage by antiandrogen treatment in female hypertensive transgenic rats. Hypertension. 2003;41:830–833. doi: 10.1161/01.HYP.0000048702.55183.89. [DOI] [PubMed] [Google Scholar]
- 52.Gangula PR, Reed L, Yallampalli C. Antihypertensive effects of flutamide in rats that are exposed to a low-protein diet in utero. Am J Obstet Gynecol. 2005;192:952–960. doi: 10.1016/j.ajog.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Protein restriction during pregnancy induces hypertension in adult female rat offspring--influence of oestradiol. Br J Nutr. 2012;107:665–673. doi: 10.1017/S0007114511003448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zanchetti A, Facchetti R, Gesana GC, Modena MG, Pirrelli A, Sega R. Menopause-related blood pressure increases and its relationship at age and body mass index: the SIMONA epidemiological study. J Hypertens. 2005;23:2269–2276. doi: 10.1097/01.hjh.0000194118.35098.43. [DOI] [PubMed] [Google Scholar]
- 55.Reckelhoff JF, Wofford M. Hypertension in women. In: Rexrode K, Wickline M, editors. Chapter in: Women and Health. 2. Elsevier Press; New York: 2012. [Google Scholar]
- 56.Gallagher PE, Ping L, Lenhart JR, Chappell MC, Brosnihan KB. Estrogen Regulation of Angiotensin-Converting Enzyme mRNA. Hypertension. 1999;33:323–328. doi: 10.1161/01.hyp.33.1.323. [DOI] [PubMed] [Google Scholar]
- 57.Gupte M, Thatcher SE, Boustany-Kari CM, Shoemaker R, Yiannikouris F, Zhang X, Karounos M, Cassis LA. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol. 2012;32:1392–1399. doi: 10.1161/ATVBAHA.112.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leung PS, Wong TP, Chung YW, Chan HC. Androgen dependent expression of AT1 receptor and its regulation of anion secretion in rat epididymis. Cell Biol Int. 2002;26:117–122. doi: 10.1006/cbir.2001.0830. [DOI] [PubMed] [Google Scholar]
- 59.Ljungman C, Kahan T, Schioler L, Hjerpe P, Hasselstrom J, Wettermark B, Bostrom KB, Manhem K. Gender differences in antihypertensive drug treatment: results from the Swedish Primary Care Cardiovascular Database (SPCCD) J Am Soc Hypertens. 2014;8:882–980. doi: 10.1016/j.jash.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 60.Lackland DT, Egan BM, Syddall HE, Barker DJ. Associations between birth weight and antihypertensive medication in black and white medicaid recipients. Hypertension. 2002;39:179–183. doi: 10.1161/hy0102.100545. [DOI] [PubMed] [Google Scholar]
- 61.Lopez-Ruiz A, Sartori-Valinotti J, Yanes LL, Iliescu R, Reckelhoff JF. Sex differences in control of blood pressure: role of oxidative stress in hypertension. Am J Physiol Heart Circ Physiol. 2008;295:H466–H474. doi: 10.1152/ajpheart.01232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Intapad S, Ojeda NB, Varney E, Royals TP, Alexander BT. Sex-specific effect of endothelin in the blood pressure response to acute angiotensin II in growth-restricted rats. Hypertension. 2015;66:1260–1266. doi: 10.1161/HYPERTENSIONAHA.115.06257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bourque SL, Gragasin FS, Quon AL, Mansour Y, Morton JS, Davidge ST. Prenatal hypoxia causes long-term alterations in vascular endothelin-1 function in aged male, but not female, offspring. Hypertension. 2013;62:753–758. doi: 10.1161/HYPERTENSIONAHA.113.01516. [DOI] [PubMed] [Google Scholar]
- 64.Ojeda NB, Hennington BS, Williamson DT, Hill ML, Betson NE, Sartori-Valinotti JC, Reckelhoff JF, Royals TP, Alexander BT. Oxidative stress contributes to sex differences in blood pressure in adult growth-restricted offspring. Hypertension. 2012;60:114–122. doi: 10.1161/HYPERTENSIONAHA.112.192955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yanes LL, Romero DG, Iles JW, Iliescu R, Gomez-Sanchez C, Reckelhoff JF. Sexual dimorphism in the renin-angiotensin system in aging spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R383–R390. doi: 10.1152/ajpregu.00510.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.