Abstract
Oxidative stress is thought to contribute to disease pathogenesis in the central nervous system (CNS) disease multiple sclerosis (MS). Myeloperoxidase (MPO), a potent peroxidase that generates toxic radicals and oxidants, is increased in the CNS during MS. However, the exact mechanism whereby MPO drives MS pathology is not known. We addressed this question by inhibiting MPO in mice with experimental autoimmune encephalomyelitis (EAE) using our non-toxic MPO inhibitor KYC. We found that therapeutic administration of KYC for five days starting at the peak of disease significantly attenuated EAE disease severity, reduced myeloid cell numbers and permeability of the blood-brain-barrier (BBB). These data indicate that inhibition of MPO by KYC restores BBB integrity thereby limiting migration of myeloid cells into the CNS that drive EAE pathogenesis. In addition, these observations indicate that KYC may be an effective therapeutic agent for the treatment of MS.
Introduction
Multiple sclerosis (MS) is thought to be a chronic inflammatory autoimmune disease of the central nervous system (CNS) that leads to both physical and cognitive disability, and eventually, early death (Lassmann 2010, Nylander & Hafler 2012, McFarland & Martin 2007). Although the pathophysiology of MS is poorly understood, one hypothesis that has emerged over recent years is that an increase in oxidative stress due to the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) contributes to CNS damage (Nylander & Hafler 2012, Gonsette 2008, Qin et al. 2007, Miljkovic & Spasojevic 2013, van Horssen et al. 2011, Castro-Borrero et al. 2012). This is supported by studies showing that during active disease in both human MS and its animal model experimental autoimmune encephalomyelitis (EAE) the CNS contains high levels of oxidized proteins, lipids and DNA (Qin et al. 2007, Ferretti et al. 2005, Haider et al. 2011, van Horssen et al. 2008, Miller et al. 2011, Vladimirova et al. 1998, Bizzozero et al. 2005, Smerjac & Bizzozero 2008, Zheng & Bizzozero 2010, Calabrese et al. 2002, Jack et al. 2007, Zeis et al. 2009). MPO is a potent pro-oxidative enzyme that converts H2O2 into highly reactive oxidants and free radicals that oxidize biomolecules and cause cellular damage (van der Veen et al. 2009). Free radicals impair blood-brain barrier (BBB) function, increase vascular leakage and lead to deposition of plasma components that, in turn, enhance inflammatory cell recruitment and CNS autoimmunity (de Vries et al. 1997). Evidence implicating MPO in MS pathogenesis includes increased levels of MPO in the plasma and in the white matter and cerebral cortex of MS patients as compared to control subjects (Minohara et al. 2006, Gray et al. 2008b). In addition, immunohistochemical studies demonstrated the presence of MPO in and around MS lesions in microphages/microglia (van der Veen et al. 2009, Nagra et al. 1997, Gray et al. 2008b, Gray et al. 2008a). In EAE, increased MPO activity in the CNS has also been reported (Forghani et al. 2012, Sajad et al. 2009).
While inhibition of MPO is considered a therapeutic strategy for MS this has not been evaluated in clinical trials because the current inhibitor classes exhibit toxicity and low specificity (Koelsch et al. 2010, Burner et al. 1999, Bekesi et al. 2005). Thus we developed a new class of MPO inhibitors and recently showed that N-acetyl lysyltyrosylcysteine amide (KYC) was a potent, highly specific and non-toxic inhibitor of MPO-dependent oxidant/free radical generation (Zhang et al. 2013a). Here we evaluated the efficacy of KYC for the treatment of MS using EAE. We found that KYC potently inhibited EAE disease severity when administered either prophylactically or therapeutically. Furthermore, we found that peak MPO levels in the CNS coincided with peak EAE disease severity. Administration of KYC starting at peak of EAE for five days resulted in a significant reduction in the level of MPO in the CNS that accompanied a similar reduction in the absolute number of macrophages and neutrophils. Of clinical relevance, the former treatment regimen was sufficient to significantly reduce BBB leakage resulting in a significant reduction in EAE disease severity. Collectively, these data demonstrate that KYC inhibition of MPO activity is a viable therapy for the treatment of MS during acute relapses that are associated with BBB breakdown. Furthermore, our data are the first to characterize a highly specific, potent and non-toxic MPO inhibitor that functions within the CNS.
Materials and Methods
Mice
C57BL/6J, B6.129X1-Mpotm1Lus/J (Mpo−/−) and B10.PL-H2u H2-T18a/(73NS)SnJ (B10.PL) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). MBP-TCR transgenic mice were described previously (Dittel et al. 1999). Upon receipt C57BL/6 and Mpo−/− mice were cohoused with mice from the Dittel colony for at least two weeks prior to EAE induction to equilibrate the microbiome of the two groups of mice. Mice were housed under SPF conditions in the Translational Biomedical Research Center of the Medical College of Wisconsin and all animal procedures were approved by the Institutional Animal Care and Use Committee.
Peptides and antibodies
Myelin oligodendrocyte glycoprotein 35–55 (MOG35–55) peptide (MEVGWYRSPFSRVVHLYRNGK) and myelin basic protein (MBP) Ac1–11 peptide (Ac-ASQKRPSQRSK) were generated by the Protein Core Laboratory of the Blood Research Institute, BloodCenter of Wisconsin. KYC was synthesized by Biomatik (Wilmington, DE). The 2.4G2 hybridoma was obtained from the American Tissue Culture Collection. Anti-mouse CD4-APC-eFluor 780 CD11b-PE, CD11b-biotin, IL-17-Alexa Fluor 647, Foxp3-PE and streptavidin-PE Cy5.5 were purchased from eBioscience (San Diego, CA). Anti-mouse IFN-γ-PE was purchased from BD Biosciences (San Diego, CA). Anti-mouse Ly-6C-APC and Ly-6G-APC-Cy7 were purchased from Biolegend (San Diego, CA). The SMI-32 antibody, which detects nonphosphorylated neurofilament-H was purchased from Covance (Emeryville, CA). Streptavidin Alexa 405 and goat anti-mouse Alex 456 (H + L) were purchased from Life Sciences Advanced Technologies (St. Petersburg, FL). Anti-MPO heavy chain was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). Anti-rabbit IgG (H + L)-HRP was purchased from Jackson ImmunoResearch (West Grove, PA). Anti-β-actin-HRP was purchased from Sigma-Aldrich (St. Louis, MO). For all experiments, the mice were age (6–8 wk) and gender matched with both sexes being utilized.
EAE induction and KYC treatment
For MOG-induced EAE, mice on the C57BL/6 background were subcutaneously immunized with 200 µg MOG35–55 peptide emulsified in an equal volume of complete Freund’s adjuvant (CFA) (Chondrex, Redmond, WA) containing 4 mg/ml mycobacterium followed by i.p. injection of 200 ng pertussis toxin (List Biological Laboratories, Campbell, CA) on days 0 and 2, as described (Ray et al. 2014). EAE was induced in B10.PL mice by i.v. adoptive transfer of 1 × 106 MBP-specific encephalitogenic T cells generated in vitro from MBP-TCR transgenic mice into sublethally irradiated (360–380 rad) B10.PL mice as described (Dittel et al. 1999, Ray et al. 2012). Clinical signs of EAE were scored daily as follows: 0, no disease; 1, limp tail; 1.5, hind limb ataxia; 2, hind limb paresis; 2.5, partial hind limb paralysis; 3, total hind limb paralysis; 4, hind and fore limb paralysis; and 5, death. Groups of mice were i.p. administered either PBS or KYC (0.3–3.0 mg/kg) daily from the indicated time points.
Cell isolation and flow cytometry
Mononuclear cells from the CNS were obtained as described (Ray et al. 2012, Ponomarev et al. 2005) and single cell suspensions were prepared from the spleen. Cells were stained with fluorochrome-conjugated antibodies and Intracellular Foxp3, staining was performed using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience, San Diego, CA) as per manufacturer’s recommended protocol. For the detection of intracellular cytokines, mononuclear cells isolated from the CNS were stimulated in vitro with PMA (50 ng/ml) and ionomycin (500 ng/ml) (Sigma-Aldrich, St. Louis, MO) for 4 h in the presence of BD GolgiStop™ (BD Biosciences, San Diego, CA). Intracellular IFN-γ and IL-17 staining was performed using the Cytofix/Cytoperm Kit (BD Biosciences, San Diego, CA). Cells were acquired on a LSRII flow cytometer (BD Biosciences, San Diego, CA) and data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Immunofluorescence
Anesthetized mice were perfused with cold PBS followed by fixative and spinal cords were isolated, fixed and frozen as previously described (Shriver & Dittel 2006). Spinal cord sections (10 µm) were stained with anti-mouse CD11b-biotin and SMI-32 followed by staining with streptavidin Alexa 405 and goat anti-mouse Alexa-546, as described (Ray et al. 2014). Isotype-matched antibodies were used as specificity controls. Laser-scanning confocal microscopy was performed using the Olympus Fluoview FV100 MPE Multiphoton Laser Scanning microscope as described (Miller et al. 2013).
MPO activity analysis
Brain tissue (250 mg) as homogenized in 1 ml of 10 mM phosphate buffer (pH 6.0) and underwent three freeze-thaw cycles after which the samples were centrifuged at 75,000 g for 1 h at 4 °C. The precipitates were suspended in the same buffer containing 0.5% HTAB and centrifuged at 75,000 g for 1 h at 4 °C. Aliquots of supernatants (100 µl) were incubated in a 96-well plates precoated with MPO antibody at 4 °C overnight. MPO peroxidase activity was determined by adding 100 µl sodium acetate buffer (10 mM, pH 6.2) containing 10 mM NaNO2, 100 µM H2O2 and 10 µM Amplex Ultra Red in each well and incubated at 37 °C for 30 min (Rymaszewski et al. 2014). MPO activity was determined using a fluorescent plate reader (excitation wavelength at 540 nm and emission wavelength at 590 nm). There is no detectable MPO activity in the brains of C57BL/6 control mice.
Western blotting
Anesthetized mice were perfused with PBS and CNS tissue were collected and stored at −80 °C. Brain tissue was extracted into a solution containing RIPA Buffer containing 1% EDTA, and 1% Protease Inhibitor Cocktail (Thermo Fisher Scientific, Inc., Waltham, MA) (1: 100; v/v). MPO levels were determined by Western blotting using an anti-MPO primary antibody and anti-rabbit IgG secondary antibody as described (Zhang et al. 2013b). To determine equal protein loading between samples, membranes were stripped and reprobed with anti-β-actin.
Evans blue BBB permeability assay
Evans blue (20 mg/kg in 100 µl PBS) (Sigma-Aldrich, St. Louis, MO) was i.v. injected into anesthetized mice and 30 minutes later the mice were perfused with 25 ml PBS and CNS tissues were collected, blotted dry and weighed. The CNS tissue was homogenized in 1 ml formamide (Sigma-Aldrich, St. Louis, MO) and were kept overnight at 55 °C for extraction of Evans blue as described (Graesser et al. 2002). For the detection of Evans blue, spectrophotometric measurement of the samples at 620 nm was performed.
Statistical analysis
Data were analyzed using GraphPad prism software (GraphPad Software, Inc., La Jolla, CA) and were presented as mean ± SEM. Statistical significance was determined using the unpaired t-test or Mann-Whitney test as indicated in the figure legends and footnotes. p-values ≤0.05 were considered significant.
Results
KYC inhibition of MPO attenuates EAE when administered both prophylactically and therapeutically
To test whether our MPO inhibitor KYC has therapeutic potential for the treatment of MS, we first determined whether its prophylactic administration attenuated EAE. EAE was induced in C57BL/6 mice by immunization with the MOG35–55 peptide, and as shown in Fig. 1A, KYC administration daily from day 0 significantly attenuated disease severity in a dose-dependent manner (Table 1). Based on these findings we chose the 3 mg/kg dosage for subsequent experiments. We next determined whether KYC has therapeutic potential and found that daily injection of KYC starting at either EAE onset (day 10) (Fig. 1B) or peak of disease (day 17) (Fig. 1C) significantly attenuated disease severity as compared to the PBS treated group (Table 1). We validated these findings in B10.PL mice using passive EAE induced by the adoptive transfer of MBP-specific encephalitogenic T cells. EAE severity was significantly attenuated when KYC was administered daily starting either on day 0 (Fig. 1D) or 15 (Fig. 1E) (Table 1). Our finding that KYC significantly attenuated EAE severity when administered after EAE onset indicates that KYC has therapeutic potential for the treatment of MS.
Figure 1. KYC treatment significantly attenuates EAE disease severity.
A–C) EAE was induced in C57BL/6 mice by immunization with MOG35–55. D, E) EAE was induced in B10.PL mice by adoptive transfer of 1 × 106 MBP-specific encephalitogenic T cells into sublethally irradiated mice. Mice were treated with KYC at 0.03 mg/kg (A), 0.3 mg/kg (A) or 3 mg/kg (A–E) or with PBS daily starting on day 0 (A, D), 10 (B), 17 (C) or 15 (E). Clinical signs of EAE were evaluated daily starting on day seven and the daily average disease score is shown. The cumulative disease score was calculated by adding the daily disease score from the first day of KYC administration to day 30. Statistical significance was determined by Mann-Whitney test comparing the 0.3 and 3 mg/kg KYC treated groups versus PBS. **p<0.05, **p<0.01, ***p<0.001. See Table 1 for additional statistical analysis and animal numbers.
Table 1.
EAE disease parameters
| Treatment group | Figure1 | Number of mice |
Peak disease score2 |
Disease score day 303 |
Cumulative disease score4 |
p value versus PBS5 |
|---|---|---|---|---|---|---|
| PBS6,8 | 1A | 5 | 2.0 ± 0 | 1.2 ± 0.1 | 23.5 ± 1 | |
| KYC 0.03 mg/kg6,8 | 1A | 5 | 2.0 ± 0 | 1.2 ± 0.2 | 22.8 ± 2 | 0.88 |
| KYC 0.3 mg/kg6,8 | 1A | 5 | 1.3 ± 0.1 | 0.7 ± 0.1 | 14 ± 1 | 0.008 |
| KYC 3 mg/kg6,8 | 1A | 5 | 1.2 ± 0.4 | 0.4 ± 0.2 | 10.4 ± 3 | 0.008 |
| PBS6,9 | 1B | 10 | 1.9 ± 0.2 | 1.6 ± 0.2 | 25.2 ± 2 | |
| KYC 3 mg/kg6,9 | 1B | 10 | 1.2 ± 0.2 | 0.8 ± 0.2 | 15.5 ± 2 | 0.006 |
| PBS6,10 | 1C | 5 | 2.4 ± 0.2 | 1.8 ± 0.2 | 29.3 ± 4 | |
| KYC 3 mg/kg6,10 | 1C | 9 | 1.8 ± 0.2 | 0.7 ± 0.1 | 16.3 ± 2 | 0.03 |
| PBS7,8 | 1D | 10 | 1.5 ± 0.5 | 0.1 ± 0.07 | 10.7 ± 1.4 | |
| KYC 3 mg/kg7,8 | 1D | 10 | 0.85 ± 0.1 | 0 ± 0 | 3.6 ± 1 | 0.0004 |
| PBS7,11 | 1E | 9 | 2.4 ± 0.1 | 0.67 ± 0.1 | 21.1 ± 1 | |
| KYC 3 mg/kg7,11 | 1E | 10 | 2.1 ± 0.07 | 0.28 ± 0.09 | 11.9 ± 0.9 | 0.0004 |
Designates the EAE graph from which the data were generated.
Peak disease score data are the mean ± SE.
Day 30 disease score data are the mean ± SE.
The cumulative disease score was calculated by adding the daily disease score from day 0 for Fig. 1A and 1D, from day 10 for Fig. 1B, from day 17 for Fig. 1C and from day 15 for Fig. 1E. Data are the mean ± SE.
The p value was calculated by Mann-Whitney test comparing the KYC treated group versus PBS for the cumulative disease score.
EAE was induced in C57BL/6 mice by immunization with MOG35–55.
EAE was induced in B10.PL mice by the adoptive transfer of 1 × 106 MBP-specific encephalitogenic T cells.
Mice were i.p. administered PBS or KYC starting on day 0.
Mice were i.p. administered PBS or KYC starting on day 10.
Mice were i.p. administered PBS or KYC starting on day 17.
Mice were i.p. administered PBS or KYC starting on day 15.
Pathogenic MPO peaks in the CNS at the height of EAE severity
Given that KYC efficacy is evident just prior to the peak of EAE, we reasoned that MPO activity would be elevated in the CNS at the height of disease. To examine this possibility, we compared MPO activity at EAE onset (days 6–10) to the peak of disease (days 18–21). As expected, MPO activity was significantly higher at the peak of EAE as compared to onset (Fig. 2A). Consistent with its role in EAE pathogenesis, MPO levels were decreased in mice undergoing recovery (day 24–28) (Fig. 2A). MPO activity was not detected in unmanipulated control mice. We next determined whether MPO activity correlated with protein levels. As shown in Fig. 2B, using Western blotting we found that MPO levels were significantly increased in PBS treated mice as compared to control mice without EAE. The administration of KYC resulted in a significant reduction in MPO levels in the CNS during EAE (Fig. 2B). These data indicate that KYC treatment attenuates EAE by reducing the level of MPO in the CNS further suggesting that MPO is pathogenic in EAE. To confirm that MPO is pathogenic during EAE, we induced EAE in Mpo−/− mice and found that EAE severity was significantly reduced as compared to WT mice (Fig. 2C). While KYC attenuated disease in WT mice it did not further reduce disease severity in Mpo−/− mice (Fig. 2C). These data support our previous findings that the primary mechanism of action of KYC is inhibition of MPO (Zhang et al. 2013a).
Figure 2. MPO levels increase in the CNS during EAE playing a pathogenic role.
EAE was induced in C57BL/6 mice (A–C) or Mpo−/− (C) mice by immunization with MOG35–55. A) MPO activity in the brain was determined at disease onset (day 6–10), peak of disease (day 18–21) and during recovery (day 24–28). A) Each data point represents one mouse. B, C) Mice were administered KYC (3 mg/kg) or PBS daily starting on day 0. B) MPO levels in the brain were determined by Western blotting on day 17 using β-actin as the loading control. The upper panel shows a representative Western blot and the bottom panel shows the quantitation (mean ± SE of 5 mice from two experiments) whereby the MPO band density was divided by the β-actin band density. Control density was normalized to 100. C) EAE was evaluated as for Fig. 1 and the data are the daily average ± SE disease score. N=10. A,B) *p≤0.05; **p=0.01, determined by unpaired t-test. C) ***p<0.001, determined by Mann-Whitney test comparing PBS treated groups.
KYC attenuates EAE by decreasing the number of CD11b+ myeloid cells
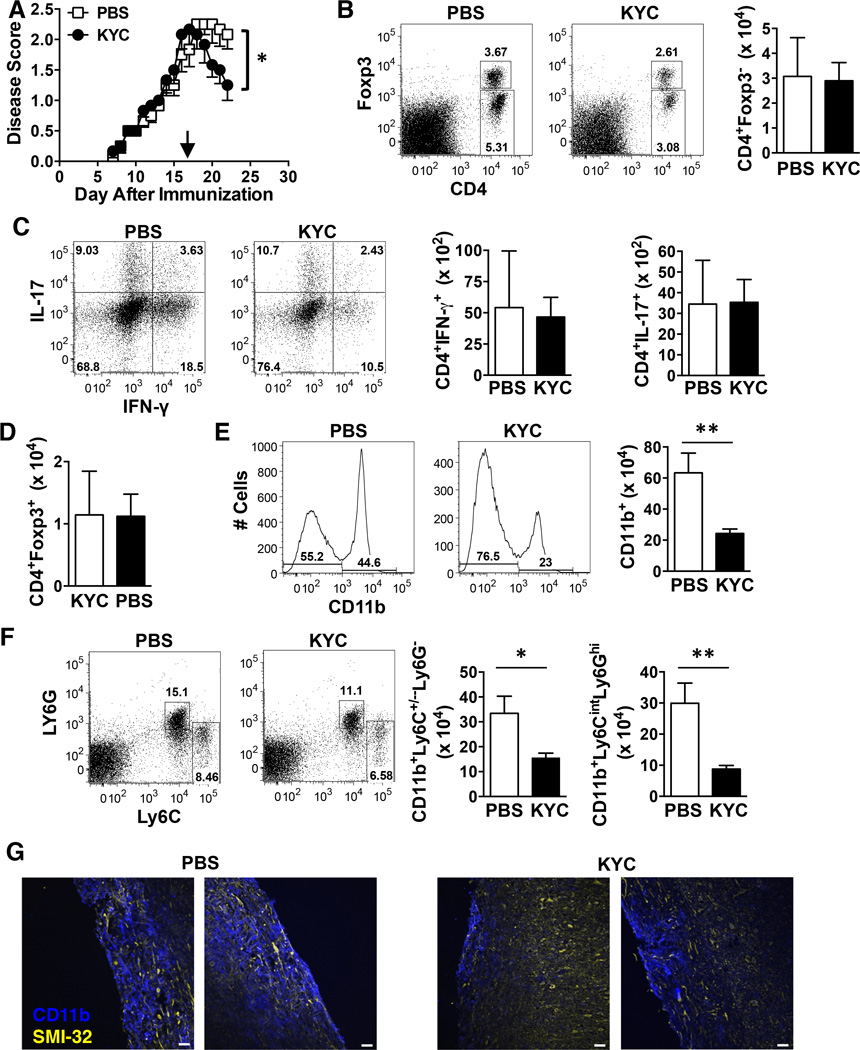
EAE initiation and pathogenesis is driven by CNS infiltrating IFN-γ and/or IL-17-producing encephalitogenic CD4 T cells as well as myeloid cells (Ransohoff & Brown 2012). Thus we assessed the impact of MPO inhibition on immune cell infiltrates by administering KYC daily starting on day 17 and quantifying immune cell infiltrates five days later when EAE severity was significantly reduced as compared to PBS group (Fig. 3A). Surprisingly, we did not observe a decrease in the absolute number of total CD4+ T cells (Fig. 3B) or those producing IFN-γ or IL-17 (Fig. 3C). The absolute number of CD4+Foxp3+ T regulatory cells (Treg) was also unchanged (Fig. 3B, D). However, CD11b+ myeloid cells were significantly reduced (Fig. 3E). Using Ly6C and Ly6G to differentiate myeloid subsets (Rose et al. 2012), we found that macrophages (Ly6C+/−Ly6G−) as well as neutrophils (Ly6CintLy6G+) were significantly decreased following KYC treatment (Fig. 3F). When we histologically examined spinal cords using the same KYC treatment regimen, reduced lesion size was evident in the KYC treated mice as determined by immunofluorescence staining for CD11b (blue) (Fig. 3G). Neuronal damage was also less evident within the lesions of KYC treated mice as assessed by staining with SMI-32 (yellow) (Fig. 3G), which detects nonphosphorylated neurofilament-H and has been associated with transected neuritis or axonal swelling in MS lesions (Trapp et al. 1998).
Figure 3. The absolute numbers of CD11b+ macrophages and neutrophils, but not CD4+ T cells are reduced in the CNS of KYC treated mice during EAE.
EAE was induced in C57BL/6 mice by immunization with MOG35–55 and KYC (3 mg/kg) or PBS was administered i.p. daily from days 17–21. A) The daily average ± SE EAE score from two experiments is shown. N = 6. B-F) On day 22 mononuclear cells were isolated from the CNS and the absolute number of total CD4 T cells (Foxp3−) (B), CD4+IFN-γ+ T cells (C), CD4+IL-17+ T cells (C), CD4+Foxp3+ Treg (D), CD11b+ myeloid cells (E), CD11b+Ly6C+/−Ly6G monocytes/macrophages (F) and CD11b+Ly6CintLy6G+ neutrophils (F) were determined by flow cytometry. Data shown are the mean ± SE from two experiments using the mice from A. Representative dots plots showing gating strategies for CD4 T cells (B) expressing Foxp3 (B) or IL-17 and IFN-γ (C) and CD11b+ myeloid cells (E) expressing Ly6C and Ly6G (F) are shown. G) Longitudinal frozen sections from the spinal cord of mice on day 22 were generated from PBS (top panels) and KYC (bottom panels) treated mice. The sections were stained with anti-CD11b (blue) and SMI-32 (yellow) and analyzed by immunofluorescence. Overlaid images are shown. Data shown are representative images from two mice of three. Scale bar, 40 µM. *p<0.05, **p<0.01, determined by the unpaired t-test.
KYC administration at peak of EAE leads to reduced CNS MPO levels and sealing of the BBB
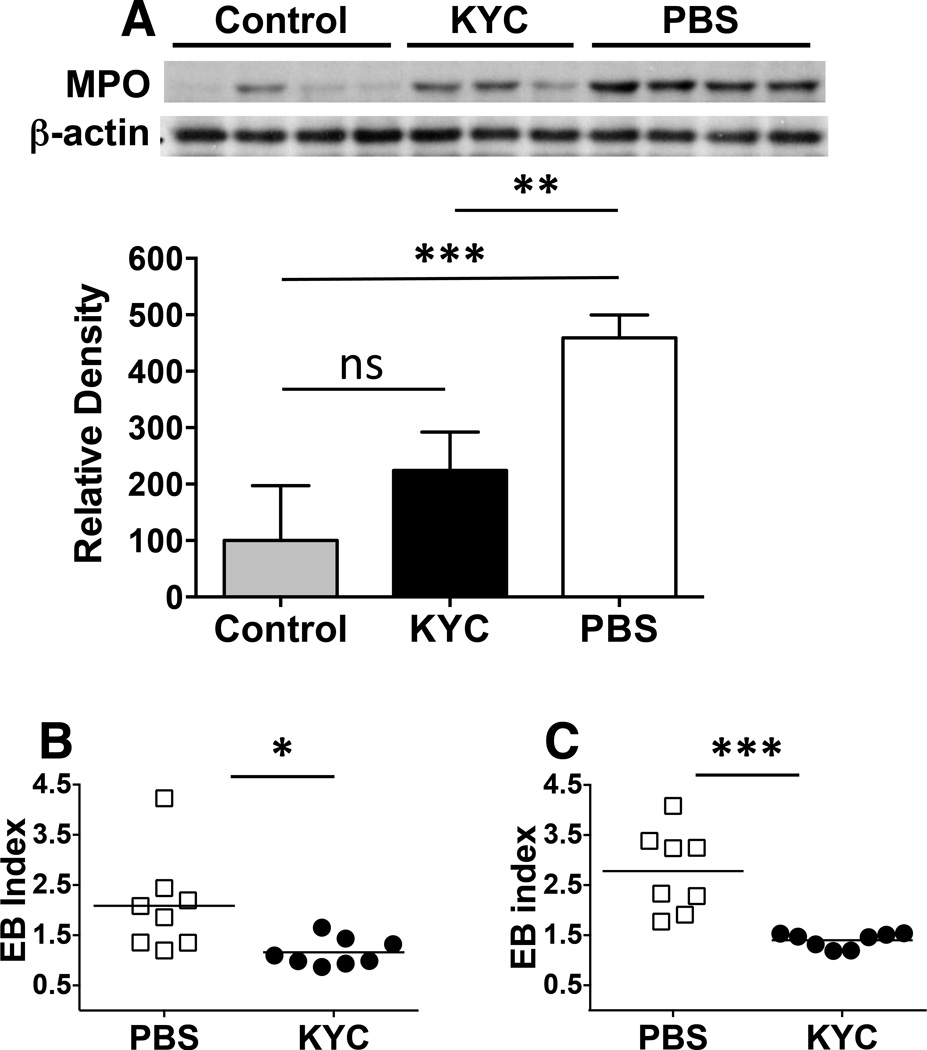
We next determined whether the loss of neutrophils and macrophages in the CNS on day 22, after five days of KYC treatment, correlated with reductions in MPO levels. Similar to data in Fig. 2B, Western blots showed that control mice expressed very low levels of MPO protein (Fig. 4A). KYC treatment for five days reduced MPO levels to that of controls, which were both significantly lower than in the PBS treated group (Fig. 4A). These data are consistent with the fact that neutrophils and macrophages are considered major sources of MPO in the CNS during EAE (Pulli et al. 2014). One mechanism whereby MPO is thought to facilitate the migration of myeloid cells into the CNS is by breakdown of the BBB, which is associated with both EAE and MS (Ullen et al. 2013, Alvarez et al. 2011). To test whether KYC reduced BBB permeability, we treated EAE mice as for Fig. 4A and performed an in vivo Evans blue permeability assay (Graesser et al. 2002). Compared to both control and PBS treated mice, the KYC group had a significant reduction in the level of Evans blue in the both the brain (Fig. 4B) and spinal cord (Fig. 4C). These data indicate that MPO is major driver of BBB leakage in CNS autoimmunity and that KYC can be used therapeutically to seal the barrier.
Figure 4. KYC administration reduces CNS MPO levels and seals the BBB.
EAE was induced in C57BL/6 mice by immunization with MOG35–55 and KYC (3 mg/kg) or PBS was administered i.p. daily from days 17–21. A) On day 22, brains were harvested and MPO levels were quantitated by Western blotting using β-actin as the loading control. The upper panel shows the Western blot including samples from two EAE experiments and the bottom panel shows the quantitation of control (n=4), KYC (n=3) and PBS (n=4) groups whereby the MPO band density was divided by the β-actin band density. Control density was normalized to 100. B, C) On day 22 BBB permeability was determined using Evans blue permeability. Data shown are the Evans blue index calculated as the fold change divided by control mice without EAE in the brain (B) and spinal cord (C). Each data point represents a single mouse from two experiments. *p<0.05, **p<0.01, ***p<0.001, ns-not significant, determined by the unpaired t-test.
Discussion
In this study, we investigated the supposition that MPO is a good therapeutic target for the treatment of MS using the EAE model. We found that administration of the MPO inhibitor KYC at the peak of disease led to a rapid attenuation of the clinical signs of EAE, which was accompanied by a significant reduction in the number of macrophages and neutrophils within the CNS. In addition, KYC administration led to a significant reduction in both the levels of MPO in the CNS and BBB leakage. Collectively these data provide strong evidence that targeting of MPO in MS has therapeutic potential.
In determining the therapeutic potential of KYC, we utilized both active immunization and adoptive transfer EAE models. We also employed preventative and therapeutic dosing regimens. Interestingly, KYC did not inhibit or delay EAE onset when administered from day 0 (Fig. 1A, D). This finding indicates that MPO plays little or no role in T cell priming in the periphery or reactivation within the CNS. Similarly, the lack of immediate effect when KYC was administered at disease onset (Fig. 1B) indicates that MPO plays no role in the early progressive stage of disease. Rather the data indicate that MPO exhibits a pathogenic role after disease initiation just prior the apex of disease (Fig. 1C). This conclusion is consistent with both EAE disease severity and MPO activity peaking between days 18–21 (Fig. 1, Fig. 2A). Based on this finding, we reasoned that targeting of MPO at the peak of disease should be sufficient to attenuate EAE severity, which is what we observed (Fig. 1C, 1E, 3A). While we did not specifically examine relapsing and remitting EAE, a previous study demonstrated that the MPO inhibitor 4-aminobenzoic acid hydrazide (ABAH) was able to reduce the severity of both the first exacerbation and the first relapse in SJL/J mice (Forghani et al. 2012). Of importance clinically, to date, we have not observed KYC toxicity in our animal studies using three different disease models (Zhang et al. 2013b, Rymaszewski et al. 2014). In addition, no toxicity was observed even after daily dosing with 1 mg/kg for up to 12 weeks or 10 mg/kg up to seven days. Also of importance clinically is that KYC attenuated EAE at the low dose of 3 mg/kg when administered daily (Fig. 1A). This is in contrast to ABAH, which required twice daily dosing at 40 mg/kg (Forghani et al. 2012). ABAH cannot be used therapeutically because of its toxicity and lack of specificity (Koelsch et al. 2010). Importantly, previous studies showed that most individuals deficient in MPO due to a genetic deficiency are essentially asymptomatic and thus KYC inhibition of MPO is not likely to induce significant side effects in MS patients.
In our previous studies, we used a variety of in vitro biochemical and cellular methods to demonstrate that KYC is a potent specific inhibitor of MPO activity (Zhang et al. 2013a). The logic of KYC’s design is as follows: Tyr by out-competing Cl− or NO2− will prevent MPO from generating HOCl and •NO2. The toxic Tyr• produced during inhibition will be rapidly scavenged by the nearby Cys. This is an efficient process for detoxifying Tyr• by forming a non-toxic disulfide end product. The Lys orientates KYC in the active site of MPO (Zhang et al. 2013a). Of importance, we have demonstrated that KYC “competitively inhibits” MPO by outcompeting MPO substrates such as Cl− for the active site and in so doing accelerates H2O2 consumption (Zhang et al. 2013a). The importance of this property is underscored by our report showing that KYC decreased the accumulation of MPO in aortas isolated from sickle cell mice to the same levels as control mice (Zhang et al. 2013b). In addition, KYC increased endothelial cell-dependent vasodilation in sickle cell mice by nearly 2-fold to ~60% of control levels (Zhang et al. 2013b). In the current study, we further tested KYC specificity in EAE Mpo−/− mice (Fig. 2C) and observed that KYC had no further inhibitory effect on disease severity confirming its specificity for MPO inhibiting in vivo. In addition, in our study Mpo−/− mice exhibited attenuated EAE as compared to WT mice (Fig. 2C) providing evidence that MPO is pathogenic in EAE. However, several studies that used a similar EAE induction protocol to ours reported that EAE severity in Mpo−/− mice was not attenuated (Steinbach et al. 2013, Brennan et al. 2001). In the Brennan, et. al, study, only 43% of WT succumbed to EAE as compared to 93% if Mpo−/− mice, indicating that MPO is protective in EAE. In contrast, in our studies 100% of both the WT and Mpo−/− mice developed EAE (Fig. 2C). Similar differences in penetrance and outcome in EAE disease has been reported for other global knockouts and can be due to different induction protocols, genetics and the host microbiota. In particular the microbiota has been shown to influence EAE incidence and severity (Lee et al. 2011, Berer et al. 2011). For this reason, we cohoused both the control and Mpo−/− mice with mice from the Dittel colony prior to EAE induction to equilibrate their microbiomes. In support of this EAE induction protocol is our studies in Il10−/− mice where we found that their T-independent immune responses were altered in a microbiome-dependent manner due to a significant increase in marginal zone B cells (Ray et al. 2015). We also found that Il10−/− mice from our colony harbored a different microbiome as compared to similar mice purchased directly from The Jackson Laboratory (Jax) and upon cohousing with mice from the Dittel colony their microbiomes shifted to that of the home colony (Ray et al. 2015). From these data and other studies in the literature demonstrating that immunodeficiencies can alter the penetrance of disease in a putatively microbiome-dependent manner (Ray et al. 2015, Ray & Dittel 2015), it is probable that since MPO is required for controlling certain bacterial and fungal infections, its absence could lead to a change in the composition of the microbiota altering EAE severity (Aratani et al. 2000). Given that the gut microbiome is now thought to modulate MS pathogenesis examining the impact of MPO-deficiency on the composition of the gut microbiota and EAE disease penetrance is of particular interest (Mielcarz & Kasper 2015).
Since it is well accepted that EAE pathogenesis is driven by CD4 Th1 and Th17 encephalitogenic T cells, we determined whether KYC administration at the peak of EAE would reduce their cell numbers in the CNS. Surprisingly, even though EAE disease severity was significantly attenuated after five days (Fig. 3A), KYC did not lead to a reduction in CD4 T cells producing IFN-γ or IL-17 (Fig. 3C). We also did not observe an increase in Treg in the KYC cohort, indicating that they likely do not play a role in EAE attenuation following MPO inhibition (Fig. 3D). Our observation that the loss of both macrophages and neutrophils (Fig. 3F) following KYC administration coincided with a significant reduction in MPO suggests that both cell types via MPO production contribute to EAE pathogenesis. This conclusion is consistent with a known role for macrophages in EAE pathogenesis (Bauer et al. 1995). In addition, a number of studies have also demonstrated an important role for neutrophils in EAE (McColl et al. 1998, Carlson et al. 2008, Steinbach et al. 2013, Christy et al. 2013, Aube et al. 2014, Miller et al. 2015). In MS, microglial cells/macrophages are prominent in MS lesions, while neutrophils are largely absent in established lesions. This is likely due to their short half-life in tissues and their inefficient migration across an intact BBB in the absence of an activated endothelium (Gorina et al. 2014). However, recently postmortem tissue from an MS patient affected by relapse following cessation of natalizumab therapy showed a prominent neutrophil presence in areas of BBB leakage (Aube et al. 2014). In addition, increased numbers of primed neutrophils have been reported in the peripheral blood of MS patients (Naegele et al. 2012, Ziaber et al. 1998).
MPO is multifunctional, thus its precise mechanism of action in EAE/MS is not completely understood. In this regard, since MPO can bind to and activate neutrophils (Lau et al. 2005, Metzler et al. 2011) the net result of limiting neutrophil activation by KYC would be reduced MPO production. In addition, it was recently shown that MPO promoted the accumulation of neutrophils in the CNS in spinal cord injury (Kubota et al. 2012). MPO has also been shown to delay the apoptosis of neutrophils through the Mac-1 integrin (CD11b/CD18) (El Kebir et al. 2008), which is also highly expressed in macrophages. Thus KYC could reduce the numbers of neutrophils and perhaps macrophages by inhibiting MPO and thereby triggering their apoptosis. One mechanism whereby MPO can facilitate the migration of myeloid cells into the CNS is by breakdown of the BBB, which is associated with both EAE and MS (Ullen et al. 2013, Alvarez et al. 2011). In this regard, neutrophils alter vascular permeability by facilitating the breakdown of the BBB (Christy et al. 2013). Interestingly, we found that only five days of KYC treatment was sufficient to completely seal the BBB (Fig. 4B, C). The inhibition of MPO and restoration of BBB integrity would in turn function to limit macrophage and neutrophil recruitment into the CNS and the death of the remaining short-lived myeloid cells would further reduce MPO levels. The longer-lived T cells would not be reduced in the five-day treatment window. In addition, since T cells do not express Mac-1, it is unlikely that KYC inhibition of MPO would alter their apoptosis by a Mac-1 mechanism.
MPO induces tissue damage by generating toxic oxidants/free radicals (Klebanoff 2005, van der Veen et al. 2009) and previous studies have shown that MPO binds to the subendothelial matrix via endothelial transcytosis (Rees et al. 2010, Tiruppathi et al. 2004). Once bound, MPO was shown to increase oxidative stress resulting in endothelial and subendothelial matrix damage (Baldus et al. 2001, Rees et al. 2010), which could be a contributing factor in BBB leakage (Ullen et al. 2013, Alvarez et al. 2011). The mechanism whereby MPO induces BBB breakdown is not clear, but a recent study in the LPS sepsis model using both in vivo and in vitro approaches reported that BBB disruption was mediated by MPO-derived oxidant chlorinated aldehydes via activation of the MAPK pathway (Ullen et al. 2013). The above findings are consistent with our studies demonstrating that KYC inhibits the formation of HOCl (Zhang et al. 2013a) and reduces BBB permeability (Fig. 4B, C). In addition, the Ullen, et al., study demonstrated that Mpo−/− mice exhibited attenuated BBB permeability in response to LPS challenge (Ullen et al. 2013). This is consistent with our studies demonstrating that Mpo−/− mice have reduced EAE severity as compared to controls (Fig. 2C). In addition, our data demonstrating the inability of KYC to further reduce EAE severity in Mpo−/− mice (Fig. 2C), suggests that KYC functions via inhibition of MPO without off-target properties. Findings here are consistent with a growing list of disease states where KYC reduced the severity of disease. In addition to our studies in sickle cell mice (Zhang et al. 2013b), KYC also decreased MPO activity and neutrophil-dependent solid tumor formation in a murine model of lung cancer (Rymaszewski et al. 2014).
Increased MPO levels are associated with MS (Nagra et al. 1997, Gray et al. 2008b, Gray et al. 2008a), which is consistent with a disrupted BBB being a prominent feature of MS. In addition breakdown of the BBB is used for diagnosis and monitoring of patients (Minagar & Alexander 2003, Charil et al. 2006). In MS, immune cell infiltration due to a breach in the BBB is thought to be a major driver of disease pathology (Minagar & Alexander 2003). Thus KYC-mediated restoration of the BBB would be beneficial for the clinical management of MS during relapses. Taken together, our data clearly demonstrate the therapeutic effect of KYC in ameliorating CNS autoimmunity by limiting the number of MPO producing myeloid cells in the CNS via restoration of BBB integrity and thus highlights the potential of this novel MPO inhibitor as a therapeutic agent for treating MS.
Acknowledgements
The authors would like to thank Sreemanti Basu, Shelley Morris and Shelby Dukes for technical support. This work was supported by NIH grants R01 AI069358, R56 AI106672 and the BloodCenter Research Foundation (BD); National MS Society grant RG 4975A1/2 (HZ, BD) and American Heart Association grant 11SDG5120015 (HZ); and NIH grant R01 HL102836 and R01 HL112270 and a generous gift from Ms. Poblocki of Elm Grove, WI (KAP).
Abbreviations
- BBB
blood-brain-barrier
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- KYC
N-acetyl lysyltyrosylcysteine amide
- MS
multiple sclerosis
- MBP
myelin basic protein
- MOG
myelin oligodendrocyte glycoprotein
- MPO
myeloperoxidase
Footnotes
Disclosures
The authors declare no scientific conflict of interests.
References
- Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Suzuki K, Maeda N, Koyama H. Differential host susceptibility to pulmonary infections with bacteria and fungi in mice deficient in myeloperoxidase. J Infect Dis. 2000;182:1276–1279. doi: 10.1086/315843. [DOI] [PubMed] [Google Scholar]
- Aube B, Levesque SA, Pare A, et al. Neutrophils mediate blood-spinal cord barrier disruption in demyelinating neuroinflammatory diseases. J Immunol. 2014;193:2438–2454. doi: 10.4049/jimmunol.1400401. [DOI] [PubMed] [Google Scholar]
- Baldus S, Eiserich JP, Mani A, et al. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Invest. 2001;108:1759–1770. doi: 10.1172/JCI12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Huitinga I, Zhao W, Lassmann H, Hickey WF, Dijkstra CD. The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia. 1995;15:437–446. doi: 10.1002/glia.440150407. [DOI] [PubMed] [Google Scholar]
- Bekesi G, Heinle H, Kakucs R, et al. Effect of inhibitors of myeloperoxidase on the development of aortic atherosclerosis in an animal model. Exp Gerontol. 2005;40:199–208. doi: 10.1016/j.exger.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- Bizzozero OA, DeJesus G, Callahan K, Pastuszyn A. Elevated protein carbonylation in the brain white matter and gray matter of patients with multiple sclerosis. J Neurosci Res. 2005;81:687–695. doi: 10.1002/jnr.20587. [DOI] [PubMed] [Google Scholar]
- Brennan M, Gaur A, Pahuja A, Lusis AJ, Reynolds WF. Mice lacking myeloperoxidase are more susceptible to experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001;112:97–105. doi: 10.1016/s0165-5728(00)00392-1. [DOI] [PubMed] [Google Scholar]
- Burner U, Obinger C, Paumann M, Furtmuller PG, Kettle AJ. Transient and steady-state kinetics of the oxidation of substituted benzoic acid hydrazides by myeloperoxidase. J Biol Chem. 1999;274:9494–9502. doi: 10.1074/jbc.274.14.9494. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Ravagna A, Bella R, Foresti R, Bates TE, Giuffrida Stella AM, Pennisi G. Nitric oxide synthase is present in the cerebrospinal fluid of patients with active multiple sclerosis and is associated with increases in cerebrospinal fluid protein nitrotyrosine and S-nitrosothiols and with changes in glutathione levels. J Neurosci Res. 2002;70:580–587. doi: 10.1002/jnr.10408. [DOI] [PubMed] [Google Scholar]
- Carlson T, Kroenke M, Rao P, Lane TE, Segal B. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp Med. 2008;205:811–823. doi: 10.1084/jem.20072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Borrero W, Graves D, Frohman TC, Flores AB, Hardeman P, Logan D, Orchard M, Greenberg B, Frohman EM. Current and emerging therapies in multiple sclerosis: a systematic review. Ther Adv Neurol Disord. 2012;5:205–220. doi: 10.1177/1756285612450936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charil A, Yousry TA, Rovaris M, et al. MRI and the diagnosis of multiple sclerosis: expanding the concept of “no better explanation”. Lance. Neurol. 2006;5:841–852. doi: 10.1016/S1474-4422(06)70572-5. [DOI] [PubMed] [Google Scholar]
- Christy AL, Walker ME, Hessner MJ, Brown MA. Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE. J Autoimmun. 2013;42:50–61. doi: 10.1016/j.jaut.2012.11.003. [DOI] [PubMed] [Google Scholar]
- de Vries HE, Kuiper J, de Boer AG, Van Berkel TJ, Breimer DD. The blood-brain barrier in neuroinflammatory diseases. Pharmacol Rev. 1997;49:143–155. [PubMed] [Google Scholar]
- Dittel BN, Merchant RM, Janeway CA., Jr Evidence for Fas-dependent and Fas-independent mechanisms in the pathogenesis of experimental autoimmune encephalomyelitis. J Immunol. 1999;162:6392–6400. [PubMed] [Google Scholar]
- El Kebir D, Jozsef L, Pan W, Filep JG. Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circ Res. 2008;103:352–359. doi: 10.1161/01.RES.0000326772.76822.7a. [DOI] [PubMed] [Google Scholar]
- Ferretti G, Bacchetti T, Principi F, Di Ludovico F, Viti B, Angeleri VA, Danni M, Provinciali L. Increased levels of lipid hydroperoxides in plasma of patients with multiple sclerosis: a relationship with paraoxonase activity. Mult Scler. 2005;11:677–682. doi: 10.1191/1352458505ms1240oa. [DOI] [PubMed] [Google Scholar]
- Forghani R, Wojtkiewicz GR, Zhang Y, et al. Demyelinating diseases: myeloperoxidase as an imaging biomarker and therapeutic target. Radiology. 2012;263:451–460. doi: 10.1148/radiol.12111593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsette RE. Neurodegeneration in multiple sclerosis: the role of oxidative stress and excitotoxicity. J Neurol Sci. 2008;274:48–53. doi: 10.1016/j.jns.2008.06.029. [DOI] [PubMed] [Google Scholar]
- Gorina R, Lyck R, Vestweber D, Engelhardt B. beta2 integrin-mediated crawling on endothelial ICAM-1 and ICAM-2 is a prerequisite for transcellular neutrophil diapedesis across the inflamed blood-brain barrier. J Immunol. 2014;192:324–337. doi: 10.4049/jimmunol.1300858. [DOI] [PubMed] [Google Scholar]
- Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest. 2002;109:383–392. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray E, Thomas TL, Betmouni S, Scolding N, Love S. Elevated activity and microglial expression of myeloperoxidase in demyelinated cerebral cortex in multiple sclerosis. Brain Pathol. 2008a;18:86–95. doi: 10.1111/j.1750-3639.2007.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray E, Thomas TL, Betmouni S, Scolding N, Love S. Elevated myeloperoxidase activity in white matter in multiple sclerosis. Neurosci Lett. 2008b;444:195–198. doi: 10.1016/j.neulet.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Haider L, Fischer MT, Frischer JM, et al. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C, Antel J, Bruck W, Kuhlmann T. Contrasting potential of nitric oxide and peroxynitrite to mediate oligodendrocyte injury in multiple sclerosis. Glia. 2007;55:926–934. doi: 10.1002/glia.20514. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- Koelsch M, Mallak R, Graham GG, et al. Acetaminophen (paracetamol) inhibits myeloperoxidase-catalyzed oxidant production and biological damage at therapeutically achievable concentrations. Biochem Pharmacol. 2010;79:1156–1164. doi: 10.1016/j.bcp.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Kubota K, Saiwai H, Kumamaru H, Maeda T, Ohkawa Y, Aratani Y, Nagano T, Iwamoto Y, Okada S. Myeloperoxidase exacerbates secondary injury by generating highly reactive oxygen species and mediating neutrophil recruitment in experimental spinal cord injury. Spine. 2012;37:1363–1369. doi: 10.1097/BRS.0b013e31824b9e77. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Axonal and neuronal pathology in multiple sclerosis: what have we learnt from animal models. Exp Neurol. 2010;225:2–8. doi: 10.1016/j.expneurol.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Lau D, Mollnau H, Eiserich JP, et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc Natl Acad Sci U S A. 2005;102:431–436. doi: 10.1073/pnas.0405193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl SR, Staykova MA, Wozniak A, Fordham S, Bruce J, Willenborg DO. Treatment with anti-granulocyte antibodies inhibits the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 1998;161:6421–6426. [PubMed] [Google Scholar]
- McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, Wahn V, Papayannopoulos V, Zychlinsky A. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielcarz DW, Kasper LH. The gut microbiome in multiple sclerosis. Curr Treat Options Neurol. 2015;17:344. doi: 10.1007/s11940-015-0344-7. [DOI] [PubMed] [Google Scholar]
- Miljkovic D, Spasojevic I. Multiple Sclerosis: Molecular Mechanisms and Therapeutic Opportunities. Antioxid Redox Signal. 2013;19:2286–2334. doi: 10.1089/ars.2012.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E, Mrowicka M, Saluk-Juszczak J, Ireneusz M. The level of isoprostanes as a non-invasive marker for in vivo lipid peroxidation in secondary progressive multiple sclerosis. Neurochem Res. 2011;36:1012–1016. doi: 10.1007/s11064-011-0442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NM, Shriver LP, Bodiga VL, Ray A, Basu S, Ahuja R, Jana A, Pahan K, Dittel BN. Lymphocytes with cytotoxic activity induce rapid microtubule axonal destabilization independently and before signs of neuronal death. ASN NEURO. 2013;5:e00105. doi: 10.1042/AN20120087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NM, Wang J, Tan Y, Dittel BN. Anti-inflammatory mechanisms of IFN-γ studied in experimental autoimmune encephalomyelitis reveal neutrophils as a potential target in multiple sclerosis. Front Neurosci. 2015;9:287. doi: 10.3389/fnins.2015.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–549. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- Minohara M, Matsuoka T, Li W, Osoegawa M, Ishizu T, Ohyagi Y, Kira J. Upregulation of myeloperoxidase in patients with opticospinal multiple sclerosis: positive correlation with disease severity. J Neuroimmunol. 2006;178:156–160. doi: 10.1016/j.jneuroim.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Naegele M, Tillack K, Reinhardt S, Schippling S, Martin R, Sospedra M. Neutrophils in multiple sclerosis are characterized by a primed phenotype. J Neuroimmunol. 2012;242:60–71. doi: 10.1016/j.jneuroim.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Nagra RM, Becher B, Tourtellotte WW, Antel JP, Gold D, Paladino T, Smith RA, Nelson JR, Reynolds WF. Immunohistochemical and genetic evidence of myeloperoxidase involvement in multiple sclerosis. J Neuroimmunol. 1997;78:97–107. doi: 10.1016/s0165-5728(97)00089-1. [DOI] [PubMed] [Google Scholar]
- Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest. 2012;122:1180–1188. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- Pulli B, Bure L, Wojtkiewicz GR, et al. Multiple Sclerosis: Myeloperoxidase Immunoradiology Improves Detection of Acute and Chronic Disease in Experimental Model. Radiology. 2014:141495. doi: 10.1148/radiol.14141495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Goswami R, Balabanov R, Dawson G. Oxidized phosphatidylcholine is a marker for neuroinflammation in multiple sclerosis brain. J Neurosci Res. 2007;85:977–984. doi: 10.1002/jnr.21206. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Basu S, Miller NM, Chan AM, Dittel BN. An Increase in Tolerogenic Dendritic Cell and Natural Regulatory T Cell Numbers during Experimental Autoimmune Encephalomyelitis in Rras−/− Mice Results in Attenuated Disease. J Immunol. 2014;192:5109–5117. doi: 10.4049/jimmunol.1302254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol. 2012;188:3188–3198. doi: 10.4049/jimmunol.1103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Basu S, Gharaibeh RZ, et al. Gut Microbial Dysbiosis Due to Helicobacter Drives an Increase in Marginal Zone B Cells in the Absence of IL-10 Signaling in Macrophages. J Immunol. 2015;195:3071–3085. doi: 10.4049/jimmunol.1500153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Dittel BN. Interrelatedness between dysbiosis in the gut microbiota due to immunodeficiency and disease penetrance of colitis. Immunol. 2015;146:359–368. doi: 10.1111/imm.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees MD, Whitelock JM, Malle E, Chuang CY, Iozzo RV, Nilasaroya A, Davies MJ. Myeloperoxidase-derived oxidants selectively disrupt the protein core of the heparan sulfate proteoglycan perlecan. Matrix Biol. 2010;29:63–73. doi: 10.1016/j.matbio.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S, Misharin A, Perlman H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry A. 2012;81:343–350. doi: 10.1002/cyto.a.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymaszewski AL, Tate E, Yimbesalu JP, Gelman AE, Jarzembowski JA, Zhang H, Pritchard KA, Jr, Vikis HG. The role of neutrophil myeloperoxidase in models of lung tumor development. Cancers. 2014;6:1111–1127. doi: 10.3390/cancers6021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajad M, Zargan J, Chawla R, Umar S, Sadaqat M, Khan HA. Hippocampal neurodegeneration in experimental autoimmune encephalomyelitis (EAE): potential role of inflammation activated myeloperoxidase. Mol Cell Biochem. 2009;328:183–188. doi: 10.1007/s11010-009-0088-3. [DOI] [PubMed] [Google Scholar]
- Shriver LP, Dittel BN. T-cell-mediated disruption of the neuronal microtubule network: correlation with early reversible axonal dysfunction in acute experimental autoimmune encephalomyelitis. Am J Pathol. 2006;169:999–1011. doi: 10.2353/ajpath.2006.050791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerjac SM, Bizzozero OA. Cytoskeletal protein carbonylation and degradation in experimental autoimmune encephalomyelitis. J Neurochem. 2008;105:763–772. doi: 10.1111/j.1471-4159.2007.05178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach K, Piedavent M, Bauer S, Neumann JT, Friese MA. Neutrophils amplify autoimmune central nervous system infiltrates by maturing local APCs. J Immunol. 2013;191:4531–4539. doi: 10.4049/jimmunol.1202613. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Naqvi T, Wu Y, Vogel SM, Minshall RD, Malik AB. Albumin mediates the transcytosis of myeloperoxidase by means of caveolae in endothelial cells. Proc Natl Acad Sci U S A. 2004;101:7699–7704. doi: 10.1073/pnas.0401712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Ullen A, Singewald E, Konya V, et al. Myeloperoxidase-derived oxidants induce blood-brain barrier dysfunction in vitro and in vivo. PLoS One. 2013;8:e64034. doi: 10.1371/journal.pone.0064034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal. 2009;11:2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- van Horssen J, Schreibelt G, Drexhage J, Hazes T, Dijkstra CD, van der Valk P, de Vries HE. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Rad Biol Med. 2008;45:1729–1737. doi: 10.1016/j.freeradbiomed.2008.09.023. [DOI] [PubMed] [Google Scholar]
- van Horssen J, Witte ME, Schreibelt G, de Vries HE. Radical changes in multiple sclerosis pathogenesis. Biochim Biophys Acta. 2011;1812:141–150. doi: 10.1016/j.bbadis.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Vladimirova O, O’Connor J, Cahill A, Alder H, Butunoi C, Kalman B. Oxidative damage to DNA in plaques of MS brains. Mult Scler. 1998;4:413–418. doi: 10.1177/135245859800400503. [DOI] [PubMed] [Google Scholar]
- Zeis T, Probst A, Steck AJ, Stadelmann C, Bruck W, Schaeren-Wiemers N. Molecular changes in white matter adjacent to an active demyelinating lesion in early multiple sclerosis. Brain Pathol. 2009;19:459–466. doi: 10.1111/j.1750-3639.2008.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Jing X, Shi Y, et al. N-acetyl lysyltyrosylcysteine amide inhibits myeloperoxidase, a novel tripeptide inhibitor. J Lipid Res. 2013a;54:3016–3029. doi: 10.1194/jlr.M038273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xu H, Weihrauch D, et al. Inhibition of myeloperoxidase decreases vascular oxidative stress and increases vasodilatation in sickle cell disease mice. J Lipid Res. 2013b;54:3009–3015. doi: 10.1194/jlr.M038281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Bizzozero OA. Accumulation of protein carbonyls within cerebellar astrocytes in murine experimental autoimmune encephalomyelitis. J Neurosci Res. 2010;88:3376–3385. doi: 10.1002/jnr.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaber J, Pasnik J, Baj Z, Pokoca L, Chmielewski H, Tchorzewski H. The immunoregulatory abilities of polymorphonuclear neutrophils in the course of multiple sclerosis. Mediators Inflamm. 1998;7:335–338. doi: 10.1080/09629359890857. [DOI] [PMC free article] [PubMed] [Google Scholar]