Abstract
Background
Recent data suggest that children of mothers who are obese before pregnancy, or who gain too much weight during pregnancy, may be at an increased risk of cognitive impairments.
Methods
Mother–infant dyads enrolled in a birth cohort study in Pittsburgh, Pennsylvania (1983–1986), were followed from early pregnancy to 14 years postpartum (n=574). Math, reading and spelling achievements were assessed at ages 6 and 10 years using the Wide Range Achievement Test-Revised, and at age 14 years using the Wechsler Individual Achievement Test Screener. Self-reported total GWG was converted to gestational age-standardised z-scores. Generalised estimating equations were used to estimate the effects of GWG and pre-pregnancy body mass index (BMI) on academic achievement at 6, 10 and 14 years, while adjusting for maternal race, child sex, parity, employment, family income, maternal intelligence, maternal depression, pre-pregnancy BMI (in GWG models only) and the home environment.
Results
The mean (SD) BMI was 23.4 (5.7) kg/m2 and the mean (SD) GWG reported at delivery was 14.4 (5.9) kg. There was a significant non-linear association between pre-pregnancy BMI and an offspring’s academic achievement. At 6, 10 and 14 years, an offspring’s academic scores were inversely associated with pre-pregnancy BMI beyond 22 kg/m2. High GWG (>1 SD) was associated with approximately 4-point lower reading (adjusted β (adjβ) −3.75, 95% CI −7.1 to −0.4) and spelling scores (adjβ −3.90, 95% CI −7.8 to −0.2), compared with GWG −1 to +1 SD.
Conclusions
Future studies in larger and socioeconomically diverse populations are needed to confirm maternal weight and weight gain as causal determinants of a child’s academic skills, and whether this effect persists into adulthood.
INTRODUCTION
Children of mothers who are obese before pregnancy, or who gain too much weight during pregnancy, are at high risk of a number of adverse short-term and long-term outcomes, including preterm birth,1 stillbirth, obesity2 and later-life cardiovascular disease.3 Recent data suggest that maternal obesity and/or mothers who gain excessive weight during pregnancy may also have children who are at increased risk of cognitive impairments (eg, deficits in intelligence4 and executive function5) and problem behaviours that are consistent with attention-deficit hyperactivity disorder.6 These deficits may interfere with academic success7; however, less is known about the impact of maternal weight and weight gain on an offspring’s academic achievement.
Academic achievement is a key outcome because it not only synthesises how behavioural and cognitive problems impact real-life functioning, but also predicts professional attainment and long-term job success.8 Four previous studies have sought to establish the association between maternal body mass index (BMI), or weight gain, and an offspring’s academic achievement,4,9–11 but only two studies adequately adjusted for socioeconomic status or other critical confounders such as the cognitive enrichment in the home. Additionally, all four studies assessed child achievement at a single time point in children 7 years or younger. Therefore, it is unclear whether underachievement related to maternal weight that is observed in kindergarten, for instance, is transient, or persists into late childhood and early adolescence.12 Our objective was to assess an offspring’s math, reading and spelling scores at ages 6, 10 and 14 years in relation to maternal pre-pregnancy BMI and gestational weight gain (GWG) in a cohort of black and white low-income mother–child pairs.
METHODS
We used data from the Maternal Health Practices and Child Development Cohort from the Magee-Womens Hospital in Pittsburgh, Pennsylvania (1983–1986). All women who drank ≥3 drinks per week, and a sample of women who used less than this amount were selected for the alcohol cohort. All women who smoked ≥2 joints per month, and a sample of women who used less than this amount were selected for the marijuana cohort. None of the women had a diagnosed substance abuse problem. A majority of these women were light marijuana and alcohol users in their first trimester (n=508 drank <3 drinks per week; n=516 smoked <2 joints per month), a time when many women do not know they are pregnant. Mother–child pairs were followed and interviewed during pregnancy and at multiple postpartum time points. Included in this analysis are postpartum assessments at ages 6, 10 and 14 years. Women provided informed, written consent, and the study was approved by the University of Pittsburgh Institutional Review Board (IRB #PRO14020264).
Pre-pregnancy weight and height were self-reported at the first study visit. We categorised pre-pregnancy BMI (weight (kg)/height (m2)) using WHO criteria.13 We classified self-reported total GWG at delivery according to gestational age-standardised z-scores, a measure of GWG that by design is uncorrelated with gestational age.14 The z-score charts were developed from serial prenatal weight measurements in a random sample of normal weight term pregnancies without complications, from the Magee-Womens Hospital (1998–2008).14 z-Scores were calculated using charts for normal weight women to allow us to evaluate whether the association between GWG z-scores and an offspring’s academic scores varied depending on pre-pregnancy BMI.
Trained assessors, blinded to maternal prenatal and current substance use, evaluated child academic achievement using the Wide Range Achievement Test-Revised (WRAT-R)15 at ages 6 and 10 years, and the Wechsler Individual Achievement Test (WIAT)16 at age 14 years. Both assess skills in math, reading and spelling. The final score on each scale is age-standardised to a mean (SD) of 100 (15), allowing for comparability across tools. We analysed all scales as continuous variables.
In addition, an offspring’s intelligence and behaviour were assessed at age 10 years. The Stanford-Binet Intelligence Scale-4th edition17 measured child intelligence (composite scale dichotomised as low IQ (≤89) vs average or above IQ (>89)).17 Parent ratings on the Child Behavior Checklist assessed offspring internalising, externalising and attention behaviours (all scales dichotomised as borderline clinical (≥67) vs average (<67)).18
At the first study visit (median 18.7 weeks, IQR 17.1–20.7), trained interviewers collected information on sociodemographic characteristics, maternal depression using the Center for Epidemiological Studies Depression Scale,19 anxiety using the Spielberger State-Trait Anxiety Personality Inventory, and the quantity and frequency of substance use during the year prior to pregnancy and during the first trimester using a questionnaire validated in this cohort.20,21 We categorised each substance into non-users throughout pregnancy, users during the first trimester, and use throughout pregnancy. At 10 years postpartum, maternal intelligence22 and the quality and quantity of support for cognitive and social development in the home environment were measured (Home Observation for Measurement of the Environment-Short Form).23
Data analysis
We tested for differences in BMI and GWG by maternal characteristics using Student t test and one-way analysis of variance (ANOVA). We used a paired t test to examine whether there was a significant and meaningful change in children’s math, reading and spelling scores across the three assessment points, and a repeated measures ANOVA to examine whether this change varied by GWG z-score group or BMI category. To visualise the longitudinal pattern of each academic score by BMI and GWG, we plotted mean math, reading and spelling scores by age. We used Student t test to examine differences in academic achievement scores by an offspring’s intelligence and behaviour scores at age 10 years (the age at which intelligence and behaviour were measured).
We fit generalised estimating equations with an exchangeable covariance structure (Gaussian family, identity link) to estimate unadjusted and adjusted β coefficients and their corresponding 95% CIs for the association between pre-pregnancy BMI or GWG, and each of the offspring’s achievement scores (math, reading and spelling). Generalised estimating equations were used to account for the intraindividual correlation of a child’s academic assessments at multiple ages, and accommodate varying data completeness over time.
We explored non-linear relationships between a child’s academic skills and maternal pre-pregnancy BMI and GWG z-score using cubic and linear splines. We selected a linear spline with a single knot at a BMI of 22 kg/m2, which was the observed point of inflection in all models. Since the relation between GWG and academic scores did not deviate from linearity, we categorised GWG as <−1 SD, −1 to +1 SD, and >+1 SD for ease of interpretation.
The age at the time of assessment (ie, 6, 10 or 14 years) was coded as a dummy variable and included in all models to account for time. Potential confounders were identified using theory-based causal diagrams.24 To select the most parsimonious model, we retained potential confounders that, if removed from the model, changed the primary exposure effect estimate by >10%.25,26 Maternal race, child sex, parity, employment, family income, maternal intelligence, maternal depression, pre-pregnancy BMI (in GWG models only), and the home environment, met our definition of confounding and were included in all adjusted models. We calculated the difference between the age the child was assessed and the standardised testing age (eg, 6.4 years minus 6 years) to account for the deviation from the standardised score, and included this variable in all models. Prenatal substance use variables were forced into all models based on a priori decisions. We separately tested effect modification by maternal race, child sex, pre-pregnancy BMI (in GWG models only), and the age at assessment (time), by including statistical interaction terms with BMI or GWG z-score (tested both as continuous and categorical for all models) in fully adjusted models. Effect modification was present when α=0.05. We plotted adjusted predicted math, reading and spelling scores, and 95% CI according to pre-pregnancy BMI, with covariates set to population means.
We re-ran our analyses after limiting to mother–child pairs with complete data and after excluding high marijuana (>1 joint a day),27 alcohol (>1 drink a day),28 cigarette (≥20 cigarettes per day),29 cocaine (any use) and illicit drug (any use) users during the first or third trimester. Analyses were conducted in Stata software, V.13.0 (StataCorp, College Station, Texas, USA).30
RESULTS
Of the 763 mother–child pairs at delivery, we excluded 65 pairs without child follow-up data, as well as 22 with missing data on BMI or GWG, and 102 with missing covariates in the final model. The final analytic sample included 574 mother–child pairs contributing 1567 observations (n=542 pairs at age 6 years, n=557 pairs at age 10 years, and n=468 pairs at age 14 years). There were no differences in maternal race, child sex, prenatal substance use or offspring’s academic scores between those with and without missing data. Mothers with a BMI >30 kg/m2 and with GWG <−1 SD were more likely to have missing data (data available on request). At the time of enrolment, about half the women were black, a majority were unmarried, unemployed, and had a family income of <US$500 per month (<US$1400 per month in 201431) (table 1). Most women reported no illicit drug use, 50% reported no marijuana use during pregnancy, and about one-third of women reported no prenatal alcohol or cigarette use (table 1).
Table 1.
Characteristics of the cohort overall, and differences by child academic scores at age 10 years, Pittsburgh, Pennsylvania (1983–1986), at enrolment or delivery (n=574 mother–child pairs)
| Overall N (%) | Math Mean (SD) | Reading Mean (SD) | Spelling Mean (SD) | |
|---|---|---|---|---|
| Enrolment | ||||
| Maternal race | ||||
| White | 276 (48.1) | 92.1 (13.3)* | 97.7 (14.5)* | 95.9 (14.1)* |
| Black | 298 (51.9) | 85.8 (12.0) | 90.9 (15.6) | 91.3 (14.6) |
| Marital status | ||||
| Never married | 388 (67.6) | 88.1 (12.3)* | 93.1 (15.8)* | 92.9 (14.7) |
| Married | 186 (32.4) | 90.3 (14.5) | 96.4 (14.5) | 94.6 (14.2) |
| Maternal employment† | ||||
| No | 420 (73.2) | 88.3 (13.1) | 93.8 (15.5) | 93.1 (14.9) |
| Yes | 154 (26.8) | 90.2 (12.8) | 95.1 (15.4) | 94.6 (13.6) |
| Family income (US$ per month) | ||||
| <500 | 351 (61.2) | 87.5 (13.0) | 92.8 (15.9) | 92.0 (14.9) |
| ≥500 | 223 (38.9) | 90.8 (12.8) | 96.2 (14.9) | 95.6 (13.9) |
| Maternal depression scale | ||||
| Not depressed <40 | 256 (44.6) | 89.1 (12.3) | 95.1 (14.8) | 94.0 (14.2) |
| Moderately depressed ≥40 | 318 (55.4) | 88.6 (13.6) | 93.4 (15.9) | 93.1 (14.8) |
| Delivery | ||||
| Prenatal alcohol use (any) | ||||
| Never used | 152 (36.5) | 88.8 (14.0) | 93.2 (16.4) | 92.4 (15.2) |
| Drank 1 trimester | 161 (28.1) | 89.4 (12.3) | 94.8 (14.6) | 94.5 (13.5) |
| Drank ≥2 trimesters | 261 (45.5) | 88.4 (13.0) | 94.3 (15.5) | 93.4 (14.9) |
| Prenatal marijuana use (any) | ||||
| Never used | 287 (50.0) | 89.0 (13.0) | 95.4 (15.3)* | 94.2 (14.2) |
| Smoked 1 trimester | 136 (23.7) | 89.7 (13.0) | 94.3 (15.3) | 93.7 (14.6) |
| Smoked ≥2 trimesters | 151 (26.3) | 87.5 (13.1) | 91.5 (15.7) | 91.8 (15.2) |
| Prenatal cigarette use (any) | ||||
| Never used | 220 (38.3) | 89.2 (12.6) | 94.3 (14.6) | 93.4 (13.4) |
| Smoked 1 trimester | 44 (7.7) | 86.6 (13.8) | 90.7 (17.8) | 91.1 (16.6) |
| Smoked ≥2 trimesters | 310 (54.0) | 88.9 (13.2) | 94.5 (15.6) | 93.9 (15.0) |
| Prenatal illicit drug use throughout pregnancy (any) | ||||
| No | 508 (88.5) | 88.5 (12.9) | 93.6 (15.5)* | 92.9 (14.5)* |
| Yes | 66 (11.5) | 91.2 (13.6) | 98.6 (14.1) | 97.5 (14.1) |
| Child sex | ||||
| Female | 288 (50.2) | 89.7 (12.8) | 95.0 (13.6) | 95.1 (13.4)* |
| Male | 286 (49.8) | 87.8 (13.3) | 93.3 (17.1) | 91.8 (15.5) |
p<0.05.
School attendance.
The mean (SD) pre-pregnancy BMI was 23.4(5.7) kg/m2, and total GWG was 14.4(5.9) kg. Mean math, reading and spelling scores did not meaningfully differ by age, and were all within the expected age-normed range of 85–115 (corresponding to a mean (SD) of 100 (15); see online supplementary table S1).15,16
Table 1 shows the differences in math, reading and spelling scores at age 10 years by maternal characteristics (results were similar for 6 and 14 years, see online supplementary table S2). At age 10 years, academic scores were significantly higher among children of white mothers and married mothers, and tended to be higher among children of working mothers and families with an income ≥US$500 per month at enrolment, compared with their counterparts. Children of mothers who did not use marijuana prenatally had significantly higher reading scores at age 10 years, and all scores were higher among children whose mothers used illicit drugs prenatally (likely explained by the racial disparity in using illicit drugs). An offspring’s academic scores did not differ by prenatal alcohol or cigarette use.
Online supplementary table S3 shows the difference in an offspring’s academic achievement by intelligence and behaviour at age 10 years. Children with average or above average intelligence, or fewer behaviour problems scored higher on the math, reading and spelling skills tests compared with children scoring lower on the intelligence test, or with a greater number of behaviour problems.
The difference in mean academic scores by pre-pregnancy BMI was similar at ages 6, 10 and 14 years (figure 1A–C). Among children of obese mothers, mean math, reading and spelling scores were 4–6 points lower at ages 6 and 14 years, and 5–6 points lower at age 10 years compared with normal weight mothers, although the level of significance varied. In unadjusted multivariable models, child reading and spelling scores were significantly lower among obese compared with normal weight mothers across ages 6, 10 and 14 years, while differences in math scores were of borderline statistical significance (table 2). After adjusting for confounders, the relationship between pre-pregnancy BMI and math, reading and spelling scores at 6, 10 and 14 years were significantly non-linear (see online supplementary figure S1). An offspring’s academic scores at 6, 10 and 14 years were inversely associated with pre-pregnancy BMI beyond 22 kg/m2. Mothers with BMI values of 26, 28 or 30 kg/m2 had children with math scores that were −1.3 (95% CI −2.2 to −0.4), 1.9 (95% CI −3.2 to −0.6) or 2.6 (95% CI −4.4 to −0.8) points lower, respectively, compared with children whose mothers had a BMI of 22 kg/m2 (table 2). Associations were similar for an offspring’s reading and spelling scores (table 2). These associations did not statistically vary by age at assessment (time).
Figure 1.
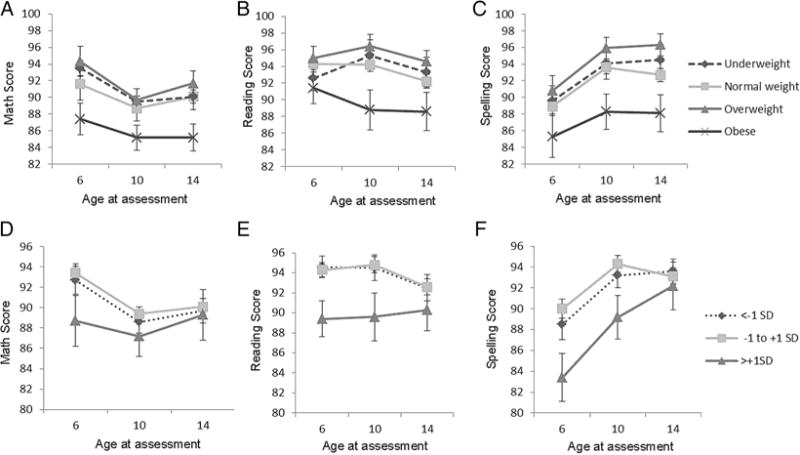
Mean (SEM) academic scores at ages 6 (n=542 pairs), 10 (n=557 pairs) and 14 years (n=468 pairs) by pre-pregnancy body mass index (A–C) and gestational weight gain z-score (D–F).
Table 2.
Unadjusted and adjusted non-linear association between pre-pregnancy body mass index (BMI) and offspring’s math, reading and spelling scores at ages 6, 10 and 14 years (n=574 unique pairs contributing 1567 observations)
| Pre-pregnancy BMI* | Math β (95% CI) |
Reading β (95% CI) |
Spelling β (95% CI) |
|---|---|---|---|
| Underweight | −0.95 (−3.9 to 1.9) | −0.06 (−3.0 to 3.0) | 0.69 (−2.5 to 3.9) |
| Normal weight | Reference | Reference | Reference |
| Overweight | 0.59 (−2.1 to 3.2) | 1.62 (−0.9 to 4.1) | 2.71 (0.01 to 5.4) |
| Obese | −5.47 (−9.2 to 1.7) | −4.63 (−8.5 to −0.8) | −4.99 (−8.9 to −1.0) |
|
| |||
| Pre-pregnancy BMI (kg/m2)† | adjβ‡ (95% CI) | adjβ‡ (95% CI) | adjβ‡ (95% CI) |
|
| |||
| 18 | −0.72 (−3.6 to 1.7) | −1.33 (−3.9 to 1.3) | −1.25 (−4.1 to 1.5) |
| 20 | −0.36 (−1.8 to 0.9) | −0.67 (−2.1 to 0.7) | −0.63 (−2.1 to 0.7) |
| 22 | Reference | Reference | Reference |
| 24 | −0.65 (−1.1 to −0.2) | −0.54 (−1.0 to −0.1) | −0.62 (−1.1 to −0.2) |
| 26 | −1.31 (−2.2 to −0.4) | −1.09 (−2.1 to −0.1) | −1.26 (−2.2 to −0.3) |
| 28 | −1.94 (−3.2 to −0.6) | −1.62 (−3.1 to −0.1) | −1.86 (−3.2 to −0.5) |
| 30 | −2.61 (−4.4 to −0.8) | −2.18 (−4.1 to −0.2) | −2.51 (−4.4 to −0.6) |
| 32 | −3.25 (−5.5 to −0.9) | −2.70 (−5.1 to −0.2) | −3.12 (−5.5 to −0.8) |
Underweight (BMI <18.5 kg/m2); normal weight (BMI 18.5–24.9 kg/m2); overweight (BMI 25–29.9 kg/m2); obese (BMI ≥30 kg/m2).
Linear spline terms with a single knot at a BMI of 22 kg/m2.
Adjusted for age, maternal race, parity, employment, family income, maternal intelligence, home environment, maternal prenatal depression and prenatal substance use (marijuana, alcohol, cigarette and illicit drugs).
The magnitude of the difference in mean academic scores by GWG z-score group did not vary significantly at ages 6, 10 and 14 years (figure 1D–F). Mean reading and spelling scores were 5–6 points lower at ages 6 and 10 years among children of mothers gaining >+1 SD compared with −1 to +1 SD. At age 14 years, children of mothers with high GWG scored a mean of 1–2 points lower on math, reading and spelling tests. Reading and spelling scores were lower with high GWG compared with mothers gaining −1 to +1 SD, while differences in math scores had a similar relation, but were only of borderline significance (table 3). After adjustment, high GWG (>+1 SD) was significantly associated with a nearly 4-point lower score in reading (adjusted β (adjβ) −3.75, 95% CI −7.1 to −0.4) and spelling (adjβ −3.90, 95% CI −7.8 to −0.2), compared with GWG −1 to +1 SD. Math scores were also lower, but this difference was not statistically significant. These associations did not statistically vary by pre-pregnancy BMI or by age at assessment, despite the appearance of attenuation in the magnitude of the effect between GWG and offspring’s achievement scores at 14 years.
Table 3.
Unadjusted and adjusted associations between gestational weight gain, and offspring’s math, reading and spelling scores at ages 6, 10 and 14 years (n=574 unique pairs)
| Math
|
Reading
|
Spelling
|
||||
|---|---|---|---|---|---|---|
| β (95% CI) | Adjβ* (95% CI) | β (95% CI) | Adjβ* (95% CI) | β (95% CI) | Adjβ* (95% CI) | |
| GWG z-score† | ||||||
| <−1 SD | 0.41 (−1.9 to 2.7) | 1.77 (−0.3 to 3.8) | 0.81 (−1.5 to 3.1) | 2.01 (−0.2 to 4.2) | 0.32 (−2.1 to 2.8) | 1.33 (−1.0 to 3.3) |
| −1 to +1 SD | Reference | Reference | Reference | Reference | Reference | Reference |
| >+1 SD | −2.47 (−6.1 to 1.1) | −2.17 (−5.6 to 1.1) | −4.39 (−7.8 to −0.9) | −3.75 (−7.1 to −0.4) | −4.41 (−8.1 to −0.7) | −3.90 (−7.8 to −0.2) |
Adjusted for age, maternal race, parity, employment, family income, maternal intelligence, home environment, maternal prenatal depression and prenatal substance use (marijuana, alcohol, cigarette and illicit drugs).
<−1 SD (<11.2 kg at 40 weeks of gestation); −1 to +1 SD (11.2–22.9 kg); >+1 SD (>22.9 kg).
None of the above findings varied by race or child sex (interaction p>0.05). Results were not meaningfully different after including other potential confounders in the models (child sex, marital status, maternal education and maternal anxiety), limiting the analysis to those with data at all three visits (n=439), or excluding heavy substance users (data available on request).
DISCUSSION
Academic performance is an indicator of a child’s general cognitive functioning, social acuity and behavioural control, and strongly predicts adult employment and work success.8 Our findings suggest that children born to obese mothers, or mothers with high GWG, have lower math, reading and spelling scores across 6, 10 and 14 years. These relations remained after adjustment for measures of cognitive stimulation in the home, socioeconomic status, prenatal depression, prenatal substance use and other confounders.
Our results on pre-pregnancy BMI confirm findings in kindergarten-aged children from two previous nationally representative studies in the USA. Data from the National Longitudinal Study of Youth (1986–2008, n=3412) found that children aged 5–7 years of obese mothers scored 2–3 points lower on math and reading portions of the Peabody Individual Achievement Test compared with children of normal weight mothers.10 In a second study of 5200 children ages 5–6 years in the Early Childhood Longitudinal Birth Cohort (2001–2008),11 children of overweight and obese mothers had a modest decrease in reading, but not math scores, on standardised tests developed for this study.11 Our work extends these findings to illustrate that associations between maternal obesity and children’s academic performance persist at 10 and 14 years, and therefore, may have long-term effects.
The existing literature on GWG and child academic performance is small, and most found no association, which conflicts with the 3–4 point lower scores we observed in an offspring’s reading and spelling skills with excessive GWG. In a study of 8704 7-year-old siblings in the Collaborative Perinatal Project (1959–1973), GWG above the 2009 Institute of Medicine guidelines was not associated with an offspring’s math or reading scores (as assessed using the WRAT, the same tool we used) compared with GWG within the guidelines, after controlling for individual factors and shared factors among siblings.9 In nearly 6000 4-year-old children from the Avon Longitudinal Study (1991–1997), GWG below the IOM guidelines was associated with a clinically insignificant decrease (<0.1 point) in an offspring’s composite academic scores.4 Previous studies used large nationally representative cohorts while we used a higher risk, low-income sample, which may explain the difference in findings. The compounding stressors associated with low SES may contribute to a more susceptible environment for excessive GWG to impact academic achievement, yet, no previous studies mentioned differences in outcomes by SES. We were unable to test effect modification by SES since the Maternal Health Practices and Child Development (MHPCD) population only represents a lower SES group of women.
Our results were generally consistent with those from studies in this cohort relating maternal BMI to domain-specific cognition (ie, child intelligence and behaviour) (S Pugh, G Richardson, J Hutcheon, et al. Gestational weight gain, pre-pregnancy body mass index and offspring behavior and attention-deficit hyperactivity disorder symptoms. Unpublished manuscript 2015).32 Unlike domain-specific cognition measurements, academic achievement synthesises how behavioural and cognitive problems impact real-life functioning. Combined, these findings suggest that lower intelligence and clinically significant problem behaviours at age 10 years due to maternal BMI and GWG translate into significantly worse functional skills. However, the associations with GWG differed in previous studies where we observed only a trend towards increasing deficits associated with high maternal GWG. While the impact of GWG on individual domains may have been too small to detect a significant difference, the totality of intelligence and behaviour impairments may have impacted academic achievement enough to detect lower scores with excessive GWG. In animal studies, offspring of mothers consuming a high-fat diet contributing to excessive weight gain during pregnancy had increased circulating proinflammatory cytokines, which can disrupt a number of fetal neurodevelopmental processes33,34 necessary for adequate cognitive and behavioural development.35,36
These results must be considered in the context of the study’s limitations. This study is observational and cannot determine causality. The pregnancy cohort is comprised of substance-using women from a lower socioeconomic status background; therefore, our results may only be generalisable to similarly disadvantaged populations. While prenatal substance use is a potential concern when assessing offspring cognition, when we examined the impact of excluding the high substance-using women on our results, our estimates remained unchanged. There is also the potential for attrition bias due to the longitudinal follow-up over 14 years. However, the retention rate was high in this cohort at 6 (88%), 10 (83%), and 14 (76%) years. This bias may be a concern, since those with and without missing data at postpartum assessments did differ by GWG and BMI; however, there were no differences by academic achievement scores, maternal race, child sex or prenatal substance use. Multiple follow-up assessments strengthened this study because we could obtain a more accurate depiction of academic skills, which tend to vary over time.37 We fit a generalised estimating equations (GEE) model as opposed to other growth curve models, because this cohort did not have an adequate number of gestational weight-gain measurements to predict gestational weight-gain trajectories. We relied on self-reported pre-pregnancy weight, height and total GWG, which may result in misclassification bias.38 However, since mothers recalled their pre-pregnancy weight at the first visit, and their weight within days of delivery, self-reported weight gain is likely close to the true value. Unfortunately, we do not have information on the validity of self-reported weight and weight gain in this population.
We used a measure of GWG that, by design, is independent of gestational age, which allows us to separate the effect of gestational age from GWG. This is especially important when studying outcomes correlated with preterm birth, such as academic performance.39,40 The objective nature and high construct validity and reliability of the WRAT-R and WIAT instils confidence that children are correctly classified. In addition, we controlled for a number of important confounders including maternal intelligence, socioeconomic status, maternal depression, prenatal substance use and child stimulation at home.
Our finding that low GWG was not associated with a child’s academic performance in the present study, or cognition in previous work (S Pugh, G Richardson, J Hutcheon, et al. Gestational weight gain, pre-pregnancy body mass index and offspring behavior and attention-deficit hyperactivity disorder symptoms. Unpublished manuscript 2015)32 is important. There is concern that low weight gain, particularly among obese women, may impair an offspring’s cognitive function.41 While our results and that of others4,9,10 suggest no relationship with low weight gain, we were limited by a mostly lean cohort and few women with very low weight gain during pregnancy. Future studies should aim to fill this knowledge gap.
Future studies in larger and socioeconomically diverse populations are needed to confirm that maternal weight and weight gain are modifiable factors related to child academic skills, and whether this effect persists into adulthood. The 2–3 point decrease in academic achievement scores that we observed with maternal obesity and excessive GWG may not be meaningful for an individual, but the downward shift in the population average may have an impact on college attendance, employment and work success.8,42
Supplementary Material
What is already known on this subject.
Children of mothers who are obese before pregnancy, or who gain too much weight during pregnancy, are at high risk of a number of adverse short-term and long-term outcomes. Recent evidence suggests children may also be at an increased risk of impaired intelligence and behaviour. However, less is known about the impact of maternal weight and weight gain on an offspring’s academic achievement, particularly in late childhood and early adolescence.
What this study adds.
Our findings suggest that children born to obese mothers or mothers with high gestational weight gain have lower math, reading and spelling scores across ages 6, 10 and 14 years, compared with normal weight mothers or mothers with average weight gain. The observed decrease in academic achievement scores may not be meaningful for an individual, but the downward shift in the population average may have an impact on college attendance, employment and work success.
Acknowledgments
Funding The study is supported by the National Institutes of Health: R01HD072008, AA06390, AA06666, DA03874, DA03209.
Footnotes
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/jech-2015-206800).
Contributors SJP, GAR, JAH, KH, MMB and LMB designed the research. GAR and NLD provided essential materials. SJP analysed the data and wrote the paper. SJP had primary responsibility for the final content. All authors critically reviewed the drafts and approved the final manuscript.
Competing interests None declared.
Ethics approval University of Pittsburgh Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.McDonald SD, Han Z, Mulla S, et al. High gestational weight gain and the risk of preterm birth and low birth weight: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2011;33:1223–33. doi: 10.1016/S1701-2163(16)35107-6. [DOI] [PubMed] [Google Scholar]
- 2.Vasudevan C, Renfrew M, McGuire W. Fetal and perinatal consequences of maternal obesity. Arch Dis Child Fetal Neonatal Ed. 2011;96:F378–82. doi: 10.1136/adc.2009.170928. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds RM, Allan KM, Raja EA, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ. 2013;347:f4539. doi: 10.1136/bmj.f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gage SH, Lawlor DA, Tilling K, et al. Associations of maternal weight gain in pregnancy with offspring cognition in childhood and adolescence: findings from the Avon Longitudinal Study of Parents and Children. Am J Epidemiol. 2013;177:402–10. doi: 10.1093/aje/kws239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buss C, Entringer S, Davis EP, et al. Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms. PLoS ONE. 2012;7:e37758. doi: 10.1371/journal.pone.0037758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brion M-J, Zeegers M, Jaddoe V, et al. Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics. 2011;127:e202–11. doi: 10.1542/peds.2010-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biederman J, Monuteaux MC, Doyle AE, et al. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. J Consult Clin Psychol. 2004;72:757–66. doi: 10.1037/0022-006X.72.5.757. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt FL. The role of general cognitive ability and job performance: why there cannot be a debate. Hum Perform. 2002;15:187–210. [Google Scholar]
- 9.Keim SA, Pruitt NT. Gestational weight gain and child cognitive development. Int J Epidemiol. 2012;41:414–22. doi: 10.1093/ije/dyr229. [DOI] [PubMed] [Google Scholar]
- 10.Tanda R, Salsberry PJ, Reagan PB, et al. The impact of prepregnancy obesity on children’s cognitive test scores. Matern Child Health J. 2013;17:222–9. doi: 10.1007/s10995-012-0964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinkle SN, Sharma AJ, Kim SY, et al. Maternal prepregnancy weight status and associations with children’s development and disabilities at kindergarten. Int J Obes (Lond) 2013;37:1344–51. doi: 10.1038/ijo.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loe IM, Feldman HM. Academic and educational outcomes of children with ADHD. J Pediatr Psychol. 2007;32:643–54. doi: 10.1093/jpepsy/jsl054. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Physical status: the use and interpretation of anthropometry. Switzerland: World Health Organization; 1995. [PubMed] [Google Scholar]
- 14.Hutcheon JA, Platt RW, Abrams B, et al. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. Am J Clin Nutr. 2013;97:1062–7. doi: 10.3945/ajcn.112.051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jastak S, Wilkinson GS. Manual for the wide range achievement test, revised. Wilmington, DE: Jastak Associates; 1984. [Google Scholar]
- 16.Pyschological Corporation. Wechsler individual achievement test screener. San Antonio, TX: Harcourt Brace Jovanovich, Inc; 1992. [Google Scholar]
- 17.Thorndike R, Hagen E, Sattler J. The Stanford-Binet Intelligence Scale. 4th. Chicago: Riverside Publishing; 1986. [Google Scholar]
- 18.Achenbach T. Manual for the child behavior checklist/4–18 and 1991 pro file. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 19.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychological Meas. 1977;1:385–401. [Google Scholar]
- 20.Robles N, Day NL. Recall of alcohol consumption during pregnancy. J Stud Alcohol. 1990;51:403–7. doi: 10.15288/jsa.1990.51.403. [DOI] [PubMed] [Google Scholar]
- 21.Day NL, Robles N. Methodological issues in the measurement of substance use. Ann N Y Acad Sci. 1989;562:8–13. doi: 10.1111/j.1749-6632.1989.tb21002.x. [DOI] [PubMed] [Google Scholar]
- 22.Brooker BH, Cyr JJ. Tables for clinicians to use to convert WAIS-R short forms. J Clin Psychol. 1986;42:982–6. [Google Scholar]
- 23.Baker P, Mott F. National Longitudinal Study of Youth-Child Handbook. State University Center for Human Resource Research; Columbus, OH: 1989. [Google Scholar]
- 24.Hernan MA, Hernandez-Diaz S, Werler MM, et al. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155:176–84. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 25.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–9. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weng HY, Hsueh YH, Messam LL, et al. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol. 2009;169:1182–90. doi: 10.1093/aje/kwp035. [DOI] [PubMed] [Google Scholar]
- 27.Day NL, Richardson GA, Goldschmidt L, et al. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol. 2011;16:169–75. doi: 10.1016/0892-0362(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 28.Day NL, Richardson GA. An analysis of the effects of prenatal alcohol exposure on growth: a teratologic model. Am J Med Genet C Semin Med Genet. 2004;127C:28–34. doi: 10.1002/ajmg.c.30013. [DOI] [PubMed] [Google Scholar]
- 29.Cornelius MD, Goldschmidt L, DeGenna N, et al. Smoking during teenage pregnancies: effects on behavioral problems in offspring. Nicotine Tob Res. 2007;9:739–50. doi: 10.1080/14622200701416971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.StataCorp. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 31.Bureau of Labor Statistics. CPI Inflation Caclulator. [cited 2013]; Available from: http://www.bls.gov/data/inflation_calculator.htm.
- 32.Pugh SJ, Richardson GA, Hutcheon JA, et al. Maternal obesity and excessive gestational weight gain are associated with components of child cognition. J Nutr. 2015;145:2562–9. doi: 10.3945/jn.115.215525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tozuka Y, Kumon M, Wada E, et al. Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem Int. 2010;57:235–47. doi: 10.1016/j.neuint.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Niculescu MD, Lupu DS. High fat diet-induced maternal obesity alters fetal hippocampal development. Int J Dev Neurosci. 2009;27:627–33. doi: 10.1016/j.ijdevneu.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oades RD. Dopamine-serotonin interactions in attention-deficit hyperactivity disorder (ADHD) Prog Brain Res. 2008;172:543–65. doi: 10.1016/S0079-6123(08)00926-6. [DOI] [PubMed] [Google Scholar]
- 36.Grayson BE, Levasseur PR, Williams SM, et al. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology. 2010;151:1622–32. doi: 10.1210/en.2009-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rockoff JE. The impact of individual teachers on student achievement: evidence from panel data. Am Econ Rev. 2004;94:247–52. [Google Scholar]
- 38.Brunner Huber LR. Validity of self-reported height and weight in women of reproductive age. Matern Child Health J. 2007;11:137–44. doi: 10.1007/s10995-006-0157-0. [DOI] [PubMed] [Google Scholar]
- 39.Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol. 2011;174:1062–8. doi: 10.1093/aje/kwr230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quigley MA, Poulsen G, Boyle E, et al. Early term and late preterm birth are associated with poorer school performance at age 5 years: a cohort study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F167–73. doi: 10.1136/archdischild-2011-300888. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy: reexamining the guidelines. Washington DC: Institute of Medicine and National Research Council of the National Academies; 2009. [PubMed] [Google Scholar]
- 42.Kuncel NR, Hezlett SA, Ones DS. Academic performance, career potential, creativity, and job performance: can one construct predict them all? J Pers Soc Psychol. 2004;86:148–61. doi: 10.1037/0022-3514.86.1.148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


