Abstract
Little is known about the frequency and patterns of hyperkalemia in clinical settings. We evaluated the association between baseline antihypertensive medications that may affect potassium levels (angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), beta-blockers, loop/thiazide diuretics, and potassium-sparing diuretics) and hyperkalemia, defined by potassium >5 mEq/L and >5.5 mEq/L, over a 3-year time period in 194,456 outpatients in the Geisinger Health System, as well as actions taken after an episode of hyperkalemia. The proportions of patients with 0, <2, 2–4, and ≥4 potassium measurements per year were 20%, 58%, 16%, and 6%. Potassium levels >5 mEq/L and >5.5 mEq/L occurred in 10.8% and 2.3% of all patients over the 3-year period; among patients with ≥4 measurements per year, corresponding values were 39.4% and 14.6%. Most cases of hyperkalemia occurred only once during follow-up. The antihypertensive medication class most strongly associated with hyperkalemia was ACEIs. Among patients with a measurement of potassium >5.5 mEq/L, only 24% were seen by a nephrologist and 5.2% were seen by a dietician during the 3-year period. Short-term actions after a potassium measurement >5.5 mEq/L included emergency room visit (3.1% within 7 days), re-measurement of potassium (44.3% with 14 days), and change in a potassium-altering medication (26.4% within 60 days). The most common medication changes were discontinuation/dose reduction of an ACEI/ARB or potassium-sparing diuretic, which occurred in 29.1% and 49.6% of persons taking these medications, respectively. In conclusion, hyperkalemia is common. Future research may enable optimal RAAS inhibitor use with improved management of hyperkalemia.
Keywords: hyperkalemia, antihypertensive, medications, potassium, renin-angiotensin-aldosterone system
Introduction
Hyperkalemia is a common electrolyte abnormality that can cause muscle weakness, paralysis, cardiac arrhythmias, and sudden cardiac death. 1,2 Renin-angiotensin-aldosterone system (RAAS) inhibitors such as angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and aldosterone antagonists can increase the risk of hyperkalemia. 3,4 These medications are commonly used and particularly beneficial in patients with conditions such as chronic kidney disease (CKD), diabetes mellitus, and atherosclerotic cardiovascular disease, each of which is also associated with higher risk of hyperkalemia.3–6
Clinical trial data suggests that the risk of hyperkalemia associated with RAAS inhibition ranges from 2%-10% in patients with hypertension, heart failure, and CKD, and that hyperkalemia risk may be slightly lower with ARBs compared to ACEIs. 7,8 However, factors such as dietary potassium counseling and laboratory monitoring for hyperkalemia likely differ in the real-world setting compared to tightly-controlled clinical trials. Suboptimal potassium monitoring practices have been noted after ACEI, ARB, and spironolactone prescription. 9,10 There is little data from clinical practice on the risk of hyperkalemia associated with RAAS inhibition, or the rate of discontinuation of RAAS inhibitors after hyperkalemia events.
Using data from a large, integrated, tertiary healthcare system, we sought to evaluate the frequency and patterns of hyperkalemia and its management in a clinical population, including frequency of potassium monitoring and risk of hyperkalemia associated with certain medication classes.
Methods
Study Population
Geisinger Health System is a large integrated rural healthcare system serving 44 counties in central and northeastern Pennsylvania. Our study population included all individuals ≥18 years of age who had an outpatient visit during which their blood pressure was measured between January 1, 2011 and October 9, 2011 in an outpatient clinic in the Geisinger Health System. Participant data was abstracted from the electronic health record for the 3 years subsequent to this index visit.
Anti-hypertensive Medication Classes
Use of medications was determined from outpatient orders and medication lists assessed at office visits. Anti-hypertensive medications were classified as ACEIs, ARBs, direct renin inhibitors, beta-blockers (BBs), potassium-sparing diuretics (including aldosterone antagonists, amiloride, and triamterene), loop or thiazide diuretics, and other blood pressure medications, which included calcium channel blockers, central agonists, direct vasodilators, and alpha-blockers. Combination pills were categorized into each of their individual components. For the purpose of determining risk factors for specific patterns of hyperkalemia, medication use was noted at the index visit date; for the purpose of determining specific actions after the onset of hyperkalemia, time-varying medication use was assessed.
Covariates
We abstracted data on age, gender, race, blood pressure, body mass index (BMI), serum creatinine, and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis coded history of hypertension, diabetes, congestive heart failure (CHF), and atherosclerotic cardiovascular disease (myocardial infarction, stroke, or peripheral vascular disease) from the Electronic Health Record. Specific coding algorithms are provided in the Online Supplement (please see http://hyper.ahajournals.org; Table S1).
Outcome – Hyperkalemia
Hyperkalemia was defined using two different cutpoints: >5.0 mEq/L and >5.5 mEq/L. For both definitions of hyperkalemia, we examined the pattern of hyperkalemia over the 3-year period, defining patterns as never, transient (only one occurrence), intermittent (more than one occurrence but ≤50% of the potassium measurements), and persistent (>50% of potassium measurements). Only outpatient potassium measurements were used.
Statistical Analysis
Because patterns of hyperkalemia can be affected by frequency of potassium measurements, we categorized patients by the frequency of serum potassium measurements (never, at least once over the 3-year period but <2 times per year, 2 to <4/year, ≥4/year). Because participants with low eGFR are at particularly high risk for hyperkalemia, we specifically evaluated this subgroup that was followed regularly, defined as having potassium measurements ≥4/year. Characteristics were compared across groups using chi-square tests and paired t-tests for categorical and continuous variables, respectively.
We then examined the proportion of individuals who experienced hyperkalemia (and the pattern of hyperkalemia) stratified by frequency of serum potassium measurements (<2/year, 2- <4/year, ≥4/year), baseline eGFR (≥90, 60–89, 45–59, 30–49, and <30 ml/min/1.73 m2), urine albumin-to-creatinine ratio (>30, 30–299, and ≥ 300 mg/g), age (<45, 45–64, ≥65 years), and number of ACEI/ARB/potassium-sparing diuretic medications. We also evaluated the proportion seeing a nephrologist and a dietician during the 3-year period within these categories.
Multinomial logistic regression was used to examine the risk relationship between anti-hypertensive medication class and patterns of hyperkalemia. Covariates selected a priori based on known associations with serum potassium included age, sex, race, eGFR, diabetes, hypertension, congestive heart failure (CHF), atherosclerotic cardiovascular disease, non-steroid anti-inflammatory drugs (NSAIDs), blood pressure, and BMI. Number of potassium measurements per year was included as a covariate with the reference category of <2 potassium checks per year. Because of similarities across category of hyperkalemia pattern, we simplified the analysis using logistic regression for the outcome of ever hyperkalemia. We also evaluated for interaction between medications classes by including a product term in adjusted analyses.
We evaluated short-term actions in participants who experienced hyperkalemia, including emergency room visit within 7 days, repeated potassium measurement within 14 days, and change in potassium-altering medication within 60 days (i.e., increase in dose or new prescription for kaliuretic diuretic or kayexalate, dose reduction or discontinuation of ACEI/ARB or potassium-sparing diuretic). We compared the short-term actions in persons who experienced a hyperkalemia event to those who did not, matching on frequency and count of potassium measurement, in unadjusted and adjusted models. For the outcome of ACEI/ARB dose reduction or discontinuation, we looked for effect modification by the presence of a medical indication for this class of medications, defined as the presence of CHF, history of atherosclerotic cardiovascular disease, a urine albumin-to-creatinine ratio ≥ 30 mg/g, urine protein-to-creatinine ratio ≥ 50 mg/g or 1+ or greater protein on urine dipstick testing.
All analyses were performed using Stata version 13.0 (College Station, TX). P-values <0.05 were considered statistically significant.
Results
Patterns of Potassium Testing
There were a total of 194,456 individuals who had an outpatient visit with a blood pressure measurement during the 2011 index period. Five percent (9,764) died during the subsequent 3 years. The median number of serum potassium measurements was 0.7 per year (IQR 0.3 – 1.7). Twenty percent of the participants had no outpatient potassium testing over the subsequent 3 years; whereas 58% had at least one potassium measurement but less than 2 per year, 16% had between 2 and 4 potassium measurements per year, and 6 % had ≥4 potassium measurements per year.
Individuals who had potassium levels checked more frequently tended to be older, more often male, and more likely to have hypertension, diabetes, atherosclerotic cardiovascular disease, congestive heart failure, eGFR <60 ml/min/1.73m2, and have proteinuria (p<0.001 for all comparisons) (Table 1). More frequent potassium testing was also associated with increased use of all antihypertensive medication classes (p<0.001 for all comparisons). Within the group of regularly-followed patients with eGFR <30 ml/min/1.73 m2 (N=1,158), 39% were taking an ACEI, ARB, or K-sparing agent.
Table 1.
Baseline characteristics of Patients Stratified by Frequency of Potassium Measurement Over A 3-Year Period, 2011–2014
| Average frequency of potassium check over the following 3 years |
Never | <2 per year | 2 - <4 per year |
4+ per year |
|---|---|---|---|---|
| Total N | 38,761 | 111,954 | 31,334 | 12,407 |
| Age, mean (SD), year | 43 (17) | 52 (17) | 65 (15) | 69 (14) |
| Female, n (%) | 23822 (61%) | 65675 (59%) | 17806 (57%) | 6662 (54%) |
| Black, n (%) | 811 (2%) | 2017 (2%) | 459 (1%) | 158 (1%) |
| Diabetes, n (%) | 2519 (6%) | 14081 (13%) | 12320 (39%) | 5641 (45%) |
| Hypertensive, n (%) | 7990 (21%) | 46564 (42%) | 23982 (77%) | 9856 (79%) |
| History of HF, n (%) | 874 (2%) | 2772 (2%) | 3014 (10%) | 2897 (23%) |
| History of atherosclerotic CVD, n (%) | 3954 (10%) | 17564 (16%) | 11994 (38%) | 6408 (52%) |
| Number of HTN meds, median (IQI) | 0 (0, 0) | 0 (0, 1) | 1 (0, 2) | 2 (0, 3) |
| Use of 4+ HTN meds, n (%) | 141 (0%) | 1683 (2%) | 1929 (6%) | 1035 (8%) |
| Use of ACEI, n (%) | 2107 (5%) | 18551 (17%) | 11210 (36%) | 3873 (31%) |
| Use of ARB, n (%) | 482 (1%) | 4392 (4%) | 3428 (11%) | 1415 (11%) |
| Use of renin inhibitors, n (%) | 2 (0%) | 29 (0%) | 26 (0%) | 16 (0%) |
| Use of other diuretics, n (%) | 2157 (6%) | 18023 (16%) | 11992 (38%) | 5342 (43%) |
| Use of K-sparing diuretics, n (%) | 283 (1%) | 2305 (2%) | 1677 (5%) | 764 (6%) |
| Use of beta blockers, n (%) | 2575 (7%) | 18477 (17%) | 12040 (38%) | 5547 (45%) |
| Use of other HTN meds, n (%) | 1255 (3%) | 9594 (9%) | 6688 (21%) | 3196 (26%) |
| Use of NSAID, n (%) | 4564 (12%) | 16406 (15%) | 5017 (16%) | 1170 (9%) |
| eGFR, mean (SD), ml/min/1.73 m2 | 96 (22) | 89 (21) | 76 (23) | 65 (27) |
| eGFR < 60, n (%) | 706 (6%) | 6777 (8%) | 7653 (25%) | 5283 (44%) |
| ACR, median (IQR), mg/g | 14 (6, 44) | 13 (6, 35) | 15 (7, 44) | 29 (11, 104) |
| Missing ACR, n (%) | 37366 (96%) | 101614 (91%) | 21687 (69%) | 7462 (60%) |
| Systolic BP, mean (SD), mmHg | 120 (16) | 124 (16) | 129 (17) | 129 (19) |
| Diastolic BP, mean (SD), mmHg | 72 (10) | 74 (10) | 73 (10) | 71 (11) |
| BMI, mean (SD), Kg/m2 | 29 (7) | 30 (7) | 32 (7) | 31 (8) |
Results are presented as mean (SD) or percentages. p<0.05 for comparisons across columns for all rows.
HF: heart failure, CVD: cardiovascular disease, NSAID: non-steroid anti-inflammatory drugs, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blockers, HTN: hypertension, eGFR: estimated glomerular filtration rate, ACR: urine albumin-to-creatinine ratio, BP: blood pressure, BMI: body mass index.
Proportion of Individuals who Experienced Hyperkalemia
Of the 155,695 participants with available potassium measurements over the 3-year period, 16,834 individuals (10.8%) had at least one potassium >5 mEq/L; 3,582 individuals (2.3%) had at least one potassium >5.5 mEq/L (please see http://hyper.ahajournals.org; Tables S2, S3, Figure S1). The 3-year risk of hyperkalemia was higher among patients with more frequent potassium measurements; 5.3%, 19.0%, and 39.4% experienced potassium >5 mEq/L among those with <2, 2 to <4, and ≥4 potassium checks per year, respectively. The 3-year risk of potassium >5.5 mEq/L was 0.6%, 3.6%, and 14.6% for those with <2, 2 to <4, and ≥4 potassium checks per year, respectively. These risks were higher in patients with eGFR <30 ml/min/1.73 m2, although patterns did not differ substantially by ACEI/ARB use in this subgroup (please see http://hyper.ahajournals.org; Tables S2, S3).
Regardless of frequency of potassium measurement, most cases of hyperkalemia were transient (Figure 1). Over the 3-year period, 13.7% of people with at least one potassium >5.0 mEq/L saw a nephrologist, and 4.4% saw a dietician. Among people with at least one potassium >5.5mEq/L, 24.2% saw a nephrologist; 5.2% saw a dietician. These proportions were generally higher among those with intermittent compared to transient hyperkalemia (please see http://hyper.ahajournals.org; Tables S4, S5).
Figure 1. Proportion Experiencing Transient, Intermittent, and Persistent Hyperkalemia over 3 Years, by Frequency of Potassium Testing.
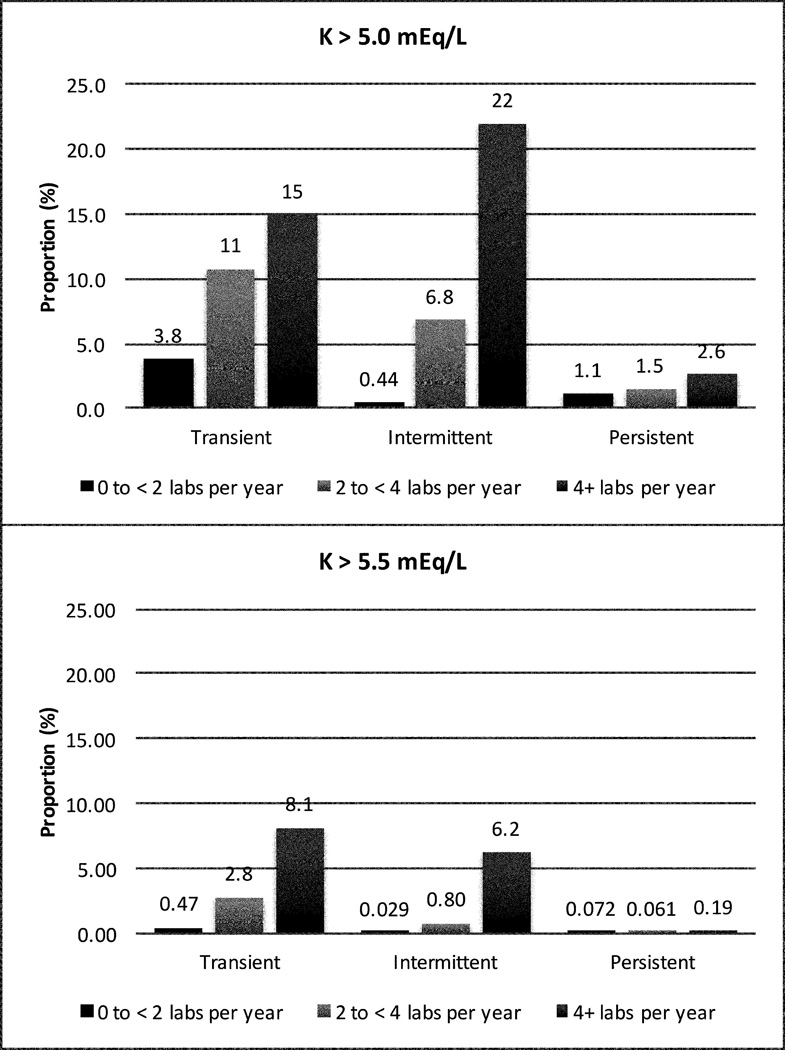
Frequency of serum potassium measurements per year was classified as <2, 2 to <4, and ≥4. Pattern of hyperkalemia was defined as transient (one occurrence), intermittent more than one occurrence but ≤50% of the time), and persistent (>50% of the time).
Risk of Hyperkalemia Associated with Baseline Characteristics
Risk of hyperkalemia increased with lower eGFR category. Among individuals with eGFR <30 ml/min/1.73m2 who had ≥4 potassium checks per year, 62.1% experienced potassium >5 mEq/L and 29.9% experienced potassium >5.5 mEq/L (Figure 2). In adjusted analyses, lower eGFR at levels both above and below 60 ml/min/1.73 m2, male sex, higher systolic blood pressure, lower BMI, and the presence of diabetes were strong and consistent risk factors for hyperkalemia of all patterns and severity (Table 2, please see http://hyper.ahajournals.org; S6, S7). Persons of black race were 46% less likely to experience potassium >5 mEq/L (HR 0.54, 95% CI: 0.44, 0.66, p<0.001), but this was not true for potassium >5.5 mEq/L (HR 1.26, 95% CI: 0.93, 1.72, p=0.1).
Figure 2. Proportion Ever Experiencing Hyperkalemia over 3-Years, by eGFR and Frequency of Potassium Testing.
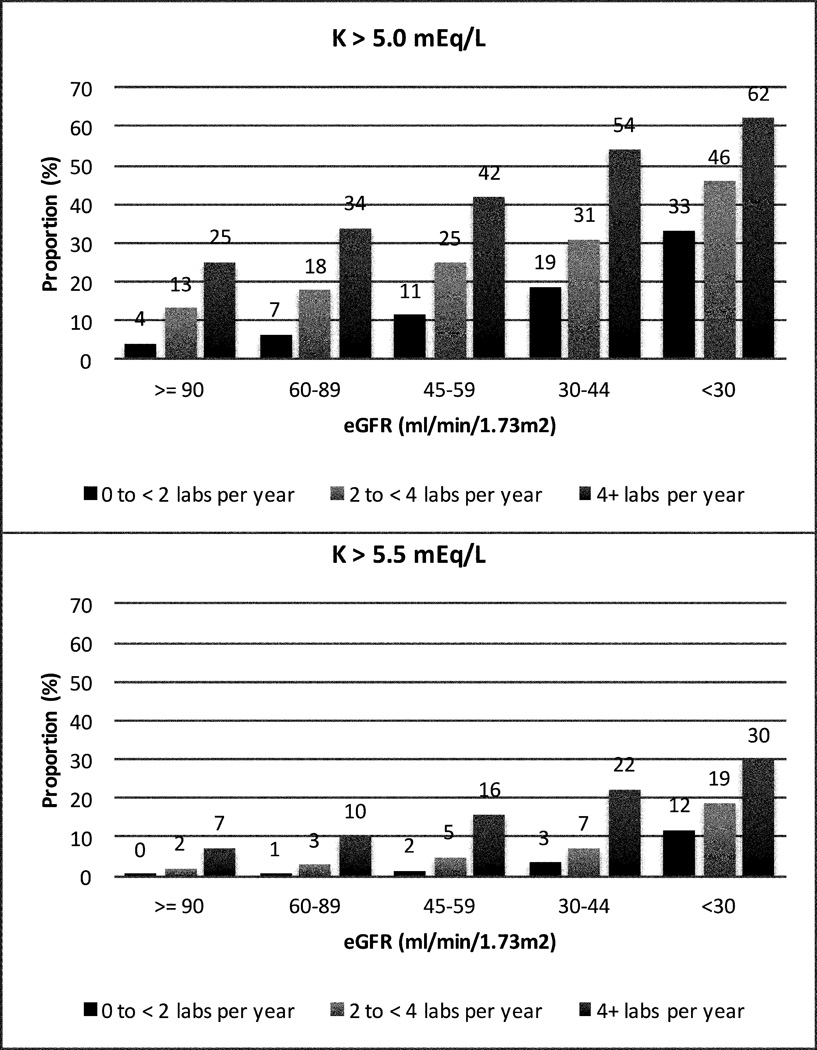
Frequency of serum potassium measurements per year was classified as <2, 2 to <4, and ≥4.
Table 2.
Baseline Risk Factors for Hyperkalemia Occurrence Over 3 Years (2011–2014)
| Variable | K >5 mEq/L | P value | K >5.5 mEq/L | P value |
|---|---|---|---|---|
| Age, per 5 years | 1.03 (1.01, 1.05) | <0.001 | 0.94 (0.91, 0.97) | <0.001 |
| Female | 0.69 (0.67, 0.72) | <0.001 | 0.67 (0.62, 0.72) | <0.001 |
| Black | 0.54 (0.44, 0.66) | <0.001 | 1.26 (0.93, 1.72) | 0.137 |
| eGFR <60, per −15ml | 1.58 (1.52, 1.63) | <0.001 | 1.73 (1.65, 1.82) | <0.001 |
| eGFR 60+, per −15ml | 1.24 (1.21, 1.26) | <0.001 | 1.34 (1.27, 1.40) | <0.001 |
| SBP, per 20 mmHg | 1.07 (1.05, 1.09) | <0.001 | 1.10 (1.06, 1.14) | <0.001 |
| BMI, per 5 kg/m2 | 0.97 (0.96, 0.98) | <0.001 | 0.94 (0.91, 0.97) | <0.001 |
| Diabetes | 1.60 (1.53, 1.66) | <0.001 | 1.62 (1.49, 1.75) | <0.001 |
| History of HF | 1.06 (0.99, 1.13) | 0.092 | 1.20 (1.08, 1.33) | <0.001 |
| History of atherosclerotic CVD | 1.17 (1.12, 1.23) | <0.001 | 1.08 (0.99, 1.18) | 0.065 |
| ACEI | 1.54 (1.47, 1.60) | <0.001 | 1.58 (1.45, 1.71) | <0.001 |
| ARB | 1.07 (1.00, 1.15) | 0.043 | 1.10 (0.97, 1.25) | 0.131 |
| K-sparing diuretics | 1.00 (0.91, 1.10) | 0.943 | 1.13 (0.96, 1.34) | 0.141 |
| Kaliuretic diuretics | 0.60 (0.57, 0.62) | <0.001 | 0.65 (0.59, 0.70) | <0.001 |
| Beta blockers | 1.13 (1.08, 1.18) | <0.001 | 1.14 (1.05, 1.24) | 0.002 |
| Other HTN meds | 0.83 (0.79, 0.87) | <0.001 | 0.95 (0.87, 1.03) | 0.232 |
| Frequency of K check | ||||
| <2 per year | Ref | Ref | Ref | Ref |
| 2 − <4 per year | 2.50 (2.39, 2.62) | <0.001 | 3.66 (3.27, 4.09) | <0.001 |
| 4+ per year | 5.72 (5.42, 6.03) | <0.001 | 11.90 (10.63, 13.31) | <0.001 |
Estimates are for multivariable model including all variables in the table.
eGFR: estimated glomerular filtration rate, SBP: systolic blood pressure, BMI: body mass index, HF: heart failure, CVD: cardiovascular disease, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blockers, HTN: hypertension.
Risk of Hyperkalemia Associated with Anti-hypertensive Medication Classes
In adjusted analyses, use of ACEIs at baseline was associated with a 54% increased risk for potassium >5 mEq/L (HR 1.54, 95% CI: 1.47–1.60, p<0.001) (Figure 3). Beta-blockers were associated with a 13% increased risk (HR 1.13, 95% CI: 1.08–1.18, p<0.001), ARBs were associated with a 7% increased risk (HR 1.07, 95% CI: 1.00–1.15, p= 0.04), and loop/thiazide diuretics were associated with a 40% decreased risk (HR 0.60, 95% CI: 0.57–0.62, p<0.001). Although much less frequently used, potassium-sparing diuretics were not associated with potassium >5 mEq/L (HR 1.00, 95% CI: 0.91–1.10, p=0.943).
Figure 3. Risk of Hyperkalemia associated with Anti-hypertensive Medication Classes.
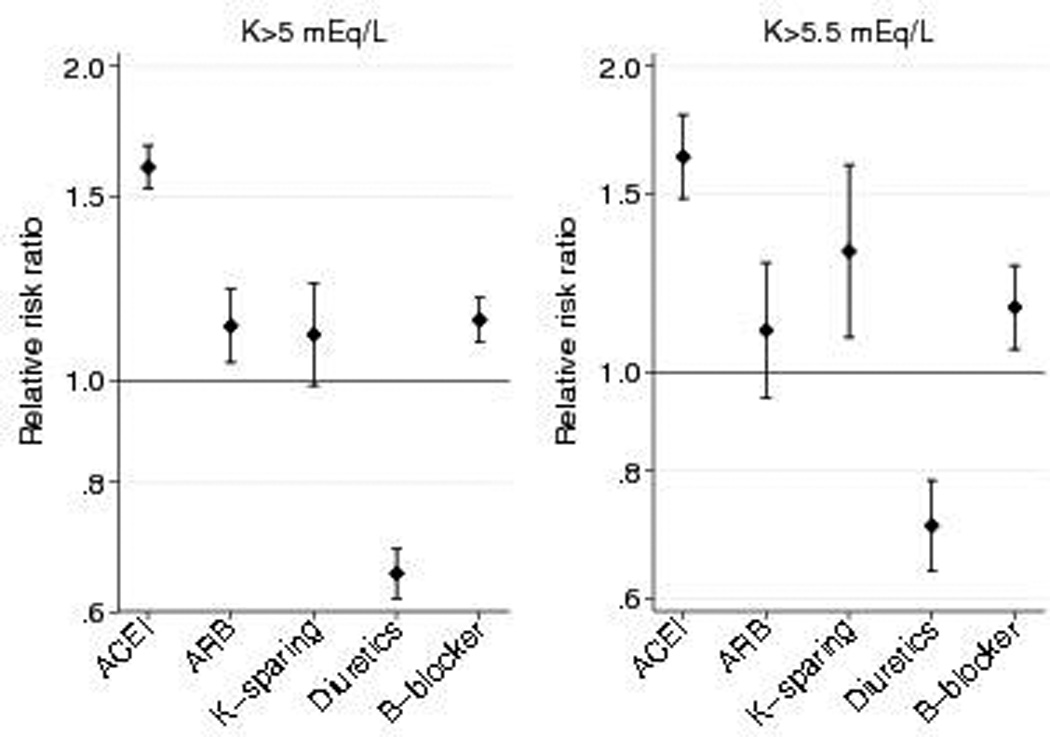
Estimates are adjusted for age, sex, race, estimated glomerular filtration rate, diabetes, hypertension, congestive heart failure. atherosclerotic cardiovascular disease, non-steroid anti-inflammatory drugs, blood pressure, BMI, and number of potassium measurements per year.
Similar associations were seen between antihypertensive medications and K >5.5 mEq/L (Table 2, Figure 3). ACEIs remained the class of antihypertensive medications most strongly associated with potassium >5.5 mEq/L (HR 1.58, 95% CI: 1.45–1.71, p<0.001). Potassium-sparing diuretics and ARBs demonstrated weak associations with K >5.5 mEq/L which were not statistically significant (potassium-sparing diuretics: HR 1.13, 95% CI: 0.96, 1.34, p=0.141; ARBs: HR 1.10, 95% CI: 0.97–1.25, p=0.131). Use of multiple ACEI/ARB/potassium-sparing diuretic medications increased risk for hyperkalemia (please see http://hyper.ahajournals.org; Tables S2, S3); no consistent interactions between medication classes with the risk of hyperkalemia were observed.
Actions Taken After Hyperkalemia Events
There were 16,834 participants who had an episode of potassium >5 mEq/L during the 3-year period (Table 3). After the first occurrence of potassium >5 mEq/L, 1.2% were seen in the emergency room within 7 days, and 18.4% had potassium levels rechecked within 14 days. Within 60 days, 12.0% had a prescribed change in a potassium-altering medication. These changes included prescription of kayexalate (0.7%), increase in dose or new prescription of a kaliuretic diuretic (5.6%), and, among those taking ACEI/ARBs (7,585) or potassium-sparing diuretics (861), discontinuation (12.0% on ACEI/ARBs and 23.0% on potassium-sparing diuretics) or dose reduction of these agents (3.0 on ACEI/ARBs and 1.4% on potassium-sparing diuretics). Among the subgroup of regularly followed persons with eGFR <30 ml/min/1.73m2, there were generally more actions taken after potassium >5 mEq/L (please see http://hyper.ahajournals.org; Table S8). For example, among the 228 patients taking ACE/ARBs at the time of hyperkalemia, 20.2% discontinued an agent and 1.8% had a dose reduction. Recurrent hyperkalemia tended to be less common in patients who had discontinuation or dose reduction of ACE/ARBs compared to those who did not have discontinuation or dose reduction (64.9% vs. 75.9%, p=0.2).
Table 3.
Actions Taken After Experiencing K >5.0, K >5.5, and K <5 mEq/L
| Action Taken | Hyperkalemia K >5.0 mEq/L (n=16,834) |
Hyperkalemia K >5.5 mEq/L (n=3,582) |
Control K <5 mEq/L (n=100,566) |
|---|---|---|---|
| Emergency room visit within 7 days | 1.2% (203/16834) | 3.1% (112/3582) | 0.7% (697/100566) |
| Repeat potassium measurement within 14 days | 18.4% (3097/16834) | 44.3% (1587/3582) | 0.0% (0/101142) |
| Prescription of kayexalate | 0.7% (111/16834) | 4.7% (171/3582) | 0.0% (2/100566) |
| Prescription or increase in kaliuretic diuretic | 5.6% (946/16834) | 9.2% (330/3582) | 2.5% (2525/100566) |
| Discontinuation of ACEI/ARB in patients taking either | 10.5% (793/7585) | 24.3% (417/1715) | 4.8% (1584/32826) |
| Dose reduction of ACEI/ARB in patients taking either | 2.6% (196/7585) | 4.8% (82/1715) | 1.2% (395/32826) |
| Discontinuation of K-sparing diuretic in patients taking med | 23.0% (198/861) | 48.5% (133/274) | 7.5% (268/3567) |
| Dose reduction of potassium- sparing diuretic in patients taking med | 1.4% (12/861) | 1.1% (3/274) | 0.5% (17/3567) |
A control group who experienced K <5 mEq/L was created, matched on frequency and count of potassium measurements to allow for comparison.
All P values <0.05 compared to control group
ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blockers,
Among the 3,582 participants who had an episode of potassium >5.5 mEq/L during the 3-year period, 3.1% were seen in the emergency room within 7 days, 44.3% had potassium levels rechecked within 14 days, and 26.4% had a prescribed change in a potassium-altering medication within 60 days. These changes included prescription of kayexalate (4.7%), increase in dose or new prescription of a kaliuretic diuretic (9.2%), and, among those taking ACEI/ARBs (1,715) or potassium-sparing diuretics (274), discontinuation (24.3% on ACEI/ARBs and 48.5% on potassium-sparing diuretics) or dose reduction of these agents (4.8% on ACEI/ARBs and 1.1% on potassium-sparing diuretics). Interestingly, dose reduction or discontinuation of ACEI/ARB was more common among those with a specific indication for this class of medication (31.4% vs. 21.3%, p<.001). Again, among the subgroup of regularly followed persons with eGFR <30 ml/min/1.73m2, there were generally more actions taken after potassium >5.5 mEq/L episode (please see http://hyper.ahajournals.org; Table S8). For example, among the 105 patients taking ACE/ARBs at the time of hyperkalemia, 35.2% discontinued an agent and 1.9% had a dose reduction. There were similar rates of recurrent hyperkalemia in those who had discontinuation or dose reduction of ACEI/ARBs and those who did not although numbers were small (48.7% vs. 56.1%, p=0.5).
In comparison, a control group matched on frequency and count of potassium measurement had low rates of these events after a measurement of potassium <5 mEq/L. For example, 0.7% of the 100,566 controls were seen in the emergency room within 7 days, 0.0% had repeat potassium measurement, and 4.7% had a prescribed change in a potassium-altering medication. These changes included prescription of kayexalate (0.0%), increase in dose or new prescription of a kaliuretic diuretic (2.5%), and, among those taking ACEI/ARBs (32,826) or potassium-sparing diuretics (3,567), discontinuation (4.8% on ACEI/ARBs and 7.5% on potassium-sparing diuretics) or dose reduction of these agents (1.2% on ACEI/ARBs and 0.5% on potassium-sparing diuretics). These differences persisted in adjusted analyses. For example, compared to a control with a potassium measurement <5 mEq/L, a person with a potassium measurement >5.5 mEq/L had 3.7-times (95% CI: 3.3–4.3) the odds of ACEI/ARB discontinuation in the next 60 days. There was no effect modification by the presence of a medical indication for this class of medications (p for interaction=0.3).
Discussion
Using data from a large, rural, integrated healthcare system, we provide a comprehensive evaluation of rates of potassium testing, patterns and risk factors of hyperkalemia, and management of hyperkalemia over a 3-year time period. We demonstrate that hyperkalemia is common, particularly among those with greater comorbidities, and that adjusted for comorbidities, ACEI use is among the strongest risk factors among classes of antihypertensive medications. We describe the short-term management of hyperkalemia in the outpatient setting, noting that the most common action is dose reduction or discontinuation of RAAS inhibition whereas there were very low rates of nephrology and dietician consultation.
We found substantial variability in the frequency of potassium testing as well as the occurrence of hyperkalemia events. Not surprisingly, more events were detected in those with more frequent potassium testing. Differences in testing frequency could reflect clinicians’ judgment about a patient’s overall health, reactions to abnormal lab tests, or variation in routine practice patterns. Since potassium is typically ordered in combination with other labs (e.g., creatinine), we were unable to determine if testing was being done specifically for potassium. The most recent Kidney Disease Improving Clinical Outcomes Clinical Practice Guideline suggests monitoring serum potassium within 1 week of initiating RAAS inhibitors. 11 Few guidelines exist that specify how often serum potassium should be monitored in different risk settings, such as after the occurrence of hyperkalemia. A minority of patients, even in the regularly followed eGFR < 30 ml/min/1.73 m2 subgroup, had potassium rechecked within 14 days in this setting. We speculate that repeat potassium measurement after an episode of hyperkalemia may be even lower in health systems without integrated laboratories and electronic health records. Strategies to improve monitoring after hyperkalemia are needed.
Comparing hyperkalemia risk and medication discontinuation in our clinical cohort to that of clinical trials is challenging, as risk differs based on study population characteristics and, as we show, the manner in which hyperkalemia risk is monitored and reported. In a large hypertension trial including patients with at least one CVD risk factor (ALLHAT), risk of potassium ≥ 5.5 mEq/L, measured after 1 year was 3.6%, 1.2%, and 1.9% for patients on lisinopril, chlorthalidone, and amlodipine, respectively. 12 In a trial comparing candesartan to placebo in patients with systolic heart failure who were intolerant to ACEIs, occurrence of potassium >6 mEq/L was approximately 3.0% in the candesartan group vs. 1.3% in the placebo group, and discontinuation of medication due to hyperkalemia occurred in 1.9% and 0.3%, respectively (p<0.001). 13 In a randomized trial comparing benazepril to placebo in patients with non-diabetic, advanced, proteinuric CKD, approximately 5% (6 enalapril, 5 placebo) developed potassium >6 mEq/L over a mean of 3.4 years, and only 1.3% required drug discontinuation. 14 Another study found similar rates of discontinuation and dose reduction of RAAS inhibitors after hyperkalemia episodes although they used a longer (390 days) time window and lacked a control group to assess actions after hyperkalemia. 15 Together, these data suggest that risk of hyperkalemia substantially limits use of RAAS inhibitors in the clinical setting, and that improvements in hyperkalemia detection and management are needed.
Hyperkalemia has many different definitions in the literature. While some may consider risk associated with hyperkalemia to be clinically significant only at potassium levels ≥ 6.0 mEq/L, 7,16 it is clear from our study that discontinuation of ACEIs/ARBs occurs commonly below this level as well (31% for K > 5.5 mEq/L), even in patients with specific indications for these drugs. Despite KDIGO recommendations, 11 patterns of hyperkalemia did not correlate with seeing a nephrologist, and relatively few patients with even persistent hyperkalemia were seen by a dietician in our cohort. In other studies, roughly 50% of patients with indications for RAAS inhibition have been reported as not taking these medications. 17–19 Our results suggest that strategies maximizing RAAS inhibition such as dietary management of hyperkalemia may be underutilized. We observed low utilization of kayexalate, which may be related to concerns about potential colonic necrosis risk that were issued on a black box warning in January 2011 by the Federal Drug Administration. Given the integral role of RAAS inhibition in reducing cardiovascular and renal risk, further research is needed to determine whether management of hyperkalemia using dietary management, kaliuretic diuretics, or potassium-binders can improve long-term patient outcomes.
Another notable finding in our study is that hyperkalemia risk associated with RAAS inhibition varies by medication class. We found that ACEIs were associated with higher risk of hyperkalemia than ARBs or potassium-sparing diuretics. This observation may be due to the relatively smaller effect of ARBs on aldosterone compared to ACEIs. 8 Two head-to-head trials of ACEIs vs. ARBs in heart failure patients (n=722 and n=768) suggest that ACEIs have a stronger effect on raising serum potassium levels than ARBs. 20,21 However, a meta-analysis of trials in albuminuric patients (4 trials with data on hyperkalemia; n=673) found no differences in hyperkalemia risk between ACEIs and ARBs. 22 More research in other large cohorts is needed to compare hyperkalemia risk between these two agents. Other antihypertensive medication classes, particularly loop/thiazide diuretics were associated with decreased risk of hyperkalemia. Our findings suggest potential hyperkalemia management strategies could include switching RAAS inhibitor class from ACEIs to ARBs or prescribing or adjusting the dose of thiazide/loop diuretics.
Although we observed a lower risk of experiencing potassium >5 mEq/L in blacks, this did not hold true for potassium >5.5 mEq/L. Our results are consistent with those in the African American Study of Kidney Disease and Hypertension (AASK) where hyperkalemia was quite rare, only occurring in 4.7% of these black, non-diabetic participants with GFRs ranging from 20–65 ml/min/1.73m2. 22 This lower risk of hyperkalemia could be driven by differences in potassium intake between blacks and whites, differences in comorbid conditions or medications, or, potentially, genetic differences in potassium handling. 23 However, our study size had few blacks; further research in more diverse populations are needed.
Our study had several strengths and limitations. First, we used data from an integrated healthcare system that captures the vast majority of outpatient and inpatient visits in a large population in a stable geographic area. Our data is longitudinal, enabling characterization of patterns of potassium testing, persistence of hyperkalemia, outpatient/inpatient/emergency room visits, and medication prescriptions. However, risk and process of care patterns from this mostly white population in a single healthcare system may not be generalizable to other populations. Furthermore, the associations reflect data as it is collected in routine care and adjustment may leave residual confounding by unmeasured indications for treatments and measurements. We were unable to identify dietary advice on lowering potassium that may have been provided by physicians. Finally, it is possible that we missed some medication changes and contact with the health system that were not documented in the electronic health record or occurred outside Geisinger.
In conclusion, hyperkalemia is a common occurrence in the outpatient setting. Substantial variability exists in monitoring of serum potassium and management of hyperkalemia, but the most common action after hyperkalemia seems to be discontinuation of RAAS inhibition. Additional studies are needed to optimize serum potassium monitoring and use of RAAS inhibitors.
“Perspectives”
Monitoring of potassium and management of hyperkalemia may differ in clinical practice from carefully controlled clinical trials. Using data from a large, integrated healthcare system, we show that hyperkalemia is common in the outpatient setting, and associated with frequency of potassium testing, lower eGFR, male sex, lower BMI, non-black race, and diabetes. Among antihypertensive medications, ACEIs were most strongly associated with hyperkalemia. Discontinuation of ACEIs/ARBs and potassium-sparing diuretics, even in individuals with specific indications for these medications, was far more common in our clinical cohort compared to what has been reported in clinical trials. Future research should evaluate strategies of optimizing RAAS inhibitor use with improved hyperkalemia detection and management.
Supplementary Material
Novelty and Significance.
-
What is new?
Hyperkalemia often occurs in patients taking certain anti-hypertensive medications in a real-world clinical setting; risk may be highest with angiotensin converting enzyme inhibitors (ACEIs).
Hyperkalemia is more common in persons in whom potassium is checked more frequently.
-
What is relevant?
Hyperkalemia risk is particularly high in patients with decreased kidney function taking renin-angiotensin system inhibitors.
Improvements are needed in retesting potassium in a timely fashion after hyperkalemia is detected.
Hyperkalemia often results in discontinuation of important anti-hypertensive medications.
Dietician counseling and nephrology consultation may be underutilized in the management of hyperkalemia.
-
Summary
Hyperkalemia occurs frequently in the outpatient setting, particularly in patients with decreased kidney function on renin-angiotensin system inhibitors. After hyperkalemia is detected, important medications like renin-angiotensin system inhibitors are often discontinued. Other means of treating hyperkalemia such as dietary counseling, nephrology consultation, and diuretic use may be underutilized. More research is needed to improve long-term outcomes through optimization of hyperkalemia management and medication use.
Acknowledgments
ARC, MEG, and JC: designed the study; YS: analyzed the data; MEG, YS, SB, and JC assisted with data analysis; and all authors: wrote the manuscript.
Sources of Funding
A.C. is supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant K23 DK106515-01. M.G. is supported by NIH/NIDDK grant K08 DK092287. M.G., J.C.were supported by the National Kidney Foundation, which receives funding from Relypsa. The CKD-PC Data Coordinating Center provided infrastructure support for this work and is funded in part by a program grant from the US National Kidney Foundation and the NIDDK (R01DK100446-01).
Footnotes
Some data presented in this study were shown in a presentation in San Diego, CA at the American Society of Nephrology Kidney Week on November 7, 2015.
Conflicts of Interest/Disclosure Statement
None
References
- 1.Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, Weir MR, Fink JC. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo J, Brunelli SM, Jensen DE, Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11:90–100. doi: 10.2215/CJN.01730215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rimmer JM, Horn JF, Gennari FJ. Hyperkalemia as a complication of drug therapy. Arch Intern Med. 1987;147:867–869. [PubMed] [Google Scholar]
- 4.Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: Causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med. 1998;158:917–924. doi: 10.1001/archinte.158.8.917. [DOI] [PubMed] [Google Scholar]
- 5.Weir MR, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol. 2010;5:531–548. doi: 10.2215/CJN.07821109. [DOI] [PubMed] [Google Scholar]
- 6.Jain N, Kotla S, Little BB, Weideman RA, Brilakis ES, Reilly RF, Banerjee S. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109:1510–1513. doi: 10.1016/j.amjcard.2012.01.367. [DOI] [PubMed] [Google Scholar]
- 7.Weir MR, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol. 2010;5:531–548. doi: 10.2215/CJN.07821109. [DOI] [PubMed] [Google Scholar]
- 8.Bakris GL, Siomos M, Richardson D, Janssen I, Bolton WK, Hebert L, Agarwal R, Catanzaro D. ACE inhibition or angiotensin receptor blockade: Impact on potassium in renal failure VAL-K study group. Kidney Int. 2000;58:2084–2092. doi: 10.1111/j.1523-1755.2000.00381.x. [DOI] [PubMed] [Google Scholar]
- 9.Shah KB, Rao K, Sawyer R, Gottlieb SS. The adequacy of laboratory monitoring in patients treated with spironolactone for congestive heart failure. J Am Coll Cardiol. 2005;46:845–849. doi: 10.1016/j.jacc.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Raebel MA, McClure DL, Simon SR, Chan KA, Feldstein A, Andrade SE, Lafata JE, Roblin D, Davis RL, Gunter MJ, Platt R. Laboratory monitoring of potassium and creatinine in ambulatory patients receiving angiotensin converting enzyme inhibitors and angiotensin receptor blockers. Pharmacoepidemiol Drug Saf. 2007;16:55–64. doi: 10.1002/pds.1217. [DOI] [PubMed] [Google Scholar]
- 11.Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. kidney inter. 2013;3(suppl):1–150. [Google Scholar]
- 12.Alderman MH, Piller LB, Ford CE, Probstfield JL, Oparil S, Cushman WC, Einhorn PT, Franklin SS, Papademetriou V, Ong ST, Eckfeldt JH, Furberg CD, Calhoun DA, Davis BR Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Clinical significance of incident hypokalemia and hyperkalemia in treated hypertensive patients in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension. 2012;59:926–933. doi: 10.1161/HYPERTENSIONAHA.111.180554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai AS, Swedberg K, McMurray JJ, Granger CB, Yusuf S, Young JB, Dunlap ME, Solomon SD, Hainer JW, Olofsson B, Michelson EL, Pfeffer MA CHARM Program Investigators. Incidence and predictors of hyperkalemia in patients with heart failure: An analysis of the CHARM program. J Am Coll Cardiol. 2007;50:1959–1966. doi: 10.1016/j.jacc.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 14.Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, Jiang JP, Liang M, Wang GB, Liu ZR, Geng RW. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354:131–140. doi: 10.1056/NEJMoa053107. [DOI] [PubMed] [Google Scholar]
- 15.Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21:s212–s220. [PubMed] [Google Scholar]
- 16.Luo J, Brunelli SM, Jensen DE, Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2015 doi: 10.2215/CJN.01730215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeh HL, Huang LY, Su S, Yang MC, Wang TC. Underuse of ACE inhibitors and angiotensin II receptor blockers among patients with diabetic nephropathy in taiwan. Health Policy. 2011;100:196–202. doi: 10.1016/j.healthpol.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Winkelmayer WC, Fischer MA, Schneeweiss S, Wang PS, Levin R, Avorn J. Underuse of ACE inhibitors and angiotensin II receptor blockers in elderly patients with diabetes. Am J Kidney Dis. 2005;46:1080–1087. doi: 10.1053/j.ajkd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Masoudi FA, Rathore SS, Wang Y, Havranek EP, Curtis JP, Foody JM, Krumholz HM. National patterns of use and effectiveness of angiotensin-converting enzyme inhibitors in older patients with heart failure and left ventricular systolic dysfunction. Circulation. 2004;110:724–731. doi: 10.1161/01.CIR.0000138934.28340.ED. [DOI] [PubMed] [Google Scholar]
- 20.Pitt B, Segal R, Martinez FA, Meurers G, Cowley AJ, Thomas I, Deedwania PC, Ney DE, Snavely DB, Chang PI. Randomised trial of losartan versus captopril in patients over 65 with heart failure (evaluation of losartan in the elderly study, ELITE) Lancet. 1997;349:747–752. doi: 10.1016/s0140-6736(97)01187-2. [DOI] [PubMed] [Google Scholar]
- 21.McKelvie RS, Yusuf S, Pericak D, Avezum A, Burns RJ, Probstfield J, Tsuyuki RT, White M, Rouleau J, Latini R, Maggioni A, Young J, Pogue J. Comparison of candesartan, enalapril, and their combination in congestive heart failure: Randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study the RESOLVD pilot study investigators. Circulation. 1999;100:1056–1064. doi: 10.1161/01.cir.100.10.1056. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg JM, Appel LJ, Bakris G, Gassman JJ, Greene T, Kendrick CA, Wang X, Lash J, Lewis JA, Pogue V, Thornley-Brown D, Phillips RA African American Study of Hypertension and Kidney Disease Collaborative Research Group. Risk of hyperkalemia in nondiabetic patients with chronic kidney disease receiving antihypertensive therapy. Arch Intern Med. 2009;169:1587–1594. doi: 10.1001/archinternmed.2009.284. [DOI] [PubMed] [Google Scholar]
- 23.Flack JM, Sica DA, Bakris G, et al. Management of high blood pressure in blacks: An update of the international society on hypertension in blacks consensus statement. Hypertension. 2010;56:780–800. doi: 10.1161/HYPERTENSIONAHA.110.152892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


