Abstract
Goals
We evaluated a cohort of patients referred to our center for presumed recurrent Clostridium difficile infection (CDI) to determine final diagnoses and outcomes.
Background
As rates of CDI have increased, more patients are diagnosed with recurrent CDI and other sequelae of the infection. Distinguishing symptomatic patients with CDI from those who are colonized with an alternative etiology of diarrheal symptoms may be challenging.
Methods
We performed a retrospective review of 117 patients referred to our center for recurrent CDI between January 2013 and June 2014. Data collected included demographics, referring provider, previous anti-CDI treatment, and significant medical conditions. Additionally we gathered data on atypical features of CDI and investigations obtained to investigate etiology of symptoms. Outcomes included rates of alternative diagnoses and the accuracy of CDI diagnosis by referral source.
Results
The mean age was 61 years and 70% were female. 29 patients (25%) were determined to have a non-CDI diagnosis. Most common alternative diagnoses included irritable bowel syndrome (18 patients: 62%) and inflammatory bowel disease (3:10 %). Age was inversely correlated with rate of non-CDI diagnosis (p=0.016). Of the remaining 88 (75%) patients with a confirmed diagnosis of CDI, 25 (28%) received medical therapy alone and 63 (72%) underwent fecal microbiota transplantation (FMT).
Conclusion
Among patients referred to our center for recurrent CDI, a considerable percentage did not have CDI, but rather an alternative diagnosis, most commonly IBS. The rate of alternative diagnosis correlated inversely with age. Providers should consider other etiologies of diarrhea in patients presenting with features atypical of recurrent CDI.
Keywords: FMT, C. difficile diagnosis, C. difficile colonization
Introduction
Over the past 15 years, Clostridium difficile infection (CDI) has increased substantially and is the leading cause of healthcare associated infection in the United States.1 The cost of CDI has placed a heavy burden on our health care system, resulting in an estimated $3.2 billion in excess health care costs annually and, more disturbingly, CDI claims at least 29,000 lives per year.2,3
Recurrent CDI occurs in approximately 25% of patients after treatment of an initial infection, with further recurrence rates of 40–60% after greater than two occurrences.4,5 While there is no uniformly successful therapy, fecal microbiota transplantation (FMT) has emerged as 90% effective, and patients often seek evaluation at a referral center for therapy; but not all of these patients actually have recurrent CDI.6 The importance of accurate diagnosis is paramount. Roughly 75% of health care associated diarrhea has been thought to be related to causes other than CDI.7 While there are multiple testing modalities, polymerase chain reaction (PCR) based assays are currently preferred because of high sensitivity and the ease and speed with which the test can be performed.8,9 However, a positive stool test does not distinguish colonized patients from those with symptomatic disease7. Asymptomatic colonization with a toxigenic strain of C. difficile is common, and patients presumed to have CDI may indeed have an alternative etiology for persistent diarrhea.10 Continued treatment with courses of antibiotics and/or FMT in these patients is ineffective and failure to diagnose an alternative etiology of diarrheal symptoms may have consequences. To date, no study has investigated the rate of non-CDI diagnosis in patients with gastrointestinal symptoms and C. difficile positive stools. We report our experience in evaluating patients referred for to our FMT center for recurrent CDI, including accuracy of diagnosis and outcomes.
Materials and Methods
We conducted a retrospective review of 117 consecutive patients referred to our center with presumed recurrent CDI between January 1st 2013 and June 30th 2014. The study was approved by the Lifespan IRB. Data were collected from initial consultation through November 2014 and included demographics, referring provider, previous anti-CDI treatment, and other significant medical conditions. Patients had a follow-up period ranging from 6 to 24 months. We noted patients who had atypical features of CDI, including non-response to anti-CDI therapy and intermittent non-progressive symptoms. We also documented those who had inappropriate testing for cure. Outcomes of interest included the frequency of alternative diagnoses and the accuracy of CDI diagnosis by the referral source. For patients deemed not to have CDI, we gathered data on the additional investigations obtained to determine the etiology for diarrhea, including fecal studies, endoscopy, histology, breath testing, and serologic testing. All referred patients did test positive for CDI by PCR on one or more occasions prior to consultation. Irritable bowel syndrome (IBS) was diagnosed on the basis of Rome guidelines: improvement of abdominal pain with defecation, onset of abdominal pain associated with frequency or change in stool.11 Post-infectious irritable bowel syndrome (PI-IBS) was distinguished from irritable bowel syndrome (IBS) on the basis of reporting symptoms consistent with IBS (per Rome guidelines) that were not present prior to the enteric infection.12 Inflammatory bowel disease (IBD) was diagnosed on the basis of endoscopic and histologic findings consistent with either Crohn’s disease or ulcerative colitis.
Data were initially recorded in a Microsoft Excel database. After completion of data collection, the data was imported into SAS (SAS version 9.4, SAS Institute, Cary, NC, USA). Generalized linear models were used to test all hypotheses (proc glimmix). Appropriate distributions were chosen based on theoretical match to the process generating the dependent variable and examination of model residuals. The models were adjusted for remaining misspecification by use of classical sandwich estimation. Mean differences with 95% confidence intervals (CI) were expressed in original scale for dissemination, using inversion of the link function where necessary. The p-values for comparisons were adjusted for alpha inflation from multiple comparisons using the Holm method to maintain family wise alpha at 0.05.
Results
Demographics, comorbidities, and prior CDI history among CDI and alternative diagnosis patients are included in table 1. Odds ratios are calculated with intervals between patients deemed to have CDI and those with alternative diagnoses. The most common overall medical comorbid conditions at presentation included gastroesophageal reflux disease (18%), IBS (19%), diabetes mellitus (15%), and solid malignancy (10%). All but 4 patients had received at least one course of vancomycin (97%). Other therapies used prior to consultation included metronidazole (92 patients; 79%), fidaxomicin (26; 22%), rifaxamin (8; 7%), probiotics (36; 31%), and FMT (4; 3%). At the time of consultation, approximately one-half of patients (48%) were being treated with at least one anti-CDI antibiotic. None were taking antibiotics for a non-CDI indication at the time of consultation. The average last positive PCR (in days prior to initial consultation) was 113 days for those with CDI and 140 days for those with alternative diagnoses.
Table 1.
Demographics, comorbidities, and prior CDI history among CDI and alternative diagnosis patients
| CDI | Alternative diagnosis | OR (95% CI) | P value (unadjusted) | |
|---|---|---|---|---|
| N | 88 | 29 | ||
| Age (mean) | 64.3 | 50.3 | 2.55 (0.48–4.58) | 0.0162*,** |
| Female/male | 2.4 | 2.2 | 0.81 (0.32–1.982 | 0.636 |
| Diarrhea at presentation? n (%) | 35 (39.7) | 10 (34.5) | 0.90 (0.38–2.16) | 0.815 |
| CDI Episodes n (%): | ||||
| 1–2 | 11 (12.5) | 15 (51.7) | 7.43 (2.64–20.95) | 0.0002**,† |
| ≥3 | 77 (87.5) | 14 (48.3) | ||
| Comorbidities and medications n (%) | ||||
| DMa | 16 (18.2) | 2 (6.9) | 0.526 (0.139–1.99) | 0.3407 |
| IBSb | 9 (10.2) | 14 (48.3) | 7.58 (2.77–20.83) | 0.0001** |
| IBDc | 12 (13.6) | 1 (3.5) | 0.22 (0.026–1.77) | 0.1517 |
| Malignancy | 12 (13.6) | 5 (17.2) | 1.22 (0.39–3.85) | 0.7361 |
| PPId | 3 (3.4) | 1 (3.5) | 0.97 (0.094–9.90) | 0.9762 |
| Immunosuppressives | 10 (11.4) | 1 (3.5) | 0.29 (0.032–2.22) | 0.2183 |
For each additional year of age, there is a 2.55% increased likelihood of having an alternative diagnosis,
p<0.05,
This denotes significance in a comparison of least square means between the two groups “1 to 2” episodes and “3 and above” episodes, which is unadjusted.
Diabetes mellitus,
irritable bowel syndrome,
inflammatory bowel disease,
proton pump inhibitor
Of the 117 patients included in the study, 29 (25%) did not have CDI, but were deemed to have an alternate etiology for their diarrhea (table 2). Establishment of alternative diagnoses was based on results of diagnostic evaluations and clinical judgment and limited by patient compliance in regards to obtaining suggested workup. In a small number of patients, a single unifying alternative diagnosis could not be established.
Table 2.
Non-CDI diagnoses
| Diagnosis | Number (percentage) |
|---|---|
| IBSa | 9 (31 %) |
| PI-IBSb | 9 (31 %) |
| IBDc | 3 (10 %) |
| Bile Salt Malabsorption | 1 (3 %) |
| Small Bowel Bacterial Overgrowth | 1 (3 %) |
| Post-Surgical Diarrhea | 1 (3 %) |
| Pancreatic Insufficiency | 1 (3 %) |
| CMLd | 1 (3 %) |
| Lactose Intolerance + PI-IBS | 1 (3 %) |
| Celiac Disease | 1 (3 %) |
| Ischemic Colitis vs IBD * | 1(3 %) |
IBS- Irritable bowel syndrome
PI-IBS- Post infectious irritable bowel syndrome
Inflammatory bowel disease
Chronic myeloid leukemia
No further workup was performed
The relationship between patient’s age and the rate of alternative diagnosis was inversely correlated and statistically significant (table 1, figure 1). There was no relationship found between gender and the rate of alternative diagnosis (table 1). For patients who received an alternative diagnosis, common atypical features of CDI included non-responsiveness to anti-CDI therapy (53%) and intermittent non-progressive symptoms (33%). There was inappropriate testing for cure in 23% of patients. Referring providers and provider accuracy are listed on table 3. Rates of alternative diagnosis did not appear to be statistically different between gastroenterologists and other providers (PCP: p=0.48, self: p=0.17, unknown=0.19). Ultimately, 88 patients (75%) were determined to have a diagnosis of recurrent CDI. Of these, 25 patients (28%) resolved with medical therapy alone and 63 (72%) underwent FMT.
Figure 1.
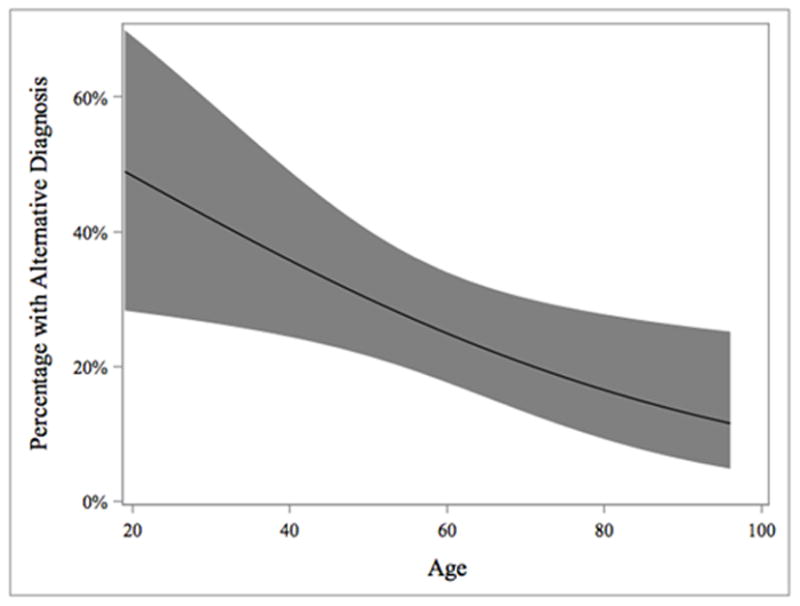
Relationship Between Age and Alternative Diagnosis
Table 3.
Referring Providers and Provider Accuracy
| Referring Provider | Number (percentage) of Patients Referred | Provider Accuracy (%) |
|---|---|---|
| Self | 40 (34 %) | 68 |
| PCP | 28 (24 %) | 75 |
| GI | 22 (18 %) | 82 |
| ID | 9 (8 %) | 100 |
| Hospitalist | 7 (6 %) | 100 |
| Family Medicine | 5 (4 %) | 80 |
| Unknown | 4 (4 %) | 50 |
| NP | 1 (1 %) | 100 |
| Surgeon | 1 (1 %) | 0 |
Of the 29 patients at initial consultation that were deemed to have an alternative etiology to explain their symptoms, 13 did not follow up (45%) and were assumed to resume care with the referring provider. The mean and median duration of follow up for the remaining 16 patients (55%) was 504 and 577 days respectively. Six (38%) had subsequent episodes of diarrhea, and 5 underwent CDI PCR testing (31%). Three (19%) of these patients with subsequent PCR testing were positive, but only one was judged to have CDI. The other two were not treated or treated without change in symptoms. Of the 29 patients deemed to have an alternative diagnosis, additional workup included further endoscopy (10 patients), serologic testing (4 patients), imaging (3 patients), stool testing (2 patients), and small intestinal bacterial overgrowth testing (2 patients).
Discussion
Recurrent CDI is clinically difficult to treat and has led to a substantial increase in referrals for FMT, which have been shown to have high cure rates in previous studies. 6,13–18 However, the utility of FMT depends on an accurate CDI diagnosis and prudent referral selection. To our knowledge, this study is the first of its kind to analyze the accuracy of presumed CDI diagnosis in a population of patients referred for FMT, and to characterize alternative diagnosis in those found not to have recurrent CDI.
Among the 117 patients referred to our center over an 18-month period, 25% did not actually have recurrent CDI, but rather an alternative etiology of their diarrhea. Only 1 of these patients later required a single course of anti-CDI therapy, but none required FMT. In our data analysis, younger age, prior diagnosis of IBS, and fewer prior CDI episodes were positively correlated with higher rates of alternative diagnosis. Common features of patients with alternative diagnoses included non-responsiveness to anti-CDI therapy and intermittent non-progressive symptoms. Many were inappropriately tested for cure after completing a course of anti-CDI therapy. In addition, fewer past episodes of CDI and prior diagnosis of IBS were positively correlated with an alternative diagnosis. This highlights the importance of vigilant assessment of symptoms, and the need to consider all etiologies of diarrhea, especially in patients with features atypical of CDI.
The most common diagnoses were IBS, PI-IBS, and IBD. PI-IBS has been documented as occurring after bacterial illnesses secondary to Campylobacter, Salmonella, Escherichia, and Shigella species, but with very limited data describing occurrence after CDI.19–21 Piche previously characterized the rate of PI-IBS after CDI in a group of 23 patients. Among these patients, 35% developed new IBS symptoms shortly after resolution of CDI.22 Another recent retrospective analysis by Guitérrez et al examined the sequelae following CDI, finding patients to be at higher risk for incident IBS, GERD and dyspepsia compared with matched controls23. This risk was sustained at 3 months and resulted in a single functional gastrointestinal sequelae for each 12 cases of CDI. It is not surprising that a prior diagnosis of IBS can result in worsened IBS symptoms following CDI because of an already dysbiotic state seen in these patients24. Our study adds to the sparse body of literature on this likely underreported cause of diarrhea following CDI.
We attempted to identify a correlation between the source of referral and ultimate CDI diagnosis, but were unable to detect any differences in rates of correct CDI diagnosis between gastroenterologists and other referral sources. However, there were two groups (infectious disease specialists and hospitalists) that correctly identified CDI for all referred patients. It is certainly possible that these two populations of providers are more likely to encounter sicker, hospitalized patients, and consequentially perhaps more accurately identify CDI. Finally, the lack of differences between the aforementioned groups highlights that high rates of alternative diagnosis is more ubiquitous than previously thought.
The high rate of misdiagnosis around recurrent CDI is not without consequence as the financial burden of anti-CDI therapy is significant. In a recent cost analysis by Varier, a financial model was used to estimate the cost of recurrent CDI.25 It was found that the cost of a 10–14 day course of vancomycin was $1347, and the cost of FMT delivered via colonoscopy was $1086. Assuming that all 29 of our patients with an alternative diagnosis received at least one unnecessary course of vancomycin, a conservative estimate of unnecessary expenditure calculates to be approximately $38,000. While the reported cost of FMT is less than that of vancomycin, the short and long-term safety profile has not been studied extensively and it is prudent to avoid FMT in patients for whom it is unlikely to be beneficial.
We did not perform FMT in patients deemed not to have recurrent CDI because limited data exists regarding efficacy for its use in other conditions. Symptoms of IBS, a particularly prevalent alternative etiology in our cohort, have not been shown to improve after FMT.26 Further, the role of FMT for patients with IBD, another prevalent diagnosis in our non-CDI cohort, remains unclear. Current evidence is limited to case reports and case series.27 We feel providers should be cautious about the use of FMT in populations of patients without recurrent CDI, as its role remains unsubstantiated.
Our study had several limitations. It was retrospective, so data collection may be incomplete. Due to the variety of referral sources and limitations of medical documentation, specific details regarding past CDI characteristics, diagnosis, and management were sometimes unavailable. Furthermore, this was a single center study. All patients were seen by a single provider, who has significant experience with FMT and CDI. It is worth noting that no patient diagnosed with an alternative diagnosis went on to receive treatment for CDI by another provider, nor were they hospitalized for treatment of CDI.
This study raises important questions that merit further research. First, we found that a substantial percentage of patients with alternative diagnoses had PI-IBS. This clinical entity, previously described in the succession of other gastrointestinal infections, has been minimally described following CDI. A positive C. difficile PCR in recently treated patients who become symptomatic poses a diagnostic dilemma for providers in accurately distinguishing recurrent CDI from PI-IBS. This is highlighted by the fact that stool PCR tests remain positive for C. difficile for up to up to 30 days after successful treatment.28 A recent review article urges providers to consider PI-IBS as a presumed etiology for symptoms rather than a presumptive diagnosis of recurrent CDI for mild symptoms, and further advocates for CDI testing to only be conducted on symptomatic patients.29 A positive stool test does not distinguish colonized patients from those with symptomatic disease.7 Second, the high rate of alternative diagnosis in our study (25%) raises the possibility that the rates of recurrent CDI in epidemiologic studies may be overestimated. Finally, our study was able to detect a significant relationship between age and the likelihood of an alternative diagnosis. This intriguing relationship between age and the syndrome of PI-IBS has been noted previously, but warrants further research to understand this association and the long-term consequences of CDI.22 It is noted that the age of diagnosis of IBS is more likely to occur before the age of 35 and that prevalence and severity is decreased in older patients.30 This may hold true for PI-IBS as well.
In conclusion, among patients referred for presumed recurrent CDI to an FMT center, a considerable percentage had an alternative diagnosis, with PI-IBS and IBS predominating. Providers should consider all possible etiologies of diarrhea in patients with features atypical of CDI.
Abbreviations
- CDI
clostridium difficile infection
- CI
confidence interval
- CML
chronic myeloid leukemia
- FMT
fecal microbiota transplant
- FDA
Food & Drug Administration
- GI
gastroenterologist
- ID
infectious disease specialist
- IBS
irritable bowel syndrome
- IND
investigational new drug
- IRB
institutional review board
- NP
nurse practitioner
- PCR
polymerase chain reaction
- PCP
primary care physician
- PI-IBS
post infectious irritable bowel syndrome
Footnotes
Specific author of contributions:
Dr. Melissa Jackson - Involved in planning, collecting and interpreting data for this study and drafting the manuscript
Dr. Sidney Olefson- Involved in planning, collecting and interpreting data for this study and drafting the manuscript
Dr. Jason Machan-Involved in statistical analyses
Dr. Colleen Kelly - Involved in study design, planning, collecting and interpreting data for this study and drafting/editing the manuscript
Potential Conflicts of Interest: Kelly C.R.: Seres health, Consultant and investigator for clinical trial., Assembly Biosciences, Inc. Research Support, NIH/NIDDK 1R21DK093839-01A1
Jackson M, Olefson S, & Machan J. None
The lead authors1 affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1.Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile Infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infection control and hospital epidemiology. 2011;32(4):387–390. doi: 10.1086/659156. [DOI] [PubMed] [Google Scholar]
- 2.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. The New England journal of medicine. 2015;372(9):825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. The American journal of gastroenterology. 2013;108(4):478–498. doi: 10.1038/ajg.2013.4. quiz 499. [DOI] [PubMed] [Google Scholar]
- 4.Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55(Suppl 2):S154–161. doi: 10.1093/cid/cis462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. The American journal of gastroenterology. 2012;107(5):761–767. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 6.van Nood E, Dijkgraaf MG, Keller JJ. Duodenal infusion of feces for recurrent Clostridium difficile. The New England journal of medicine. 2013;368(22):2145. doi: 10.1056/NEJMc1303919. [DOI] [PubMed] [Google Scholar]
- 7.Koo HL, Van JN, Zhao M, et al. Real-time polymerase chain reaction detection of asymptomatic Clostridium difficile colonization and rising C. difficile-associated disease rates. Infection control and hospital epidemiology. 2014;35(6):667–673. doi: 10.1086/676433. [DOI] [PubMed] [Google Scholar]
- 8.EC, EC, Johnson DA. Clinical update for the diagnosis and treatment of Clostridium difficile infection. World journal of gastrointestinal pharmacology and therapeutics. 2014;5(1):1–26. doi: 10.4292/wjgpt.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goudarzi M, Seyedjavadi SS, Goudarzi H, Mehdizadeh Aghdam E, Nazeri S. Clostridium difficile Infection: Epidemiology, Pathogenesis, Risk Factors, and Therapeutic Options. Scientifica. 2014;2014:916826. doi: 10.1155/2014/916826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. The American journal of gastroenterology. 2015;110(3):381–390. doi: 10.1038/ajg.2015.22. quiz 391. [DOI] [PubMed] [Google Scholar]
- 11.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 12.Beatty JK, Bhargava A, Buret AG. Post-infectious irritable bowel syndrome: mechanistic insights into chronic disturbances following enteric infection. World journal of gastroenterology : WJG. 2014;20(14):3976–3985. doi: 10.3748/wjg.v20.i14.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53(10):994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 14.Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. The American journal of gastroenterology. 2012;107(7):1079–1087. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 15.Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9(12):1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon SS, Brandt LJ. Treatment of refractory/recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol. 2010;44(8):562–566. doi: 10.1097/MCG.0b013e3181dac035. [DOI] [PubMed] [Google Scholar]
- 17.Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol. 2012;46(2):145–149. doi: 10.1097/MCG.0b013e318234570b. [DOI] [PubMed] [Google Scholar]
- 18.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. The New England journal of medicine. 2011;364(5):422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 19.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47(6):804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connor BA. Sequelae of traveler’s diarrhea: focus on postinfectious irritable bowel syndrome. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;41(Suppl 8):S577–586. doi: 10.1086/432956. [DOI] [PubMed] [Google Scholar]
- 21.Borgaonkar MR, Ford DC, Marshall JK, Churchill E, Collins SM. The incidence of irritable bowel syndrome among community subjects with previous acute enteric infection. Digestive diseases and sciences. 2006;51(5):1026–1032. doi: 10.1007/s10620-006-9348-1. [DOI] [PubMed] [Google Scholar]
- 22.Piche T, Vanbiervliet G, Pipau FG, et al. Low risk of irritable bowel syndrome after Clostridium difficile infection. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2007;21(11):727–731. doi: 10.1155/2007/262478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutierrez RL, Riddle MS, Porter CK. Increased Risk of Functional Gastrointestinal Sequelae Following Clostridium difficile infection among Active Duty United States Military Personnel (1998–2010) Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.07.059. [DOI] [PubMed] [Google Scholar]
- 24.Ohman L, Simren M. Intestinal microbiota and its role in irritable bowel syndrome (IBS) Current gastroenterology reports. 2013;15(5):323. doi: 10.1007/s11894-013-0323-7. [DOI] [PubMed] [Google Scholar]
- 25.Varier RU, Biltaji E, Smith KJ, et al. Cost-effectiveness analysis of treatment strategies for initial Clostridium difficile infection. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20(12):1343–1351. doi: 10.1111/1469-0691.12805. [DOI] [PubMed] [Google Scholar]
- 26.Borody TJ, George L, Andrews P, et al. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? The Medical journal of Australia. 1989;150(10):604. doi: 10.5694/j.1326-5377.1989.tb136704.x. [DOI] [PubMed] [Google Scholar]
- 27.Ianiro G, Bibbo S, Scaldaferri F, Gasbarrini A, Cammarota G. Fecal microbiota transplantation in inflammatory bowel disease: beyond the excitement. Medicine. 2014;93(19):e97. doi: 10.1097/MD.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surawicz CM, McFarland LV, Greenberg RN, et al. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000;31(4):1012–1017. doi: 10.1086/318130. [DOI] [PubMed] [Google Scholar]
- 29.Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. Jama. 2015;313(4):398–408. doi: 10.1001/jama.2014.17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clinical epidemiology. 2014;6:71–80. doi: 10.2147/CLEP.S40245. [DOI] [PMC free article] [PubMed] [Google Scholar]


